Abstract
Background
Hyperphosphataemia is an independent risk factor for accelerated cardiovascular disease in chronic kidney disease (CKD), although the mechanism for this is poorly understood. We investigated the effects of sustained exposure to a high-phosphate environment on endothelial function in cellular and preclinical models, as well as in human subjects.
Methods
Resistance vessels from rats and humans (± CKD) were incubated in a normal (1.18 mM) or high (2.5 mM) phosphate concentration solution and cells were cultured in normal- (0.5 mM) or high-phosphate (3 mM) concentration media. A single-blind crossover study was performed in healthy volunteers, receiving phosphate supplements or a phosphate binder (lanthanum), and endothelial function measured was by flow-mediated dilatation.
Results
Endothelium-dependent vasodilatation was impaired when resistance vessels were exposed to high phosphate; this could be reversed in the presence of a phosphodiesterase-5-inhibitor. Vessels from patients with CKD relaxed normally when incubated in normal-phosphate conditions, suggesting that the detrimental effects of phosphate may be reversible. Exposure to high-phosphate disrupted the whole nitric oxide pathway with reduced nitric oxide and cyclic guanosine monophosphate production and total and phospho endothelial nitric oxide synthase expression. In humans, endothelial function was reduced by chronic phosphate loading independent of serum phosphate, but was associated with higher urinary phosphate excretion and serum fibroblast growth factor 23.
Conclusions
These directly detrimental effects of phosphate, independent of other factors in the uraemic environment, may explain the increased cardiovascular risk associated with phosphate in CKD.
Keywords: cardiovascular risk, chronic kidney disease, endothelial function, nitric oxide, phosphate
INTRODUCTION
Hyperphosphataemia is an independent risk factor for accelerated cardiovascular disease (CVD) in chronic kidney disease (CKD) [1–7]. CVD is more prevalent in CKD patients than in the general population and is the biggest single contributor to markedly reduced life expectancy, with a predominance of sudden death and heart failure, rather than coronary artery disease [8–10]. The relationship between conventional CV risk factors and CV events in CKD has proved difficult to define and focus has turned to non-traditional risk factors and therapeutic targets, such as hyperphosphataemia [5, 11–14]. Based on observational data linking elevated phosphate with adverse outcomes, most patients with advanced CKD receive phosphate-lowering treatment with oral phosphate binders, although we lack specific evidence on the cardiac and survival benefit of this therapeutic strategy [4, 6, 15–23]. There is also strong evidence that ‘normal’ phosphate levels at the upper limit of the reference range increase the CV risk in other populations, including recipients of a renal transplant, healthy individuals or individuals without CKD but with pre-existing CV disease [12, 14, 22, 24]. One explanation may be that serum phosphate is a poor measure of total body phosphate, as phosphate is largely an intracellular anion. This is reflected in CKD, where the CV risk is increased before phosphate levels rise above the reference range—phosphate seems to be a continuous risk factor, although a phosphate level within the reference range is largely ignored in CKD [15].
Phosphate levels can be easily manipulated. Current means of modification may be suboptimal in terms of risk reduction and because there is limited information about phosphate's mechanism of action as a CV risk factor, it has proved difficult to determine the best approach to altering phosphate levels [25]. The established view is that elevated phosphate contributes to vascular calcification and thus CVD [26–28]. However, alternative mechanisms include direct effects of phosphate on endothelial function, and effects mediated by regulators of phosphate homoeostasis, including fibroblast growth factor 23 (FGF-23), a phosphaturic hormone implicated in cardiac hypertrophy, another common feature of CKD [4, 28–31]. In reality it is likely to be a combination of these effects that contributes to CV risk in CKD. Defining the isolated actions of elevated phosphate independent of other abnormalities of the uraemic environment is challenging.
Emerging evidence supports direct effects of phosphate on vascular function. Shuto et al. demonstrated impaired endothelial dysfunction in healthy males following a single high-phosphate content meal, consistent with an acute effect of phosphate [32]. In the same study, endothelium-dependent vasodilatation was impaired in rat aortic rings exposed acutely to high-phosphate concentration. In a preclinical rat model of adenine-induced CKD, aortic rings from rats fed a low-phosphate diet for 16 days exhibited significantly improved endothelium-dependent vasodilatation compared with animals fed standard rat chow [28]. In cells in culture, there is evidence of disruption of the nitric oxide (NO) pathway upon exposure to both a low- and high-phosphate environment, although the authors attributed this to the concomitant reduction in intracellular calcium [33].
Overall, these elegant studies support direct, acute effects of phosphate on vascular cells and function but they do not explore more chronic effects that are relevant to CKD, nor the mechanisms or potential for new therapeutic approaches.
Endothelial dysfunction is a feature of CKD and may contribute to CV risk, probably via disruption of the NO pathway [32, 34, 35]. We hypothesized that phosphate has direct effects on endothelial function via dysregulation of the NO pathway and this explains the association between phosphate and CV risk. We show, for the first time, in translational cell to animal to human studies, the direct effects of prolonged exposure to elevated phosphate concentration on vascular and endothelial function, specifically examining the isolated actions of elevated phosphate independent of other effects of the uraemic environment.
MATERIALS AND METHODS
For detailed materials and methods, see Supplementary Methods online.
Vessel studies
Rat mesenteric vessels were utilized and human resistance vessels came from subcutaneous abdominal fat, removed prior to the use of diathermy or the harmonic scalpel, at the beginning of surgery. Twelve-week-old male Wistar-Kyoto rats were sacrificed in accordance with the Animals Scientific Procedures Act 1986. Live kidney donors (LKDs) undergoing nephrectomy for living kidney donation and patients with CKD undergoing live donor renal transplant were identified. Blood samples were collected the day prior to surgery. All myography experiments were performed on a four-chamber wire myograph. Experiments were conducted after storage of the vessels at 4°C in a normal- (1.18 mM) or high-phosphate (2.5 mM) concentration solution for 16 h (Supplementary data, Table S1).
Statistical analysis
All responses are expressed as mean ± standard error of the mean (SEM) and comparison made between the areas under the curve (AUC) of groups with Student's t test, unless otherwise stated. For comparisons between maximal vasodilation or contractile responses, an unpaired Student's t test (rat vessels) or an ANOVA with Tukey's post hoc analysis (human vessels) was used. The median L100 (rat vessels only) and the median vessel lengths were compared with a Mann–Whitney U test. Statistical analysis was performed in SPSS v 19 (IBM, Armonk, NY, USA).
Cell culture
Cell media was either of standard phosphate concentration (0.5 mM) or custom formulated (phosphate concentration 3 mM). Cells were grown in standard phosphate concentration medium until they reached 90% confluence. At the first passage, they were divided into standard and high-phosphate concentration cells and grown in the appropriate media from that point.
Clinical study
This was a single-blind crossover study with healthy volunteers without CKD. Volunteers were screened to ensure they were ‘healthy’ prior to inclusion. Each participant attended three visits. At visit one, patients were randomized to receive either 500 mg phosphate or 1000 mg of lanthanum three times daily for 2 weeks. The participants knew which tablet they were taking but the investigator did not. After 2 weeks, patients attended for the second visit. After a further 2-week washout period, patients received the other drug for 2 weeks, before attending for the final visit. Supplementary data, Figure S1 illustrates the study visit protocol. At each visit, the same measures were taken and pill counts were performed to assess compliance.
Serum and plasma samples were sent to the laboratory and blood was stored at −80°C for FGF-23, cyclic guanosine monophosphate (cGMP) and vitamin D analysis. Prior to each visit, a 24 h urine collection was obtained.
Endothelial function was measured using flow-mediated dilatation (FMD) and measurements were standardized [36]. All recordings and analyses were performed by one investigator. Vascular stiffness was measured using the SphygmoCor® Vx system and standardized [37].
Statistical analysis
Data were assessed for normality. Univariate and multivariate linear regression models were constructed with change in FMD from baseline as the measure of outcome. The crossover nature of the study utilizing the same patients for each arm was taken into account and randomization order was included in the model. One-third of the images were randomly selected and reanalysed to test reproducibility and intra-observer variability.
RESULTS
We exposed cells and vessels to two phosphate concentrations, normal and high (0.5 and 3 mM in cell lines and 1.18 and 2.5 mM in vessels). For context, 0.5 mM is the standard concentration of phosphate in the conventional cell culture medium suited to the cells utilized, 0.8–1.4 mM is the normal plasma range in humans, whilst 2.5–3 mM is at the extreme of the CKD spectrum.
Effect of phosphate on the function of rat and human vessels in vitro
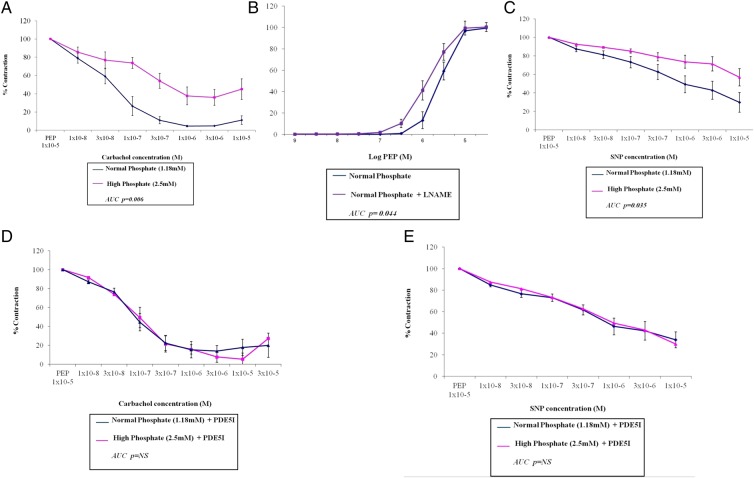
Table 1 shows the numbers of rat mesenteric vessels used and their normalized internal diameter (L100), which was not significantly different between the groups. In phenylephrine (PEP) pre-constricted vessels, the endothelium-dependent vasodilator carbachol was less effective in high- compared with normal-phosphate conditions (Figure 1A; maximum vasodilatation 64 ± 9% versus 95 ± 1%; P < 0.001). When we assessed basal NO production using the NO synthase inhibitor L-NAME (LN), we found that in normal-phosphate vessels, LN shifted the concentration–response curve to PEP significantly to the left compared with contraction in the absence of LN (Figure 1B). In high-phosphate conditions, no significant shift was observed (EC50 + LN: 4.5 ± 2.1 × 10−6 M versus EC50-LN: 5.3 ± 2.8 × 10−6 M; data not shown). To assess endothelium-independent vasodilatation, we used the NO donor sodium nitroprusside (SNP). Vasodilatation to SNP was reduced in high- versus normal-phosphate conditions (Figure 1C; maximal vasodilatation 45 ± 22% versus 73 ± 26; P = 0.02). Adding zaprinast, a phosphodiesterase (PDE5) inhibitor (1 × 10−5 M for 1 h), to increase levels of cGMP reversed the effects of high phosphate, improving vasodilator responses to carbachol and SNP (Figure 1D and E).
Table 1.
Comparison of numbers of vessels studied for each drug and the corresponding size of the vessels
| Incubated in standard phosphate | Incubated in high phosphate | P | |
|---|---|---|---|
| Phenylephrine | |||
| Number | 12 | 10 | |
| L100 | 366.5 (292.7–422.3) | 401.6 (226.3–525.4) | NS |
| Carbachol | |||
| Number | 12 | 10 | |
| L100 | 366.5 (292.7–422.3) | 401.6 (226.3–525.4) | NS |
| L-Name | |||
| Number | 12 | 10 | |
| L100 | 366.5 (292.7–422.3) | 401.6 (226.3–525.4) | NS |
| SNP | |||
| Number | 11 | 9 | |
| L100 | 391.9 (311.2–504.3) | 396.9 (267.5–525.3) | NS |
| Carbachol and PDE5I | |||
| Number | 10 | 13 | |
| L100 | 387.2 (324.2–412.1) | 384.1 (341–411.6) | NS |
| SNP and PDE5I | |||
| Number | 10 | 13 | |
| L100 | 387.2 (324.2–412.1) | 384.1 (341–411.6) | NS |
Number is the number of vessels for which a complete concentration response curve was obtained. L100 is the normalized internal diameter of the vessels with comparison by the Mann–Whitney U test.
PDE5I, phosphodiesterase-5 inhibitor (zaprinast); SNP, sodium nitroprusside; NS, not significant.
FIGURE 1.
Rat mesenteric vessels incubated in a high-phosphate concentration (2.5 mM) solution have impaired endothelium-dependent and -independent vasodilatation, an effect that can be reversed when the vessels are co-incubated with a phosphodiesterase-5 inhibitor (PDE5I), zaprinast. Vessels were incubated for 16 h in either a normal (1.18 mM) or high (2.5 mM) phosphate concentration solution and all responses are expressed as mean ± SEM. (A and C) Vasodilatation to increasing concentrations of carbachol or SNP, respectively, expressed as a % of maximal contraction with PEP 1 × 10−5 M. (B) Contraction with PEP in the presence and absence of L-NAME. (D and E) Vasodilatation to increasing concentrations of carbachol or SNP, respectively, in the presence of zaprinast (PDE5I) expressed as a % of maximal contraction with PEP 1 × 10−5 M. n = 10–13 vessels (see Table 1).
These data are consistent with impaired endothelium-dependent and -independent vasodilation and reduced basal NO production in high-phosphate conditions, effects that are potentially reversible.
To determine whether these findings could be translated to humans, we studied resistance vessels from LKDs and patients with CKD stage 5 (Table 2). Vessels were obtained at the time of live donor nephrectomy or at the time of transplantation. Comparisons were made between subjects with and without CKD. The former had higher serum phosphate and FGF-23 levels. Eighty-nine per cent (n = 8) received a phosphate binder. No patient was taking drugs that interfere with NO metabolism. Table 3 details the vessels used.
Table 2.
Demographics of the study population
| Parameter | Live donor | CKD | P |
|---|---|---|---|
| Age (years) | 48.8 ± 10.2 | 44.6 ± 14.5 | NS |
| Male sex (%) | 64 | 44 | |
| Systolic BP | 134 ± 16 | 134 ± 24 | NS |
| BMI | 29.5 ± 3.6 | 25.4 ± 3.4 | 0.02 |
| Dialysis (%) | 0 | 44 | |
| eGFR (mL/min/1.73 m2) | 95.8 ± 21 | 8.0 ± 3.0 | <0.001 |
| Phosphate (mmol/L) | 1.1 ± 0.1 | 1.7 ± 0.6 | 0.003 |
| Adjusted calcium (mmol/L) | 2.4 ± 0.1 | 2.5 ± 0.1 | NS |
| FGF-23 (RU/mL) | 45.8 (40.5–51.7) | 1644 (1135.7–1750.4) | <0.001 |
| On any medication (%) | 18 | 100 | |
| Calcium-containing phosphate binder (%) | 0 | 33 | |
| Non-calcium-containing phosphate binder (%) | 0 | 67 | |
| Alfacalcidol (%) | 0 | 78 | |
| Cinacalcet (%) | 0 | 0 | |
| Antihypertensive (%) | 18 | 89 |
BP, blood pressure; BMI, body mass index; NS, not significant.
Live donor, vessels from live donor nephrectomy; CKD, vessels from recipients of live kidney transplant. Demographics for the patients whose vessels were used are shown in columns two to four. Live donor, n = 14 and CKD, n = 14.
Table 3.
Comparison of numbers of vessels studied for each patient group
| CKD |
Live donor |
||||
|---|---|---|---|---|---|
| Normal | High | Normal | High | ||
| Length | 4.76 ± 0.36 | 4.52 ± 0.49 | 4.59 ± 0.35 | 4.51 ± 0.44 | |
| Diameter | 494.3 (339–700) | 460.9 (340–792) | 534.2 (286–879) | 438.4 (297–684) | |
| Phenylephrinea | 12 | 9 | 13 | 12 | |
| Carbachola | 12 | 9 | 11 | 12 | |
| SNPa | 12 | 9 | 13 | 12 | |
Measurements are in microns.
The number of vessels in which a complete concentration response curve was obtained for each drug and the corresponding size of the vessels are presented. Comparison was made with a one-way ANOVA.
aThese values represent the number of vessels used (n).
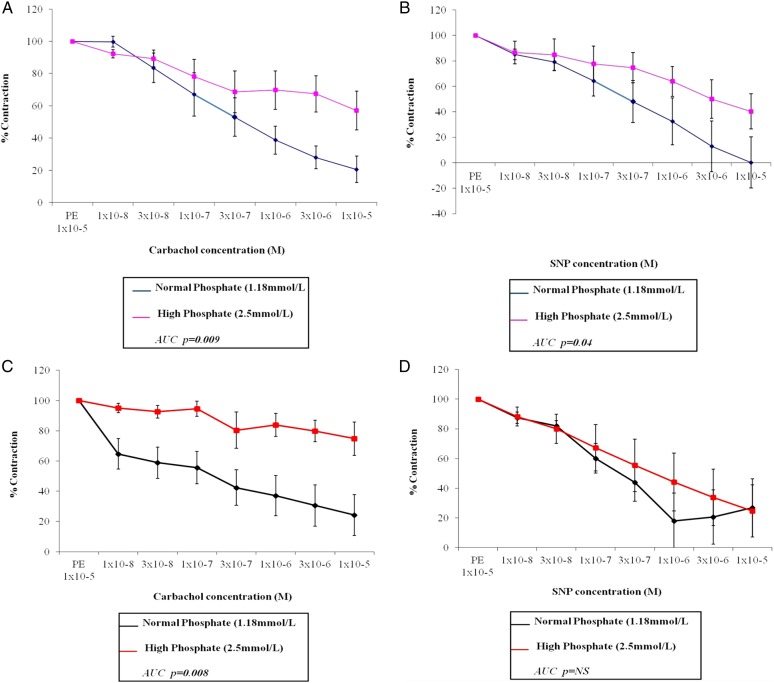
In LKD vessels, in high-phosphate compared with normal-phosphate conditions, vasodilatation to carbachol was impaired (Figure 2A; maximum vasodilatation 42.9 ± 12% versus 79.4 ± 8.2%; P = 0.003). Similarly, high-phosphate LKD vessels vasodilated less well to SNP compared with normal-phosphate vessels (Figure 2B; maximum vasodilatation 59.7 ± 13.8% versus 99.8 ± 20.2%; P = 0.02). Impaired vasodilation with carbachol was also observed in CKD vessels incubated in high- versus normal-phosphate conditions (Figure 2C; maximum vasodilatation 25.3 ± 11.1% versus 75.7 ± 13.6%; P < 0.001).
FIGURE 2.
Human resistance vessels from patients with (C) and without (A) CKD incubated in a high-phosphate concentration (2.5 mM) solution have impaired endothelium-dependent vasodilatation. Endothelium-independent vasodilatation is impaired in vessels from humans without CKD (B) but preserved in those with CKD (D). Vessels were incubated for 16 h in either a normal (1.18 mM) or high (2.5 mM) phosphate concentration solution and all responses are expressed as mean ± SEM. (A and C) Vasodilatation to increasing concentrations of carbachol expressed as a % of contraction with PEP 1 × 10−5 M in vessels from living kidney donors without CKD (A) and from patients with CKD stage 5 (C). (B and D) Vasodilatation to increasing concentrations of SNP expressed as a % of contraction with PEP 1 × 10−5 M in vessels from living kidney donors without CKD (B) and from patients with CKD stage 5 (D). n = 9–14 vessels (see Table 3).
Interestingly, there was no significant difference in either AUC or maximal relaxation between the CKD and the LKD vessels incubated in normal-phosphate conditions, supporting the notion that the effects of phosphate may be reversible. There was no significant difference in relaxation to SNP between the CKD vessels incubated in high- or normal-phosphate conditions (Figure 2D). In LKD with normal renal function, both endothelium-dependent and -independent vasodilation are impaired, consistent with the effects observed in our preclinical model. In CKD resistance vessels endothelium-dependent vasodilation was blunted in high-phosphate conditions, but when incubated ex vivo in a normal-phosphate solution, the relaxation response was similar to that of healthy LKD vessels.
Effect of phosphate on the NO pathway
Since the data above, in isolated vessels, suggest a direct and reversible effect of phosphate on the endothelium mediated by disruption of both basal and stimulated NO production, we next explored the NO pathway further by examining the effects of phosphate in cell lines.
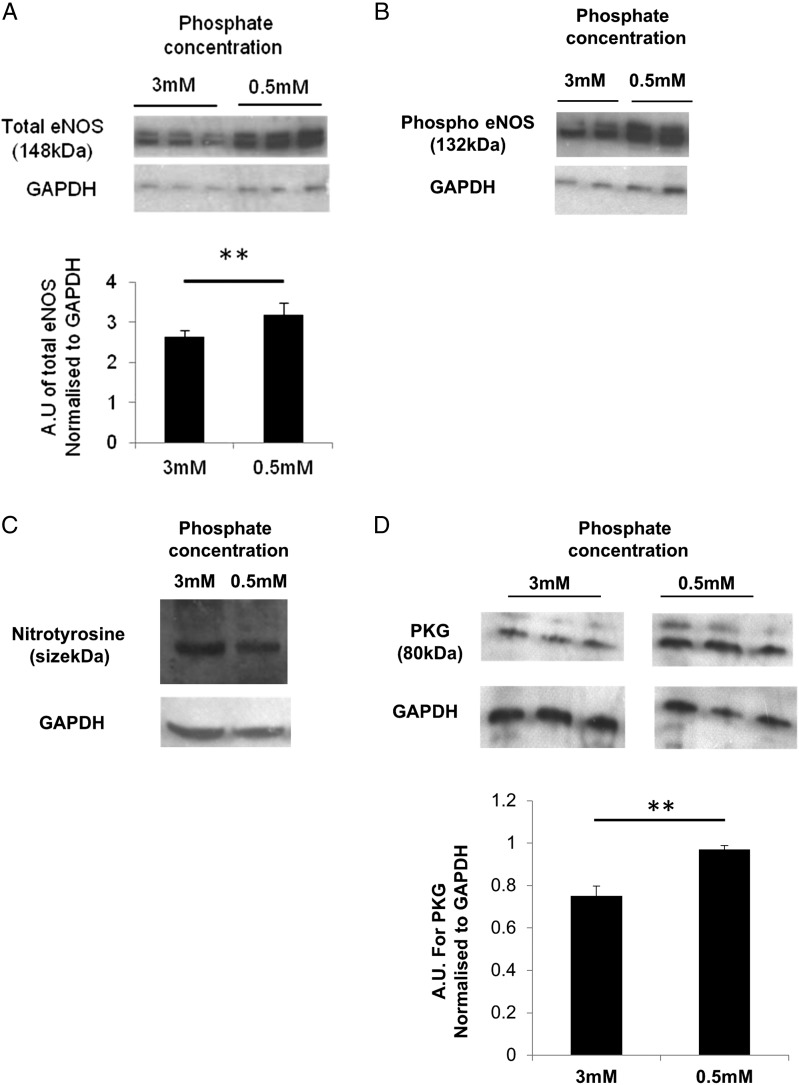
We measured endothelial nitric oxide synthase (eNOS) expression and NO production in human umbilical vein endothelial cells (HUVECs), as well as nitrotyrosine expression to investigate the role of reactive oxygen species (ROS) and free radicals in the high-phosphate environment. Total and phospho eNOS (normalized to GAPDH) and NO levels (data not shown) were significantly reduced in HUVECs cultured in high-phosphate conditions (Figure 3A and B). There was evidence of increased free radical formation in a high-phosphate environment as there was increased expression of nitrotyrosine (Figure 3C).
FIGURE 3.
HUVECs express reduced total (A) and phospho (B) eNOS and increased nitrotyrosine (C) when cultured in high-phosphate concentration medium (3 mM). PKG expression is reduced in rat VSMCs cultured in high- compared with normal-phosphate concentration medium. **P < 0.05. HUVECs and VSMCs were cultured in 0.5 mM or 3 mM phosphate concentration medium. Ten µg of protein was fractionated on SDS page gels. Primary antibodies for Total eNOS (1:1000), Phospho eNOS (1:200) (both Cell Signalling Technology), nitrotyrosine (1:500, R & D Systems), PKG (1:200, Enzo Life Sciences) and protein expression normalized to GAPDH. Densitometry was performed. In (A)–(D), top panels show representative gel from cells cultured in 0.5 or 3 mM phosphate concentration medium and bottom panels show GAPDH. The panels were all taken from a single gel and each band represents one well from separate six-well plates.
The smooth muscle cell is vital for a contractile response instigated via an intact NO pathway. In view of the impaired endothelium-independent vasodilatation to SNP observed in our resistance vessels, we measured cGMP levels in vascular smooth muscle cells and the resistance vessels exposed to normal and high-phosphate environments and protein kinase G (PKG), a cGMP-dependent kinase implicated in the relaxation of smooth muscle.
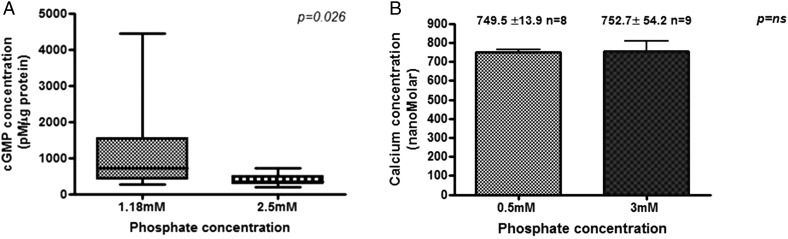
In rat vascular smooth muscle cells (VSMCs), cultured in high-phosphate conditions, PKG expression was significantly reduced (Figure 3D). In rat mesenteric vessels, cGMP was higher in vessels incubated in low- versus high-phosphate conditions (715.6(418–1541) pM/µg versus 330.6 (284.1–500.1) pM/µg; P = 0.0026; Figure 4A). In a high-phosphate environment, in support of our hypothesis that there is disruption in the entire NO pathway from the endothelial cell to the smooth muscle cell, we demonstrate reduced eNOS and PKG and increased nitrotyrosine expression, and reduced NO and cGMP production.
FIGURE 4.
cGMP concentration is significantly reduced in rat vessels incubated for 24 h in a high-phosphate concentration solution (A), whereas the intracellular calcium level in HUVECs is unchanged in a high-phosphate environment (B). (A) Rat mesenteric resistance vessels were incubated in a 1.18 mM (n = 11) or 2.5 mM (n = 11) phosphate concentration solution for 24 h. Results are expressed as cGMP pM/µg protein. Analysis was by the Mann–Whitney U test. (B) Cells were plated in glass-bottomed plates and cytosolic loading of FURA-2-AM was achieved by incubating the cells with FURA-2-AM. Values are mean ± SEM and are from at least four experiments with the number of cells included indicated by (n).
Although these data are consistent with a direct effect of phosphate, we measured intracellular calcium to exclude an indirect effect mediated by reduction in intracellular calcium. In HUVECs, intracellular calcium measurements were not significantly different between cells cultured in high- versus normal-phosphate conditions (Figure 4B; 749.5 ± 13.9 nM versus 752 ± 54.2 nM; P = not significant); rat VSMCs showed the same pattern (228.3 ± 22.0 nM versus 278 ± 22.4 nM).
Effects of sustained phosphate loading in healthy human volunteers
To determine whether the observations on NO and endothelial function in cellular and preclinical models are of physiological relevance in man, we performed a study of phosphate loading and phosphate binders (lanthanum) in healthy subjects, using FMD to assess endothelial function. Healthy volunteers, rather than CKD patients, were selected initially to allow study of the sole effects of phosphate, independent of the uraemic milieu.
We recruited 19 volunteers: 63% (n = 12) were female and the mean age was 42.2 ± 14.3 years (Table 4). At baseline, mean estimated glomerular filtration rate (eGFR) was 102 ± 10 mL/min/1.73 m2, serum phosphate level was 1.05 ± 0.18 mmol/L and the fractional excretion of urinary phosphate (FeP) was 14.3 ± 3.4%. Participants were normoglycaemic, with normal mean lipid values. The median FMD was 8.4% (6.2–11.6%) post-cuff inflation and 17.7% (13.4–23.2%) post-GTN spray.
Table 4.
Participant demographics at baseline, following lanthanum and phosphate
| Parameter | Baseline | Post-lanthanum | Post-phosphate | P-value |
|---|---|---|---|---|
| Age (years) | 42.2 ± 14.3 | |||
| Male sex | 36.8% (n = 7) | |||
| BMI | 26.0 ± 4.1 | 26.3 ± 3.9 | 26.3 ± 3.8 | NS |
| Creatinine (µM) | 66.4 ± 6.3 | 65.8 ± 6.8 | 65.8 ± 6.6 | NS |
| Systolic BP (mmHg) | 123.1 ± 15.8 | 122.9 ± 10.3 | 119.8 ± 16.8 | NS |
| Diastolic BP (mmHg) | 74.5 ± 10.5 | 75.2 ± 9.4 | 74.1 ± 12.1 | NS |
| Adjusted calcium (mmol/L) | 2.35 ± 0.07 | 2.36 ± 0.05 | 2.34 ± 0.09 | NS |
| Phosphate (mmol/L) | 1.05 ± 0.18 | 1.03 ± 0.18 | 1.06 ± 0.16 | NS |
| Vitamin D3 (nmol/L) | 48.2 ± 23.3 | 40.3 ± 20.6 | 45.6 ± 25.8 | NS |
| PTH (pg/mL) | 5.9 ± 2.1 | 5.8 ± 1.4 | 6.4 ± 2.3 | NS |
| FGF-23 (RU/mL) | 49.7 (45.9–69.1) | 59.1 (38.2–73.4) | 66.6 (50.0–84.9) | 0.028 |
| FGF-23% change | −1 (−19.8–21.7) | 19.6 (3.1–38.9) | 0.004 | |
| FeP (%) | 14.3 ± 3.4 | 11.4 ± 4.3 | 28.4 ± 9.2 | <0.001 |
| Urinary phosphate (mmol/day) | 28.4 ± 14.7 | 23.2 ± 12.3 | 54.9 ± 19.2 | <0.001 |
| Urinary cGMP (nmol/L) | 472.8 (312–645.4) | 530.6 (288.2–756.4 | 501.1 (274.9–674.0) | NS |
| Urinary FGF-23 (RU/mL) | 46.1 (26.6–288.2) | 139.5 (31.3–360.6) | 227.9 (39.4–405.8) | NS |
| FMD post-cuff (%) | 8.4 (6.2–11.6) | 6.6 (3.4–10.3) | 3.4 (2.6–5.3) | <0.001 |
| FMD post-cuff % change | −23.5 (−59 to â 0.2) | −58.1 (−71.9 to â 43.4) | <0.001 | |
| FMD post-GTN (%) | 17.7 (13.4–23.2) | 17.2 (12.3–23.7) | 16.3 (12.1–17.7) | NS |
| PWV (m/s) | 7.4 ± 1.9 | 7.3 ± 1.7 | 7.1 ± 1.6 | NS |
| AI@75 | 12.8 ± 12 | 12.5 ± 13.1 | 9.9 ± 12.6 | NS |
BMI, body mass index; BP, blood pressure; PTH, parathyroid hormone; FeP, fractional excretion of urinary phosphate; FMD, flow-mediated dilatation; PWV, pulse wave velocity; AI@75, aortic augmentation index corrected to a heart rate of 75 beats per minute; NS, not significant. P-value is in comparison with baseline values.
Statistically significant differences (P < 0.05) between baseline and subsequent measures are indicated in bold. % change refers to the % change in a particular parameter when compared with the value obtained at baseline.
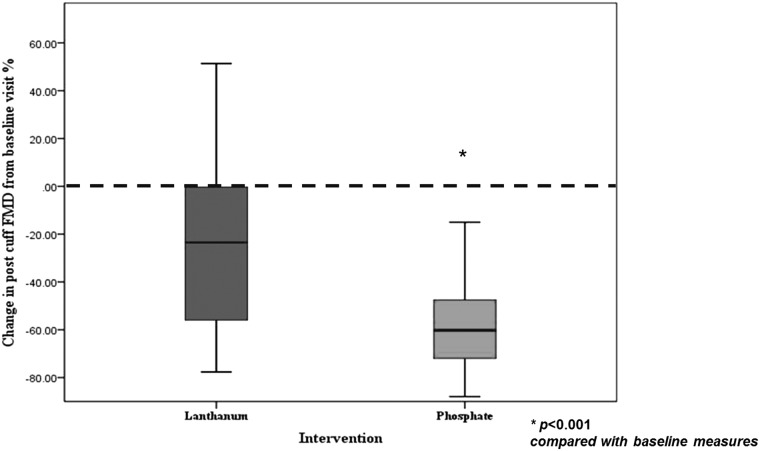
Table 4 shows the effect of each intervention. Phosphate supplementation was associated with a significant reduction in FMD [Figure 5; 3.4% (2.6–5.3%); P < 0.001]. With lanthanum, FMD also fell [Figure 5; 6.6% (3.4–10.3%); P = 0.033]. Post-GTN, as expected, there was significant increase in vessel diameter but there was no significant effect of phosphate supplementation or binding on GTN-mediated dilatation; randomization order was not a predictor of either FMD or GTN-mediated dilatation. In univariate analyses, the change in the serum phosphate level from baseline, change in serum FGF-23 from baseline and FeP were significant predictors of FMD. In a multiple regression model (Table 5), the inclusion of serum FGF-23 and urinary FeP was significantly associated with the change in FMD from baseline [F(2,54) = 10.1; P < 0.001; r2 = 0.28]. Inclusion of systolic blood pressure, serum phosphate, age, sex, urinary FGF-23, urinary calcium or urinary cGMP levels did not increase the amount of variation of change in FMD explained by the model. By recoding the three drug categories (no drug, lanthanum, phosphate), we included effects of lanthanum and oral phosphate in a multivariate model with the change in FMD as a % of the baseline visit as the dependent variable. The change in FeP and that in serum FGF-23 were included as covariates, resulting in an improved model [F(4,52) = 18.3; P < 0.001; r2 = 0.59].
FIGURE 5.
Change in FMD from baseline visit measures, following intervention with lanthanum and phosphate expressed as a % change from the baseline visit.
Table 5.
Multiple regression model with post-cuff FMD as the outcome measure
| Variable | B | Confidence interval |
P-value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| FeP | −1.1 | −1.9 | −0.2 | 0.014 |
| Serum FGF-23 | −0.5 | −0.7 | −0.2 | 0.002 |
Urinary phosphate and serum FGF-23 are significant predictors of post-cuff FMD.
Urinary cGMP were measured as a marker of endothelial function, and correlated inversely with FeP, consistent with disruption of the NO pathway (r = −0.39; P 0.003). In univariate analysis, serum phosphate, age, male sex and FeP were associated with urinary cGMP levels. Overall, at the end of the treatment phases of phosphate supplementation and lanthanum treatment, there was no significant difference in mean serum phosphate, parathyroid hormone (PTH), vitamin D, adjusted calcium or magnesium levels compared with baseline visit values. Serum FGF-23 levels increased significantly following phosphate supplementation and fell, albeit not significantly, following lanthanum, compared with baseline.
DISCUSSION
This paper investigates the independent, direct actions of phosphate in cells, vessels and human subjects, in order to understand the potential effects of phosphate as a CV risk factor. The prevailing view has been that elevated serum phosphate increases CV risk by promoting vascular calcification, and more recently that increased levels of FGF-23 contribute to CV risk by promoting cardiac hypertrophy. Our data provide strong support for an additional direct effect of phosphate resulting in endothelial dysfunction, mediated via disruption of the NO pathway.
Direct actions of phosphate and the NO pathway
The ex vivo studies demonstrate that sustained exposure to phosphate, independent of other features of the uraemic environment, causes impaired endothelium-dependent vasodilatation in resistance vessels. This supports a direct detrimental effect of phosphate, and this appears to be mediated by NO, with evidence of reduced basal and stimulated NO production. Endothelium-independent vasodilation is also impaired in rat and human LKD vessels exposed to high-phosphate concentration, suggesting disruption of the whole NO pathway. Adding zaprinast improved both endothelium-dependent and independent vasodilatation in vessels incubated in high-phosphate conditions. Zaprinast inhibits cGMP breakdown, increasing cGMP levels, and is beneficial in pulmonary hypertension where endothelial dysfunction is present [38, 39]. This could be a potential therapeutic option.
Our cellular models support the concept of disruption to the NO pathway with reduced basal NO, reduced expression of phosphorylated and total eNOS and PKG, lower concentrations of cGMP and increased nitrotyrosine expression. Some of these effects may be reversible. The finding that CKD vessels incubated in normal-phosphate conditions relaxed as well as LKD vessels did, with carbachol is consistent with a degree of adaptability and reversibility of the effects of phosphate in CKD.
Actions of phosphate in the uraemic environment
In CKD vessels, endothelium-dependent relaxation was impaired, but only with incubation in a high-phosphate environment. In a normal-phosphate environment, CKD vessels relax as well as LKD vessels. Unlike the LKD and rat vessels, endothelium-independent relaxation did not appear to be impaired in CKD.
These findings may reflect adaptive changes during chronic exposure to high-phosphate concentration in the uraemic environment, altering the responses to subsequent changes in phosphate. Our results conflict with a previous study from our group in human uraemic resistance vessels that showed impaired endothelium-dependent and, to a lesser extent, independent function in CKD [40]. In the present study, CKD vessels showed endothelial dysfunction, compared with normal vessels, with sustained exposure to high-phosphate concentration similar to uraemic levels. In contrast, the study of Morris et al. used no added phosphate [40]. It is likely that the effects of extracellular phosphate are mediated via changes in intracellular phosphate, which will equilibrate with time. Removing vessels from a CKD environment will also ‘wash out’ circulating factors, which may include asymmetric dimethylarginine and ROS [41, 42]. Phosphate itself might cause increased ROS, given that nitrotyrosine (a product of tyrosine nitration mediated by ROS) was increased in cells cultured in high-phosphate conditions. The timing of such effects and the timescale of their reversibility is unclear and requires further study.
Physiological relevance in humans
In vivo, we have shown similar effects to the in vitro studies: sustained oral phosphate loading causes endothelial dysfunction. Serum phosphate is <1% of total body phosphate and did not change significantly during treatment. The serum FGF-23 level rose by around 20% during phosphate loading and urinary phosphate excretion by 50% compared with baseline. This suggests that compliance with study medication demonstrates phosphate homoeostasis and highlights the limitations of serum phosphate as a measure of total body phosphate. In early CKD, serum phosphate is maintained within the reference range and rises consistently only when eGFR drops below 30 mL/min/1.73 m2, although in population studies, serum phosphate—even within the ‘normal’ range—is linked to CV risk. It may be that serum phosphate is an insensitive, homoeostatically regulated marker of total body phosphate which, in turn, influences endothelial function. A more precise estimate of risk may be derived from serum phosphate, FGF-23 levels and urinary phosphate levels—accounting for phosphate intake and total body excess. This is supported by our findings associating elevated serum FGF-23 and urinary phosphate excretion with poorer endothelial function. FeP is likely to be a better indicator of actual phosphate leak per nephron than standard 24 h urinary phosphate, by accounting for serum phosphate levels [43]. In the clinical setting, it may be possible to use a phosphate:creatinine ratio or a spot FeP to provide additional information about phosphate intake and activation of phosphaturic mechanisms. Whether such detail has any clinical benefit would need to be investigated in a clinical trial. Several small studies have demonstrated an association between FGF-23 and dietary phosphate intake in healthy volunteers with increased phosphate intake over a few days resulting in increased levels of serum FGF-23 [44, 45]. Increased levels of FGF-23 correlate with increased serum phosphate, urinary excretion of phosphate and vitamin D levels [44]. Urinary phosphate excretion alone or in combination with measures of FGF-23 may be a better surrogate for total body phosphate than the serum phosphate level.
CKD patients may respond differently to sustained phosphate loading and thus results from our clinical study cannot be extrapolated to the CKD population. Our study was not powered to assess the direct effects of either phosphate supplementation or lanthanum and so no firm conclusions about lanthanum and endothelial function can be drawn. We chose lanthanum as a non-calcium-containing ‘single tablet’ binder, without well-described effects on confounding factors, for example the lipid-lowering properties of sevelamer [18]. Further studies on the direct effects of lanthanum on vascular function, and comparison of different classes of binder or diet, are merited.
Naturally, there are limitations of our work. We deliberately used the extreme ends of the phosphate spectrum (not least because standard concentration medium contains 0.5 mM phosphate), but dose response experiments using concentrations of phosphate in between 0.5 mM and 3 mM would be of value. We acknowledge that our findings are likely to form only part of the explanation for the increased CV risk associated with phosphate and it seems probable that vascular calcification and FGF-23 and PTH also play a role. However, to help tease out the isolated effects of phosphate, we deliberately chose to study it alone and not look at effects of its key regulators, i.e. FGF-23 and PTH in vivo and in vitro. Similarly, this is why we used healthy volunteers for our clinical study.
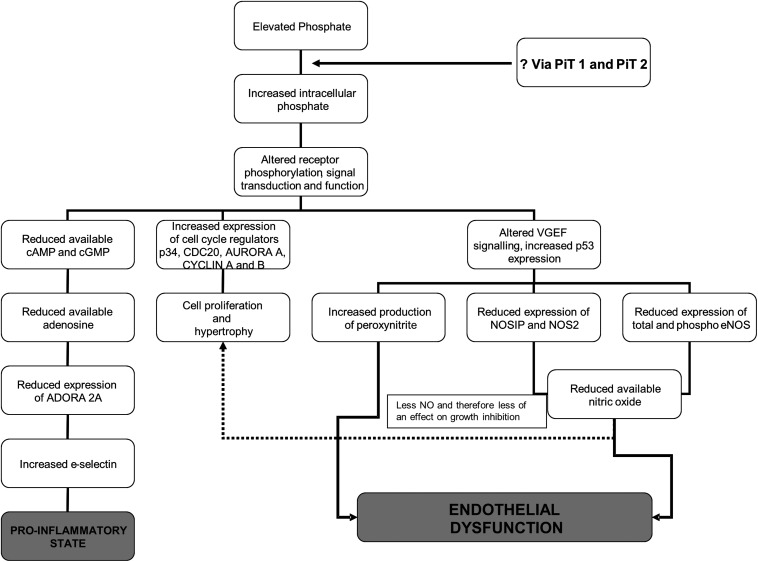
In conclusion, these studies show that phosphate has directly detrimental effects on the endothelium and vasculature, effects that are likely to be mediated by disruption of the whole NO pathway (Figure 6). Some of the effects may be reversible, either by manipulation of phosphate or pharmacologically, for example by inhibiting PDE5 (Figure 6). We have previously shown direct effects of phosphate on inflammation, leading to a pro-inflammatory state [46].
FIGURE 6.
Possible mechanism of action of phosphate as a cardiovascular risk factor causing both a pro-inflammatory state and endothelial dysfunction. Using evidence presented in this manuscript and previous studies, we propose this as the possible mechanism of action of phosphate resulting in endothelial dysfunction and a pro-inflammatory state. Additionally, we have some evidence of an effect of phosphate on cell growth (data not yet published).
The clinical study presented here shows that the in vitro effects are of functional relevance and likely to play a role in the CV risk associated with high-phosphate concentration. It is our impression that intracellular, rather than extracellular phosphate is central to the observed effects. Drugs which inhibit cellular phosphate uptake may offer an alternative strategy to target adverse effects of hyperphosphataemia in CKD. Future work should consider the measurement of intracellular phosphate.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mrs Elisabeth Beattie and Dr Craig Daly for their help with the design of the myography experiments, Dr Dianne Hillyard and Dr William Sands for their guidance with laboratory techniques and Dr Lesley Anderson for teaching the FMD technique (all from The University of Glasgow). These studies were funded in part by grants from The British Heart Foundation (British Heart Foundation Junior Clinical Research Fellowship, K.K.S.) and Darlinda's Charity for Renal Research.
CONFLICT OF INTEREST STATEMENT
Shire Pharmaceuticals, who market lanthanum, provided pill boxes for the human study. K.K.S., L.D., R.K.P., S.K., G.L.S., M.J.C. and C.D. report no competing interests. P.B.M. received a travel grant from Sanofi. A.G.J. has been a member of the steering, data monitoring and end-point committees of numerous international multi-centre studies and has received direct or institutional support from Novartis, Astellas, Roche, Pfizer, Otsuka and Genzyme in the field of nephrology and transplantation. The work presented here has not been published previously, in whole or part, except in abstract form.
REFERENCES
- 1. Betriu A, Martinez-Alonso M, Arcidiacono MV. et al. Prevalence of subclinical atheromatosis and associated risk factors in chronic kidney disease: the NEFRONA study. Nephrol Dial Transplant 2014; 29: 1415–1422 [DOI] [PubMed] [Google Scholar]
- 2. Ganesh SK, Stack AG, Levin NW. et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–2138 [DOI] [PubMed] [Google Scholar]
- 3. Kestenbaum B, Sampson JN, Rudser KD. et al. Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 2005; 16: 520–528 [DOI] [PubMed] [Google Scholar]
- 4. Achinger SG, Ayus JC. Left ventricular hypertrophy: is hyperphosphatemia among dialysis patients a risk factor? J Am Soc Nephrol 2006; 17(12 Suppl 3): S255–S261 [DOI] [PubMed] [Google Scholar]
- 5. Tonelli M, Pfeffer MA. Kidney disease and cardiovascular risk. Annu Rev Med 2007; 58: 123–139 [DOI] [PubMed] [Google Scholar]
- 6. Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 7. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 8. Fellstrom B, Jardine AG, Holdaas H. et al. The effects of rosuvastatin versus placebo on cardiovascular outcomes in patients with end stage renal disease on haemodialysis—results of the AURORA study. NDT Plus 2009; 2(Suppl 2): ii1514 [Google Scholar]
- 9. Jardine AG, Fellstrom B, Logan JO. et al. Cardiovascular risk and renal transplantation: post hoc analyses of the Assessment of Lescol in Renal Transplantation (ALERT) Study. Am J Kidney Dis 2005; 46: 529–536 [DOI] [PubMed] [Google Scholar]
- 10. Foley RN, Parfrey PS, Sarnak MJ. Epidemiology of cardiovascular disease in chronic renal disease. J Am Soc Nephrol 1998; 9(12 Suppl): S16–S23 [PubMed] [Google Scholar]
- 11. Sarnak MJ, Levey AS, Schoolwerth AC. et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 2003; 42: 1050–1065 [DOI] [PubMed] [Google Scholar]
- 12. Stevens KK, Morgan IR, Patel RK. et al. Serum phosphate and outcome at one year after deceased donor renal transplantation. Clin Transplant 2011; 25: E199–E204 [DOI] [PubMed] [Google Scholar]
- 13. Cirillo M, Botta G, Chiricone D. et al. Glomerular filtration rate and serum phosphate: an inverse relationship diluted by age. Nephrol Dial Transplant 2009; 24: 2123–2131 [DOI] [PubMed] [Google Scholar]
- 14. Connolly GM, Cunningham R, McNamee PT. et al. Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 2009; 87: 1040–1044 [DOI] [PubMed] [Google Scholar]
- 15. Bellasi A, Mandreoli M, Baldrati L. et al. Chronic kidney disease progression and outcome according to serum phosphorus in mild-to-moderate kidney dysfunction. Clin J Am Soc Nephrol 2011; 6: 883–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chue CD, Edwards NC, Moody WE. et al. Serum phosphate is associated with left ventricular mass in patients with chronic kidney disease: a cardiac magnetic resonance study. Heart 2012; 98: 219–224 [DOI] [PubMed] [Google Scholar]
- 17. Block GA. Control of serum phosphorus: implications for coronary artery calcification and calcific uremic arteriolopathy (calciphylaxis). Curr Opin Nephrol Hypertens 2001; 10: 741–747 [DOI] [PubMed] [Google Scholar]
- 18. Cozzolino M, Rizzo MA, Stucchi A. et al. Sevelamer for hyperphosphataemia in kidney failure: controversy and perspective. Ther Adv Chronic Dis 2012; 3: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yilmaz MI, Sonmez A, Saglam M. et al. Comparison of calcium acetate and sevelamer on vascular function and fibroblast growth factor 23 in CKD patients: a randomized clinical trial. Am J Kidney Dis 2012; 59: 177–185 [DOI] [PubMed] [Google Scholar]
- 20. Voormolen N, Noordzij M, Grootendorst DC. et al. , High plasma phosphate as a risk factor for decline in renal function and mortality in pre-dialysis patients. Nephrol Dial Transplant 2007; 22: 2909–2916 [DOI] [PubMed] [Google Scholar]
- 21. Tonelli M, Pannu N, Manns B. Oral phosphate binders in patients with kidney failure. N Engl J Med 2010; 362: 1312–1324 [DOI] [PubMed] [Google Scholar]
- 22. Tonelli M, Sacks F, Pfeffer M. et al. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation 2005; 112: 2627–2633 [DOI] [PubMed] [Google Scholar]
- 23. Toussaint ND, Pedagogos E, Tan SJ. et al. Phosphate in early chronic kidney disease: associations with clinical outcomes and a target to reduce cardiovascular risk. Nephrology (Carlton) 2012; 17: 433–444 [DOI] [PubMed] [Google Scholar]
- 24. Foley RN, Collins AJ, Herzog CA. et al. Serum phosphorus levels associate with coronary atherosclerosis in young adults. J Am Soc Nephrol 2009; 20: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zoccali C, Mallamaci F. Moderator's view: phosphate binders in chronic kidney disease patients: a clear ‘No’ at the moment, but stay tuned. Nephrol Dial Transplant 2016; 31: 196–199 [DOI] [PubMed] [Google Scholar]
- 26. Giachelli CM. The emerging role of phosphate in vascular calcification. Kidney Int 2009; 75: 890–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shanahan CM, Crouthamel MH, Kapustin A. et al. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res 2011; 109: 697–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Van TV, Watari E, Taketani Y. et al. Dietary phosphate restriction ameliorates endothelial dysfunction in adenine-induced kidney disease rats. J Clin Biochem Nutr 2012; 51: 27–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gutierrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Faul C, Amaral AP, Oskouei B. et al. FGF23 induces left ventricular hypertrophy. J Clin Invest 2011; 121: 4393–4408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gutierrez OM, Januzzi JL, Isakova T. et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation 2009; 119: 2545–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shuto E, Taketani Y, Tanaka R. et al. Dietary phosphorus acutely impairs endothelial function. J Am Soc Nephrol 2009; 20: 1504–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng A, Wu T, Zeng C. et al. Adverse effects of simulated hyper- and hypo-phosphatemia on endothelial cell function and viability. PLoS One 2011; 6: e23268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thambyrajah J, Landray MJ, McGlynn FJ. et al. Abnormalities of endothelial function in patients with predialysis renal failure. Heart 2000; 83: 205–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Annuk M, Soveri I, Zilmer M. et al. Endothelial function, CRP and oxidative stress in chronic kidney disease. J Nephrol 2005; 18: 721–726 [PubMed] [Google Scholar]
- 36. Thijssen DH, Black MA, Pyke KE. et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol 2011; 300: H2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Bortel LM, Duprez D, Starmans-Kool MJ. et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens 2002; 15: 445–452 [DOI] [PubMed] [Google Scholar]
- 38. Keswani AN, Peyton KJ, Durante W. et al. The cyclic GMP modulators YC-1 and zaprinast reduce vessel remodeling through antiproliferative and proapoptotic effects. J Cardiovasc Pharmacol Ther 2009; 14: 116–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sebkhi A, Strange JW, Phillips SC. et al. Phosphodiesterase type 5 as a target for the treatment of hypoxia-induced pulmonary hypertension. Circulation 2003; 107: 3230–3235 [DOI] [PubMed] [Google Scholar]
- 40. Morris ST, McMurray JJ, Spiers A. et al. Impaired endothelial function in isolated human uremic resistance arteries. Kidney Int 2001; 60: 1077–1082 [DOI] [PubMed] [Google Scholar]
- 41. Ando R, Ueda S, Yamagishi S. et al. Involvement of advanced glycation end product-induced asymmetric dimethylarginine generation in endothelial dysfunction. Diab Vasc Dis Res 2013; 10: 436–441 [DOI] [PubMed] [Google Scholar]
- 42. Brunet P, Gondouin B, Duval-Sabatier A. et al. Does uremia cause vascular dysfunction? Kidney Blood Press Res 2011; 34: 284–290 [DOI] [PubMed] [Google Scholar]
- 43. Stevens KK, Patel RK, Methven S. et al. Proteinuria and outcome after renal transplantation: ratios or fractions? Transplantation 2013; 96: 65–69 [DOI] [PubMed] [Google Scholar]
- 44. Burnett SM, Gunawardene SC, Bringhurst FR. et al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 2006; 21: 1187–1196 [DOI] [PubMed] [Google Scholar]
- 45. Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab 2006; 91: 3144–3149 [DOI] [PubMed] [Google Scholar]
- 46. Stevens KK, McQuarrie EP, Sands W. et al. Fibroblast growth factor 23 predicts left ventricular mass and induces cell adhesion molecule formation. Int J Nephrol 2011; 2011: 297070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.