Abstract
Background
Artificial sweeteners are widely replacing caloric sweeteners. Data on long-term impact of artificially sweetened beverage (ASB) consumption during pregnancy on offspring obesity risk are lacking. We prospectively investigated intake of ASBs and sugar-sweetened beverages (SSBs) during pregnancy in relation to offspring growth through age 7 years among high-risk children born to women with gestational diabetes.
Methods
In a prospective study of 918 mother-singleton child dyads from the Danish National Birth Cohort, maternal dietary intake was assessed by a food frequency questionnaire during pregnancy. Offspring body mass index z-scores (BMIZ) and overweight/obesity status were derived using weight and length/height at birth, 5 and 12 months and 7 years. Linear regression and Poisson regression with robust standard errors were used, adjusting for major risk factors.
Results
Approximately half of women reported consuming ASBs during pregnancy and 9% consumed daily. Compared to never consumption, daily ASB intake during pregnancy was positively associated with offspring large-for-gestational age [adjusted relative risk (aRR) 1.57; 95% CI: 1.05, 2.35 at birth], BMIZ (adjusted β 0.59; 95% CI: 0.23, 0.96) and overweight/obesity (aRR 1.93; 95% CI; 1.24, 3.01) at 7 years. Per-serving-per-day substitution of ASBs with water during pregnancy was related to a lower overweight/obesity risk at 7 years (aRR 0.83; 95% CI: 0.76, 0.91), whereas SSB substitution with ASBs was not related to a lower risk (aRR 1.14; 95% CI: 1.00, 1.31).
Conclusions
Our findings illustrated positive associations between intrauterine exposure to ASBs and birth size and risk of overweight/obesity at 7 years. Data with longer follow-up are warranted.
Keywords: Artificially sweetened beverages, sugar-sweetened beverages, pregnancy, obesity
Introduction
The global epidemic of childhood obesity remains a growing public health concern due to its short- and long-term adverse health sequelae.1,2 Despite the overall plateauing trends in some countries, the prevalence of more severe forms of obesity continues to increase among certain subgroups.3 In particular, offspring born to women with gestational diabetes (GDM), the most common metabolic pregnancy complication affecting approximately 16% of pregnancies worldwide,4,5 represent a high-risk phenotype which may serve as a unique model to study the early origins of obesity.6 Further, accumulating evidence has linked nutritional perturbations during pregnancy to fetal development and obesity risk in later life.7 Thus, it is of great clinical and public health significance to identify modifiable intrauterine dietary factors which may potentially interrupt the vicious cycle between maternal pathophysiology and offspring obesity.8
Pregnancy is characterized by an increase in plasma volume and accretion of amniotic fluid.9 Despite the compensation by gestational haemodynamic changes, there is an increased demand in fluid intake. Due to the health concern related to sugar-sweetened beverages (SSBs) in the general population,10 artificially sweetened beverages (ASBs) have been considered as potential healthier alternatives, although emerging evidence has suggested the contrary. Specifically, despite the lack of acute toxicity, accumulating experimental data in animal models have questioned the advisability of artificial sweeteners, as evidenced by alterations in the gut microbiome,11 increase in intestinal glucose absorption12 and stimulation of sweet-taste receptors.13 However, epidemiological data on ASB consumption during pregnancy in relation to offspring longitudinal growth and obesity risk over life span are limited. To date, only one study has reported a positive association between ASB, but not SSB, consumption during pregnancy with offspring growth at 1 year.14 Longitudinal studies on long-term impact of ASB on offspring growth beyond infancy are lacking. Further, despite the possibility of examining the association related to substituting SSBs or ASBs with other types of beverages in observational studies,15 no such data are available; this however might be informative in terms of identifying healthier alternatives.
To address these critical data gaps, we prospectively investigated the associations between maternal consumption of ASBs and SSBs during pregnancy, and offspring growth and risk of overweight/obesity through age 7 years among high-risk offspring born to pregnancies complicated by GDM. Furthermore, we examined offspring risk of overweight/obesity associated with substituting SSBs or ASBs with an equal serving of alternative beverages (fruit juices or water) during pregnancy.
Methods
Study population and design
The study data were based on the Danish National Birth Cohort (DNBC), a longitudinal cohort of 101 042 pregnancies (91 827 women) in Denmark (1996–2002).16 Briefly, Danish-speaking pregnant women residing in Denmark were recruited by general practitioners at the first antenatal visit around gestational weeks 6–12. Enrolled participants completed four computer-assisted telephone interviews at gestational weeks 12 and 30, and 6 and 18 months postpartum, which collected data on socio-demographic, perinatal and clinical factors. In addition, a validated food frequency questionnaire (FFQ) was administered at 25 weeks of gestation.17 When the offspring was 7 years old, a follow-up questionnaire about the child’s health and development was delivered by mail or electronically to the parents. The study was approved by the Danish National Committee on Biomedical Research Ethics. Informed consent was obtained from all participants.
As part of the ongoing Diabetes and Women’s Health Study,18 we identified 1379 pregnancies complicated by GDM, documented from the study interviews at 30 weeks of gestation or 6 months postpartum and/or the Danish National Patient Registry in the DNBC. The World Health Organization (WHO) criteria19 or local clinical practices20 were applied for GDM diagnosis. We sequentially excluded pregnancies with missing dietary data from FFQ (n = 346), implausible daily energy intake (<4000 or >20 000 kJ/day; n = 14), missing data on offspring growth at birth and all follow-ups (n = 2), multiple gestations (n = 24), pre-existing diabetes (n = 57) and recurrent GDM (n = 18), rendering a pool of 918 mother-child dyads as our analytical sample. At follow-up, we included data from offspring with complete anthropometric data at birth (n = 918), 5 months (n = 589), 12 months (n = 575) or 7 years (n = 531) in the final analysis. Maternal and offspring characteristics at baseline did not substantially differ between those lost to follow-up due to missing offspring anthropometric data and those retained, except that women lost to any follow-up were more likely to have a lower socioeconomic status. The mean consumption of ASBs or SSBs during pregnancy (serving/day) did not differ between the lost and retained groups.
Exposure assessment
Maternal dietary intakes during pregnancy were assessed by an FFQ self-administered at 25 weeks of gestation, which collected information on habitual dietary intake during the previous month.17 Consumptions of ASBs and SSBs were determined by reported frequency of sugar-free/light and sugar-sweetened soft drinks, respectively, with response categories ranging from never to ≥ 8 servings/day (d). Servings of ASBs and SSBs were standardized with the assumption that one serving was equivalent to 1 cup (250 ml or 261 g).21 Dietary intakes and macronutrient contents of individual food items were quantified in grams per day based on standard portion sizes and the Danish Food Composition Tables version 6.02.22 The FFQ has been validated against a 7-day weighted food record and several biomarkers, and demonstrated as a useful instrument for analyses of energy, food and nutrient levels.23,24 Further, a randomly selected sample of 103 women completed the FFQ a second time at 33–35 weeks of gestation. For both beverage variables, Spearman’s correlation between intakes reported in the first and second FFQ was approximately 0.7.25
Outcome measures
Offspring birthweight and length were extracted from the Danish Medical Birth Registry. Ponderal index at birth was calculated as birthweight in kg/birth length in m3. Large-for-gestational age (LGA) was defined as a birthweight greater than the sex- and gestational age-specific 90th percentile based on the entire DNBC population. During the 6-month postpartum interview, the mother referred to the Child’s Book and reported child’s weight and recumbent length measured by the general practitioner at the 5-month postnatal visit. Similarly, child’s weight and recumbent length at 12 months were reported during the 18-month postpartum interview. At the 7-year follow-up questionnaire, child’s weight and height were reported by the parent(s) based on measurements obtained by the general practitioner, school nurse or parent(s). Age- and sex-specific body mass index z-scores (BMIZ) were calculated using the WHO Child Growth Standards for children aged < 5 years26 and WHO Growth Reference for those aged ≥ 5 years.27 Childhood overweight/obesity was classified using age- and sex-specific WHO cutoffs [≥ 85th percentile for children aged < 5 years26 and ≥ two standard deviations (SD) for those aged ≥ 5 years27].
Covariates
A comprehensive list of a priori selected covariates was considered in the analyses. These include, first, the following maternal non-dietary covariates obtained from interviews at gestational weeks 12 and 30: maternal age (continuous), socioeconomic status determined by the highest familial employment level [high (medium-to-high-level professionals), middle (skilled workers), low (unskilled workers, others)], pre-pregnancy BMI (< 25.0, 25.0–29.9, ≥30.0 kg/m2; derived from self-reported pre-pregnancy weight and height), smoking during pregnancy (yes, no), and moderate-to-vigorous physical activity during pregnancy (ever, never). Second, covariates include maternal dietary covariates from FFQ: intakes of total energy, desserts and sweets, oil/margarine/butter, potato, processed meat, refined grains and whole grains (all continuous). Third, covariates include offspring characteristics from: the Medical Birth Registry, postpartum 6- and 18-month questionnaires and 7-year follow-up questionnaire [sex (male, female), breastfeeding duration ≥ 6 months (yes, no), ASB consumption ≥ 1/week (yes, no), SSB consumption ≥ 1/week (yes, no) and physical activity ≥ 2 h/weekday (yes, no) at 7 years]. All models were mutually adjusted for maternal intake of both beverage types (ASBs and SSBs) during pregnancy. Given the relatively low frequency of missing values for categorical variables in the full sample (range 0–4%), we assigned a separate category for missing values as necessary.
Statistical analysis
Distributions of maternal and offspring characteristics across ASB or SSB categories were assessed by analysis of variance (ANOVA) for continuous variables and χ2 test for categorical variables. Multivariable linear regression models estimated the beta coefficients (β) and 95% confidence intervals (CIs) for offspring ponderal index at birth and BMIZ at follow-up in association with maternal ASB or SSB consumption during pregnancy, after adjustment for aforementioned covariates. Similarly, Poisson regression with robust standard errors calculated relative risks (RR) and 95% CIs for offspring risk of LGA at birth and overweight/obesity at follow-up. Further, nonparametric cubic spline regression examined the association between beverage intake on a continuum (in serving/d) and offspring risk of overweight/obesity. Further, assuming the observed association between maternal ASB consumption and offspring overweight/obesity reflected a true effect, we used the substitution regression models28,29 to examine whether ASBs could serve as healthier alternatives for SSBs. We also assessed whether substitution of SSBs or ASBs with an equal serving of alternative beverages (fruit juices or water) might have any beneficial effect on mitigating offspring risk of overweight/obesity. Specifically, Poisson regression estimated adjusted RRs (aRRs) and 95% CIs for the substitution association using the computed difference in β coefficients and variances, and the covariance between the two beverage types of interest.28
To minimize the potential reverse causality impact, we performed a priori subgroup analyses by known risk factors for GDM including maternal pre-pregnancy obesity status, age and smoking during pregnancy. Further, to examine the potential effect modification by offspring sex and early life factors (breastfeeding, diet and physical activity), we included a cross-product of the potential modifier and maternal ASB consumption. Accordingly, we conducted stratified analyses by sex and breastfeeding duration (<, ≥ 6 months), with a P-for-interaction < 0.20.
To further test the robustness of the findings, we conducted several sensitivity analyses restricted to women who did not receive medications for GDM treatment (93%), women without hypertensive complications during pregnancy (91%) or women who delivered at term (≥ 37 gestational weeks; 94%), respectively. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
In total, 45.4% of women reported consuming ASBs during pregnancy, with 9.3% consuming ASBs daily; 68.7% reported consuming SSBs, with 9.2% consuming SSBs daily (Table 1). Across the increasing maternal ASB categories, women tended to have a higher pre-pregnancy BMI and energy density of proteins, but a lower total energy intake, glycaemic index and glycaemic load. Maternal SSB consumption was associated with a younger maternal age and a lower energy density of proteins, but a higher total energy, energy density of carbohydrates, and glycaemic index and load. Offspring characteristics at birth did not vary by maternal ASB or SSB intake, respectively. Also notably, compared with offspring of women with SSB intake ≥ 1/d, their counterparts of women with ASB intake ≥ 1/d were more likely to be boys (55% vs 44%) and had a slightly higher mean birthweight (3747.5 g vs 3603.2 g).
Table 1.
Maternal and infant characteristics according to frequency of maternal beverage consumption during pregnancy
| Frequency of maternal beverage consumption during pregnancy |
|||||
|---|---|---|---|---|---|
| Never | < 1/wk | 1–6/wk | ≥1/d | P-valuea | |
| Artificially sweetened beverages | |||||
| Total, no. (%) | 501 (54.6) | 127 (13.8) | 205 (22.3) | 85 (9.3) | |
| Maternal characteristics, mean (SD)b | |||||
| Age at index child birth, y | 31.5 (4.5) | 31.4 (4.8) | 30.7 (4.5) | 31.5 (4.4) | 0.22 |
| Socioeconomic status, no. (%) | 0.13 | ||||
| High | 237 (47.3) | 58 (45.7) | 82 (40.0) | 38 (44.7) | |
| Middle | 143 (28.5) | 29 (22.8) | 73 (35.6) | 22 (25.9) | |
| Low | 99 (19.8) | 34 (26.8) | 44 (21.5) | 22 (25.9) | |
| Missing or unknown | 22 (4.4) | 6 (4.7) | 6 (2.9) | 3 (3.5) | |
| Pre-pregnancy body mass index, kg/m2 | 26.5 (5.4) | 28.4 (6.1) | 28.6 (5.7) | 30.5 (7.3) | < 0.001 |
| Parity ≥ 1, no. (%) | 287 (59.7) | 75 (61.5) | 119 (59.8) | 49 (59.8) | 0.99 |
| Smoke, no. (%) | 73 (14.6) | 16 (12.7) | 36 (17.6) | 23 (27.1) | 0.021 |
| Hypertensive complications, no. (%)c | 24 (5.0) | 8 (6.5) | 12 (6.2) | 5 (6.2) | 0.88 |
| Any MVPA during pregnancy, no. (%) | 120 (25.2) | 33 (27.3) | 53 (27.2) | 16 (21.3) | 0.76 |
| Total energy intake, kcal/d | 2452.9 (630.8) | 2366.0 (640.8) | 2333.0 (649.9) | 2199.4 (556.7) | < 0.001 |
| Carbohydrate, %E | 54.1 (5.9) | 54.0 (6.1) | 53.8 (6.0) | 52.6 (6.0) | 0.4 |
| Protein, %E | 15.2 (2.6) | 16.0 (2.8) | 16.1 (2.3) | 16.5 (2.1) | < 0.001 |
| Total fat, %E | 30.3 (5.9) | 29.7 (6.0) | 29.7 (6.1) | 30.5 (6.3) | 0.5 |
| Glycaemic indexd | 90.9 (85.8) | 70.3 (62.1) | 66.9 (40.4) | 57.1 (32.9) | < 0.001 |
| Glycaemic loadd | 298.9 (348.7) | 221.7 (279.4) | 200.9 (164.5) | 153.6 (116.7) | < 0.001 |
| Desserts and sweets, g/d | 37.2 (25.4) | 36.9 (41.8) | 33.9 (27.4) | 28.6 (26.9) | < 0.001 |
| Oil/butter/margarine, g/d | 29.4 (22.3) | 25.6 (18.8) | 23.9 (18.6) | 21.7 (18.6) | < 0.001 |
| Potato, g/d | 139.7 (94.3) | 144.4 (83.0) | 131.3 (84.7) | 112.1 (68.7) | 0.02 |
| Processed meat, g/d | 16.0 (12.5) | 18.4 (13.9) | 18.4 (13.1) | 18.5 (12.9) | 0.01 |
| Refined grains, g/d | 88.1 (51.3) | 79.8 (42.5) | 86.7 (49.1) | 83.9 (48.4) | 0.68 |
| Whole grains, g/d | 146.8 (88.0) | 161.0 (87.7) | 156.6 (90.7) | 167.0 (81.9) | 0.02 |
| Offspring characteristics at birth, mean (SD)b | |||||
| Male, no. (%) | 239 (47.7) | 66 (52.0) | 106 (51.7) | 47 (55.3) | 0.49 |
| Gestational age, wks | 39.5 (1.7) | 39.4 (1.7) | 39.6 (1.7) | 39.4 (1.7) | 0.69 |
| Birthweight, g | 3684.1 (568.3) | 3750.1 (595.3) | 3753.9 (653.5) | 3747.5 (680.1) | 0.33 |
| Pponderal index, kg/m3 | 25.4 (2.5) | 25.8 (2.4) | 25.7 (2.9) | 26.0 (2.7) | 0.1 |
| Sugar-sweetened beverages | |||||
| Total, no. (%) | 287 (31.3) | 197 (21.5) | 350 (38.1) | 84 (9.2) | |
| Maternal characteristics, mean (SD)b | |||||
| Age at index child birth, y | 31.9 (4.4) | 31.8 (4.7) | 30.9 (4.6) | 30.0 (4.2) | 0.002 |
| Socioeconomic status, no. (%) | 0.53 | ||||
| High | 125 (43.6) | 88 (44.7) | 167 (47.7) | 35 (41.7) | |
| Middle | 84 (29.3) | 64 (32.5) | 95 (27.1) | 24 (28.6) | |
| Low | 68 (23.7) | 36 (18.3) | 72 (20.6) | 23 (27.4) | |
| Missing or unknown | 10 (3.5) | 9 (4.6) | 16 (4.6) | 2 (2.4) | |
| Pre-pregnancy body mass index, kg/m2 | 28.4 (6.5) | 27.2 (5.4) | 27.3 (5.6) | 27.5 (5.9) | 0.23 |
| Parity ≥1, no. (%) | 180 (65.0) | 115 (60.5) | 190 (56.7) | 45 (54.9) | 0.15 |
| Smoke, no. (%) | 51 (17.8) | 29 (14.8) | 51 (14.6) | 17 (20.2) | 0.48 |
| Hypertensive complications, no. (%)c | 17 (6.2) | 13 (6.8) | 14 (4.2) | 5 (6.2) | 0.56 |
| Any MVPA during pregnancy, no. (%) | 67 (25.2) | 54 (28.7) | 85 (25.4) | 16 (20.5) | 0.56 |
| Total energy intake, kcal/d | 2218.7 (598.5) | 2393.5 (673.5) | 2470.5 (604.5) | 2638.6 (639.0) | < 0.001 |
| Carbohydrate, %E | 53.9 (6.3) | 53.5 (5.8) | 53.5 (5.7) | 56.6 (5.9) | < 0.001 |
| Protein, %E | 16.8 (2.3) | 15.9 (2.4) | 15.1 (2.4) | 13.6 (2.2) | < 0.001 |
| Total fat, %E | 29.0 (6.4) | 30.2 (5.6) | 31.0 (5.8) | 29.5 (5.6) | < 0.001 |
| Glycaemic indexd | 65.5 (48.6) | 68.8 (48.9) | 74.1 (46.5) | 175.4 (154.5) | < 0.001 |
| Glycaemic loadd | 185.7 (177.9) | 215.8 (211.9) | 235.5 (208.3) | 642.0 (625.6) | < 0.001 |
| Desserts and sweets, g/d | 23.6 (23.4) | 38.9 (35.9) | 41.1 (25.2) | 46.1 (28.7) | < 0.001 |
| Oil/butter/margarine, g/d | 21.0 (18.0) | 26.0 (22.1) | 31.6 (21.7) | 29.9 (18.8) | < 0.001 |
| Potato, g/d | 133.8 (85.4) | 130.4 (86.7) | 137.1 (90.7) | 151.1 (97.0) | 0.22 |
| Processed meat, g/d | 17.7 (13.2) | 17.8 (15.2) | 16.1 (11.0) | 17.6 (13.6) | 0.67 |
| Refined grains, g/d | 79.3 (45.1) | 89.7 (54.5) | 88.0 (48.6) | 94.0 (52.7) | 0.03 |
| Whole grains, g/d | 171.2 (91.2) | 157.6 (93.5) | 142.8 (81.3) | 120.5 (77.8) | < 0.001 |
| Offspring characteristics at birth, mean (SD)b | |||||
| Male, no. (%) | 150 (52.3) | 107 (54.3) | 164 (46.9) | 37 (44.0) | 0.2 |
| Gestational age, wks | 39.5 (1.6) | 39.7 (1.5) | 39.5 (1.7) | 39.4 (2.1) | 0.57 |
| Birthweight, g | 3704.4 (607.9) | 3818.0 (555.7) | 3691.7 (623.6) | 3603.2 (576.2) | 0.07 |
| Ponderal index, kg/m3 | 25.5 (2.7) | 25.8 (2.6) | 25.5 (2.6) | 25.5 (2.7) | 0.29 |
Wks, weeks; y, years; MVPA, moderate-to-vigorous physical activity; %E, percent of total energy intake.
aP-values were obtained by ANOVA for continuous variables and by χ2 test for categorical variables.
bValues are mean (SD) unless otherwise specified.
cIncluded pre-gestational hypertension, gestational hypertension, pre-eclampsia and eclampsia.
dValue is energy-adjusted.
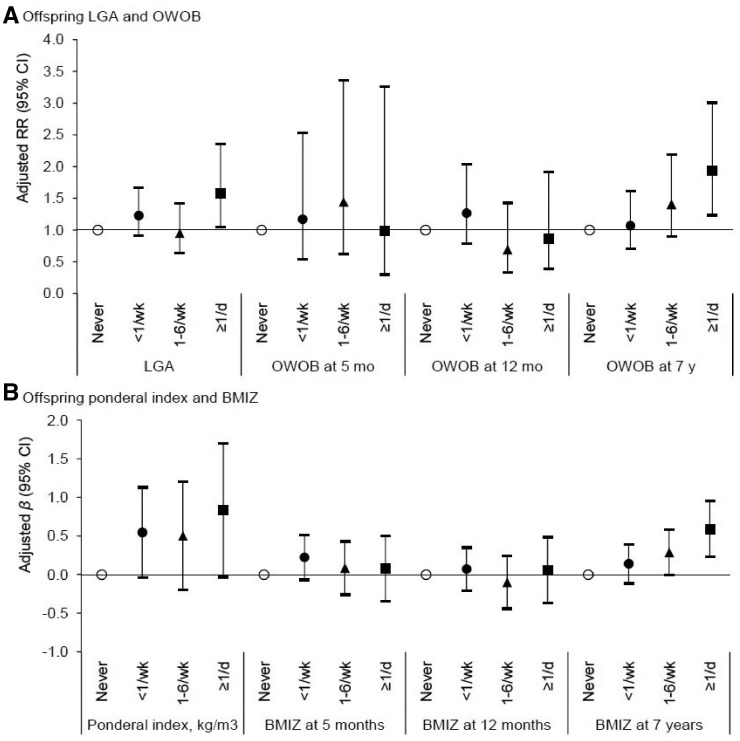
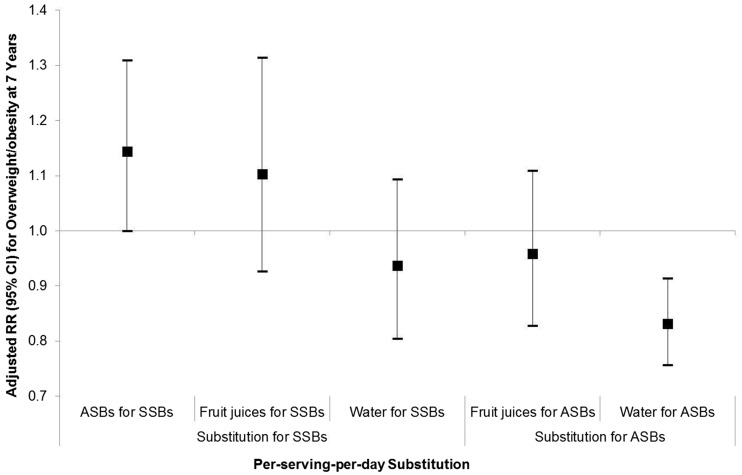
After adjusting for major maternal and offspring covariates, daily ASB consumption during pregnancy compared with never was positively associated with offspring risk of LGA (aRR, 1.57; 95% CI, 1.05–2.35; Figure 1) and overweight/obesity at 7 years (aRR, 1.93; 95% CI, 1.24–3.01; Table 2), but not during infancy (Figure 1). Consistently for continuous growth outcomes, ASB consumption ≥ 1/d was associated with a 0.59 SD increase (95% CI, 0.23–0.96) in offspring BMIZ at 7 years (Table 2) but not at earlier ages (Figure 1), except for a marginally significant 0.83 kg/m3 increase (95% CI, −0.04 to 1.70) in ponderal index at birth (P = 0.06). Further, cubic spline regression curve illustrated a dose-response relationship between per-serving-per day consumption of ASBs during pregnancy and offspring overweight/obesity at 7 years (Supplementary Figure 1, available as Supplementary data at IJE online). In contrast, no significant associations were observed for SSB consumption with offspring growth at birth, in infancy (Supplementary Figure 2, available as Supplementary data at IJE online) or at 7 years (Supplementary Table 1, available as Supplementary data at IJE online). Notably, per-serving-per-day substitution of SSBs with ASBs was not associated with a reduced but with an increased risk of offspring overweight/obesity at 7 years (aRR, 1.14; 95% CI, 1.00–1.31; although being marginally significant Figure 2), whereas per-serving-per-day substitution of ASBs with water was significantly associated with a 17% reduced risk (aRR, 0.83; 95% CI, 0.76–0.91).
Figure 1.
Maternal consumption of artificially sweetened beverages and offspring growth and risk of overweight or obesity from birth through age 7 years. Point estimates for β for ponderal index and body mass index z-scores (BMIZ), and relative risk (RR) for large-for-gestational age (LGA) and overweight or obesity (OWOB) were adjusted for maternal: pre-pregnancy body mass index, age, socioeconomic status, smoking during pregnancy; maternal intakes of total energy, desserts and sweets, oil/margarine/butter, potato, processed meat, refined grains, whole grains and sugar-sweetened beverages during pregnancy, and physical activity during pregnancy; and offspring sex, breastfeeding duration, consumption of artificially and sugar-sweetened beverages at 7 y (only for outcomes at 7 y), and physical activity at 7 y (only for outcomes at 7 y).
Table 2.
Maternal consumption of artificially sweetened beverages during pregnancy and offspring BMIZ and risk of overweight or obesity at 7 years of age
| Artificially sweetened beverage consumption | Crude | Model 1: maternal pre-pregnancy BMIa | Model 2: maternal non-dietary and dietary covariatesb | Model 3: maternal and offspring covariatesc |
|---|---|---|---|---|
| BMIZ at 7 years | β(95% CI)d | |||
| Never | 0 [Reference] | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| < 1/wk | 0.29 (−0.00 to 0.58) | 0.19 (−0.05 to 0.43) | 0.15 (−0.10 to 0.39) | 0.14 (−0.11 to 0.39) |
| 1–6/wk | 0.30 (0.05 to 0.55) | 0.24 (−0.04 to 0.53) | 0.31 (0.02 to 0.59) | 0.29 (−0.01 to 0.58) |
| ≥ 1/d | 0.74 (0.39 to 1.09) | 0.60 (0.26 to 0.94) | 0.59 (0.24 to 0.95) | 0.59 (0.23 to 0.96) |
| Overweight or obesity at 7 years | Relative risk (95% CI)d | |||
| Never | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| < 1/wk | 1.28 (0.85 to 1.91) | 1.11 (0.74 to 1.67) | 1.06 (0.70 to 1.59) | 1.07 (0.71 to 1.61) |
| 1–6/wk | 1.35 (0.85 to 2.16) | 1.27 (0.81 to 1.99) | 1.36 (0.87 to 2.12) | 1.41 (0.90 to 2.20) |
| ≥ 1/d | 2.29 (1.53 to 3.42) | 1.93 (1.29 to 2.89) | 1.88 (1.21 to 2.92) | 1.93 (1.24 to 3.01) |
aAdjusted for maternal pre-pregnancy BMI.
bAdjusted for covariates in model 1 and maternal age, socioeconomic status, smoking during pregnancy, intakes of total energy, desserts and sweets, oil/margarine/butter, potato, processed meat, refined grains, and whole grains during pregnancy, and physical activity during pregnancy.
cAdjusted for covariates in model 2 and offspring sex, breastfeeding duration, consumption of artificially and sugar-sweetened beverages at 7 years, and physical activity at 7 years.
dAll models are adjusted for maternal intake of sugar-sweetened beverages during pregnancy.
Figure 2.
Risk of offspring overweight or obesity at 7 years associated with substitution of maternal per-serving-per-day consumption of SSBs or ASBs with alternative beverages during pregnancy. ASBs indicates artificially sweetened beverages; SSBs, sugar-sweetened beverages. Relative risks (RR) and 95% confidence intervals (CI) were adjusted for: maternal pre-pregnancy body mass index, age, socioeconomic status, smoking during pregnancy; maternal intakes of total energy, desserts and sweets, oil/margarine/butter, potato, processed meat, refined grains and whole grains during pregnancy, and physical activity during pregnancy; and offspring sex, breastfeeding duration, consumption of ASBs and SSBs at 7 y, and physical activity at 7 y.
In the stratified analyses, the associations were more prominent among boys and offspring who were breastfed < 6 months (P-for-interaction = 0.09 and 0.18, respectively; Supplementary Table 2, available as Supplementary data at IJE online). No significant interaction was evident by maternal pre-pregnancy obesity status, age or smoking during pregnancy (Supplementary Table 2), nor by offspring physical activity, beverage consumption or other dietary factors (i.e. fruits, vegetables and sweets) at 7 years (data not shown). Further, sensitivity analyses showed robust results even among women who did not take any medications for GDM, or had no hypertensive complications during pregnancy or delivered at term (Supplementary Table 3, available as Supplementary data at IJE online).
Discussion
In this prospective study, daily maternal ASB consumption during pregnancy compared with never was associated with a 1.57-fold increase in LGA risk at birth, a 0.59-SD increase in BMIZ at 7 years and a 1.93-fold increase in overweight/obesity risk at 7 years after adjustment for major maternal and offspring risk factors, among high-risk offspring born to women with GDM. Notably, results remained robust even after adjustment for pre-pregnancy BMI and other major risk factors of GDM. Furthermore, substituting one-serving-per-day of SSBs with an equivalent serving of ASBs was not associated with a decreased risk of offspring overweight/obesity at 7 years, whereas ASB substitution with water was significantly associated with a reduced risk.
Our findings are biologically plausible. The high-intensity artificial sweeteners compared with glucose or sucrose may exacerbate glucose intolerance at a greater magnitude via alterations of gut microbiota,11 increase intestinal glucose absorption through apical glucose transporter 212 and promote excessive intake and weight gain via dysregulation of sweet taste and caloric reward.30 Such maternal metabolic perturbations during the critical developmental window of gestation may in turn predispose offspring to obesity and metabolic disorders in later life.31 Moreover, we observed sex-specific associations of ASB consumption during pregnancy, with male but not female offspring risk of overweight/obesity at 7 years. Despite that the mechanisms underlying the observed sexual dimorphism remain to be elucidated, our findings are consistent with animal data; male mice exhibited greater weight gain and decreased insulin sensitivity compared with females in response to chronic lifetime exposure to aspartame starting in utero.32 In addition, the metabolic perturbations among male offspring might be partially attributable to sex-specific alterations in the gut microbiome,33 which has been implicated in the artificial sweeteners-induced glucose tolerance.11 On the other hand, no significant associations were observed for SSB consumption during pregnancy and offspring growth herein and previously.14 As observed in our study and previously,14 women who consumed more ASBs during pregnancy were different from those who consumed more SSBs in terms of several key characteristics. Specifically, the former compared with the latter had relatively greater pre-pregnancy BMI but lower total daily energy intake during pregnancy, suggesting the intention of women at higher risk for GDM to seek for ‘healthier’ alternatives of SSBs during pregnancy. Since we have adjusted for these major risk factors in the multivariable models, they could be indicators of other unmeasured risk factors, which could partially contribute to the null findings for SSB consumption. Further, notably, only 9% of pregnant women reported daily SSB consumption in our study during 1996–2002, which was remarkably lower than the consumption of a Canadian pregnant population (23.4%) in 2009–1214 and a contemporary US non-pregnant population (59%).34 Therefore, the null findings for SSBs in the present study could also be partially attributed to the limited variation in SSB exposure.
To our knowledge, only one study in a Canadian population has investigated the associations between maternal ASB and SSB consumption during pregnancy and offspring growth at 1 year, with positive associations observed only for ASBs but not SSBs.14 In the present study, the positive associations were pronounced at birth and extended to 7 years but not during infancy. The discordant findings in age-specific associations could be partially attributable to the difference in the underlying study population and offspring growth pattern. Also notably, the validity of using BMIZ as an indicator of paediatric adiposity in infancy remains debatable.35 Studies with more objective measures of paediatric adiposity are warranted. Further, our data suggest that unfavourable early life factors (i.e. suboptimal breastfeeding duration) may exacerbate the potential adverse effects of intrauterine ASB exposure on childhood risk of overweight/obesity. It is plausible that the subtle metabolic alteration due to fetal programming may become more apparent later in life encountered by a second hit or challenge, resulting in latency between intrauterine exposure and disease phenotype.36
A major strength of this study is the prospective and longitudinal follow-up of offspring growth through 7 years of age. Further, our results are robust against a series of sensitivity analyses. For instance, to reduce potential confounding from pre-pregnancy BMI, we included it in multivariable models and observed significant associations even after its adjustment. Moreover, stratified analysis illustrated robust findings; even among non-obese women, maternal ASB consumption was related to higher offspring risk of overweight/obesity at 7 years. Notably, our findings could be of particular relevance to the high-risk population of GDM women who may be particularly health conscious and be seeking ‘healthier’ alternatives for SSBs during pregnancy.
Several potential limitations of the study need to be noted. First, offspring weight and height at 7 years were reported by parents based on measurements made by general practitioner, school nurse or parent(s). Despite the inevitable measurement errors, parent-reported and measured data are highly correlated (height r = 0.942, weight 0.925, BMI 0.813; P < 0.001).37 Second, loss to follow-up due to missing offspring anthropometric data may have reduced statistical power and resulted in a lower proportion of women from the high-risk group (i.e. low SES group), which may consequently underestimate the true effect sizes. Third, estimates from the substitution analyses should be interpreted with caution due to the underlying assumption of causality between maternal beverage consumption and risk of offspring overweight/obesity. Nonetheless, given that an effective long-term, population-based dietary intervention study to assess the causal relationship would be costly and pragmatically challenging, such data from intervention studies are lacking and therefore carefully conducted observational studies could serve as a reasonable approach to assessing the association. Further, our findings suggest that pregnancy is an important window of susceptibility to ASBs in relation to risk of offspring obesity. However, notably, despite the richness and uniqueness of our data, dietary data during pregnancy were collected between 1996 and 2002. The shifting beverage landscape with an overall increasing trend of ASB consumption during the past decades38,39 necessitates further investigation using more contemporary data. Moreover, although results were robust even after additional adjustment of childhood factors (breastfeeding, and dietary intake and physical activity at 7 years), we could not completely rule out the possibility of residual confounding due to other postnatal obesogenic risk factors across early childhood (e.g. food and eating environment, other eating behaviours, and psychosocial factors). Nonetheless, these postnatal factors could be in the downstream pathway between diet during pregnancy and offspring outcomes during childhood; adjustment for them could underestimate the true effect sizes, and therefore investigation on their mediating roles may be warranted to identify potential effect modifiers. Finally, the study is generalizable to Danish women with GDM and their children. Future investigations among other high-risk racial/ethnic groups are warranted.
In conclusion, our study fills the critical data gap in the literature with a longitudinal follow-up of offspring growth from birth through age 7 years, among high-risk offspring born to women with GDM. In this prospective cohort, higher maternal ASB consumption during pregnancy was positively related to LGA at birth and offspring BMIZ and risk of overweight/obesity at 7 years. Our findings further raise the questionability of promoting ASBs as ‘healthier’ alternatives for SSBs, particularly among high-risk pregnant women. Future studies among other populations with longer follow up beyond early childhood are warranted.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract # HHSN275201000020C). The DNBC is supported by grants from the Danish Research Council # 09‐067124 (Center for Fetal Programming) and 09‐075611.
Key Messages
Among high-risk children born to women with gestational diabetes, maternal daily consumption of artificially sweetened beverages compared with never consumption during pregnancy was associated with a higher body mass index z-score and increased risk of overweight/obesity at 7 years.
Per-serving-per-day substitution of sugar-sweetened beverages with artificially sweetened beverages was not related to a lower risk of overweight/obesity at 7 years, whereas substitution of artificially sweetened beverages with water was related to a lower risk.
Given the widespread use of artificially sweetened beverages, further investigation with longer follow-up beyond early childhood is warranted.
Supplementary Material
Acknowledgements
We are grateful to all the participants in the Danish National Birth Cohort, as part of the ongoing Diabetes and Women’s Health Study, and to the whole study team, including but not limited to research scientists, staff and volunteers.
Author Contributions
Y.Z. and C.Z. conceptualized and designed the analysis. Y.Z. analysed data and drafted the manuscript. S.F.O., T.I.H., L.G.G, C.G., A.A.B. and C.Z. contributed to acquisition, interpretation of data analyses, and critical revision of the manuscript. P.M, S.R., S.N.H., E.H.Y., J.E.C. and F.B.H. contributed to the interpretation of data analyses and critical revision of the manuscript. Y.Z. and C.Z. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of interest: None declared.
References
- 1. Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents:the Bogalusa Heart Study. J Pediatr 2007;150:12–17 e2. [DOI] [PubMed] [Google Scholar]
- 2. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes 2011;35:891–98. [DOI] [PubMed] [Google Scholar]
- 3. Skinner AC, Skelton JA. Prevalence and trends in obesity and severe obesity among children in the United States, 1999–2012. JAMA Pediatr 2014;168:561–66. [DOI] [PubMed] [Google Scholar]
- 4. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141–46. [DOI] [PubMed] [Google Scholar]
- 5. Zhu Y, Zhang C. Prevalence of gestational diabetes and risk of progression to type 2 diabetes: a global perspective. Curr Diab Rep 2016;16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oken E, Gillman MW. Fetal origins of obesity. Obes Res 2003;11:496–506. [DOI] [PubMed] [Google Scholar]
- 7. Parlee SD, MacDougald OA. Maternal nutrition and risk of obesity in offspring: the Trojan horse of developmental plasticity. Biochim Biophys Acta 2014;1842:495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Catalano PM, Hauguel-De Mouzon S. Is it time to revisit the Pedersen hypothesis in the face of the obesity epidemic? Am J Obstet Gynecol 2011;204:479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hytten F. Blood volume changes in normal pregnancy. Clin Haematol 1985;14:601–12. [PubMed] [Google Scholar]
- 10. Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Suez J, Korem T, Zeevi D. et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181–86. [DOI] [PubMed] [Google Scholar]
- 12. Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol 2007;582:379–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown RJ, Walter M, Rother KI. Ingestion of diet soda before a glucose load augments glucagon-like peptide-1 secretion. Diabetes Care 2009;32:2184–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azad MB, Sharma AK, de Souza RJ. et al. Association between artificially sweetened beverage consumption during pregnancy and infant body mass index. JAMA Pediatr 2016. [DOI] [PubMed] [Google Scholar]
- 15. Pereira MA, Gillman MW. Maternal consumption of artificially sweetened beverages and infant weight gain:Causal or casual? JAMA Pediatr 2016;170:662–70. [DOI] [PubMed] [Google Scholar]
- 16. Olsen J, Melbye M, Olsen SF. et al. The Danish National Birth Cohort – its background, structure and aim. Scand J Public Health 2001;29:300–07. [DOI] [PubMed] [Google Scholar]
- 17. Olsen SF, Mikkelsen TB, Knudsen VK. et al. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2007;21:76–86. [DOI] [PubMed] [Google Scholar]
- 18. Zhang C, Hu FB, Olsen SF. et al. Rationale, design, and method of the Diabetes & Women’s Health study – a study of long-term health implications of glucose intolerance in pregnancy and their determinants. Acta Obstet Gynecol Scand 2014;93:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1, Diagnosis and Classification of Diabetes Mellitus. 1999. http://apps.who.int/iris/handle/10665/66040 (25 March 2016, date last accessed).
- 20. Kuhl C. Glucose metabolism during and after pregnancy in normal and gestational diabetic women. 1. Influence of normal pregnancy on serum glucose and insulin concentration during basal fasting conditions and after a challenge with glucose. Acta Endocrinol (Copenh) 1975;79:709–19. [PubMed] [Google Scholar]
- 21. Ambrosini GL, Oddy WH, Huang RC, Mori TA, Beilin LJ, Jebb SA. Prospective associations between sugar-sweetened beverage intakes and cardiometabolic risk factors in adolescents. Am J Clin Nutr 2013;98:327–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Food Institute (Denmark). Danish Food Composition Databank. 2007. http://www.foodcomp.dk/v6/fcdb_default.asp (25 March 2016, date last accessed).
- 23. Mikkelsen TB, Olsen SF, Rasmussen SE, Osler M. Relative validity of fruit and vegetable intake estimated by the food frequency questionnaire used in the Danish National Birth Cohort. Scand J Public Health 2007;35:172–79. [DOI] [PubMed] [Google Scholar]
- 24. Mikkelsen TB, Osler M, Olsen SF. Validity of protein, retinol, folic acid and n-3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr 2006;9:771–78. [DOI] [PubMed] [Google Scholar]
- 25. Halldorsson TI, Strom M, Petersen SB, Olsen SF. Intake of artificially sweetened soft drinks and risk of preterm delivery: a prospective cohort study in 59,334 Danish pregnant women. Am J Clin Nutr 2010;92:626–33. [DOI] [PubMed] [Google Scholar]
- 26. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards:Length/height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for-age:Methods and Development. Geneva: WHO, 2006. [Google Scholar]
- 27. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pan A, Sun Q, Bernstein AM. et al. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65:1220–28S;discussion 1229–31S. [DOI] [PubMed] [Google Scholar]
- 30. Davidson TL, Martin AA, Clark K, Swithers SE. Intake of high-intensity sweeteners alters the ability of sweet taste to signal caloric consequences: implications for the learned control of energy and body weight regulation. Q J Exp Psychol (Hove) 2011;64:1430–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nelson SM, Matthews P, Poston L. Maternal metabolism and obesity: modifiable determinants of pregnancy outcome. Hum Reprod Update 2010;16:255–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Collison KS, Makhoul NJ, Zaidi MZ. et al. Gender dimorphism in aspartame-induced impairment of spatial cognition and insulin sensitivity. PLoS One 2012;7:e31570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kozyrskyj AL, Kalu R, Koleva PT, Bridgman SL. Fetal programming of overweight through the microbiome: boys are disproportionately affected. J Dev Orig Health Dis 2016;7:25–34. [DOI] [PubMed] [Google Scholar]
- 34. Chen L, Hu FB, Yeung E, Willett W, Zhang C. Prospective study of pre-gravid sugar-sweetened beverage consumption and the risk of gestational diabetes mellitus. Diabetes Care 2009;32:2236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001;2:141–47. [DOI] [PubMed] [Google Scholar]
- 36. Heindel JJ, Balbus J, Birnbaum L. et al. Developmental origins of health and disease: Integrating environmental influences. Endocrinology 2015;156:3416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Cauwenberghe J, Delvaux I, Michels N. et al. Validity of parentally reported versus measured weight, length and waist in 7- to 9-year-old children for use in follow-up studies. Eur J Pediatr 2014;173:921–28. [DOI] [PubMed] [Google Scholar]
- 38. Storey M. The shifting beverage landscape. Physiol Behav 2010;100:10–14. [DOI] [PubMed] [Google Scholar]
- 39. Sylvetsky AC, Rother KI. Trends in the consumption of low-calorie sweeteners. Physiol Behav 2016;164:446–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.