Abstract
Over the past two decades it has become clear that the glomerular podocyte is a key cell in preventing albuminuria, kidney failure and cardiovascular morbidity. Understanding the key pathways that protect the podocyte in times of glomerular stress, which can also be therapeutically manipulated, are highly attractive. In the following review we assess the evidence that the peroxisome proliferator activating receptor (PPAR) agonists are beneficial for podocyte and kidney function with a focus on PPAR-γ. We explain our current understanding of the mechanisms of action of these agonists and the evidence they are beneficial in diabetic and non-diabetic kidney disease. We also outline why these drugs have not been widely used for kidney disease in the past but they may be in the future.
Keywords: kidney disease, podocyte, PPAR-γ, PPAR-α, thiazolidinediones
INTRODUCTION
Podocytes are highly specialized, terminally differentiated cells that form a major constituent of the kidney's glomerular filtration barrier (GFB). Their interdigitating foot processes and slit diaphragm are two key components that allow for this specialized functioning. Damage to the podocyte is a common pathological event in many glomerular diseases [1]. Research efforts have focused on finding protective pathways in this cell to exploit, and therapeutic agents such as the peroxisome proliferator activating receptor (PPAR) agonists have shown great promise. We will describe the molecular mechanisms of the thiazolidinedione (TZD)–PPAR interaction, with particular focus on PPAR-γ (the best characterized isoform within this group of nuclear hormone receptors). Through this we will explain why manipulation of these pathways may enhance podocyte and kidney health and be therapeutically attractive.
THE PPARs
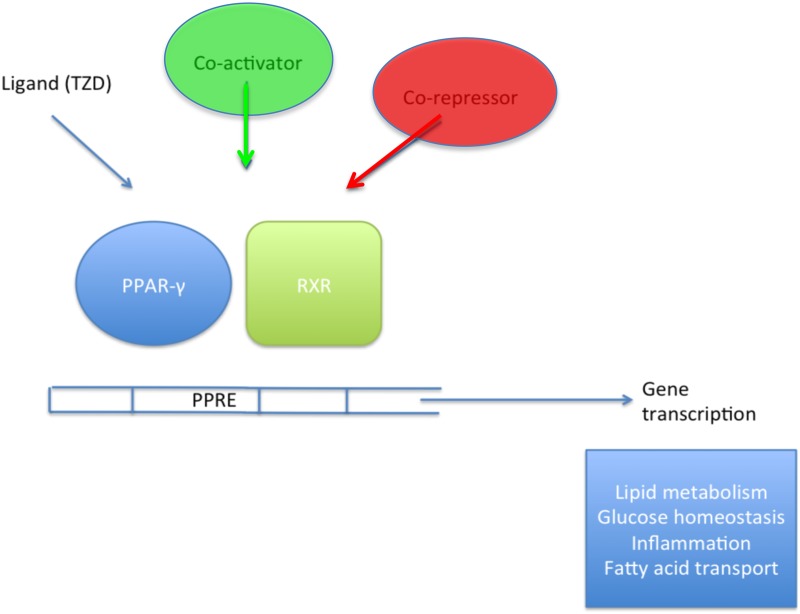
The PPARs are a group of nuclear hormone receptors and ligand-activated transcription factors. They work through heterodimerization with the retinoid x receptor (RXR) to activate gene cassettes involved in a wide variety of tissue-specific cellular processes [2, 3] (Table 1). Ligands (synthetic and endogenous) bind to a specific domain within the PPAR (this is the case for all subtypes) and facilitate a conformational change that allows the recruitment of cofactors that, in turn modulate PPAR activity and effect gene transcription (Figure 1) [12].
Table 1.
| Isoform | Expression profile | Chromosomal location | Synthetics ligands | Natural ligands | Renal distribution |
|---|---|---|---|---|---|
| PPAR-α | Adipose | C22 (human) | Fibrates: clofibrate, benzafibrate | Fatty acids | Proximal tubules |
| Liver | |||||
| Heart | C15 (mouse) | TZDs: KRP-297 | Eicosanoid derivatives | Medullary thick ascending limb | |
| Muscle | |||||
| Renal cortex | NSAIDs (partial agonists): ibuprofen, indomethacin | Podocytes (mouse and human) | |||
| Lung | |||||
| Placenta | |||||
| Intestine | Phenylacetic acid derivatives (potent agonists) | ||||
| Pancreas | |||||
| Skeletal muscle | |||||
| PPAR-β/δ | Ubiquitous | C6 (human) | GW-501516 | Fatty acids | Ubiquitous |
| C17 (mouse) | Phenylacetic acid derivatives (potent agonist) | Eicosanoid derivatives | |||
| PPAR-γ | Adiposea | C3 (human) | TZDs | Fatty acids | Glomeruli |
| Renal medulla | Medullary collecting duct | ||||
| Bladder | C6 (mouse) | −Rosiglitzoneb | Eicosanoid derivatives | Pelvic urothelium | |
| Skeletal muscle | − Ciglitazone | PUFAs in particular: | Mesangial cells | ||
| Liver | − Troglitazone | − Arachidonic acid | Podocytes (mouse and human) | ||
| Heart | − Englitazone | − Linoleic acid | |||
| Macrophages | − Linolenic acid | ||||
| Vascular smooth muscle | NSAIDs (partial agonist): | Xenobiotics | |||
| Malignant epithelial cells | − Ibuprofen | ||||
| Endothelium | − Indomethacin | ||||
| Bone marrow | Phenylacetic acid derivatives (potent agonist) | ||||
| GW2570 (potent)c |
aAdipose is the predominant site of PPAR-γ expression and Rosiglitazone has high affinity for the PPAR-γ receptor.
bBinding with high affinity, the rest do not bind so strongly.
cPPAR-γ agonist with anti-diabetic activity in humans
FIGURE 1.
Inside the nucleus, a simplified illustration of the mechanism through which PPARs activate gene transcription using PPAR-γ as an example. On ligand binding, the protein undergoes a conformational change that allows binding of transcriptional co-activators. In the absence of a ligand, the receptor remains bound to transcriptional co-repressors, resulting in target genes being silenced.
The first PPAR to be cloned (from rat hepatocytes) was PPAR-α. Three further subtypes (β, δ and γ) of the receptor were subsequently cloned from frog (Xenopus) cDNA [13]. They each have different tissue expression profiles within the body, however, the kidney expresses all four [14] (Table 1). All PPARs have been detected in rodent and human podocytes [15].
Specific mutations in each of the PPARs have been detected that cause a variety of human phenotypes as illustrated in Table 2.
Table 2.
Genotype–phenotype correlations with some specific PPAR mutations illustrating the importance of these transcription factors in a variety of contexts [16]
| Mutations in humans | Specific examples | Phenotype |
|---|---|---|
| PPAR-α | LEU162VAL | Susceptibility to hyperapobetalipoproteinaemia |
| PPAR-β/δ | SNPs (rs1053049) | |
| GLY482SER | Insulin resistance | |
| Polymorphism 87T/C | Altered cholesterol metabolism | |
| PPAR-γ | P115Q | Obesity |
| 1-BP deletion 472A | ||
| GLN286PRO | Colon cancer | |
| PRO467LEU | Partial familial lipodystrophy type 3 | |
| 3-BP deletion/1-BP insertion NT553 | Insulin resistance | |
| Polymorphism PRO12ALA (13% of Caucasians) | Improved insulin sensitivity + lower BMI |
PRO467L, proline to leucine mutation; BP, base pair.
Data from Online Mendelian Inheritance in Man and Atlas of Genetics and Cytogenetics by Astarci and Banerjee.
PPAR-γ
The PPAR-γ gene is located on chromosome 3 in humans (locus 3p25.2) and extends over 100 kb with nine exons that give rise to three transcripts (γ1, γ2 and γ3). Alternate transcription start sites and splicing generates the three transcripts [4]. Six exons are common to all three transcripts. The γ1 and γ3 transcripts give rise to the same protein because their additional exons located at the 5′ terminal (A1 and A2 for γ1 and A2 for γ3) remain untranslated. γ3 mRNA expression is limited to white adipose tissue in humans and its functional significance is uncertain.
The PPAR-γ protein, located in the cellular nucleus, contains 505 amino acids and has a molecular weight of 57.6 kDa. There are four domains within the protein: A/B (involved in transcriptional regulation), C (DNA binding), D (hinge region) and E/F (ligand binding). Two of these domains are highly conserved: one for DNA binding (DBD, made up of two zinc finger motifs and located at the N-terminal end of the protein; this allows specific interaction with the PPAR regulatory element common to all PPAR-responsive genes), and one for ligand binding (LBD, a structure containing α helices and β sheets, located at the C-terminus, that allows binding of natural and synthetic ligands to the PPAR). Activation of the receptor can be liganddependent [via the activation function 2 (AF2) domain at the C-terminus] or ligandindependent (via the AF1 domain at the N-terminus).
PPAR-γ2 has an additional 28 amino acids at its N-terminus, which enhances its ability for ligand-independent activation properties when compared with the γ1 isoform.
From transcription through to functioning protein, PPAR-γ is subjected to a number of complex processes and interactions. At the transcriptional level, these include epigenetic modifications such as promoter region methylation and histone acetylation. More recently, microRNAs have been shown to exert control over the stability and translation of PPAR mRNA [17, 18]. miR-128 has been identified as having direct interaction with PPAR-γ [19, 20]; for example, in a recent study of ischaemia–reperfusion (IR) injury in cardiac myocytes, the activation of PPAR-γ expression was increased by miR-128 inhibition and conversely, the reduction in apoptosis induced by miR-128 inhibition in IR-injured cells was blocked by the specific PPAR-γ inhibitor GW9662 [21].
Post-translational modifications are well described and include phosphorylation, acetylation, ubiquitination and sumoylation [12]. Phosphorylation of PPAR-γ can inhibit or increase its transcriptional activity depending on where that modification occurs. As an example, phosphorylation of serine 112 by MAP kinase at the N-terminus of the γ2 isoform or the corresponding serine 82 positioned at the N-terminus of the γ1 isoform reduces the transcriptional activity of PPAR-γ [5, 6, 22]. Conversely, phosphorylation at the same serine 112 by the cyclin-dependent kinase family members 5 and 9 (CDK5/9) increases PPAR-γ transcriptional activity [12].
The expression of PPAR-γ is regulated by a number of factors, including insulin, glucocorticoids and tumour necrosis factor a (TNF-α) in adipose tissue, with transfection studies in HepG2 cells, for example, showing insulin producing an almost 2-fold increase in the protein's transcriptional activity through mechanisms involving mitogen-activated protein kinase (MAPK) phosphorylation [5]. Insulin has also been shown to have a synergistic effect with ligand-dependent activation of PPAR-γ on the expression of aP2, a recognized target gene of PPAR-γ [23].
PPAR-γ1 is the predominant isoform in humans. Under normal physiological conditions, the γ2 isoform is limited to expression in adipose, but it can be induced in other tissues on exposure to a high-fat diet [12, 24]. The glomerulus is reported to be one of the major sites of PPAR-γ action within the kidney [14]. Quantitative mass spectrometry–based proteomics experiments on freshly isolated murine podocytes have previously identified a specific overrepresentation of both γ and α isoforms of the receptor [25], lending support to their relevance in this cell.
PPAR-γ is best known for its abilities to regulate pathways linked to adipocyte differentiation and metabolism [12]. However, it is now known that it is also important for podocyte function through the use of in vitro and cell-specific transgenic knockout (KO) models [26, 27]. This has provided an opportunity for research to focus on targeted therapy for disease involving GFB disruption.
PPAR-γ ACTION THROUGHOUT THE BODY
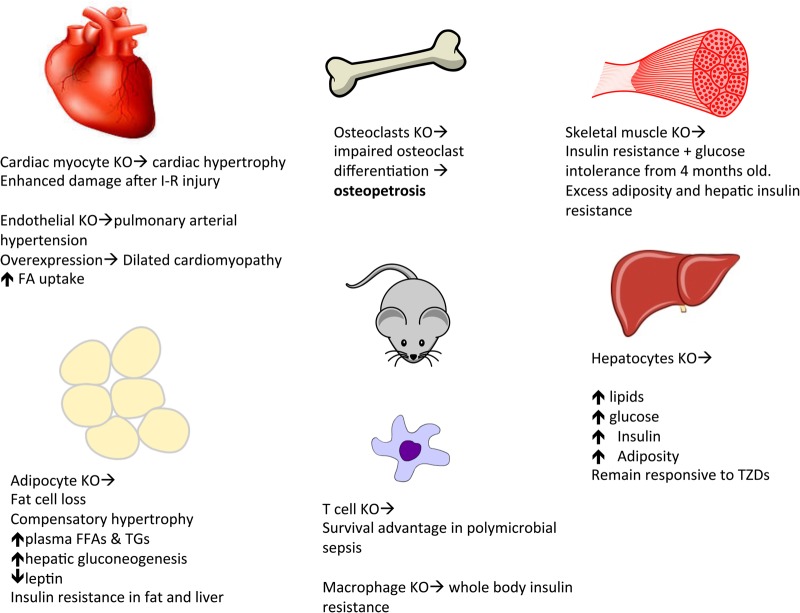
PPAR-γ null mice die in utero due to placental defects (specifically due to the failure of trophoblastic differentiation) [28]; however, a whole body KO that can be rescued from embryonic lethality through genetic modification allowing PPAR-γ expression to be maintained only in trophoblastic cells [36] has been reported, showing severe lipodystrophy, insulin resistance and hypotension. Through the generation of cell specific transgenic mouse models it has become clear that PPAR gamma has important actions throughout the body (Figure 2).
FIGURE 2.
Tissue-specific knockout (KO) of PPAR-γ: skeletal muscle [29], hepatic [30], cardiac I-R (ischaemia–reperfusion injury) [31]; adipose [32]. Osteoclasts: Tie2Cre/flox mouse model (specific PPAR-γ gene deletion) [27], endothelial cells [33], T cells [34]. In the liver of the ob/ob mouse (genetically predestined to obesity) and the lipoatrophic mouse (AZIP) examples, KO of PPAR-γ has been shown to remedy the associated hepatic steatosis [35] but at the same time worsen the triglyceride clearance and total body insulin resistance.
The murine PPAR-γ heterozygote is protected from the hepatic steatosis induced by a high-fat diet and is also more insulin sensitive (an unexpected consequence of a functional decrease in gene expression, when considering that agonists of PPAR-γ enhance insulin sensitivity) [37, 38] and less susceptible to the colon cancer carcinogen azoxymethane (through mechanisms involving the suppression of β catenin and altered regulation of the β catenin/adenomatous polyposis coli pathway [39]). Heterozygosity for PPAR-γ has also been shown to have a protective effect in diabetic nephropathy [40].
In humans, loss-of-function/dominant-negative mutations in PPAR-γ cause partial lipodystrophy, insulin resistance and hypertension [41, 42].
PPAR-γ IN THE KIDNEY
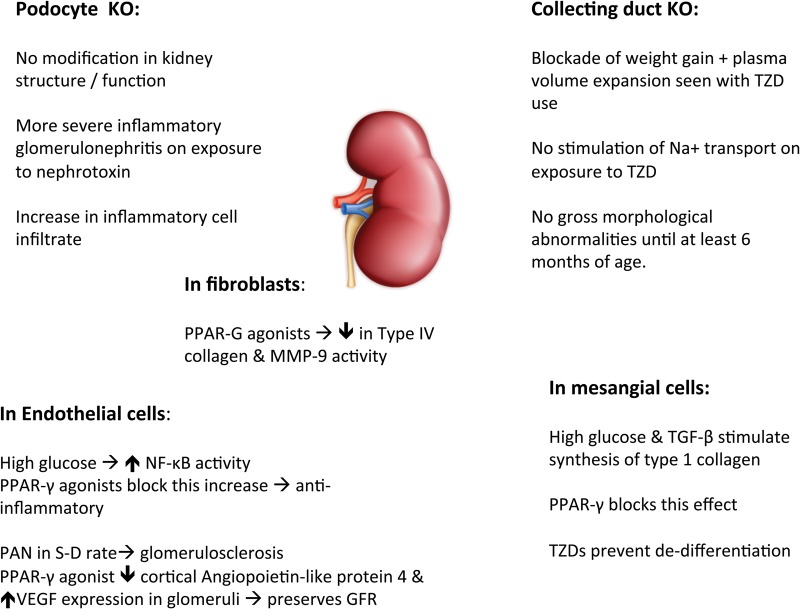
Using the Cre-lox system for investigating the effects of specific cell knock-down has provided insight into the role of PPAR-γ within the kidney (Figure 3).
FIGURE 3.
Collecting duct knockout → blockade of the body weight gain and plasma volume expansion associated with TZD use highlighting the importance of PPAR-γ-regulated fluid resorption in the distal nephron. These KO mice had no morphological abnormality by 6 months of age [43, 44]. Mesangial cells [45], endothelial cells [46] and pioglitazone reduces progression of glomerulosclerosis in Sprague–Dawley rats treated with PAN [10], fibroblasts [47] and PPAR-γ.
PPAR-γ IN THE PODOCYTE
Of particular interest is recent research suggesting a protective role for podocyte PPAR-γ in inflammatory crescentic glomerulonephritis [48]. In this study, the podocyte-specific PPAR-γ-deficient mice [generated by crossing the podocin-Cre mouse (which expresses Cre-recombinase exclusively in podocytes) with the B6.129S6-Ppargtm1.1Mgn/Mmmh strain on a C57BL6/J background] given nephrotoxin developed a more severe glomerulonephritis with a 2- to 3-fold increase in crescent formation compared with wild-type controls (littermates with no deletion of PPAR-γ alleles in any cells). This was associated with significantly accentuated periglomerular infiltration of T cells and macrophages and a 30% increase in mRNA expression of monocyte chemoattractant protein-1 and interleukin 6 (inflammatory cytokines) in the renal cortex. TZD treatment was less effective at alleviating the nephritis in the podocyte PPAR-γ KOs, showing that many of its effects are through this receptor.
In the same study, kidney biopsy specimens from patients with rapidly progressive glomerulonephritis were analysed, showing PPAR-γ to be absent from the nuclei of the cells forming crescents but present in normal glomerular cells.
Mechanistically, the induction of PPAR-γ by the nuclear factor erythroid 2-related factor (NRF2) has been described. The renal phenotypes of the podocyte-specific PPAR-γ KO and NRF2 KO are similar after exposure to a nephrotoxic insult. PPAR-γ in this study was shown to have reduced expression within the glomeruli of the NRF2 KO. In these mice, TZDs were able to alleviate the effects of the nephrotoxin-induced glomerulonephritis. In this model of acute inflammatory crescentic nephritis, TZDs work principally through PPAR-γ (in the podocyte-specific KO, the TZDs had little effect) and therefore the NRF2–PPAR-γ pathway appears to play an important role in the prevention of oxidant-induced glomerular injury.
ACTIVATING PPAR-γ THROUGH NATURAL : AND SYNTHETIC LIGANDS
Synthetic agonists of PPAR-γ and endogenous agonists (such as fatty acid derivatives) have overlapping binding sites in the ligand binding pocket of the PPAR-γ LBD. Synthetic agonists compete with endogenous ligand for binding. More recently, it has become apparent that agonists can bind at an alternative site and facilitate a conformational change in the receptor that influences transcriptional activity. Understanding more about this particular mechanism may lead to the exploitation of synthetic agonists that have a more favourable side-effect profile than those currently in use (the TZDs). The TZDs do not display significant alternate binding site functional effects [49].
TZD BENEFITS AND SIDE EFFECTS
TZDs are full agonists of PPAR-γ. They have strong insulin sensitizing actions and have been used very effectively to restore metabolic control in the treatment of type 2 diabetes [50]. The ability of the TZDs to enhance insulin sensitivity relies on their ability to modulate the activities of adipocytes and skeletal muscle cells. TZDs oppose the effects of TNF-α (pro-inflammatory cytokine) in adipocytes (probably through mechanisms involving the suppression of transcription factor NF-κB), as well as increasing the expression of the GLUT4 transporter, which is essential for the uptake of glucose into cells, and inducing the production of the insulin-sensitizing hormone adiponectin [12]. Through the enhancement of PI3-kinase activity and phosphorylation of AKT, the TZDs increase the utilization of glucose by skeletal muscle. They also inhibit the effects of resistin (an adipocyte-derived hormone that elevates blood glucose) [3].
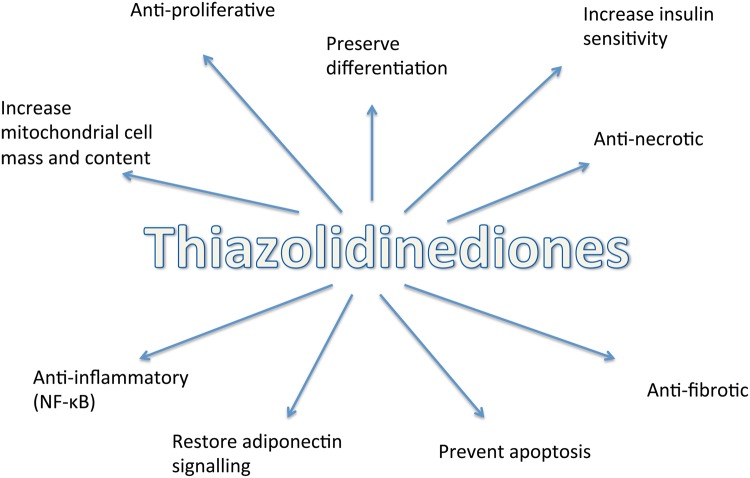
At a cellular level, the TZDs have anti-inflammatory, anti-proliferative, anti-fibrotic and anti-apoptotic functions. Systemically they have an influence on haemodynamics and exert a mildhypotensive effect in both animals and humans [14, 51]. TZD-induced mitochondrial biogenesis has been shown to promote cell integrity and sustain survival in a number of cell types, including T lymphocytes and neurons [33, 52] (Figure 4).
FIGURE 4.
Effects of TZDs at a cellular level [11].
Although tolerated by the majority of patients, clinical use of the TZDs has been hampered by an unfavourable side-effects profile with links to an increase in cardiovascular morbidity and mortality, carcinogenesis, hepatotoxicity and a reduction in bone mineral density [12]. Therefore, the focus on newer and more selective agents is of significant importance if their protective effects are to be exploited. Interestingly, anti-proteinuric agents including angiotensin receptor blockers such as irbesartan and telmisartan (characterized as selective PPAR-γ modulators in 2005 [34]) and angiotensin-converting enzyme inhibitors (used widely in the treatment of renal disease) are known to have partial PPAR-γ agonist activity [9, 53].
TZD OFF-TARGET (PPAR-γ INDEPENDENT) : EFFECTS
These are poorly understood, but involvement of the MAPKs and the glucocorticoid receptor (GR) has been suggested.
Treatment with a combination of TZD and dexamethasone (possibly due to the synergistic effects on GR phosphorylation) has been shown to protect podocytes from puromycin aminonucleoside (PAN)–induced injury (through improving cytoskeletal integrity and cell viability) [54]. It may therefore be that the TZDs exert an important influence on podocyte function and structure in part through this receptor.
There is evidence that connects PPAR-γ with other receptors in the nuclear hormone family. The manipulation of these specific pathways may provide new targets for future therapeutic advance. For example, signalling pathways of the vitamin D receptor (VDR) and the PPARs are interconnected in a number of cancer cell lines including melanoma and human breast cancer cells (in the latter, PPAR-γ actively competes with the VDR for RXR binding/heterodimerization and suppresses vitamin D signalling) [55, 56]. The bile acid receptor farnesoid X (FXR), another nuclear hormone receptor, has been shown to regulate adipocyte differentiation in part through its interaction with PPAR-γ. FXR homozygous KO mice are resistant to the effects of rosiglitazone on adipocyte differentiation [57]. FXR ligands upregulate PPAR-γ mRNA in hepatic stellate cells and in rodent models of liver fibrosis, with this counter-regulatory action affording an anti-fibrotic effect [58].
MAPKs are a family of serine/threonine protein kinases involved in a wide range of vital cellular processes, including apoptosis, survival and motility [59]. Their activation has been implicated in the progression of various glomerulopathies. Rosiglitazone has been shown to deactivate several of the MAPKs (c-Jun/Erk1/2 and p38) [54]. Inhibition of p38 and Erk1/2 MAPKs in a rodent model of PAN-induced nephrotic syndrome and adriamycin nephropathy (in which podocyte damage is observed) has been linked to an improvement in podocyte health (through inhibition of actin reorganization) and a reduction in proteinuria [60].
TZD RENAL AND SYSTEMIC ACTIONS
There is evidence that the TZDs reduce the progression of early diabetic nephropathy [61], the most common cause of renal failure in the developed world.
Using the example of the apolipoprotein E KO mouse that develops diabetes after exposure to STZ treatment, subsequent treatment with a PPAR-γ agonist markedly attenuates the hallmark changes within the glomeruli and tubules that are typical of nephropathy in this model. This effect is seen independently of changes in insulin, glucose and blood pressure reduction [62].
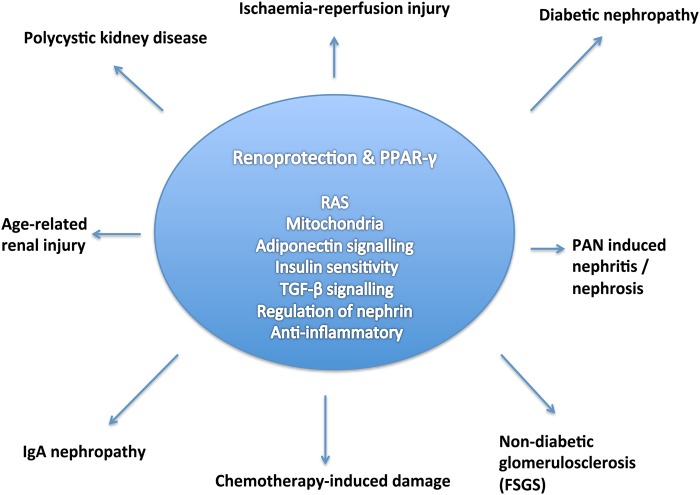
TZDs also appear to have protective roles in kidney disease more widely, for example, in non-diabetic glomerulosclerosis [63], focal segmental glomerulosclerosis [64], nephrotic syndrome [65], polycystic kidney disease [66] and in acute inflammatory crescentic glomerulonephritis [48] (Figure 5).
FIGURE 5.
Outline of the protective mechanisms through which PPAR-γ has been suggested to work and the renal diseases influenced as a result. PAN, puromycin aminonucleoside; FSGS, focal segmental glomerulosclerosis; TGF, transforming growth factor; RAS, renin–angiotensin system.
TZD protective roles span outside of renal disease and include beneficial effects in, for example, stroke [67] and skin cancer [68] (Table 3).
Table 3.
The varying protective and damaging effects of PPAR-γ activation
| System | Disease/model | Outcome | Reference |
|---|---|---|---|
| Central nervous system | Stroke | PPAR-γ agonist promotes cerebral protection | [83] |
| Cerebral malaria | Improved neurological outcomes and survival | [84] | |
| Vascular | Mouse model of hind limb ischaemia | PPAR-γ agonist promoted neovascularization | [85] |
| Gastrointestinal | Mouse model of IR injury | More severe disease in PPAR-γ-deficient mice, amelrioated by PPAR-γ ligand | [86] |
| Respiratory | Emphysema/mice exposed to chronic smoke | PPAR-γ downregulated in myeloid dendritic cells of smokers lungs | [87] |
| Mice exposed to chronic smoke | PPAR-γ agonist reverses emphysema in mice | ||
| Immune system | Polymicrobal sepsis | PPAR-γ activation induces T cell apoptosis → reduced survival | [88] |
| Renal | Type 2 diabetes | PPAR-γ agonists anti-albuminuric, produced a stabilization in eGFR and exerted a significant hypotensive effect | [89] |
| FSGS | [90] | ||
| Non-diabetic renal disease (overweight adults) | [91] |
IR, ischaemia–reperfusion injury; FSGS, focal segmental glomerulosclerosis.
TZD PODOCYTE EFFECTS
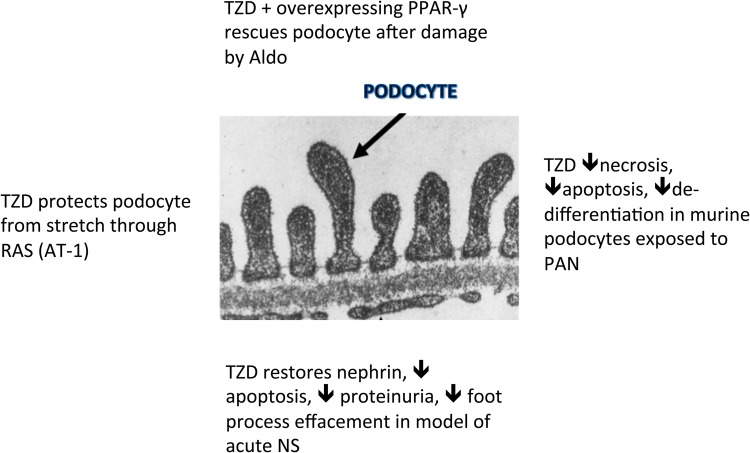
TZDs have been shown to increase the expression of PPAR-γ in podocytes as well as other glomerular and tubular cells at both the mRNA and protein levels [11, 69]. TZDs have also been shown to be podocyte protective in rodent models of nephropathy (aldosterone [70]/adriamycin [71]/puromycin aminonucleoside [11]) and glomerular capillary hypertension [72] (Figure 6).
FIGURE 6.
TZD podocyte-protective effects explained. After stretch: through RAS blockade [72]. In PAN nephritis: through restoration in balance of pro-apoptotic (caspase 3) and anti-apoptotic (Bcl-xl) molecules and reduction in pro-inflammatory TGF-β expression [11]. After damage by aldosterone [70]: overexpression of PPAR-γ/use of rosiglitazone, rescues podocytes through decreased ROS and maintenance of cell morphology (restores nephrin expression). Both are on-target effects (blocked by small interfering PPAR-γ RNA). In acute nephrosis [65], podocytes cause a decrease in the expression of nephrin, phosphorylated Akt and α-actinin 4 and an increase in apoptosis. PPAR-γ agonists given around the time of the injury produce a reversal of these effects as well as a reduction in proteinuria, a decrease in desmin and an improvement in foot process effacement [63].
Interestingly, PPAR-γ activation in the podocyte seems to be a key protective response after exposure to injury, with the up-regulation in expression of PPAR-γ seen in a wide variety of kidney diseases [11, 73].
THE FUTURE
Therapeutic advances and an improved understanding of PPAR-γ and its downstream molecular pathways have drawn attention to new PPAR-γ-based drugs, which are hopefully free of the side effects of the established TZDs (fluid retention, weight gain, cardiovascular morbidity, liver failure, cancer) [74–77]. A good example is the antidiabetic SR1664, which works completely separate from the typical transcriptional agonism associated with other PPAR-γ-mediated effects. SR1664 acts through blockade of the Cdk-5-mediated phosphorylation of PPAR-γ at serine 273 (a phosphorylation that is induced by obesity). It improves insulin sensitivity in insulin-resistant mice. It comes without the unwanted (TZD-associated) side effects of fluid retention and weight gain, including a lack of reduced bone cell mineralization of cells in culture [78]. By exploiting this phosphorylation pathway, there is perhaps new hope on the horizon for PPAR-γ-based drugs.
Targeting more than one of the PPAR isoforms simultaneously with agents such as the glitazars also provides opportunity. Aleglitazar, muraglitazar and saroglitazar target both PPAR-γ and PPAR-α and have been shown to improve insulin sensitivity and improve lipid profiles in the context of type 2 diabetes mellitus [79]. The use of muraglitazar, a strong agonist of PPAR-γ with moderate PPAR-α effects, was found to be associated with significant cardiovascular side effects and excess all-cause mortality [80] and as a result was never approved for clinical use. Saroglitazar has not demonstrated any of the adverse side effects described in association with other PPAR-γ agonists, and although its long-term cardiovascular safety has not been established yet, it has been approved for use by the official medicines regulatory authority in India for the treatment of dyslipidaemia in type 2 diabetes [81, 82].
CONCLUSIONS
PPAR-γ controls a large array of important cellular processes through the transcriptional regulation of specific gene cassettes. It has actions that are tissue and cell specific. The use of cell-specific transgenic models is helping us understand the complexities. It is clear that manipulation of PPAR-γ-related pathways (both on- and off-target) may be of great advantage to the podocyte in conditions of disease. We know that the TZDs, full agonists of the receptor, have a significant renoprotective effect in the context of diabetic nephropathy. The real challenges for the future surround understanding which are the key cells or tissues through which PPAR-γ exerts its actions and the development of new selective modulators of PPAR-γ with favourable side-effect profiles, and a focused research effort into the off-target mechanisms of its actions. Modulating this receptor may still have great therapeutic potential in preventing kidney disease.
ACKNOWLEDGEMENTS
This work was supported by a Senior Clinical Fellowship to R.J.C. (MR/K010492/1) and also through a Wellcome Trust clinical primer awarded to C.P.
CONFLICT OF INTEREST STATEMENT
The results presented in this paper have not been published previously in whole or part, except in abstract form.
REFERENCES
- 1. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 2003; 83: 253–307 [DOI] [PubMed] [Google Scholar]
- 2. Peroxisome Proliferator-Activated Receptors: from metabolic control to epidermal wound healing. http://www.smw.ch/docs/pdf200x/2002/07/smw-09939.PDF (15 April 2016, date last accessed) [DOI] [PubMed]
- 3. Kota BP, Huang TH-W, Roufogalis BD. An overview on biological mechanisms of PPARs. Pharmacol Res 2005; 51: 85–94 [DOI] [PubMed] [Google Scholar]
- 4. Fajas L, Auboeuf D, Raspé E et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem 1997; 272: 18779–18789 [DOI] [PubMed] [Google Scholar]
- 5. Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev 1999; 20: 649–688 [DOI] [PubMed] [Google Scholar]
- 6. Elbrecht A, Chen Y, Cullinan CA et al. Molecular cloning, expression and characterization of human peroxisome proliferator activated receptors gamma 1 and gamma 2. Biochem Biophys Res Commun 1996; 224: 431–437 [DOI] [PubMed] [Google Scholar]
- 7. Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med 2002; 53: 409–435 [DOI] [PubMed] [Google Scholar]
- 8. Guan Y, Breyer MD. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int 2001; 60: 14–30 [DOI] [PubMed] [Google Scholar]
- 9. Ernsberger P, Koletsky RJ. Metabolic actions of angiotensin receptor antagonists: PPAR-gamma agonist actions or a class effect? Curr Opin Pharmacol 2007; 7: 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang H-C, Ma L-J, Ma J et al. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int 2006; 69: 1756–1764 [DOI] [PubMed] [Google Scholar]
- 11. Kanjanabuch T, Ma L-J, Chen J et al. PPAR-gamma agonist protects podocytes from injury. Kidney Int 2007; 71: 1232–1239 [DOI] [PubMed] [Google Scholar]
- 12. Ahmadian M, Suh JM, Hah N et al. PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 2013; 19: 557–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dreyer C, Krey G, Keller H et al. Control of the peroxisomal beta-oxidation pathway by a novel family of nuclear hormone receptors. Cell 1992; 68: 879–887 [DOI] [PubMed] [Google Scholar]
- 14. Ruan X, Zheng F, Guan Y. PPARs and the kidney in metabolic syndrome. Am J Physiol Renal Physiol 2008; 294: F1032–F1047 [DOI] [PubMed] [Google Scholar]
- 15. Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature 2000; 405: 421–424 [DOI] [PubMed] [Google Scholar]
- 16. Atlas of Genetics and Cytogenetics in Oncology and Haematology. http://atlasgeneticsoncology.org/Genes/PPARGID383ch3p25.html (7 January 2016, date last accessed) [Google Scholar]
- 17. Peyrou M, Ramadori P, Bourgoin L et al. PPARs in liver diseases and cancer: epigenetic regulation by microRNAs. PPAR Res 2012; 2012: 757803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leader JE, Wang C, Fu M et al. Epigenetic regulation of nuclear steroid receptors. Biochem Pharmacol 2006; 11: 1589–1596 [DOI] [PubMed] [Google Scholar]
- 19. Motohashi N, Alexander MS, Casar JC et al. Identification of a novel microRNA that regulates the proliferation and differentiation in muscle side population cells. Stem Cells Dev 2012; 21: 3031–3043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Povero D, Panera N, Eguchi A et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cells via microRNA targeting peroxisome proliferator-activated receptor-γ. Cell Mol Gastroenterol Hepatol 2015; 1: 646–663.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng XC, Li L, Wen H et al. MicroRNA-128 inhibition attenuates myocardial ischemia/reperfusion injury-induced cardiomyocyte apoptosis by the targeted activation of peroxisome proliferator-activated receptor gamma. Mol Med Rep 2016; 14: 129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger J, Moller DE. The mechanism of action of PPARs. http://www.annualreviews.org/doi/pdf/10.1146/annurev.med.53.082901.104018 (7 January 2016, date last accessed)
- 23. Zhang B, Berger J, Zhou G et al. Insulin- and mitogen-activated protein kinase-mediated phosphorylation and activation of peroxisome proliferator-activated receptor gamma. J Biol Chem 1996; 271: 31771–31774 [DOI] [PubMed] [Google Scholar]
- 24. Rogue A, Spire C, Brun M et al. Gene expression changes induced by PPAR gamma agonists in animal and human liver. PPAR Res 2010; 2010: 325183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boerries M, Grahammer F, Eiselein S et al. Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 2013; 83: 1052–1064 [DOI] [PubMed] [Google Scholar]
- 26. Son N-H, Park T-S, Yamashita H et al. Cardiomyocyte expression of PPARγ leads to cardiac dysfunction in mice. J Clin Invest 2007; 117: 2791–2801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan Y, Chong L-W, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med 2007; 13: 1496–1503 [DOI] [PubMed] [Google Scholar]
- 28. Parast MM, Yu H, Ciric A et al. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PLoS One 2009; 4: e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hevener AL, He W, Barak Y et al. Muscle-specific Pparg deletion causes insulin resistance. Nat Med 2003; 9: 1491–1497 [DOI] [PubMed] [Google Scholar]
- 30. Gavrilova O, Haluzik M, Matsusue K et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 2003; 278: 34268–34276 [DOI] [PubMed] [Google Scholar]
- 31. Hobson MJ, Hake PW, O'Connor M et al. Conditional deletion of cardiomyocyte peroxisome proliferator-activated receptor γ enhances myocardial ischemia-reperfusion injury in mice. Shock 2014; 41: 40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. He W, Barak Y, Hevener A et al. Adipose-specific peroxisome proliferator-activated receptor gamma knockout causes insulin resistance in fat and liver but not in muscle. Proc Natl Acad Sci USA 2003; 100: 15712–15717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Strum JC, Shehee R, Virley D et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis 2007; 11: 45–51 [DOI] [PubMed] [Google Scholar]
- 34. Schupp M, Clemenz M, Gineste R et al. Molecular characterization of new selective peroxisome proliferator-activated receptor modulators with angiotensin receptor blocking activity. Diabetes 2005; 54: 3442–3452 [DOI] [PubMed] [Google Scholar]
- 35. Matsusue K, Haluzik M, Lambert G et al. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest 2003; 111: 737–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Duan SZ, Ivashchenko CY, Whitesall SE et al. Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J Clin Invest 2007; 117: 812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kubota N, Terauchi Y, Miki H et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell 1999; 4: 597–609 [DOI] [PubMed] [Google Scholar]
- 38. Lowell BB. PPARgamma: an essential regulator of adipogenesis and modulator of fat cell function. Cell 1999; 99: 239–242 [DOI] [PubMed] [Google Scholar]
- 39. Girnun GD, Smith WM, Drori S et al. APC-dependent suppression of colon carcinogenesis by PPARgamma. Proc Natl Acad Sci USA 2002; 99: 13771–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollex RL, Mamakeesick M, Zinman B et al. Peroxisome proliferator-activated receptor γ polymorphism Pro12Ala is associated with nephropathy in type 2 diabetes. J Diabetes Complicat 2007; 21: 166–171 [DOI] [PubMed] [Google Scholar]
- 41. Hegele RA. Monogenic forms of insulin resistance: apertures that expose the common metabolic syndrome. Trends Endocrinol Metab 2003; 14: 371–377 [DOI] [PubMed] [Google Scholar]
- 42. Tsai Y-S, Kim H-J, Takahashi N et al. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARgamma. J Clin Invest 2004; 114: 240–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang T. Kidney-specific gene targeting: insight into thiazolidinedione-induced fluid retention. Nephrology 2006; 11: 201–206 [DOI] [PubMed] [Google Scholar]
- 44. Zhang H, Zhang A, Kohan DE et al. Collecting duct-specific deletion of peroxisome proliferator-activated receptor blocks thiazolidinedione-induced fluid retention. Proc Natl Acad Sci USA 2005; 102: 9406–9411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zheng F, Fornoni A, Elliot SJ et al. Upregulation of type I collagen by TGF-beta in mesangial cells is blocked by PPARgamma activation. Am J Physiol Renal Physiol 2002; 282: F639–F648 [DOI] [PubMed] [Google Scholar]
- 46. Ohga S, Shikata K, Yozai K et al. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activation. Am J Physiol Renal Physiol 2007; 292: F1141–F1150 [DOI] [PubMed] [Google Scholar]
- 47. Zafiriou S, Stanners SR, Saad S et al. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol 2005; 16: 638–645 [DOI] [PubMed] [Google Scholar]
- 48. Henique C, Bollee G, Lenoir O et al. Nuclear factor erythroid 2-related factor 2 drives podocyte-specific expression of peroxisome proliferator-activated receptor essential for resistance to crescentic GN. J Am Soc Nephrol 2016; 27: 172–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hughes TS, Giri PK, de Vera IMS et al. An alternate binding site for PPARγ ligands. Nat Commun 2014; 5: 3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saltiel AR, Olefsky JM. Thiazolidinediones in the treatment of insulin resistance and type II diabetes. Diabetes 1996; 45: 1661–1669 [DOI] [PubMed] [Google Scholar]
- 51. Dormandy JA, Charbonnel B, Eckland DJ et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005; 366: 1279–1289 [DOI] [PubMed] [Google Scholar]
- 52. Jo S-H, Yang C, Miao Q et al. Peroxisome proliferator-activated receptor promotes lymphocyte survival through its actions on cellular metabolic activities. J Immunol 2006; 177: 3737–3745 [DOI] [PubMed] [Google Scholar]
- 53. Goyal SN, Bharti S, Bhatia J et al. Telmisartan, a dual ARB/partial PPAR-γ agonist, protects myocardium from ischaemic reperfusion injury in experimental diabetes. Diabetes Obes Metab 2011; 13: 533–541 [DOI] [PubMed] [Google Scholar]
- 54. Agrawal S, Guess AJ, Benndorf R et al. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol Pharmacol 2011; 80: 389–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sertznig P, Seifert M, Tilgen W et al. Peroxisome proliferator-activated receptor (PPAR) and vitamin D receptor (VDR) signaling pathways in melanoma cells: priming new therapeutic targets? J Steroid Biochem Mol Biol 2010; 121: 383–386 [DOI] [PubMed] [Google Scholar]
- 56. Alimirah F, Peng X, Yuan L et al. Crosstalk between the peroxisome proliferator-activated receptor γ (PPARγ) and the vitamin D receptor (VDR) in human breast cancer cells: PPARγ binds to VDR and inhibits 1α,25-dihydroxyvitamin D3 mediated transactivation. Exp Cell Res 2012; 318: 2490–2497 [DOI] [PubMed] [Google Scholar]
- 57. Abdelkarim M, Caron S, Duhem C et al. The farnesoid X receptor regulates adipocyte differentiation and function by promoting peroxisome proliferator-activated receptor-gamma and interfering with the Wnt/beta-catenin pathways. J Biol Chem 2010; 285: 36759–36767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fiorucci S, Rizzo G, Antonelli E et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther 2005; 315: 58–68 [DOI] [PubMed] [Google Scholar]
- 59. Arthur JSC, Ley SC. Mitogen-activated protein kinases in innate immunity. Nat Rev Immunol 2013; 13: 679–692 [DOI] [PubMed] [Google Scholar]
- 60. Koshikawa M, Mukoyama M, Mori K et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J Am Soc Nephrol 2005; 16: 2690–2701 [DOI] [PubMed] [Google Scholar]
- 61. Sarafidis PA, Stafylas PC, Georgianos PI et al. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis 2010; 55: 835–847 [DOI] [PubMed] [Google Scholar]
- 62. Calkin AC, Giunti S, Jandeleit-Dahm KA et al. PPAR-alpha and -gamma agonists attenuate diabetic kidney disease in the apolipoprotein E knockout mouse. Nephrol Dial Transplant 2006; 21: 2399–2405 [DOI] [PubMed] [Google Scholar]
- 63. Ma LJ, Marcantoni C, Linton MF et al. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int 2001; 59: 1899–1910 [DOI] [PubMed] [Google Scholar]
- 64. Peyser A, Machardy N, Tarapore F et al. Follow-up of phase I trial of adalimumab and rosiglitazone in FSGS: III. Report of the FONT study group. BMC Nephrol 2010; 11: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zuo Y, Yang H-C, Potthoff SA et al. Protective effects of PPARγ agonist in acute nephrotic syndrome. Nephrol Dial Transplant 2012; 27: 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dai B, Liu Y, Mei C et al. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci (Lond) 2010; 119: 323–333 [DOI] [PubMed] [Google Scholar]
- 67. Yin K-J, Fan Y, Hamblin M et al. KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 2013; 136(Pt 4): 1274–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sahu RP, DaSilva SC, Rashid B et al. Mice lacking epidermal PPARγ exhibit a marked augmentation in photocarcinogenesis associated with increased UVB-induced apoptosis, inflammation and barrier dysfunction. Int J Cancer 2012; 131: E1055–E1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Yang H-C, Deleuze S, Zuo Y et al. The PPARgamma agonist pioglitazone ameliorates aging-related progressive renal injury. J Am Soc Nephrol 2009; 20: 2380–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhu C, Huang S, Yuan Y et al. Mitochondrial dysfunction mediates aldosterone-induced podocyte damage: a therapeutic target of PPARγ. Am J Pathol 2011; 178: 2020–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu H-F, Guo L-Q, Huang Y-Y et al. Thiazolidinedione attenuate proteinuria and glomerulosclerosis in adriamycin-induced nephropathy rats via slit diaphragm protection. Nephrology (Carlton) 2010; 15: 75–83 [DOI] [PubMed] [Google Scholar]
- 72. Miceli I, Burt D, Tarabra E et al. Stretch reduces nephrin expression via an angiotensin II-AT(1)-dependent mechanism in human podocytes: effect of rosiglitazone. Am J Physiol Renal Physiol 2010; 298: F381–F390 [DOI] [PubMed] [Google Scholar]
- 73. Lepenies J, Hewison M, Stewart PM et al. Renal PPARγ mRNA expression increases with impairment of renal function in patients with chronic kidney disease. Nephrology (Carlton) 2010; 15: 683–691 [DOI] [PubMed] [Google Scholar]
- 74. Crunkhorn S. Diabetes: safer PPARγ-targeted drugs on the horizon? Nat Rev Drug Discov 2011; 10: 814. [DOI] [PubMed] [Google Scholar]
- 75. Colmers IN, Bowker SL, Majumdar SR et al. Use of thiazolidinediones and the risk of bladder cancer among people with type 2 diabetes: a meta-analysis. CMAJ 2012; 184: E675–E683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rizos CV, Elisaf MS, Mikhailidis DP et al. How safe is the use of thiazolidinediones in clinical practice? Expert Opin Drug Saf 2009; 8: 15–32 [DOI] [PubMed] [Google Scholar]
- 77. Bortolini M, Wright MB, Bopst M et al. Examining the safety of PPAR agonists – current trends and future prospects. Expert Opin Drug Saf 2013; 12: 65–79 [DOI] [PubMed] [Google Scholar]
- 78. Choi JH, Banks AS, Kamenecka TM et al. Antidiabetic actions of a non-agonist PPARγ ligand blocking Cdk5-mediated phosphorylation. Nature 2011; 477: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Munigoti SP, Harinarayan CV. Role of Glitazars in atherogenic dyslipidemia and diabetes: two birds with one stone? Indian J Endocrinol Metab 2014; 18: 283–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Nissen SE, Wolski K, Topol EJ. Effect of muraglitazar on death and major adverse cardiovascular events in patients with type 2 diabetes mellitus. JAMA 2005; 294: 2581–2586 [DOI] [PubMed] [Google Scholar]
- 81. Jain MR, Giri SR, Trivedi C et al. Saroglitazar, a novel PPARα/γ agonist with predominant PPARα activity, shows lipid-lowering and insulin-sensitizing effects in preclinical models. Pharmacol Res Perspect 2015; 3: e00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Agrawal R. The first approved agent in the Glitazar's Class: Saroglitazar. Curr Drug Targets 2014; 15: 151–155 [DOI] [PubMed] [Google Scholar]
- 83. Yin K-J, Fan Y, Hamblin M et al. KLF11 mediates PPARγ cerebrovascular protection in ischaemic stroke. Brain 2013; 136: 1274–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Serghides L, McDonald CR, Lu Z et al. PPARγ agonists improve survival and neurocognitive outcomes in experimental cerebral malaria and induce neuroprotective pathways in human malaria. PLoS Pathog 2014; 10: doi:10.1371/journal.ppat.1003980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Nagahama R, Matoba T, Nakano K et al. Nanoparticle-mediated delivery of pioglitazone enhances therapeutic neovascularization in a murine model of hindlimb ischemia. Arterioscler Thromb Vasc Biol 2012; 32: 2427–2434 [DOI] [PubMed] [Google Scholar]
- 86. Nakajima A, Wada K, Miki H et al. Endogenous PPARγ mediates anti-inflammatory activity in murine ischemia-reperfusion injury, Gastroenterology 2001; 120: 460–469 [DOI] [PubMed] [Google Scholar]
- 87. Shan M, You R, Yuan X et al. Agonistic induction of PPARγ reverses cigarette smoke-induced emphysema. J Clin Invest 2014; 124: 1371–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schmidt MV, Paulus P, Kuhn A-M et al. Peroxisome proliferator-activated receptor γ-induced T cell apoptosis reduces survival during polymicrobial sepsis. Am J Respir Crit Care Med 2011; 184: 64–74 [DOI] [PubMed] [Google Scholar]
- 89. Miglio G, Rosa AC, Rattazzi L et al. Protective effects of peroxisome proliferator-activated receptor agonists on human podocytes: proposed mechanisms of action. Br J Pharmacol 2012; 167: 641–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Joy M, Gipson D, Dike M et al. Clin J Am Soc Neph 2009; 39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kincaid-Smith P, Fairley KF, Farish S et al. Reduction of proteinuria by rosiglitazone in non-diabetic renal disease. Nephrology 2008; 13: 58–62 [DOI] [PubMed] [Google Scholar]