Abstract
Aims
Transthyretin cardiac amyloidosis (ATTR-CA) has been reported in patients with aortic stenosis (AS) but its prevalence and phenotype are not known. We examine elderly patients with severe symptomatic AS undergoing transcatheter aortic valve replacement (TAVR) and determine the prevalence and phenotype of ATTR-CA non-invasively.
Methods and results
We performed technetium-99m pyrophosphate (99mTc-PYP) cardiac scintigraphy prospectively on patients who underwent TAVR, to screen for ATTR-CA. Transthoracic echocardiography and speckle-strain imaging were performed. We assessed the association of several parameters with ATTR-CA using multivariable logistic regression and constructed receiver operating curves to evaluate the best predictors of ATTR-CA. Among 151 patients (mean age 84 ± 6 years, 68% men), 16% (n = 24) screened positive for ATTR-CA with 99mTc-PYP scintigraphy. Compared with patients without ATTR-CA, ATTR-CA patients had a thicker interventricular septum (1.3 vs. 1.1 cm, P = 0.007), higher left ventricular (LV) mass index (130 vs. 98 g/m2, P = 0.002), and lower stroke volume index (30 vs. 36 mL/m2, P = 0.009). ATTR-CA patients had advanced diastolic dysfunction with higher E/A ratio (2.3 vs. 0.9, P = 0.001) and lower deceleration time (176 vs. 257 ms, P < 0.0001); impairment in systolic function with lower ejection fraction (48% vs. 56%, P = 0.011), myocardial contraction fraction (26 vs. 41, P < 0.0001), and average of lateral and septal mitral annular tissue Doppler S’ (4.0 vs. 6.6 cm/s, P < 0.0001). While ATTR-CA patients had more impaired global longitudinal strain (−12 vs. −16%, P = 0.007), relative apical longitudinal strain was the same regardless of ATTR-CA diagnosis (0.98 vs. 0.98, P = 0.991). Average S’ best predicted ATTR-CA in multivariable logistic regression (odds ratio 16.67 per 1 cm/s decrease with AUC 0.96, 95% confidence interval 0.90–0.99, P = 0.002) with a value ≤6 conferring 100% sensitivity for predicting a positive 99mTc-PYP amyloid scan.
Conclusions
Transthyretin cardiac amyloidosis is prevalent in 16% of patients with severe calcific AS undergoing TAVR and is associated with a severe AS phenotype of low-flow low-gradient with mildly reduced ejection fraction. Average tissue Doppler mitral annular S’ of < 6 cm/s may be a sensitive measure that should prompt a confirmatory 99mTc-PYP scan and subsequent testing for ATTR-CA. Prospective assessment of outcomes after TAVR is needed in patients with and without ATTR-CA.
Keywords: Transthyretin cardiac amyloidosis, Aortic stenosis, TAVR, Low-flow low-gradient, Strain
Introduction
Aortic stenosis (AS) is present in up to 3% of patients ≥75 years old1 and in symptomatic patients, confers a mean survival of 1.8 years when medically managed.2,3 Several flow-gradient patterns of severe AS have been elucidated,4 each with different management strategies because the degree of ventricular recovery after aortic valve replacement (AVR) and clinical outcomes differ.5,6 Among patients with an aortic valve area (AVA) ≤1.0 cm2, specific subgroups include Stage D1: ‘High-gradient AS’ with a mean gradient (MG) ≥40 mmHg or peak velocity (Vmax) ≥4 m/s; Stage D2: ‘Classical low-flow, low-gradient (LF-LG) AS with reduced left ventricular ejection fraction’ (LVEF) (MG <40 mmHg or Vmax <4 m/s with LVEF <50%); and Stage D3: ‘LF-LG AS with normal LVEF or paradoxical low-flow AS’ (MG <40 mmHg or Vmax ≤4 m/s with LVEF ≥50%).4 Despite benefiting from AVR compared with medical therapy, patients with low-flow AS have the worst outcomes, but the reasons for this discrepancy are not fully elucidated.
In low-flow AS with or without a preserved LVEF (Stages D2 and D3), the reduced forward stroke volume may occur in the setting of severe concentric remodelling, diastolic dysfunction, reduced longitudinal myocardial shortening and atrial fibrillation.7 These findings bear a striking resemblance to transthyretin cardiac amyloidosis (ATTR-CA), the most common cause of restrictive cardiomyopathy in older adults.8 In ATTR-CA, extracellular deposition of fibrils composed of destabilized wild-type (ATTRwt) or mutant (ATTRm) transthyretin protein leads to diastolic dysfunction, arrhythmias, and clinical heart failure.9 ATTRwt deposits have been reported in 25% of surgically removed heart valves from adults >80 years old.10 Identifying ATTR-CA among patients with severe AS may be important as there are a number of disease modifying therapies for ATTR-CA in clinical trials.9
Classically, a low electrocardiographic voltage-mass ratio (VMR)11 and echocardiographic parameters12–14 have characterized cardiac amyloidosis. Myocardial contraction fraction (MCF), a novel volumetric measure of myocardial shortening, may also be useful in identifying this disease.15 However, these are not sufficiently sensitive or specific to know that cardiac amyloidosis is not being missed. Nuclear cardiac imaging with bone seeking radioisotopes such as technetium-99m pyrophosphate (99mTc-PYP) have shown excellent diagnostic accuracy for ATTR-CA.16–18 A recent multicentre international collaboration validated these radioisotopes for the non-invasive diagnosis of ATTR-CA, with a reported 100% specificity when combined with the absence of a monoclonal protein to rule out light-chain (AL) amyloid, the other major form of cardiac amyloidosis.19
In this study, we used 99mTc-PYP cardiac imaging to examine elderly patients with severe AS undergoing transcatheter aortic valve replacement (TAVR) and determine the prevalence and phenotype of ATTR-CA non-invasively.
Methods
Study design and population
We prospectively recruited patients with severe calcific AS undergoing TAVR at Columbia University’s Centre for Interventional Vascular Therapy between December 2014 and July 2016. Patients provided informed consent in accordance with Columbia’s IRB. Inclusion criteria were patients ≥65 years old with severe symptomatic AS. Exclusion criteria included patients with AS due to congenital or rheumatic heart disease, patients unable to provide informed consent or lie still for 10 min under the camera. Patients underwent transthoracic echocardiography with strain rate imaging at the time of TAVR evaluation and a 99mTc-PYP scan within 30 days after TAVR. Echocardiographers and nuclear cardiologists were blinded to patients’ clinical information.
Clinical and laboratory measures
Biochemistry analyses were measured prior to TAVR and included troponin I, brain natriuretic peptide (BNP), estimated glomerular filtration rate (eGFR), and modified body mass index (mBMI) [albumin x BMI] reflecting autonomic dysfunction and malabsorption.20 In patients who scanned positive for ATTR-CA, we ruled out AL cardiac amyloid by a normal serum free light chain ratio (0.26–1.65) on Freelite assay and absence of an abnormal monoclonal band on immunofixation of serum or urine.
Technetium-99m pyrophosphate planar cardiac imaging for diagnosis of transthyretin cardiac amyloidosis
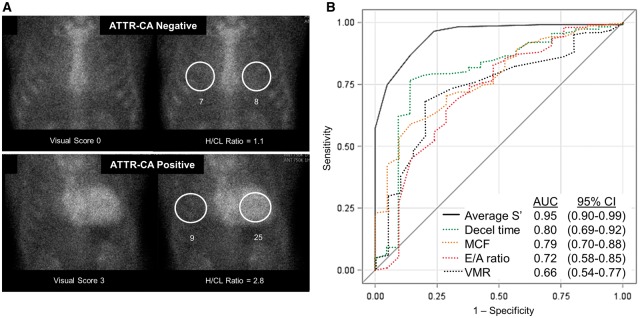
All patients underwent a 99mTc-PYP cardiac scan within 30 days after TAVR according to previously described technique21: myocardial tracer uptake was analysed using the semi-quantitative visual score (range 0–3) and quantitative heart-to-contralateral (H/CL) ratio of total counts in a region of interest (ROI) over the heart divided by counts in an identical size ROI over the contralateral chest to normalize for soft tissue and ribs. ATTR-CA was defined by diffuse 99mTc-PYP uptake, visual score ≥2 and H/CL ≥1.5 based on prior multicentre data on the accuracy of 99mTc-PYP for diagnosis of ATTR-CA.18
Echocardiography and speckle strain imaging
Doppler echocardiography was performed using commercially available ultrasound systems at the time of TAVR evaluation. Standard measurements of cardiac size and function, and classification of LV geometry were performed per American Society of Echocardiography guidelines.22 Pulse wave and tissue Doppler were used to assess diastolic and biventricular function in the apical four-chamber view. Average mitral annular s’ was calculated from tissue Doppler at the lateral and septal mitral annulus. Myocardial contraction fraction was calculated as the ratio of stroke volume to myocardial volume, which were calculated from linear dimensions in the parasternal long-axis view.15 Speckle strain imaging was performed using TomTec Image-ArenaTM software in patients with adequate endomyocardial border definition from transthoracic echocardiography in the 4-, 3-, and 2-chamber apical views. Regional longitudinal strain (LS) was determined in 17 segments of the LV as per ASE guidelines.22 Global LS was calculated as the average LS of these 17 segments. Relative apical LS was calculated as average apical LS/[(average basal LS + average mid LS)/2].13 The VMR was calculated as the ratio of electrocardiographic voltage by Sokolov Lyon criteria to the echocardiographically derived cross sectional area.11
Statistical analysis
Analyses were performed using Statistical Analysis Software (SAS) (v9.4, Cary, NC, USA) and R (R Core Team 2014). Continuous variables were presented as mean ± SD or median and categorical variables were summarized as counts (frequency percentages). χ2 or Fisher exact test (for small cell counts) compared categorical variables and Wilcoxon rank-sum tests compared continuous variables between patients with and without ATTR-CA. Two-sided P-values were used throughout all analyses. Univariable and multivariable logistic regressions evaluated for factors associated with the outcome of ATTR-CA. All non-collinear variables with P < 0.25 in univariable analysis were included in a multivariable regression model, and several model selection methods were employed (backward, forward, and stepwise). Internal validation of the final model was assessed using bootstrap method (1000 repetitions) with the optimism corrected C-statistic.23 Using systolic or diastolic echocardiographic parameters as well as the VMR as diagnostic markers for ATTR-CA, we estimated and compared the area under the corresponding receiver operating curve (ROC) curve (C-statistic) with corresponding 95% confidence interval (CI). Reproducibility of echocardiographic strain rate imaging was assessed in terms of inter- and intra-observer variability using interclass correlation values for the global LS for a subset of subjects (n = 20).
Results
Patient population
The study population (Table 1) included 151 patients. They were elderly (mean age 84 ± 6 years), 68% men, with severe cardiac symptoms (75% had New York Heart Association functional class >2) and frequent comorbid conditions. On average, cardiac biomarkers were elevated, troponin I 0.07 (0.02–0.22) ng/mL and BNP 305 (146–847) pg/mL, with mean LVEF 55 ± 15%.
Table 1.
Baseline characteristics in elderly patients with severe symptomatic AS with and without ATTR-CA
| All | No ATTR-CA | ATTR-CA | ||
|---|---|---|---|---|
| (n = 151) | (n = 127) | (n = 24) | P-value | |
| Socio-demographic variables | ||||
| Age, years | 83.7 ± 6.2 | 83.3 ± 6.3 | 86.3 ± 5.7 | 0.038 |
| Race | ||||
| White | 141 (93.4%) | 118 (92.9%) | 23 (95.8%) | |
| Black | 6 (4.0%) | 5 (3.9%) | 1 (4.2%) | 0.678 |
| Asian | 4 (2.7%) | 4 (3.2%) | 0 (0%) | |
| Male sex | 102 (67.6%) | 80 (63.0%) | 22 (91.7%) | 0.005 |
| BMI, kg/m2 | 26.8 ± 5.0 | 27.0 ± 5.3 | 25.5 ± 2.7 | 0.225 |
| Modified BMI, kg·g/m2·dL | 106.1 ± 24.6 | 107.6 ± 25.2 | 98.1 ± 19.9 | 0.084 |
| New York Heart Association (N, %) | ||||
| I | 8 (5.3%) | 8 (6.3%) | 0 (0%) | |
| II | 30 (19.9%) | 24 (18.9%) | 6 (25%) | 0.195 |
| III | 99 (65.6%) | 81 (63.8%) | 18 (75%) | |
| IV | 14 (9.3%) | 14 (11.0%) | 0 (0%) | |
| Severe symptomatic AS stage | ||||
| D1: High gradient AS | 117 (79.1%) | 102 (82.2%) | 15 (62.5%) | |
| D2: Low-flow, low-gradient AS with reduced LVEF | 20 (13.5%) | 13 (10.5%) | 7 (29.2%) | 0.045 |
| D3: Low-flow, low-gradient AS with normal LVEF | 11 (7.4%) | 9 (7.3%) | 2 (8.3%) | |
| Clinical and ECG parameters | ||||
| Hypertension | 129 (85.4%) | 107 (84.3%) | 22 (91.7%) | 0.530 |
| Coronary artery disease | 96 (63.6%) | 78 (61.4%) | 18 (75%) | 0.252 |
| Cerebrovascular disease | 19 (12.6%) | 17 (13.4%) | 2 (8.3%) | 0.739 |
| Atrial fibrillation/flutter | 64 (42.4%) | 54 (42.5%) | 10 (41.7%) | 0.938 |
| Carpal tunnel syndrome | 11 (7.3%) | 7 (5.5%) | 4 (16.7%) | 0.075 |
| Systolic blood pressure, mmHg | 140.9 ± 25.4 | 143.6 ± 25.1 | 125.3 ± 21.5 | 0.009 |
| Diastolic blood pressure, mmHg | 64.5 ± 13.9 | 65.1 ± 14.3 | 61.1 ± 10.6 | 0.310 |
| Low voltage | 7 (4.9%) | 4 (3.33%) | 3 (12.5%) | 0.091 |
| Voltage-to-mass ratio | 1.3 ± 0.8 | 1.4 ± 0.8 | 1.0 ± 0.6 | 0.028 |
| QRS duration, ms | 113.4 ± 31.0 | 110.7 ± 30.7 | 127.4 ± 29.8 | 0.017 |
| Right bundle branch block | 28 (19.4%) | 19 (15.8%) | 9 (37.5%) | 0.023 |
| Need for permanent pacemaker post-TAVR | 22 (14.9%) | 18 (14.4%) | 4 (17.4%) | 0.751 |
| Laboratory values | ||||
| Troponin I, ng/mL | 0.07 (0.02–0.22) | 0.06 (0.02–0.14) | 0.11 (0.05–0.40) | 0.430 |
| Troponin T, ng/dL | 0.07 (0.05–0.11) | 0.06 (0.05–0.08) | 0.18 (0.06–0.33) | 0.181 |
| BNP, pg/mL | 304.5 (146–847) | 275 (124–722) | 522 (302–1023) | 0.041 |
| NT pro-BNP, ng/dL | 2003 (714–3226) | 1896 (514–2932) | 3220 (1092–19 007) | 0.148 |
| Estimated GFR, mL/min | 67.7 ± 29.2 | 69.6 ± 29.8 | 57.6 ± 23.8 | 0.066 |
| Albumin, g/dL | 4.0 ± 0.5 | 4.0 (3.6–4.3) | 3.9 (3.6–4.1) | 0.134 |
| Presence of Clone | 19 (32.2%) | 14 (30.4%) | 5 (38.5%) | 0.738 |
| 99mTc-PYP cardiac imaging | ||||
| Visual score ≥2 | 24 (15.9%) | 0 (0%) | 24 (100%) | <0.0001 |
| Heart-to-contralateral ratio | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.7 ± 0.2 | <0.0001 |
Values are presented as mean ± SD, median (inter-quartile range), or n (%).
ATTR-CA, transthyretin cardiac amyloidosis; BNP, brain natriuretic peptide; 99mTc-PYP, technetium pyrophosphate; BNP, brain natriuretic peptide; GFR, glomerular filtration rate; ECG, electrocardiogram; BMI, body mass index; AS, aortic stenosis; LVEF, left ventricular ejection fraction; SD, standard deviation.
Transthyretin cardiac amyloidosis prevalence and phenotype among patients undergoing transcatheter aortic valve replacement
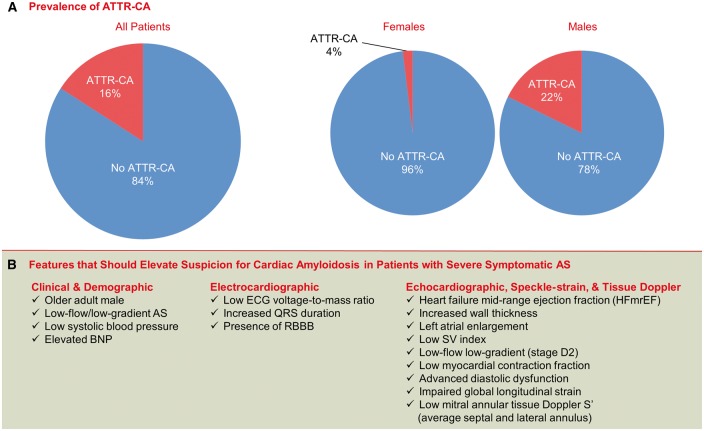
Transthyretin cardiac amyloidosis was found in 24 subjects (16%, 95% CI 10–23%) (Figure 1A, Table 1). Compared with patients without ATTR-CA, those with ATTR-CA were more likely to be male, with 22% of male subjects screening positive for ATTR-CA (95% CI 14–31%). Subjects with ATTR-CA had lower systolic blood pressure before TAVR and higher BNP but had no difference in troponin I, eGFR, or mBMI compared with subjects without ATTR-CA. With respect to conduction disease, patients with ATTR-CA also had lower VMR and higher incidence of right bundle branch block but did not have an increased need for placement of a permanent pacemaker after TAVR implantation compared with patients without ATTR-CA.
Figure 1.
Predictors of ATTR-CA in elderly patients undergoing transcatheter aortic valve replacement. Quantitative assessment of technetium-99m pyrophosphate myocardial uptake (A) is shown in a patient with (bottom) and without ATTR-CA (top) with corresponding H/CL ratio. ROC curves for predictors of ATTR-CA (B). DT, deceleration time; H/CL, heart-to-contralateral ratio; MCF, myocardial contraction fraction; VMR, voltage-mass ratio; ATTR-CA, transthyretin cardiac amyloidosis.
Echocardiographic features of transthyretin cardiac amyloidosis in transcatheter aortic valve replacement patients
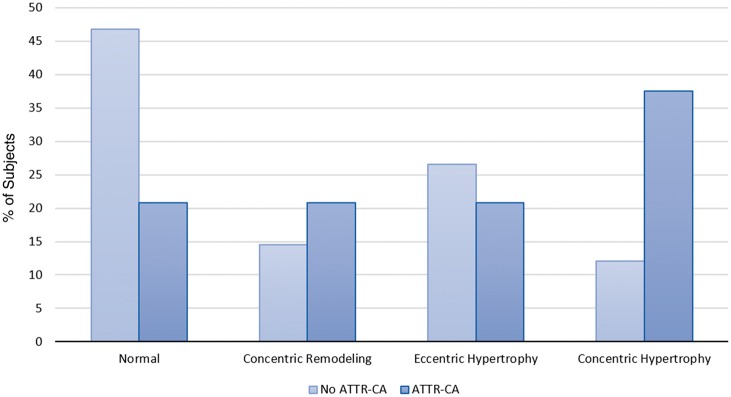
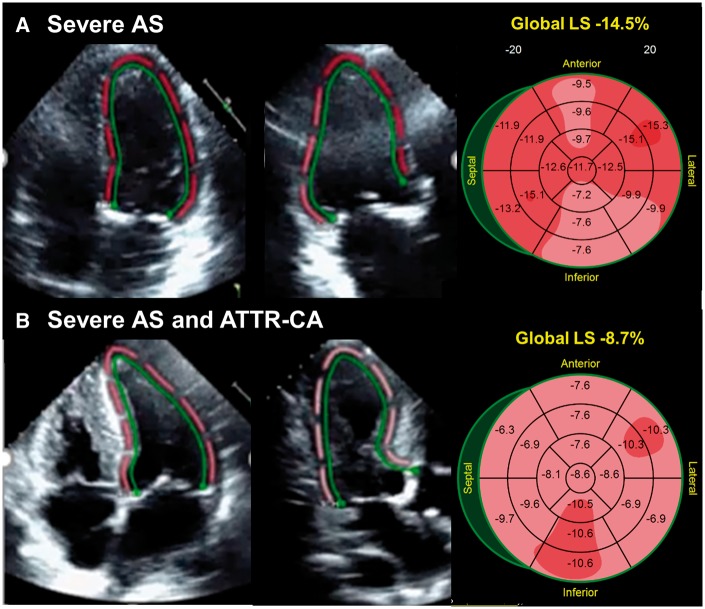
Baseline echocardiographic assessment (Table 2) showed patients with ATTR-CA had a similar AVA and a trend towards lower MG and lower peak velocity compared with subjects without ATTR-CA. A greater percentage of patients with ATTR-CA had heart failure with mid-range ejection fraction24 (48% vs. 56%, P = 0.011) with lower average mitral annular S’ (4.0 ± 1.1 vs. 6.6 ± 1.5 cm/s, P < 0.0001) and reduced MCF (26 ± 10 vs. 41 ± 16, P < 0.0001). In addition, they had a thicker interventricular septal wall (1.3 ± 0.3 vs. 1.1 ± 0.2 cm, P = 0.007), higher LV mass index (130 ± 44 vs. 98 ± 25 g/m2, P = 0.002), with lower stroke volume index (SVI) (30 ± 11 vs. 36 ± 10 mL/m2, P = 0.009) consistent with increased incidence of concentric hypertrophy (37.5% vs. 12.1%, P = 0.0009) (Figure 2). Patients with ATTR-CA were nearly 3× more likely to have low-flow low-gradient AS (Stage D2) (29.2% vs. 10.5%, P = 0.045) than patients without ATTR-CA. Patients with ATTR-CA had a higher E/A ratio [2.3 (1.10–3.10) vs. 0.9 (0.70–1.70), P = 0.001] and lower deceleration time [176 (144–198) vs. 257 (209–313) ms, P < 0.0001] consistent with grade III diastolic dysfunction. Strain rate imaging revealed more severe impairment in global LS in patients with ATTR-CA compared with patients without ATTR-CA (−12 vs. −16%, P = 0.007), which persisted throughout the LV basal, mid, and apical segments (Figure 3). However, no significant difference in relative apical LS was observed in patients with and without ATTR-CA (0.98 vs. 0.98, P = 0.991). Corresponding bulls-eye plots of segmental strain revealed a patchy distribution of impaired LS consistent with severe AS.13
Table 2.
Baseline echocardiographic parameters in patients with severe AS with and without ATTR-CA
| All | No ATTR-CA | ATTR-CA | ||
|---|---|---|---|---|
| (n = 151) | (n = 127) | (n = 24) | P-value | |
| 2D and Doppler variables | ||||
| Aortic valve area, cm2 | 0.77 ± 0.18 | 0.77 ± 0.19 | 0.80 ± 0.16 | 0.358 |
| Mean gradient, mmHg | 40.1 ± 13.9 | 41.1 ± 13.8 | 35.2 ± 13.9 | 0.060 |
| Peak velocity, cm/s | 4.2 ± 0.7 | 4.3 ± 0.7 | 4.0 ± 0.7 | 0.078 |
| LV ejection fraction, % | 54.8 ± 15.0 | 56.1 ± 14.1 | 47.6 ± 17.6 | 0.011 |
| Interventricular septal wall, cm | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.3 ± 0.3 | 0.007 |
| LV posterior wall, cm | 0.9 ± 0.3 | 0.9 ± 0.2 | 1.1 ± 0.4 | <0.001 |
| LV mass index, g/m2 | 103.1 ± 31.3 | 97.9 ± 25.4 | 129.8 ± 43.6 | 0.002 |
| Relative wall thickness | 0.40 ± 0.10 | 0.38 ± 0.12 | 0.49 ± 0.21 | 0.017 |
| Stroke volume index, mL/m2 | 34.7 ± 9.9 | 35.7 ± 9.6 | 29.9 ± 10.5 | 0.009 |
| Myocardial contraction fraction, % | 38.6 ± 15.7 | 41.0 ± 15.5 | 26.4 ± 10.1 | <0.0001 |
| Left atrial dimension, cm | 4.5 ± 0.7 | 4.4 ± 0.7 | 5.0 ± 0.7 | 0.002 |
| Diastolic function | ||||
| E wave, cm/s | 93.7 ± 31.1 | 95.4 ± 31.1 | 84.4 ± 30.7 | 0.140 |
| A wave, cm/s | 84.3 ± 37.8 | 90.7 ± 36.0 | 50.7 ± 28.8 | <0.0001 |
| E/A ratio | 1 (0.75–2.10) | 0.90 (0.70–1.70) | 2.30 (1.10–3.10) | 0.001 |
| Deceleration time, ms | 250 (191–306) | 257 (209–313) | 176 (144–198) | <0.0001 |
| E/e’ ratio | 16 (12–20) | 16 (12–20) | 19 (15–26) | 0.075 |
| LA volume index, mL/m2 | 50.2 ± 17.1 | 49.2 ± 17.2 | 55.5 ± 15.8 | 0.108 |
| Tissue Doppler and strain systolic function | ||||
| Average mitral annular S’, cm/s | 6.2 ± 1.7 | 6.6 ± 1.5 | 4.0 ± 1.1 | <0.0001 |
| Right ventricular S’, cm/s | 10.9 ± 3.1 | 11.3 ± 2.9 | 8.5 ± 2.8 | <0.0001 |
| Basal LS (%) | −14.0 ± 4.6 | −14.8 ± 4.2 | −11.6 ± 5.0 | 0.007 |
| Mid LS (%) | −14.0 ± 4.6 | −14.8 ± 4.2 | −11.6 ± 5.0 | 0.008 |
| Apical LS (%) | −13.8 ± 4.6 | −14.6 ± 4.3 | −11.4 ± 4.9 | 0.009 |
| Global LS (%) | −14.9 ± 4.7 | −15.7 ± 4.3 | −12.4 ± 5.2 | 0.007 |
| Relative apical LS | 0.98 ± 0.04 | 0.98 ± 0.03 | 0.98 ± 0.06 | 0.991 |
Values are presented as mean ± SD or median (inter-quartile range).
ATTR-CA, transthyretin cardiac amyloidosis; LA, left atrium; LS, longitudinal strain; LV, left ventricular; AS, aortic stenosis; 2D, two dimensional; SD, standard deviation.
Figure 2.
Left ventricular geometry among patients undergoing transcatheter aortic valve replacement with and without ATTR-CA. P-value comparing patients with ATTR-CA vs. no ATTR-CA. ATTR-CA, transthyretin cardiac amyloidosis.
Figure 3.
Speckle-strain imaging in elderly patients with severe symptomatic AS with and without ATTR-CA. Bullseye plots demonstrate relative apical sparing is the same whether amyloid is present or not. AS, aortic stenosis; ATTR-CA, transthyretin cardiac amyloidosis.
Using logistic regression models, significant univariable predictors of ATTR-CA included older age, male gender, a higher natriuretic peptide level, a lower LVEF, increased left atrial dimension, an interventricular septal wall thickness of ≥1.2 cm, a SVI <35 mL/m2, decreased MCF, a lower average mitral annular S’ and higher E/A ratio (Table 3). In multivariable logistic regression using forward, backward, and step-wise selection models that considered all non-collinear significant univariable predictors of ATTR-CA, only average mitral annular S’ remained significantly associated with ATTR-CA with odds ratio 5.0 per 1 cm/s decrease, 95% CI 2.56–9.09, P < 0.0001. In bootstrap validation, the final model generated an optimism-corrected area under the curve (AUC) almost identical to the value obtained using original data (0.947). In ROC analysis, while deceleration time, MCF, E/A ratio, and VMR predicted ATTR-CA with AUCs ranging 0.6–0.8, the average tissue Doppler mitral annular S’ was the strongest predictor with an AUC 0.95, P < 0.0001 (Figure 1B). Dichotomizing the average mitral annular S’ around the population median of ≤6 vs. >6 cm/s was 100% sensitive but only 57% specific for ATTR-CA by 99mTc-PYP scan.
Table 3.
Predictors of ATTR-CA among elderly patients undergoing TAVR
| N | OR | 95% CI | C-statistic | P-value | |
|---|---|---|---|---|---|
| Univariable | |||||
| Age, per 1 year increase | 151 | 1.09 | (1.01–1.18) | 0.635 | 0.034 |
| Gender, male vs. female | 151 | 6.46 | (1.45–28.72) | 0.643 | 0.014 |
| Modified BMI, per 1 kg·dL/m2·g increase | 151 | 0.98 | (0.96–1.00) | 0.603 | 0.085 |
| Coronary artery disease, yes vs. no | 151 | 1.89 | (0.70–5.08) | 0.568 | 0.210 |
| Elevated BNPa, yes vs. no | 119 | 2.96 | (1.07–8.19) | 0.627 | 0.037 |
| Systolic blood pressure, per 1 mmHg increase | 151 | 1.00 | (0.98–1.02) | 0.466 | 0.893 |
| QRS duration, per 1 ms increase | 142 | 1.02 | (1.01–1.03) | 0.672 | 0.021 |
| Albumin, per 1 g/dL increase | 151 | 0.55 | (0.22–1.36) | 0.597 | 0.195 |
| Mean gradient, per 1 mmHg increase | 148 | 0.97 | (0.93–1.00) | 0.617 | 0.061 |
| LVEF, per 1% decrease | 151 | 1.04 | (1.01–1.06) | 0.640 | 0.014 |
| LA dimension, per 1 mm increase | 141 | 1.10 | (1.03–1.17) | 0.702 | 0.003 |
| IVSD ≥1.2 vs. <1.2 cm | 147 | 4.10 | (1.52–11.03) | 0.664 | 0.005 |
| SV index <35 vs. ≥35 mL/m2 | 147 | 4.53 | (1.68–12.21) | 0.676 | 0.003 |
| Severe symptomatic AS stage | 0.605 | 0.058 | |||
| Stage D2 vs. D1 | 148 | 3.66 | (1.26–10.64) | 0.017 | |
| Stage D3 vs. D1 | 148 | 1.51 | (0.30–7.68) | 0.619 | |
| MCF, per 1 unit decrease | 147 | 1.10 | (1.05–1.15) | 0.789 | <0.0001 |
| Average mitral annular S’, per 1 cm/s decrease | 136 | 5.0 | (2.56–9.09) | 0.947 | <0.0001 |
| E/A ratio, per 1 unit increase | 136 | 2.08 | (1.39–3.11) | 0.719 | 0.0004 |
| Model selection: ‘final’ modelb | |||||
| Average mitral annular S’, per 1 cm/s decrease | 136 | 5.0 | (2.56–9.09) | 0.719 | <0.0001 |
ATTR-CA, transthyretin cardiac amyloidosis; TAVR, transcatheter aortic valve replacement; OR, odds ratio; CI, confidence interval; BNP, brain natriuretic peptide; BMI, body mass index; LVEF, left ventricular ejection fraction; LA, left atrium; AS, aortic stenosis; IVSD, interventricular septum at diastole.
Elevated BNP was designated for patients if BNP ≥300 pg/mL or NT proBNP ≥2000 pg/mL.
The ‘final’ model shows the unanimous recommendations of several model building strategies (backward, forward, and stepwise) that considered all non-collinear predictors with P < 0.25 from univariable regression.
It should be noted that five patients who had a positive 99mTc-PYP scan who met formal diagnostic criteria for ATTR-CA also had an abnormal monoclonal protein by international consensus criteria.19 Of these, two had a known lymphoproliferative disorder, e.g. monoclonal gammopathy of undetermined significance based on bone marrow biopsy and were followed by a haematologist. All five patients ultimately chose not to undergo tissue biopsy to rule out the possibility of AL CA.
Discussion
This prospective cohort study is the first to report: (i) a 16% prevalence of ATTR-CA in elderly patients undergoing TAVR, (ii) an echocardiographic phenotype of low-flow low-gradient AS with reduced LVEF and severe diastolic dysfunction in this patient population, and (iii) reduced myocardial systolic mechanics with average tissue Doppler mitral annular S’ independently associated with ATTR-CA.
Implications for severe as
The 16% prevalence of ATTR-CA that we report corroborates the 6–12% reported in smaller retrospective studies in patients with severe AS.25,26 The slightly higher prevalence is likely because our study population was older and exclusively underwent TAVR, not surgical AVR. Our findings also corroborate the 13% reported prevalence in a population of hospitalized patients with heart failure with preserved ejection fraction (HFpEF) and increased wall thickness.27 Given the high prevalence of calcific AS in the general population and the increasing frequency of TAVR in high and intermediate surgical risk older adults, it may be prudent to screen patients in whom there is suspicion of concomitant severe AS and ATTR-CA because management and prognosis of ATTR-CA differs from that for other cardiomyopathies. Prior reports have suggested the potential detrimental impact of ATTR-CA in patients with severe AS. At 2-year follow-up among 146 patients who underwent surgical AVR, the presence of ATTR-CA was associated with death with a hazard ratio of 9.5 (95% CI 2.5–35.8, P = 0.001).26 In a retrospective study of 171 patients with ATTR-CA with and without AS, mortality was the same irrespective of AVR, suggesting that ATTR-CA may have a deleterious contribution to outcomes.28 Whether ATTR-CA affects mortality synergistically with severe AS or as the primary driver needs further exploration in large cohorts.
The current study also suggests that 99mTc-PYP cardiac imaging can be used to screen for ATTR-CA in at risk populations. In Europe, 99mTc-DPD cardiac imaging has proven to be an effective screening tool for ATTR-CA, both among patients undergoing TAVR and HFpEF.25,27 Screening for ATTR-CA may identify ATTR-CA early in the setting of severe AS and/or HFpEF when emerging therapies may have greater benefit. Our data suggest that echocardiography may be an initial screen for ATTR-CA in this high risk, older population, with severe calcific AS. Patients diagnosed with ATTR-CA had specific features including male sex, concentric LV hypertrophy, severe diastolic dysfunction, and reduced myocardial mechanics (Figure 4). One would expect a Stage D3 low-flow AS with a preserved LVEF phenotype initially in patients with both AS and ATTR-CA that transitions to Stage D2 low-flow low-gradient AS with a low LVEF as systolic dysfunction ensues. The best independent echocardiographic predictor of ATTR-CA was an average mitral annular S’ ≤6 cm/s. The high sensitivity (100%) of a low mitral annular S ≤6 cm/s may enable clinicians to rule out ATTR-CA in subjects with velocities >6 and consider further testing with 99mTc-PYP cardiac imaging in the rest. While endomyocardial biopsy and tissue typing with histological staining and precursor protein confirmation using mass spectrometry remain the gold standard for diagnosis of ATTR-CA,29 these procedures often delay diagnosis and may not be appropriate in frail elderly adults, including those already undergoing TAVR. A positive radioisotope scan, such as 99mTc-PYP, in the absence of an abnormal monoclonal protein is 100% specific for ATTR-CA without the need for invasive heart biopsy. Furthermore, 99mTc-PYP cardiac imaging may have prognostic in addition to diagnostic importance in patients with ATTR-CA.18
Figure 4.
Prevalence and phenotype of ATTR-CA among patients undergoing transcatheter aortic valve replacement at our institution. ATTR-CA, transthyretin cardiac amyloidosis.
Potential mechanism of non-apical sparing in concomitant severe as and amyloid
Speckle strain imaging has demonstrated impaired LS at the base and mid LV with sparing of the apical segments as a predictive feature of cardiac amyloidosis.13 Cardiac magnetic resonance imaging data have demonstrated that the base and mid-LV segments are twice as thick in amyloid compared with non-amyloid controls, whereas apical thickness only increased by 26%.13 The apical sparing phenotype in ATTR-CA may reflect lower extracellular amyloid deposition at the apex, less resistance to myocardial deformation, and increased myocyte contraction relative to the other segments. In our cohort of patients with concomitant severe, symptomatic AS and ATTR-CA, apical sparing by speckle strain could not predict ATTR-CA. It is possible that the stress and afterload imposed on the ventricle by a severely calcified and stenotic aortic valve masks the sparing of apical strain that is otherwise detected in a patient with pure ATTR-CA without AS.
Limitations
This study was subject to the referral bias of an academic medical centre. Strain analysis was not performed universally as it could only be obtained in patients with adequate endocardial wall definition. While all patients underwent a 99mTc-PYP scan, cardiac biopsy was not performed given safety risks in this frail older population already undergoing TAVR. However, 99mTc-PYP is an excellent test for diagnosing advanced forms of ATTR-CA and may replace the need for endomyocardial biopsy.19 In the current study, of the five patients with ATTR-CA who had an abnormal monoclonal protein, two met the formal diagnostic criteria and had a known MGUS, but ultimately all five chose not to undergo tissue biopsy to formally rule out AL cardiac amyloidosis. The diagnostic utility of 99mTc-PYP cardiac imaging in early disease has not yet been studied, raising the possibility that ATTR-CA could have been missed among severe AS patients with early cardiac amyloidosis.
Conclusions
In this study of elderly patients with severe calcific AS undergoing TAVR, ATTR cardiac amyloidosis was prevalent at a rate of 16% and associated with a phenotype of low-flow, low gradient AS with a reduced LVEF. An average tissue Doppler mitral annular S’ of 6 cm/s or lower may be a sensitive measure that should prompt a confirmatory 99mTc-PYP scan and subsequent testing for ATTR-CA. Prospective assessment of outcomes after TAVR is needed in patients with and without ATTR-CA.
Acknowledgements
We thank David and Landon Storrs for their generous gift to support Cardiac Amyloidosis research. We also thank the Nurse Practitioners of the Heart Center Critical Care Unit and the Physician Assistants of the Heart Valve Team for their extraordinary care of these patients.
Conflict of interest: A.C. received salary support from the ACC/Merck and New York Academy of Medicine Fellowships in Cardiovascular Disease. S.K. receives consulting fees from Edwards Lifesciences and Medtronic but not in the past 12 months. R.H. is part of the Core Lab consortium for the PARTNER Trial but receives no direct compensation from industry. M.S.M. receives funding from an NIH K24 AG036778 Midcareer Mentoring Award in Geriatric Cardiology and his institution receives funding for research and serving on advisory boards and DSMBs from Pfizer Inc., Alnylam Pharmaceuticals Inc., ISIS Pharmaceuticals and Prothena Inc. D.L.N., N.H., O.K.K., A.D., J.R., C.C., T.N., T.V., I.G., M.B.L., S.B., and R.M. have no disclosures.
References
- 1. Thaden JJ, Nkomo VT, Enriquez-Sarano M.. The global burden of aortic stenosis. Prog Cardiovasc Dis 2014;56:565–571. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M; ESC Committee for Practice Guidelines (CPG); Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology; European Association for Cardio-Thoracic Surgery. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1–S44. [DOI] [PubMed] [Google Scholar]
- 3. Clark MA, Arnold SV, Duhay FG, Thompson AK, Keyes MJ, Svensson LG, Bonow RO, Stockwell BT, Cohen DJ.. Five-year clinical and economic outcomes among patients with medically managed severe aortic stenosis results from a medicare claims analysis. Circ-Cardiovasc Qual 2012;5:697–704. [DOI] [PubMed] [Google Scholar]
- 4. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:e57–e185. [DOI] [PubMed] [Google Scholar]
- 5. Dayan V, Vignolo G, Magne J, Clavel MA, Mohty D, Pibarot P.. Outcome and impact of aortic valve replacement in patients with preserved LVEF and low-gradient aortic stenosis. J Am Coll Cardiol 2015;66:2594–2603. [DOI] [PubMed] [Google Scholar]
- 6. Bavishi C, Balasundaram K, Argulian E.. Integration of flow-gradient patterns into clinical decision making for patients with suspected severe aortic stenosis and preserved LVEF: a systematic review of evidence and meta-analysis. JACC Cardiovasc Imaging 2016;9:1255–1263. [DOI] [PubMed] [Google Scholar]
- 7. Pibarot P, Dumesnil JG.. Paradoxical low-flow, low-gradient aortic stenosis: new evidence, more questions. Circulation 2013;128:1729–1732. [DOI] [PubMed] [Google Scholar]
- 8. Connors LH, Sam F, Skinner M, Salinaro F, Sun F, Ruberg FL, Berk JL, Seldin DC.. Heart failure resulting from age-related cardiac amyloid disease associated with wild-type transthyretin: a prospective, observational cohort study. Circulation 2016;133:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castano A, Drachman BM, Judge D, Maurer MS.. Natural history and therapy of TTR-cardiac amyloidosis: emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev 2015;20:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kristen AV, Schnabel PA, Winter B, Helmke BM, Longerich T, Hardt S, Koch A, Sack FU, Katus HA, Linke RP, Dengler TJ.. High prevalence of amyloid in 150 surgically removed heart valves–a comparison of histological and clinical data reveals a correlation to atheroinflammatory conditions. Cardiovasc Pathol 2010;19:228–235. [DOI] [PubMed] [Google Scholar]
- 11. Carroll JD, Gaasch WH, McAdam KP.. Amyloid cardiomyopathy: characterization by a distinctive voltage/mass relation. Am J Cardiol 1982;49:9–13. [DOI] [PubMed] [Google Scholar]
- 12. Authors/Task Force members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H.. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 13. Phelan D, Collier P, Thavendiranathan P, Popovic ZB, Hanna M, Plana JC, Marwick TH, Thomas JD.. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–1448. [DOI] [PubMed] [Google Scholar]
- 14. Quarta CC, Solomon SD, Uraizee I, Kruger J, Longhi S, Ferlito M, Gagliardi C, Milandri A, Rapezzi C, Falk RH.. Left ventricular structure and function in transthyretin-related versus light-chain cardiac amyloidosis. Circulation 2014;129:1840–1849. [DOI] [PubMed] [Google Scholar]
- 15. King DL, El-Khoury Coffin L, Maurer MS.. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol 2002;40:325–329. [DOI] [PubMed] [Google Scholar]
- 16. Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F, Maurer MS. ( 99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rapezzi C, Quarta CC, Guidalotti PL, Pettinato C, Fanti S, Leone O, Ferlini A, Longhi S, Lorenzini M, Reggiani LB, Gagliardi C, Gallo P, Villani C, Salvi F.. Role of (99m)Tc-DPD scintigraphy in diagnosis and prognosis of hereditary transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2011;4:659–670. [DOI] [PubMed] [Google Scholar]
- 18. Castano A, Haq M, Narotsky DL, Goldsmith J, Weinberg RL, Morgenstern R, Pozniakoff T, Ruberg FL, Miller EJ, Berk JL, Dispenzieri A, Grogan M, Johnson G, Bokhari S, Maurer MS.. Multicenter study of planar technetium 99m pyrophosphate cardiac imaging: predicting survival for patients with ATTR cardiac amyloidosis. JAMA Cardiol 2016;1:880–889. [DOI] [PubMed] [Google Scholar]
- 19. Gillmore JD, Maurer MS, Falk RH, Merlini G, Damy T, Dispenzieri A, Wechalekar AD, Berk JL, Quarta CC, Grogan M, Lachmann HJ, Bokhari S, Castano A, Dorbala S, Johnson GB, Glaudemans AW, Rezk T, Fontana M, Palladini G, Milani P, Guidalotti PL, Flatman K, Lane T, Vonberg FW, Whelan CJ, Moon JC, Ruberg FL, Miller EJ, Hutt DF, Hazenberg BP, Rapezzi C, Hawkins PN.. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation 2016;133:2404–2412. [DOI] [PubMed] [Google Scholar]
- 20. Suhr O, Danielsson A, Holmgren G, Steen L.. Malnutrition and gastrointestinal dysfunction as prognostic factors for survival in familial amyloidotic polyneuropathy. J Intern Med 1994;235:479–485. [DOI] [PubMed] [Google Scholar]
- 21. Bokhari S, Morgenstern R, Weinberg R, Kinkhabwala M, Panagiotou D, Castano A, DeLuca A, Andrew K, Jin Z, Maurer MS.. Standardization of 99mTechnetium pyrophosphate imaging methodology to diagnose TTR cardiac amyloidosis. J Nucl Cardiol 2016; doi: 10.1007/s12350-016-0610-4. [DOI] [PubMed] [Google Scholar]
- 22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 23. Efron B, Tibshirani R.. Improvements on cross validation: the .632+ bootstrap method. J Am Stat Assoc 1997;92:548–560. [Google Scholar]
- 24. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Member. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 25. Longhi S, Lorenzini M, Gagliardi C, Milandri A, Marzocchi A, Marrozzini C, Saia F, Ortolani P, Biagini E, Guidalotti PL, Leone O, Rapezzi C.. Coexistence of degenerative aortic stenosis and wild-type transthyretin-related cardiac amyloidosis. JACC Cardiovasc Imaging 2016;9:325–327. [DOI] [PubMed] [Google Scholar]
- 26. Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, Roberts N, Hutt DF, Rowczenio DM, Whelan CJ, Ashworth MA, Gillmore JD, Hawkins PN, Moon JC.. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis: prevalence and prognosis in patients undergoing surgical aortic valve replacement. Circ Cardiovasc Imaging 2016;9: [DOI] [PubMed] [Google Scholar]
- 27. González-López E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral F, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L, Garcia-Pavia P.. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 28. Sperry BW, Jones BM, Vranian MN, Hanna M, Jaber WA.. Recognizing transthyretin cardiac amyloidosis in patients with aortic stenosis: impact on prognosis. JACC Cardiovasc Imaging 2016;9:904–906. [DOI] [PubMed] [Google Scholar]
- 29. Satoskar AA, Efebera Y, Hasan A, Brodsky S, Nadasdy G, Dogan A, Nadasdy T.. Strong transthyretin immunostaining: potential pitfall in cardiac amyloid typing. Am J Surg Pathol 2011;35:1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]