Abstract
Aims
In patients with aortic stenosis (AS), risk stratification for aortic valve replacement (AVR) relies mainly on valve-related factors, symptoms and co-morbidities. We sought to evaluate the prognostic impact of a newly-defined staging classification characterizing the extent of extravalvular (extra-aortic valve) cardiac damage among patients with severe AS undergoing AVR.
Methods and results
Patients with severe AS from the PARTNER 2 trials were pooled and classified according to the presence or absence of cardiac damage as detected by echocardiography prior to AVR: no extravalvular cardiac damage (Stage 0), left ventricular damage (Stage 1), left atrial or mitral valve damage (Stage 2), pulmonary vasculature or tricuspid valve damage (Stage 3), or right ventricular damage (Stage 4). One-year outcomes were compared using Kaplan–Meier techniques and multivariable Cox proportional hazards models were used to identify 1-year predictors of mortality. In 1661 patients with sufficient echocardiographic data to allow staging, 47 (2.8%) patients were classified as Stage 0, 212 (12.8%) as Stage 1, 844 (50.8%) as Stage 2, 413 (24.9%) as Stage 3, and 145 (8.7%) as Stage 4. One-year mortality was 4.4% in Stage 0, 9.2% in Stage 1, 14.4% in Stage 2, 21.3% in Stage 3, and 24.5% in Stage 4 (Ptrend < 0.0001). The extent of cardiac damage was independently associated with increased mortality after AVR (HR 1.46 per each increment in stage, 95% confidence interval 1.27–1.67, P < 0.0001).
Conclusion
This newly described staging classification objectively characterizes the extent of cardiac damage associated with AS and has important prognostic implications for clinical outcomes after AVR.
Keywords: Aortic stenosis, Aortic valve, Aortic valve replacement, Transcatheter aortic valve replacement, Transcatheter aortic valve implantation, Classification, Staging
Introduction
Current recommendations for aortic valve replacement (AVR) in patients presenting with aortic stenosis (AS) rely solely on the presence of two criteria: (i) The demonstration of a severe stenosis based on a grading system that comprises specific valvular criteria, including peak aortic velocity (Vmax), mean transvalvular gradient, and aortic valve area or aortic valve area index (AVAi), and (ii) the presence or absence of symptoms (dyspnea, heart failure, angina, or syncope) related to AS.1,2 Additionally, risk stratification of patients for AVR is currently based on the presence of comorbidities.1,2 There are no recommendations regarding the importance of anatomical or functional cardiac consequences of AS as a component of the AVR decision algorithm, other than reduced left ventricular (LV) systolic function defined as a LV ejection fraction <50%, although the literature clearly documents that the presence of cardiac damage holds prognostic significance. In this report, from a large contemporary population of patients with symptomatic severe AS undergoing AVR (either surgery or transcatheter AVR), we sought to (i) better characterize the prevalence of concomitant cardiac damage at the time of AVR, (ii) categorize the different types of cardiac damage (LV dysfunction, left atrial enlargement, pulmonary hypertension, and right ventricular dysfunction) into distinct stages of cardiac disease involvement, and (iii) assess the impact of these stages on survival and the risk of adverse outcomes after AVR.
Methods
Study population
The design of the Placement of Aortic Transcatheter Valves (PARTNER) 2 trial has previously been described, including a detailed description of eligibility criteria and procedural methods.3,4 Data from the PARTNER 2A (N = 2032) and PARTNER 2B (N = 671) trials were pooled for this analysis. The PARTNER 2 Trials were approved by the institutional review board of each participating site and all patients provided written informed consent.
Definitions
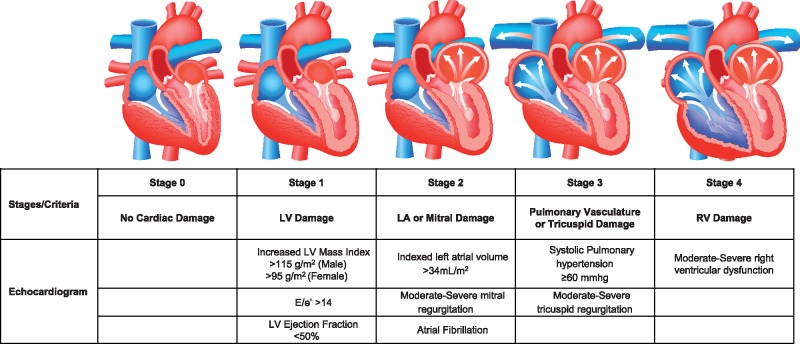
Patients were categorized into five stages (independent, not additive) depending on the presence or absence of extravalvular (extra aortic valve) cardiac damage or dysfunction as detected by transthoracic echocardiography before AVR—Stage 0: No other cardiac damage detected; Stage 1: LV damage as defined by presence of LV hypertrophy (LV mass index >95 g/m2 for women, >115 g/m2 for men),5 severe LV diastolic dysfunction (E/e′ > 14),6 or LV systolic dysfunction (LV ejection fraction <50%); Stage 2: LA or mitral valve damage or dysfunction as defined by the presence of an enlarged left atrium (>34 mL/m2), the presence of atrial fibrillation, or the presence of moderate or severe mitral regurgitation; Stage 3: Pulmonary artery vasculature or tricuspid valve damage or dysfunction as defined by the presence of systolic pulmonary hypertension (systolic pulmonary arterial pressure ≥60 mmHg) or the presence of moderate or severe tricuspid regurgitation7,8; and Stage 4: RV damage as defined by the presence of moderate or severe RV dysfunction (Figure 1).5,6,9,10 Patients were hierarchically classified in a given stage (worst stage) if at least one of the proposed criteria was met within that stage. These criteria were chosen based on their broad acceptance, prior validation as markers of abnormal cardiac function, their simplicity of acquisition, and their potential for future clinical external generalizability.1,2,5,6 The classification algorithm as well as the statistical models were defined fully a priori. Frailty was defined as the presence of at least two of the following criteria: (i) Katz index of independence in activities of daily living <6; (ii) 15-m walk time ≥24 s, (iii) serum albumin <3.8 g/dL, and (iv) grip strength <13 kg (women) or <26 kg (men).11
Figure 1.
Cardiac stratification of aortic stenosis based on the extent of cardiac damage. LA, left atrial; LV, left ventricular; RV, right ventricular.
Transthoracic echocardiograms were obtained at baseline and follow-up using a uniform image acquisition protocol. All studies were analysed by a central core laboratory with quality and measurement methodology previously reported.12,13 All adverse events were adjudicated by an independent committee.
Statistical analysis
Continuous data are presented as mean ± standard deviation and were compared between groups using the Student’s t-test or the Wilcoxon rank sum test, as appropriate. Categorical variables were presented as count and per cent, and compared using the χ2 or the Fisher exact test. We estimate time-to-event data using Kaplan–Meier techniques.
The unadjusted and adjusted risk of dying within 1 year according to stage of cardiac damage was assessed using Cox proportional hazards regression. Models were stratified by study (PARTNER 2A vs. PARTNER 2B) and treatment assignment (transcatheter vs. surgical), with the following variables included for multivariable adjustment: STS score, frailty, age, sex, O2-dependent chronic obstructive pulmonary disease (O2-COPD), coronary artery disease, renal insufficiency, prior myocardial infarction, prior coronary artery bypass grafting, diabetes, Vmax, and AVAi. In separate models, we assessed whether the variables STS score, frailty, O2-COPD, Vmax, or AVAi moderated the effect of stage of cardiac damage on mortality by including interaction terms between these variables and the stage of cardiac damage in the Cox models. We also tested for statistical interaction between the staging variable and study or treatment assignment; these latter two models were not stratified by study or treatment assignment. To determine the incremental value of the staging system in regards to 1-year mortality predictability, we calculated the net reclassification index (NRI) and the integrated discriminatory index (IDI) for the stage variable by comparing Cox models including vs. excluding the staging variable in addition to other patient characteristics.
To account for missing variables, we conducted separate sensitivity analyses using multiple imputations and generated 40 imputed datasets. In addition to the individual components of the staging algorithm, the following variables were included in the imputation model: Age, STS score, renal insufficiency, coronary artery disease, previous coronary artery bypass grafting, O2-COPD, Katz frailty index, 15-foot walk time, serum albumin, grip strength, study, treatment assignment, Vmax, and AVAi.
Results
Study population
From the PARTNER 2A (n = 1114) and PARTNER 2B (n = 547) trials, the primary study population comprised 1661 patients who had complete echocardiographic assessments at baseline, which allowed for patient staging (see Supplementary material online, Figure S1). A total of 1107 patients underwent transcatheter AVR, and 554 patients underwent surgical AVR. At the time of AVR, 47 (2.8%) patients were in Stage 0 (no cardiac damage), 212 (12.8%) patients were in Stage 1 (LV damage), 844 (50.8%) patients were in Stage 2 (LA or mitral valve damage), 413 (24.9%) patients were in Stage 3 (pulmonary vasculature or tricuspid valve damage), and 145 (8.7%) patients were in Stage 4 (RV damage) (Table 1). Baseline and procedural characteristics according to stage are presented in see Supplementary material online, Table S1. In general, patients in more advanced stages were older, were more often male, had higher body mass index, higher STS score and EuroSCORE, more often had diabetes, more often had previous myocardial infarction or coronary artery bypass grafting, and were more often frail and had O2-COPD. Rates of each individual cardiac damage component within each stage are presented in see Supplementary material online, Table S2, and rates of each prior stages within each stages are presented in see Supplementary material online, Figure S2.
Table 1.
Incidence of cardiac damage stages and their individual components
| Stages of cardiac damage | |
| Stage 0 (no cardiac damage) | 47/1661 (2.8%) |
| Stage 1 (left ventricular damage) | 212/1661 (12.8%) |
| Stage 2 (left atrial or mitral valve damage) | 844/1661 (50.8%) |
| Stage 3 (pulmonary vasculature or tricuspid valve damage) | 413/1661 (24.9%) |
| Stage 4 (right ventricular damage) | 145/1661 (8.7%) |
| Individual components of cardiac damage types among the study population | |
| Stage 1: Left ventricular damage | 1437/1654 (86.9%) |
| Increased left ventricular mass index (>115 g/m2 male; >95 female g/m2) | 1095/1586 (66.6%) |
| E/e′ >14 | 948/1375 (67.3%) |
| Ejection fraction <50% | 479/1436 (32.1%) |
| Stage 2: Left atrial or mitral valve damage | 1343/1661 (80.9%) |
| Indexed left atrial volume >34 mL/m2 | 1104/1590 (69.4%) |
| Moderate-severe mitral regurgitation | 425/1638 (20.7%) |
| Atrial fibrillation | 683/1661 (41.1%) |
| Stage 3: Pulmonary vasculature or tricuspid valve damage | 475/1654 (28.7%) |
| Pulmonary hypertension ≥60 mmHg | 140/1613 (8.7%) |
| Moderate-severe tricuspid regurgitation | 423/1648 (25.7%) |
| Stage 4: Right ventricular damage | 145/1661 (8.7%) |
| Moderate-severe right ventricular dysfunction | 145/1661 (8.7%) |
Outcomes
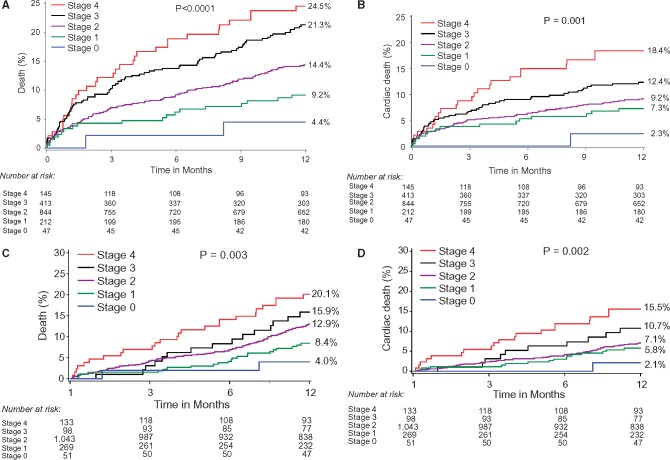
One-year outcomes after AVR stratified by stage of cardiac damage are presented in Table 2 and Figure 2. At 1 year, all-cause death (Figure 2A) and cardiac death (Figure 2B) significantly increased with each stage of worsening cardiac damage. Landmark analysis demonstrated consistent relationship between extent of cardiac damage prior to AVR and the occurrence of death or cardiac death (Figure 2C and D) beyond 30 days. Supplementary material online, Figure S3 presents 1-year adjusted survival curves for death and cardiac death, with consistent results. Supplementary material online, Figure S4 presents 1-year outcomes after excluding patients with prior coronary artery bypass grafting and O2-COPD. Results of all sensitivity analyses were consistent with the main analysis.
Table 2.
One-year outcomes
| Stage 0 (n = 47) | Stage 1 (n = 212) | Stage 2 (n = 844) | Stage 3 (n = 413) | Stage 4 (n = 145) | P-value (trend) | |
|---|---|---|---|---|---|---|
| All-cause death | 4.4% (2) | 9.2% (19) | 14.4% (116) | 21.3% (85) | 24.5% (33) | <0.001 |
| Cardiovascular death | 2.3% (1) | 7.3% (15) | 9.2% (73) | 12.4% (48) | 18.4% (24) | <0.001 |
| Non-cardiovascular death | 2.2% (1) | 2.1% (4) | 5.7% (43) | 10.2% (37) | 7.5% (9) | 0.0004 |
| Rehospitalization | 6.7% (3) | 17.0% (34) | 16.4% (128) | 21.1% (79) | 26.7% (34) | 0.0006 |
| Any stroke | 2.1% (1) | 6.4% (13) | 8.8% (71) | 6.9% (27) | 8.3% (11) | 0.51 |
| Major stroke | 0.0% (0) | 3.0% (6) | 6.8% (54) | 4.6% (18) | 5.3% (7) | 0.38 |
| Minor stroke | 2.1% (1) | 3.4% (7) | 2.2% (18) | 2.3% (9) | 3.0% (4) | 0.86 |
| Death, rehospitalization, and stroke | 11.0% (5) | 25.5% (53) | 28.9% (236) | 34.8% (140) | 37.3% (51) | <0.001 |
Values are % (n) or mean ± standard deviation. Values are estimated by Kaplan–Meier method, and do not account for competing risks.
Figure 2.
One-year outcomes after aortic valve replacement according to the extension of cardiac damage. (A) One-year all-cause death; (B) 1-year cardiac death; (C) all-cause death with 30-day landmark analysis (D) cardiac death with 30-day landmark analysis.
Predictors of 1-year death
After multivariable analysis and when tested in multiple models, stage of cardiac damage was shown to be one of the strongest predictors of 1-year death, with an adjusted mortality hazard of ∼1.45 with each increase in stage (Table 3). Frailty and O2-COPD were the only other identifiable predictors of 1-year death after AVR. Stage of cardiac damage had incremental value over other covariates for prediction of 1-year death after AVR, with NRI and IDI consistently showing improvement after the addition of the staging variable to several different nested models (see Supplementary material online, Table S3). In particular, the addition of stage of cardiac damage as a co-variable was shown to reclassify a significant proportion of patients compared with STS (NRI = 0.149) and frailty (NRI = 0.126) variables in regard to 1-year mortality prediction. No interaction was detected between AVR strategy (surgical AVR vs. transcatheter AVR) (Pinteraction = 0.28), STS (Pinteraction = 0.13), frailty (Pinteraction = 0.98), O2-COPD (Pinteraction = 1.00), AVAi (Pinteraction = 0.75), or Vmax (Pinteraction = 0.87) and stage of cardiac damage in regard to the occurrence of 1-year death.
Table 3.
One-year predictors of mortality among patients with severe aortic stenosis undergoing aortic valve replacement
| Variables | Unadjusted hazard ratio (95% confidence interval) | P-value | Adjusted hazard ratio (95% confidence interval) | P-value |
|---|---|---|---|---|
| Model 1 | ||||
| Stage of cardiac damage (by each stage increase) | 1.46 (1.27–1.67) | <0.0001 | 1.41 (1.20–1.66) | <0.0001 |
| Society of Thoracic Surgeons score (by each 1% increase) | 1.05 (1.02–1.08) | 0.0002 | 1.03 (0.99–1.07) | 0.10 |
| Frailty | 1.98 (1.48–2.64) | <0.0001 | 1.83 (1.35–2.49) | <0.0001 |
| Age (by each 10 years increase) | 1.13 (0.95–1.35) | 0.17 | 1.13 (0.89–1.43) | 0.32 |
| Male sex | 1.14 (0.89–1.47) | 0.28 | 1.25 (0.92–1.70) | 0.15 |
| O2-chronic obstructive pulmonary disease | 1.90 (1.31–2.76) | <0.0001 | 1.99 (1.24–3.17) | 0.004 |
| Renal insufficiency | 1.37 (0.90–2.08) | 0.14 | 0.79 (0.46–1.35) | 0.39 |
| Coronary artery disease | 0.89 (0.69–1.15) | 0.37 | 0.95 (0.69–1.29) | 0.73 |
| Previous coronary artery bypass graft | 0.91 (0.68–1.22) | 0.55 | 0.95 (0.65–1.37) | 0.77 |
| Vmax (by 0.5 m/s increase) | 0.91 (0.82–1.01) | 0.074 | 0.91 (0.80–1.04) | 0.17 |
| Aortic valve area index (by 0.1 cm2 decrease) | 0.96 (0.84–1.10) | 0.53 | 0.99 (0.84–1.16) | 0.88 |
| Model 2 | ||||
| Stage of cardiac damage (by each stage increase) | 1.46 (1.27–1.67) | <0.0001 | 1.41 (1.20–1.65) | <0.0001 |
| STS (by each 1% increase) | 1.05 (1.02–1.08) | <0.0001 | 1.03 (1.00–1.07) | 0.09 |
| Frailty | 1.98 (1.48–2.64) | <0.0001 | 1.81 (1.34–2.44) | <0.0001 |
| Age (by each 10 years increase) | 1.13 (0.95–1.35) | 0.17 | 1.07 (0.84–1.36) | 0.60 |
| Male sex | 1.14 (0.89–1.47) | 0.28 | 1.23 (0.92–1.66) | 0.17 |
| O2-chronic obstructive pulmonary disease | 1.90 (1.31–2.76) | 0.0008 | 2.01 (1.28–3.15) | 0.003 |
| Renal insufficiency | 1.37 (0.90–2.08) | 0.14 | 0.80 (0.47–1.36) | 0.41 |
| Previous myocardial infarction | 1.27 (0.94–1.71) | 0.11 | 1.25 (0.89–1.77) | 0.20 |
| Previous coronary artery bypass graft | 0.91 (0.68–1.22) | 0.55 | 0.90 (0.63–1.30) | 0.58 |
| Diabetes | 0.97 (0.74–1.26) | 0.80 | 0.89 (0.65–1.21) | 0.46 |
| Model 3 | ||||
| Stage of cardiac damage (by each stage increase) | 1.46 (1.27–1.67) | <0.0001 | 1.44 (1.23–1.70) | <0.0001 |
| Frailty | 1.98 (1.48–2.64) | <0.0001 | 1.82 (1.34–2.47) | 0.0001 |
| STS (by each 1% increase) | 1.05 (1.02–1.08) | 0.0002 | 1.03 (1.00–1.07) | 0.07 |
| Age (by each 10 years increase) | 1.13 (0.95–1.35) | 0.17 | 1.10 (0.86–1.41) | 0.43 |
| Male sex | 1.14 (0.89–1.47) | 0.28 | 1.26 (0.93–1.71) | 0.13 |
| O2-chronic obstructive pulmonary disease | 1.90 (1.31–2.76) | 0.0008 | 1.99 (1.25–3.17) | 0.004 |
| Renal insufficiency | 1.37 (0.90–2.08) | 0.14 | 0.77 (0.45–1.32) | 0.34 |
| Previous myocardial infarction | 1.27 (0.94–1.71) | 0.11 | 1.32 (0.93–1.87) | 0.12 |
| Previous coronary artery bypass graft | 0.91 (0.68–1.22) | 0.55 | 0.89 (0.62–1.29) | 0.55 |
| Diabetes | 0.97 (0.74–1.26) | 0.80 | 0.91 (0.67–1.25) | 0.56 |
| Aortic valve area index (by 0.1 cm2 decrease) | 0.96 (0.84–1.10) | 0.53 | 1.03 (0.89–1.19) | 0.68 |
Sensitivity analysis
Baseline characteristics and outcomes of patients included and excluded in the present study are presented in Supplementary material online, Tables S4 and S5. Stage of cardiac damage showed a strong unadjusted and adjusted association with mortality after multiple imputation of missing data (see Supplementary material online, Table S6), consistent with the main analysis.
Discussion
The current report, derived from 1661 patients who underwent comprehensive echocardiographic assessment before AVR, demonstrated a strong relationship between the extent of cardiac damage at baseline and 1-year survival after AVR. The proposed anatomic and functional cardiac damage staging system, was shown to be one of the strongest predictors of mortality. For each stage increment, 1-year mortality risk increased by ∼45%. Our report also demonstrated that this new staging system had significant incremental value for prediction of 1-year survival over several well-established predictors of worse outcomes after AVR, including patient frailty and the STS score.
Current guidelines recommend risk stratification of patients with AS using the integration of different variables including the severity of AS, the presence or absence of AS-related symptoms, and the presence of other risk factors such as STS score, frailty, or the compromise of other major organ systems (e.g. kidney disease, lung disease)1,2; however, no clear recommendation exists on how to incorporate the extent of consequential (or associated) cardiac damage in clinical decision making related to AS. Given the strong association demonstrated in this study between advanced staging of cardiac damage and worse clinical outcomes after AVR, consideration of the stage of AS-related cardiac damage in future recommendations for risk stratification might be useful.
Notably, we demonstrated that the extent of cardiac damage remains one of the strongest independent predictors of 1-year mortality post-AVR after controlling for important prognostic factors such as STS score and the presence of frailty, coronary artery disease, renal disease, or O2-COPD. After adjustment, only stage of cardiac damage, frailty, and O2-COPD remain predictors of 1-year mortality. This finding is important and may have identified meaningful variables that could be easily incorporated in a simple risk-prediction model beyond the standard STS. In the current report, a high proportion of patients enrolled in the two-pooled studies were at particularly high risk, with multiple comorbidities. Therefore, it is expected that in a lower risk population (i.e. with minimal comorbidities), the extent of cardiac damage at baseline could play an even greater role in risk prediction. Similarly, extent of cardiac damage was a considerably stronger predictor of adverse outcomes after AVR than both Vmax and AVAi, underscoring the fact that the valvular haemodynamic burden is effectively corrected by AVR whereas the detrimental impact of extravalvular consequences of AS often persists after AVR.
It is important to note that patients classified with more advanced stages also demonstrated higher non-cardiac mortality. This finding is not surprising since patients with more advanced cardiac disease are known to be more vulnerable to any other new insult (such as infection, bleeding, trauma, etc.), and to have a decreased physiological reserve to fight any additional disease process. That being said, identifying the exact cause of death among elderly patients with multiple co-morbidities could be challenging, with often an initial event triggering a cascade of consequences, ultimately leading to death.
Interestingly, the natural evolution or ‘propagation’ of AS associated cardiac damage doesn't seem to occur systematically in a sequential fashion. Indeed, among patients with more advanced stages (3 or 4), lower than expected proportion of patients cumulated damage from earlier stages (see Supplementary material online, Figure S2). For instance, among the 145 patients presenting with moderate to severe RV failure, only 25% presented with severe pulmonary hypertension, and ∼75% with left atrial enlargement (see Supplementary material online, Table S2). These finding are intriguing and may suggest that the extension of cardiac damages related to a LV pressure overload such as AS may not always be sequential (LV, LA, pulmonary vasculature, and then RV dysfunction), and may vary based on patient susceptibility or genetic predisposition.14–16 In this regard, recent studies demonstrated that the occurrence of LV hypertrophy or LV dysfunction may impact RV function early, either by ventricular interdependence, by contiguous extension of the pathological response to pressure overload, or by systemic hormonal response to left ventricle overload or genetic predisposition to hypertrophy affecting both ventricles.17–19 Further prospective mechanistic investigations related to the natural evolution of cardiac damages among patients with AS are needed to better characterize these hypothesis-generating findings.
Strengths and limitations
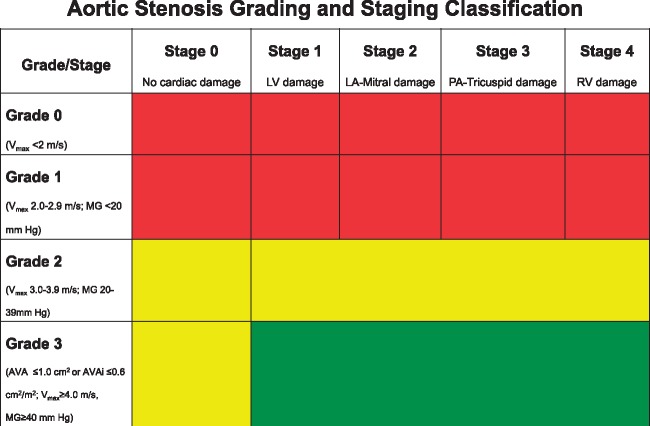
The current report has several strengths. It was derived from a substantial number of patients who underwent comprehensive echocardiographic analysis before AVR evaluated by an independent echocardiographic core laboratory, and it describes for the first time a simple and intuitive physiologic classification, reflecting the natural evolution of AS and bearing prognostic implications. Additionally, outside the current report, the proposed classification may have potential clinical and research utility: (i) It may improve clinical risk stratification of patients prior to AVR; (ii) it provides a tool for clinician to better communicate to patients risks, benefits, and expected prognostic after AVR; (iii) by acknowledging some of the technical challenges, variability, and discordances in echocardiographic acquisition of currently recommended severity grading criteria,20–29 the proposed multi-parametric stratification system may synergistically help to better define the optimal timing of AVR by focusing on the consequences and mechanical repercussion of AS (Figure 3); and (iv) it may represent a standardized tool to better describe, stratify, and quantify cardiac damage during a randomized trial or other research work, either at baseline or following diverse treatment or therapeutic strategies.
Figure 3.
Combination of the newly proposed cardiac damage staging classification and the valvular grading severity classification for patients presenting with aortic stenosis. Green represent a potential Class I aortic valve replacement (AVR) indication, yellow represents a potential Class IIa or IIb AVR indication, and red indicates a potential Class III AVR indication. AVA, aortic valve area; AVAi, aortic valve area index; LA, left atrial; MG, mean gradient; PA, pulmonary artery; RV, right ventricular.
The current report has many limitations. First, the described staging classification was retrospectively and not prospectively studied; future ongoing trials will be able to prospectively confirm the prognostic value of this new classification scheme (PARTNER 3 [NCT02675114], TAVR UNLOAD [NCT02661451], PROGRESSA [NCT01679431], EARLY TAVR NCT03042104). Second, a substantial amount of echocardiographic data were missing, leading to the exclusion of a high proportion of patients. That being said, our analysis is by far the largest cohort of patients with core laboratory adjudicated echocardiogram paired with independent events adjudication, making this manuscript unique. Third, the current staging system infers a direct causal role between the presence of AS and the detected cardiac damage. While concomitant comorbidities and diseases (e.g. severe coronary artery disease, severe lung disease) may co-exist and the detected cardiac damage may not be completely due to the AS, given the strong association with mortality, one could argue that this cardiac-oriented classification still has value when applied in a real-world setting. Indeed, patients with severe AS and several concomitant diseases (e.g. chronic obstructive pulmonary disease, ischaemic cardiomyopathy) are much more vulnerable to any new ‘insult’ (e.g. pneumonia), and any potential deterioration or extent of the already existent cardiac damage may not be well tolerated, leading to poor outcomes, even after successful AVR. However, after excluding patients with severe lung disease and prior coronary artery bypass grafting (severe CAD), our results were unchanged (see Supplementary material online, Figure S3), supporting the value of this classification among all patients with AS. Fourth, more detailed and granular detection of cardiac damage involving different imaging modalities exist. These include detection of reduced LV strain,30–33 LA strain,34 or RV strain35–37 by speckle tracking echocardiography and the presence of myocardial fibrosis by magnetic resonance imaging.38 When available, these findings could be incorporated into the appropriate anatomical staging level (i.e. reduced LV strain in Stage 1, reduced LA strain in Stage 2), resulting in the expansion and improvement of the proposed staging system. Similarly, serum biomarkers, with specific capability to identify LV, LA, or RV overload, could also eventually complement this classification.39–42 Fifth, outcomes were available up to 1 year only; however, longer-term follow-up (up to 5 years) would have most likely amplified the observed difference in mortality between each level of cardiac damage. Finally, the concept behind the proposed staging system could be adapted and applied to other valvular disease.
In conclusion, this new staging classification characterizes the extent of anatomical and functional cardiac damage associated with AS prior to AVR and has prognostic implications post-AVR. Further studies are needed to prospectively validate this classification across different AS severities and to better define how it could be integrated to existing grading severity system in guiding AVR timing for patients with AS
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The PARTNER 2 Trial was funded by Edwards Lifesciences.
Conflict of interest: P.G. has received consultant fees and speaker fees from Edwards Lifesciences. P.P. holds the Canada research Chair in Valvular Heart Diseases, Canadian Institutes of Health research, Ottawa, ON, Canada, has Core Lab contracts with Edwards Lifesciences for which he receives no direct compensation, and is a consultant for St Jude Medical. M.J.M. is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. R.R.M. has received grants from Edwards Lifesciences and St Jude Medical, is a consultant for Abbott Vascular, Cordis, and Medtronic, and holds equity in Entourage Medical. W.A.J. has Core Lab contracts with Edwards Lifesciences for which he receives no direct compensation. L.G.S. holds equity in Cardiosolution and Valvexchange, intellectual property with Postthorax, and is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. E.M.T. is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. V.H.T. is a consultant for Edwards Lifesciences, Abbott, Sorin Medical, St Jude Medical, and DirectFlow. V.B. is a consultant for Edwards Lifesciences and Abbott Vascular. H.C.H. has received grants from Edwards Lifesciences, St Jude Medical, Medtronic, Boston Scientific, Abbott Vascular, Gore, Siemens, Cardiokinetix, and Mitraspan, is a consultant for Edwards Lifesciences and Siemens, and holds equity in Microinterventional Devices. W.Y.S. is a consultant for Microinterventional Devices. D.J.C. has received research support from Edwards Lifesciences, Medtronic, and Boston Scientific and is a consultant for Edwards Lifesciences and Medtronic. B.R.L. was supported by K23 HL116660, serves on the advisory board of Roche Diagnostics, and has received grants from Edwards Lifesciences and Roche Diagnostics. M.C.A. is a consultant for Claret Medical. P.S.D. has Core Lab contracts with Edwards Lifesciences for which she receives no direct compensation. R.T.H. has Core Lab contracts with Edwards Lifesciences for which she receives no direct compensation and is a consultant for Philips Healthcare, St Jude Medical and Boston Scientific. S.K.K. is a consultant for Edwards Lifesciences and holds equity in Thubrikar Aortic Valve, Inc. C.R.S. is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. D.C.M. is supported by research grant R01 NHLBI #HL67025, has received consulting fees from Abbott Vascular, St Jude Medical, and Medtronic, and is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. J.G.W. is a consultant for Edwards Lifesciences and a member of the PARTNER Trial Executive Committee, for which he receives no direct compensation. M.B.L. is a member of the PARTNER Trial Executive Committee for which he receives no direct compensation. The other authors report no relevant relationships with industry to disclose.
Supplementary Material
References
- 1. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2438–2488. [DOI] [PubMed] [Google Scholar]
- 2. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M.. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 3. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG; PARTNER 2 Investigators. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 4. Webb JG, Doshi D, Mack MJ, Makkar R, Smith CR, Pichard AD, Kodali S, Kapadia S, Miller DC, Babaliaros V, Thourani V, Herrmann HC, Bodenhamer M, Whisenant BK, Ramee S, Maniar H Jr, Kereiakes D, Xu K, Jaber WA, Menon V, Tuzcu EM, Wood D, Svensson LG, Leon MB.. A randomized evaluation of the SAPIEN XT transcatheter heart valve system in patients with aortic stenosis who are not candidates for surgery. JACC Cardiovasc Interv 2015;8:1797–1806. [DOI] [PubMed] [Google Scholar]
- 5. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU.. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 6. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Popescu BA, Waggoner AD.. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 7. Lindman BR, Maniar HS, Jaber WA, Lerakis S, Mack MJ, Suri RM, Thourani VH, Babaliaros V, Kereiakes DJ, Whisenant B, Miller DC, Tuzcu EM, Svensson LG, Xu K, Doshi D, Leon MB, Zajarias A.. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valves II inoperable cohort. Circ Cardiovasc Interv 2015;8:e002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang M, Liu X, Lin C, He Y, Cai X, Xu Q, Hu P, Gao F, Jiang J, Lin X, Zhu Q, Wang L, Kong H, Yu Y, Wang J.. Meta-analysis of outcomes and evolution of pulmonary hypertension before and after transcatheter aortic valve implantation. Am J Cardiol 2017;119:91–99. [DOI] [PubMed] [Google Scholar]
- 9. Cavalcante JL, Simon MA, Chan SY.. Comprehensive right-sided assessment for transcatheter aortic valve replacement risk stratification: time for a change. J Am Soc Echocardiogr 2017;30:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz LA, Rozenbaum Z, Ghantous E, Kramarz J, Biner S, Ghermezi M, Shimiaie J, Finkelstein A, Banai S, Aviram G, Ingbir M, Keren G, Topilsky Y.. Impact of right ventricular dysfunction and tricuspid regurgitation on outcomes in patients undergoing transcatheter aortic valve replacement. J Am Soc Echocardiogr 2017;30:36–46. [DOI] [PubMed] [Google Scholar]
- 11. Green P, Arnold SV, Cohen DJ, Kirtane AJ, Kodali SK, Brown DL, Rihal CS, Xu K, Lei Y, Hawkey MC, Kim RJ, Alu MC, Leon MB, Mack MJ.. Relation of frailty to outcomes after transcatheter aortic valve replacement (from the PARTNER trial). Am J Cardiol 2015;116:264–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Douglas PS, Waugh RA, Bloomfield G, Dunn G, Davis L, Hahn RT, Pibarot P, Stewart WJ, Weissman NJ, Hueter I, Siegel R, Lerakis S, Miller DC, Smith CR, Leon MB.. Implementation of echocardiography core laboratory best practices: a case study of the PARTNER I trial. J Am Soc Echocardiogr 2013;26:348–358.e343. [DOI] [PubMed] [Google Scholar]
- 13. Hahn RT, Pibarot P, Stewart WJ, Weissman NJ, Gopalakrishnan D, Keane MG, Anwaruddin S, Wang Z, Bilsker M, Lindman BR, Herrmann HC, Kodali SK, Makkar R, Thourani VH, Svensson LG, Akin JJ, Anderson WN, Leon MB, Douglas PS.. Comparison of transcatheter and surgical aortic valve replacement in severe aortic stenosis: a longitudinal study of echocardiography parameters in cohort A of the PARTNER trial (placement of aortic transcatheter valves). J Am Coll Cardiol 2013;61:2514–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lindman BR, Clavel MA, Mathieu P, Iung B, Lancellotti P, Otto CM, Pibarot P.. Calcific aortic stenosis. Nat Rev Dis Primers 2016;2:16006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Potus F, Ruffenach G, Dahou A, Thebault C, Breuils-Bonnet S, Tremblay E, Nadeau V, Paradis R, Graydon C, Wong R, Johnson I, Paulin R, Lajoie AC, Perron J, Charbonneau E, Joubert P, Pibarot P, Michelakis ED, Provencher S, Bonnet S.. Downregulation of MicroRNA-126 contributes to the failing right ventricle in pulmonary arterial hypertension. Circulation 2015;132:932–943. [DOI] [PubMed] [Google Scholar]
- 16. Thanassoulis G, Campbell CY, Owens DS, Smith JG, Smith AV, Peloso GM, Kerr KF, Pechlivanis S, Budoff MJ, Harris TB, Malhotra R, O’Brien KD, Kamstrup PR, Nordestgaard BG, Tybjaerg-Hansen A, Allison MA, Aspelund T, Criqui MH, Heckbert SR, Hwang SJ, Liu Y, Sjogren M, van der Pals J, Kalsch H, Muhleisen TW, Nothen MM, Cupples LA, Caslake M, Di Angelantonio E, Danesh J, Rotter JI, Sigurdsson S, Wong Q, Erbel R, Kathiresan S, Melander O, Gudnason V, O’Donnell CJ, Post WS; CHARGE Extracoronary Calcium Working Group. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med 2013;368:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Friedberg MK, Redington AN.. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 2014;129:1033–1044. [DOI] [PubMed] [Google Scholar]
- 18. Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, Roger VL, Redfield MM.. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation 2014;130:2310–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Todiere G, Neglia D, Ghione S, Fommei E, Capozza P, Guarini G, Dell’omo G, Aquaro GD, Marzilli M, Lombardi M, Camici P, Pedrinelli R.. Right ventricular remodelling in systemic hypertension: a cardiac MRI study. Heart 2011;97:1257–1261. [DOI] [PubMed] [Google Scholar]
- 20. Berthelot-Richer M, Pibarot P, Capoulade R, Dumesnil JG, Dahou A, Thebault C, Le Ven F, Clavel MA.. Discordant grading of aortic stenosis severity: echocardiographic predictors of survival benefit associated with aortic valve replacement. JACC Cardiovasc Imaging 2016;9:797–805. [DOI] [PubMed] [Google Scholar]
- 21. Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M.. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329–2338. [DOI] [PubMed] [Google Scholar]
- 22. Genereux P, Stone GW, O’Gara PT, Marquis-Gravel G, Redfors B, Giustino G, Pibarot P, Bax JJ, Bonow RO, Leon MB.. Natural history, diagnostic approaches, and therapeutic strategies for patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol 2016;67:2263–2288. [DOI] [PubMed] [Google Scholar]
- 23. Mehrotra P, Flynn AW, Jansen K, Tan TC, Mak G, Julien HM, Zeng X, Picard MH, Passeri JJ, Hung J.. Differential left ventricular outflow tract remodeling and dynamics in aortic stenosis. J Am Soc Echocardiogr 2015;28:1259–1266. [DOI] [PubMed] [Google Scholar]
- 24. Kamperidis V, van Rosendael PJ, Katsanos S, van der Kley F, Regeer M, Al Amri I, Sianos G, Marsan NA, Delgado V, Bax JJ.. Low gradient severe aortic stenosis with preserved ejection fraction: reclassification of severity by fusion of Doppler and computed tomographic data. Eur Heart J 2015;36:2087–2096. [DOI] [PubMed] [Google Scholar]
- 25. Ng AC, Yiu KH, Ewe SH, van der Kley F, Bertini M, de Weger A, de Roos A, Leung DY, Schuijf JD, Schalij MJ, Bax JJ, Delgado V.. Influence of left ventricular geometry and function on aortic annular dimensions as assessed with multi-detector row computed tomography: implications for transcatheter aortic valve implantation. Eur Heart J 2011;32:2806–2813. [DOI] [PubMed] [Google Scholar]
- 26. Clavel MA, Malouf J, Messika-Zeitoun D, Araoz PA, Michelena HI, Enriquez-Sarano M.. Aortic valve area calculation in aortic stenosis by CT and Doppler echocardiography. JACC Cardiovasc Imaging 2015;8:248–257. [DOI] [PubMed] [Google Scholar]
- 27. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N.. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients with apparently normal left ventricular function. Heart 2010;96:1463–1468. [DOI] [PubMed] [Google Scholar]
- 28. Simard L, Cote N, Dagenais F, Mathieu P, Couture C, Trahan S, Bosse Y, Mohammadi S, Page S, Joubert P, Clavel MA.. Sex-related discordance between aortic valve calcification and hemodynamic severity of aortic stenosis: is valvular fibrosis the explanation? Circ Res 2017;120:681–691. [DOI] [PubMed] [Google Scholar]
- 29. Lancellotti P, Magne J, Donal E, Davin L, O’Connor K, Rosca M, Szymanski C, Cosyns B, Pierard LA.. Clinical outcome in asymptomatic severe aortic stenosis: insights from the new proposed aortic stenosis grading classification. J Am Coll Cardiol 2012;59:235–243. [DOI] [PubMed] [Google Scholar]
- 30. Yingchoncharoen T, Gibby C, Rodriguez LL, Grimm RA, Marwick TH.. Association of myocardial deformation with outcome in asymptomatic aortic stenosis with normal ejection fraction. Circ Cardiovasc Imaging 2012;5:719–725. [DOI] [PubMed] [Google Scholar]
- 31. Kusunose K, Goodman A, Parikh R, Barr T, Agarwal S, Popovic ZB, Grimm RA, Griffin BP, Desai MY.. Incremental prognostic value of left ventricular global longitudinal strain in patients with aortic stenosis and preserved ejection fraction. Circ Cardiovasc Imaging 2014;7:938–945. [DOI] [PubMed] [Google Scholar]
- 32. Dahl JS, Videbaek L, Poulsen MK, Rudbaek TR, Pellikka PA, Moller JE.. Global strain in severe aortic valve stenosis: relation to clinical outcome after aortic valve replacement. Circ Cardiovasc Imaging 2012;5:613–620. [DOI] [PubMed] [Google Scholar]
- 33. Dahou A, Bartko PE, Capoulade R, Clavel MA, Mundigler G, Grondin SL, Bergler-Klein J, Burwash I, Dumesnil JG, Senechal M, O’Connor K, Baumgartner H, Pibarot P.. Usefulness of global left ventricular longitudinal strain for risk stratification in low ejection fraction, low-gradient aortic stenosis: results from the multicenter True or Pseudo-Severe Aortic Stenosis study. Circ Cardiovasc Imaging 2015;8:e002117.. [DOI] [PubMed] [Google Scholar]
- 34. Galli E, Fournet M, Chabanne C, Lelong B, Leguerrier A, Flecher E, Mabo P, Donal E.. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: a 2D speckle-tracking echocardiographic study. Eur Heart J Cardiovasc Imaging 2016;17:533–541. [DOI] [PubMed] [Google Scholar]
- 35. Cavalcante JL, Rijal S, Althouse AD, Delgado-Montero A, Katz WE, Schindler JT, Crock F, Harinstein ME, Navid F, Gleason TG, Lee JS.. Right ventricular function and prognosis in patients with low-flow, low-gradient severe aortic stenosis. J Am Soc Echocardiogr 2016;29:325–333. [DOI] [PubMed] [Google Scholar]
- 36. Dahou A, Clavel MA, Capoulade R, Bartko PE, Magne J, Mundigler G, Bergler-Klein J, Burwash I, Mascherbauer J, Ribeiro HB, O’Connor K, Baumgartner H, Senechal M, Dumesnil JG, Rosenhek R, Mathieu P, Larose E, Rodes-Cabau J, Pibarot P.. Right ventricular longitudinal strain for risk stratification in low-flow, low-gradient aortic stenosis with low ejection fraction. Heart 2016;102:548–554. [DOI] [PubMed] [Google Scholar]
- 37. Galli E, Guirette Y, Feneon D, Daudin M, Fournet M, Leguerrier A, Flecher E, Mabo P, Donal E.. Prevalence and prognostic value of right ventricular dysfunction in severe aortic stenosis. Eur Heart J Cardiovasc Imaging 2015;16:531–538. [DOI] [PubMed] [Google Scholar]
- 38. Barone-Rochette G, Pierard S, De Meester de Ravenstein C, Seldrum S, Melchior J, Maes F, Pouleur AC, Vancraeynest D, Pasquet A, Vanoverschelde JL, Gerber BL.. Prognostic significance of LGE by CMR in aortic stenosis patients undergoing valve replacement. J Am Coll Cardiol 2014;64:144–154. [DOI] [PubMed] [Google Scholar]
- 39. Clavel MA, Malouf J, Michelena HI, Suri RM, Jaffe AS, Mahoney DW, Enriquez-Sarano M.. B-type natriuretic peptide clinical activation in aortic stenosis: impact on long-term survival. J Am Coll Cardiol 2014;63:2016–2025. [DOI] [PubMed] [Google Scholar]
- 40. Lindman BR, Breyley JG, Schilling JD, Vatterott AM, Zajarias A, Maniar HS, Damiano RJ Jr, Moon MR, Lawton JS, Gage BF, Sintek MA, Aquino A, Holley CL, Patel NM, Lawler C, Lasala JM, Novak E.. Prognostic utility of novel biomarkers of cardiovascular stress in patients with aortic stenosis undergoing valve replacement. Heart 2015;101:1382–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR.. High-sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J 2014;35:2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goodman A, Kusunose K, Popovic ZB, Parikh R, Barr T, Sabik JF, Rodriguez LL, Svensson LG, Griffin BP, Desai MY.. Synergistic utility of brain natriuretic peptide and left ventricular strain in patients with significant aortic stenosis. J Am Heart Assoc 2016;5:e002561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.