Abstract
Introduction
Tyrosine kinase inhibition of the epidermal growth factor receptor (EGFR) is the standard in the first line treatment of patients with advanced non-small–cell lung cancer (NSCLC) harbouring EGFR activating mutations. Here we aim to discern efficacy and toxicity measures through a meta-analysis of published studies that could aid treatment selection.
Materials And Methods
We performed a meta-analysis of the main randomized clinical trials evaluating the currently approved EGFR-TKIs in first-line of treatment of EGFR-positive advanced NSCLC. Cochrane guidelines were used for statistical analysis.
Results
3,179 patients were included. All EGFR TKIs showed improved outcomes with respect to ORR and PFS when compared to standard platinum-doublet chemotherapy. Comparative ORR for gefitinib, erlotinib and afatinib were 52.1%, 67.3% and 61.6% respectively. HRs for PFS were 0.62 (95% CI, 0.38–1.00) for gefitinib, 0.28 (95% CI, 0.17–0.45) for erlotinib and 0.40 (95% CI, 0.20–0.83) for afatinib. HRs for OS were not statistically significant for any agent.
Conclusions
Our results suggest similar clinical efficacy and higher toxicity of Afatinib treatment. As this still remains the agent with best CSF penetration, we suggest its use is limited to patients presenting with brain metastasis. We suggest the use of Gefitinib in patients without CNS involvement. Faced with the impossibility to dose-reduce Gefitinib, Erlotinib represents a tolerable and effective alternative to Afatinib and Gefitinib if response to EGFR inhibition is considered still effective.
Keywords: non-small cell lung cancer, epidermal growth factor receptor, tyrosine kinase inhibitors
INTRODUCTION
Non-small-cell lung cancer (NSCLC) is the major cause of cancer-related death worldwide [1]. The Epidermal Growth Factor Receptor (EGFR), a transmembrane glycoprotein, is mutated in approximately 10–15% of European patients, more frequently in women, adenocarcinoma type and never-smokers [2]. When the EGFR gene is mutated, (most commonly with exon 19 deletions or exon 21 L858R point mutation), constitutive receptor activation influences the cell cycle, the apoptotic pathway and the production of inflammatory agents [3]. This understanding of EGFR signalling led to the development of specific tyrosine-kinase inhibitors (TKIs) [4], which reached three generations: gefitinib and erlotinib (first); afatinib, dacomitinib, and neratinib (second); rociletinib, HM61713, osimertinib and others (third). The last generation overcomes the threonine-to-methionine substitution (T790M) in exon 20 of the EGFR gene, responsible for 50% of resistance mechanisms to first line anti-EGFR therapy with first and second generation agents [5]. Only gefitinib, erlotinib, and afatinib are approved by Food and Drug Administration (FDA) thus far for the first line setting [6–8].
In patients whose tumours harbours an activating EGFR mutation, EGFR TKIs should be used as first-line therapy [6–9], whereas for the rest of NSCLC cases, standard treatment currently consists of platinum-based doublet chemotherapy. Gefitinib, erlotinib and afatinib show higher response rates and longer progression free survival than chemotherapy in those patients, as tested in several clinical trials exhibiting consistent results [10–20], all of them favouring the target therapy.
Since there are several similar drugs targeting the EGFR mutation in NSCLC first line setting, the critical question emerging is which one should be best for this setting. Our analysis presents the findings of a network meta-analysis, attempting to access the main outcomes among EGFR TKIs in NSCLC, exploiting the data of clinical trials with gefitinib, erlotinib and afatinib. Recently, the Lux-Lung 7 study reported longer PFS and similar OS when comparing Afatinib with Gefitinib, but a triple arm comparison of all these agents is unlikely to occur. Here we aimed to provide an indirect comparison among these drugs which may contribute to guide the drug choice for physicians.
MATERIALS AND METHODS
For this comparative meta-analysis, we performed computerized searches of the Medline. Embase, Scopus and Information Sciences Institute (ISI) databases up to August 14, 2016, using the following terms: “gefitinib” OR “afatinib” OR “erlotinib” AND “NSCLC” OR “lung cancer” OR “epidermal growth factor”. These searches were complemented by examining review articles. Only articles published in English, available in full text and reporting results of randomized, double-arm, phase III clinical trials comparing EGFR-TKIs with chemotherapy regimens were included. The most recent –updated- data of the studies were used for the meta-analysis. For gefitinib, erlotinib and afatinib, only first line treatments were considered due to the paucity of trials comparing these agents to chemotherapy in second line. There were no time restrictions in the search. Exclusion criteria were: trials with patients presenting Eastern Cooperative Oncology Group (ECOG) performance status > 2 and those including EGFR TKI plus chemotherapy versus chemotherapy (Effectiveness of EGFR-TKIs may be obscured in this setting). Case reports or patient series, which report few patients, were excluded. All abstracts were screened twice and unrelated studies were excluded.
For included trials, we extracted data on: title, first author, year of publication, study design (inclusion and exclusion criteria), patient’s characteristics (median patient age, stage of disease, performance status, gender, smoking status, histology, tissue-assessed EGFR mutation), treatment schedules and line of treatment, outcomes from the trial, incidence of adverse events, demographic data. If the study was updated, main outcomes were extracted from the last published article. Data extraction was done independently by two of the authors and divergences were resolved by consensus with a third author.
The primary outcome of this meta-analysis was objective response rate (ORR). Second outcomes were progression free survival (PFS), overall survival (OS) and incidence of adverse events (AE). Summary measures were risk ratio (95% confidence interval [CI]; 95% PI) for ORR and AE and hazard ratio for OS and PFS.
ORR was defined as the proportion of patients who presented complete or partial response, assessed by Response Evaluation Criteria in Solid Tumours (RECIST) [21] in most of the studies. The time of assessment varied for each trial. PFS was the time, in months, from the randomization until disease progression, or death. OS was the time, in months, from the randomization to death. AE could be any unfavourable and unintentional sign, symptom, or disease temporarily associated with the use of the drugs, without any judgment about causality or relationship to them. Relevant adverse events of all grades related by two or more studies were condensed by each EGFR TKI arm and compared as meta-estimation with another EGFR TKI.
Statistical analysis was directed by Cochrane Guidelines [22]. We combined the risk ratios from each study using the random-effects model (Mantel-Haenzsel) [23]. For the hazard ratios, the Inverse Variance method was used. The heterogeneity between trials was estimated by the I2 statistic. We used the Review Manager version 5.3.5.
RESULTS
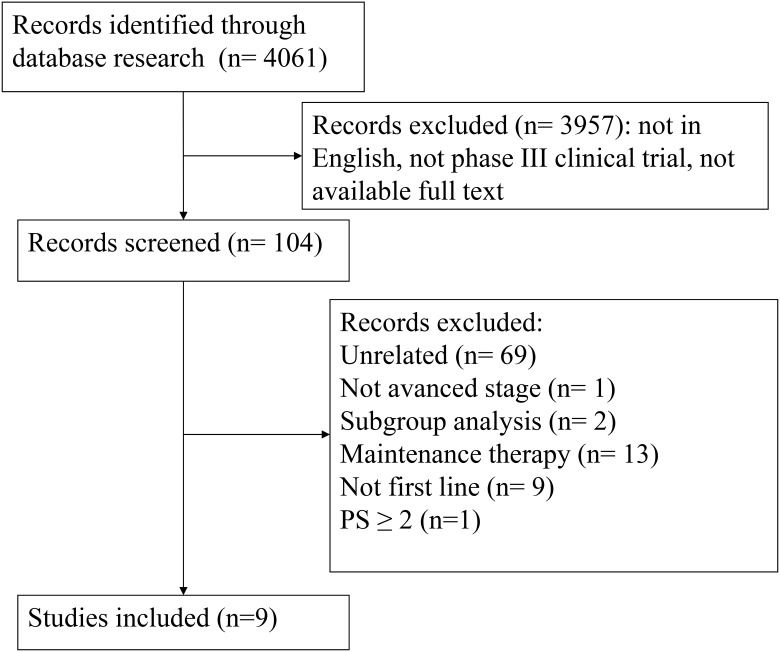
As shown in the flow chart of the meta-analysis (Figure 1), 09 eligible studies were identified. All of them were included in the current analysis (Table 1), totalizing 3,179 patients. NEJ002 [15]; WJTOG3405 [16], First-SIGNAL [17], and IPASS [14] evaluated gefitinib as first-line treatment to, respectively, carboplatin plus paclitaxel, cisplatin plus docetaxel, cisplatin plus gemcitabine, and carboplatin plus paclitaxel; LUX-Lung 3 [24] and LUX-Lung 6 [19] compared afatinib as first-line treatment with cisplatin plus pemetrexed and gemcitabine, respectively. EURTAC [20], OPTIMAL [12] and ENSURE [25] compared first-line erlotinib with cisplatin plus docetaxel, gemcitabine plus carboplatin, and cisplatin plus gemcitabine, respectively. NEJ002, IPASS, and OPTIMAL published updated outcomes, so 12 reports were used in total for this meta-analysis.
Figure 1. Study selection.
Table 1. Patient demographics and disease characteristics of included studies.
| Study | First author | Population | Line | Treatment arms | Response criteria |
|---|---|---|---|---|---|
| ENSURE (2015) | Wu | Chemotherapy-naïve patients from China,
Malaysia, and the Philippines with stage IIIB/IV EGFR mutation-positive NSCLC |
First | Erlotinib 150 mg/day (n =
110) Gemcitabine 1000 mg/m2plus cisplatin 75 mg/m2 every 3 weeks (n = 107) |
RECIST |
| LUX-Lung 6 (2014) | Wu | Patients with previously untreated stage IIIB or IV lung adenocarcinoma and EGFR mutation-positives | First | Afatinib 40 mg/day (n =
242) Gemcitabine 1000 mg/m2plus cisplatin 75 mg/m2 every 3 weeks (n = 122) |
RECIST |
| LUX-Lung 3 (2013) | Sequist | Treatment-naïve patients with advanced lung adenocarcinoma and EGFR mutation-positives | First | Afatinib 40 mg/day (n =
230) Cisplatin 75 mg/m2 and pemetrexed 500 mg/m2 (n = 115) |
RECIST |
| EURTAC (2012) | Rosell | European patients with stage IIIB or IV NSCLC and EGFR mutations who had no history of chemotherapy for metastatic disease | First | Erlotinib 150 mg/day (n =
86) Cisplatin 75 mg/m2 plus docetaxel 75 mg/m2 or cisplatin 75 mg/m2 plus gemcitabine 1250 mg/m2 (n= 87) |
RECIST |
| First-SIGNAL (2012) | Han | Chemotherapy-naïve and never-smokers patients with stage IIIB or IV adenocarcinoma of the lung | First | Gefitinib 250 mg/day (n =
159) Gemcitabine 1,250 mg/m2 plus cisplatin 75 mg/m2 every 3 weeks (n = 150) |
WHO |
| OPTIMAL (2011) | Zhou and Wu | Chinese patients with stage IIIB or IV NSCLC and a confirmed activating mutation of EGFR, without receiving therapy for metastatic disease | First | Erlotinib 150 mg/day (n =
82) Gemcitabine 1000 mg/m2plus carboplatin AUC = 5 every 3 weeks (n = 72) |
RECIST |
| NEJ002 (2010) | Maemondo | Japanese patients with metastatic
NSCLC and EGFR mutations who had not previously received chemotherapy |
First | Gefitinib 250 mg/day (n =
114) Carboplatin AUC = 6 plus paclitaxel 200 mg/m2 every 3 weeks (n = 114) |
RECIST |
| WJTOG3405 (2009) | Mitsudomi | Patients with advanced or recurrent NSCLC harbouring an activating mutation of the EGFR | First | Gefitinib 250 mg/day (n =
88) Cisplatin 80 mg/m2 plus docetaxel 60 mg/m2 (n = 89) |
RECIST |
| IPASS (2009) | Mok | Asian, nonsmokers or light smokers patients with stage IIIB or IV adenocarcinoma of the lung who had no previous chemotherapy | First | Gefitinib 250 mg/day (n =
609) Carboplatin AUC = 5 or 6 plus paclitaxel 200 mg/m2 every 3 weeks (n = 608) |
RECIST |
Abbreviations: NSCLC, non-small-cell lung cancer; EGFR, epidermal growth factor receptor; AUC, area under the curve; RECIST, response evaluation criteria in Solid tumors; WHO, world health organization.
Patients’ characteristics are summarized in Table 2. More patients were female (2,315 of 3,179 [72.8%]), never smokers (2,606 of 3,179 [81.9%]), with performance status from 0 to 1 (2,974 of 3,179 [93.5%]) and had tumours of adenocarcinoma histology (3,068 of 3,179 [96.5%]). Disease stage was not summarized because of differences in evaluation among studies.
Table 2. Patient demographics and disease characteristics of included studies.
| Characteristic | Gefitinib (n = 968) | Control (n = 958) | Erlotinib (n = 278) | Control (n = 266) | Afatinib (n = 472) | Control (n = 237) | |
|---|---|---|---|---|---|---|---|
| Sex | Male | 213 (22%) | 210 (21.9%) | 104 (37.4%) | 90 (33.8%) | 170 (36%) | 77 (32.5%) |
| Female | 755 (78%) | 748 (78.1%) | 174 (62.6%) | 176 (66.2%) | 302 (64%) | 160 (67.5%) | |
| Age (median)† | 60.5 | 60 | 59.8 | 60 | 59.7 | 59.5 | |
| Smoking status | Never smoker | 866 (89.5%) | 842 (87.9%) | 195 (70.1%) | 187 (70.3%) | 336 (71.2%) | 180 (75.9%) |
| Previous or current smoker | 102 (10.5%) | 116 (12.1%) | 140 (50.4%) | 79 (29.7%) | 136 (28.8%) | 57 (24.1%) | |
| ECOG | 0–1 | 892 (92.1%) | 877 (91.5%) | 252 (90.6%) | 245 (92.1%) | 472 (100%) | 236 (99.6%) |
| 2 | 76 (7.9%) | 81 (8.5%) | 26 (9.4%) | 21 (7.9%) | 0 (0%) | 1 (0.4%) | |
| Histologic diagnosis | Adenocarcinoma | 926 (95.7%) | 934 (97.5%) | 258 (92.8%) | 241 (90.6%) | 472 (100%) | 237 (100%) |
| Other | 39 (4%) | 20 (2.1%) | 20 (7.2%) | 25 (9.4%) | 0 (0%) | 0 (0%) | |
| Unknown | 3 (0.3%) | 3 (0.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| EGFR mutation | Positive | 358 (37%) | 345 (36%) | 278 (100%) | 266 (100%) | 472 (100%) | 237 (100%) |
| Negative | 118 (12.2%) | 112 (11.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Unknown | 492 (50.8%) | 501 (52.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviations: ECOG, Eastern Collaborative Oncology Group; EGFR, epidermal growth factor receptor.
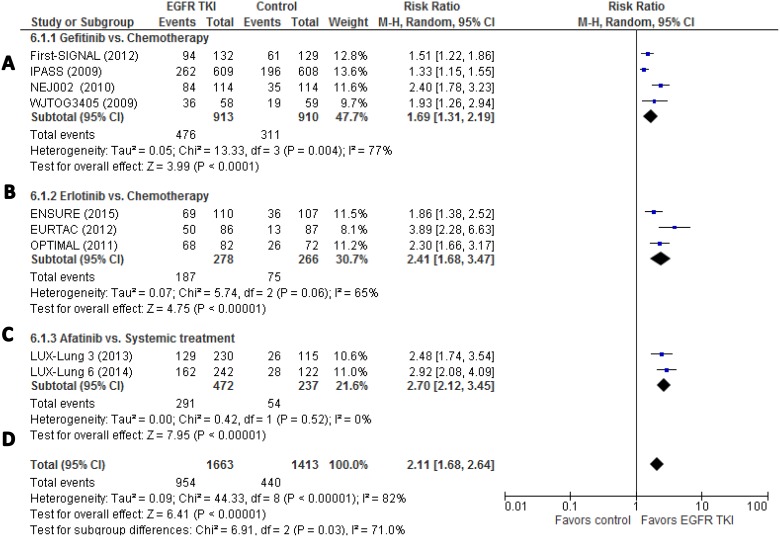
The risk ratio of objective response rate (ORR) is shown in Figure 2. For gefitinib versus chemotherapy as first-line treatment, 52.1% (476 out of 913) of patients treated with gefitinib showed complete or partial response against 34.2% (311 out of 910) of patients treated with chemotherapy, and the pooled risk ratio (RR) was 1.69 (95% CI, 1.31–2.19; p < 0.0001). For afatinib versus chemotherapy, RR was 2.70 (95% CI, 2.12–3.45; p < 0.0001); 61.6% (291 of 472) of patients in the afatinib arm had response, compared to 22.8% (54 out of 237) in the chemotherapy arm. For erlotinib versus chemotherapy, RR was 2.41 (95% CI, 1.68–3.47; p < 0.0001). ORR was 67.3% (187 patients of 278 for the erlotinib arm and 28.2% (75 out of 266) for the chemotherapy arm. Heterogeneity was high between studies (I2 = 78%).
Figure 2.
(A–D) Individual study and meta-estimate risk ratio of objective response ratio for gefitinib, afatinib, and erlotinib. ORR, overall response rate; PFS, progression-free-survival; OS, overall survival.
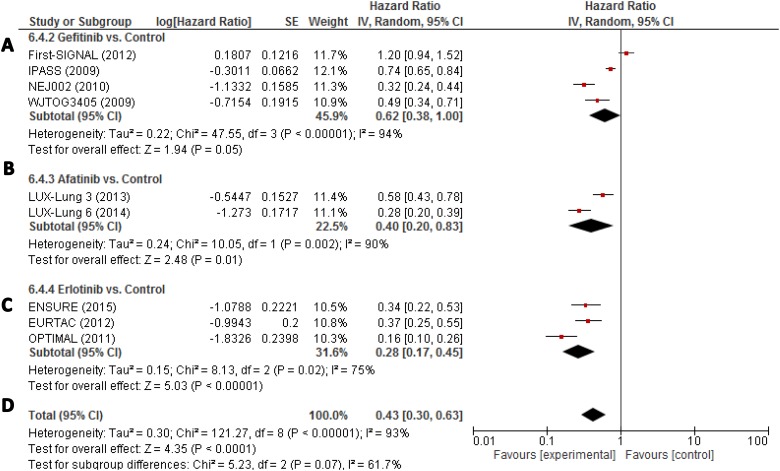
In terms of progression free survival (PFS), the pooled hazard ratio (HR) for gefitinib as first-line HR was 0.62 (95% CI, 0.38–1.00 (Figure 3). In the afatinib analysis, HR was 0.40 (95% CI, 0.20–0.83). In the erlotinib one, HR was 0.28 (95% CI, 0.17–0.45). Heterogeneity was high (I2 = 93%).
Figure 3.
(A–D) Individual study hazard ratios with pooled estimation for progression-free survival for gefitinib, erlotinib, and afatinib. ORR, overall response rate; PFS, progression-free-survival; OS, overall survival.
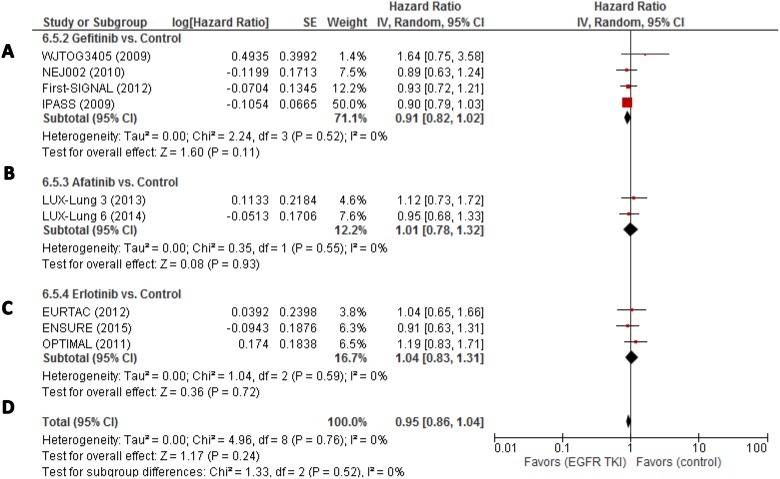
Accessing overall survival (OS), heterogeneity was very low (I2 = 0%). For Gefitinib HR was 0.91 (95% CI, 0.82–1.02; p = 0.11) (Figure 4). For afatinib, HR was 1.01 (95% CI, 0.78–1.32; p = 0.93) and 1.04 (95% CI, 0.83–1.31; p = 0.72) for erlotinib.
Figure 4.
(A–D) Individual study hazard ratios with pooled estimation for overall survival for gefitinib, erlotinib, and afatinib. ORR, overall response rate; PFS, progression-free-survival; OS, overall survival.
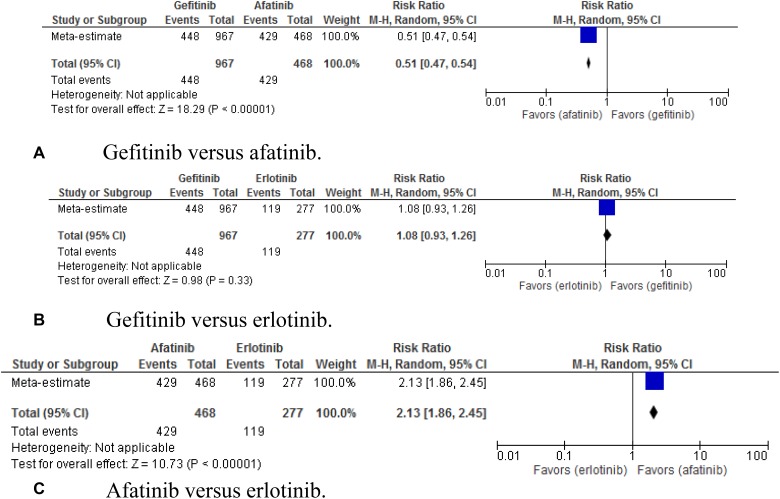
Most common adverse events of EGFR TKIs [26] were analysed (Figures 5–8). Diarrhoea of any grade was a common side effect for these patients. Comparing gefitinib with afatinib, RR was 0.51 (95% CI, 0.47–0.54; p < 0.00001); gefitinib with erlotinib, RR was 1.00 (95% CI, 0.93–1.26; p = 0.03); and afatinib with erlotinib, RR was 2.13 (95% CI, 1.86–2.45; p < 0.00001).
Figure 5.
(A–C) Pooled risk ratio of gefitinib, erlotinib, and afatinib indirectly compared for the ocurrence of diahrrea. EGFR, epidermal growth factor receptor; ORR, overall response rate; PFS, progression-free-survival; OS, overall survival.
Figure 8.
(A–C) Pooled risk ratio of gefitinib, erlotinib, and afatinib indirectly compared for the ocurrence of paronychia.
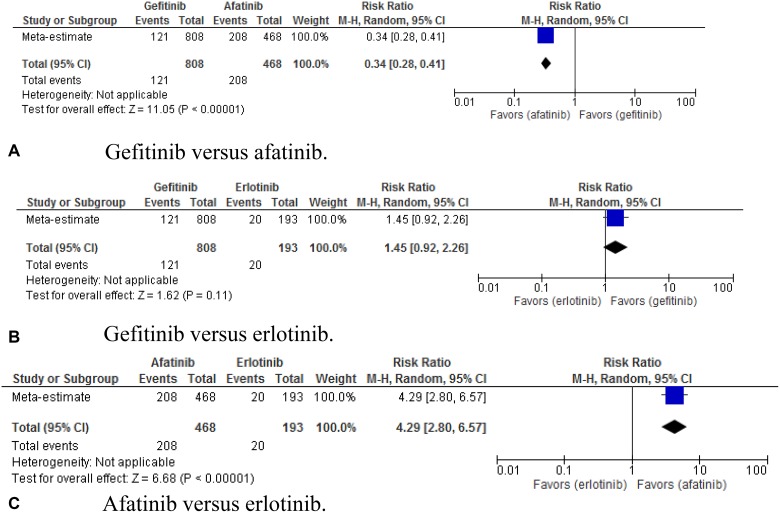
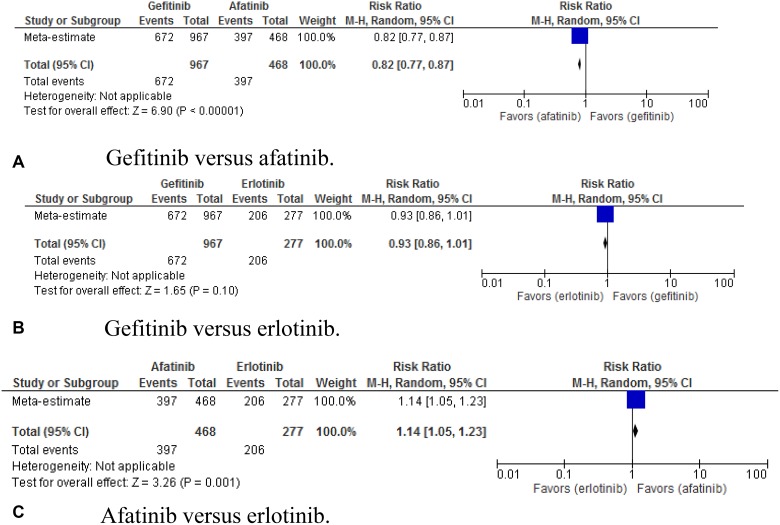
The incidence of skin rash was also observed. In the indirect comparison, gefitinib versus afatinib showed RR of 0.82 (95% CI, 0.77–0.87; p < 0.00001). For gefitinib versus erlotinib, RR was 0.93 (95% CI, 0.86–1.01; p = 0.10). For afatinib versus erlotinib, RR was 1.14 (95% CI, 1.05 – 1.23; p = 0.001) (Figure 6).
Figure 6.
(A–C) Pooled risk ratio of gefitinib, erlotinib, and afatinib indirectly compared for the ocurrence of skin rash.
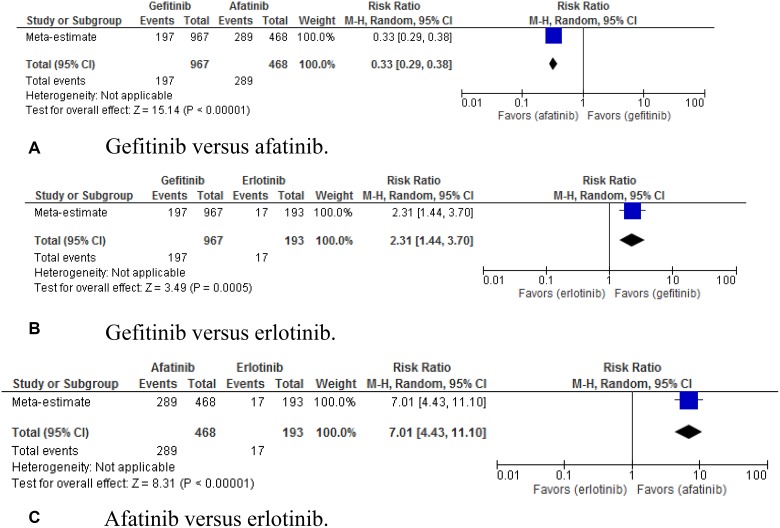
For the occurrence of stomatitis (Figure 7), the pooled RR for gefitinib versus afatinib was 0.33 (95% CI, 0.29–0.38; p < 0.00001); gefitinib versus erlotinib, 2.31 (95% CI, 1.44–3.70; p = 0.00015); afatinib versus erlotinib, 7.01 (95% CI, 4.43–11.10; p < 0.00001).
Figure 7.
(A–C) Pooled risk ratio of gefitinib, erlotinib, and afatinib indirectly compared for the ocurrence of stomatitis.
Paronychia was also accessed (Figure 8). The indirect comparison showed RR of 0.34 (95% CI, 0.28–0.41; p < 0.00001) for gefitinib versus afatinib, 1.45 (95% CI, 0.92–2.26; p = 0.11) for gefitinib versus erlotinib, and 4.29 (95% CI, 2.80–6.57; p < 0.00001) for afatinib versus erlotinib.
DISCUSSION
Currently, the landscape of NSCLC treatment is changing. Most recently, the use of EGFR TKI agents for patients harbouring activating mutations of EGFR (exons 18–21) is the standard of care. Several drugs have been approved in this setting, including gefitinib, erlotinib and recently afatinib. In this meta-analysis, gefitinib, erlotinib, and afatinib were superior in terms of objective response rate and progression free survival than platinum-based chemotherapy, but, as expected due to the cross-over effect, there was no statistically significant differences in terms of OS for either of the three drugs. Overall, gefitinib had the most consistent efficacy profile from a statistical point of view, and erlotinib had the best efficacy profile in terms of comparative improvement of PFS.
Our results challenge the recently reported results of LUX-Lung 7 [27], a phase 2b trial comparing afatinib with gefitinib as first-line treatment in patients harboring EGFR mutations, that showed improvement in PFS and ORR with afatinib over gefitinib. Nevertheless, previous meta-analysis [28–30] evaluating first-line therapies of EGFR TKIs in EGFR mutation positive patients had not confirmed the results of this study. Although LUX-LUNG 7 is the only prospective, randomized clinical trial, it also harboured several drawbacks, including the small number of events, the lack of statistical power and the three co-primary endpoints. Our meta-analysis, on the other hand, is a retrospective collective analysis of data, but includes a large number of patients and possesses robust statistical power.
Afatinib was more likely to be related to adverse events, as expected because of its irreversible binding to ATP site of EGFR, HER2 and HER4, in contrast to the reversible nature of binding of gefitinib and erlotinib. [31–32]. Differences between gefitinib and erlotinib were not statistically significant, except for paronychia, which was more frequent with erlotinib.
Limitations of our study include its retrospective nature and the indirect comparison between gefitinib, erlotinib and afatinib, since there is a paucity of head-to-head clinical trials, with the exception of LUX-LUNG 7; the high heterogeneity obtained during the data analysis; and the relative paucity of studies evaluating afatinib. Strengths of our study included the large number of patients, the robust statistical design and the broader range of therapies included, as we present data on the three approved first-line drugs.
Future studies are warranted to associate each type of EGFR-activating mutation to the efficacy of a specific treatment and to compare new drugs, as osimertinib, with first and second generation TKIs.
In conclusion, gefitinib, erlotinib, and afatinib are effective in the treatment of NSCLC in terms of progression free survival and objective response rate. Gefitinib had the most consistent efficacy profile from a statistical point of view, and erlotinib had the best efficacy profile in terms of comparative improvement of PFS. As Afatinib still remains the agent with best CSF penetration, we suggest its use is limited to patients presenting with brain metastasis. We suggest the use of Gefitinib in patients without CNS involvement. Faced with the impossibility to dose-reduce Gefitinib, Erlotinib represents a tolerable and effective alternative to Afatinib and Gefitinib if response to EGFR inhibition is considered still effective.
Acknowledgments
Logistic provision from the Cearense School of Oncology, Ceará Cancer Institute, Haroldo Juaçaba Hospital, Fortaleza, Ceará, Brazil, and The Clatterbridge Cancer Centre NHS Foundation Trust, Wirral & Liverpool, England, United Kingdom.
Footnotes
CONFLICTS OF INTEREST
RAM has received honoraria from Pfizer Advisory Board, Zodiac advisory board, AstraZeneca, Novartis, National Science Centre, Krakow, Poland, and educational grant from Pierre Fabre, Amgem. RAM is ad hoc consultant at Ministry of Health, Brasília, Brazil. The other authors have no conflicts of interest in this manuscript. GM has received honoraria from Bristol Myers Squibb, Roche and AstraZeneca.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;5:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;9:2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 3.Tsiambas E, Lefas AY, Georgiannos SN, Ragos V, Fotiades PP, Grapsa D, Stamatelopoulos A, Kavantzas N, Patsouris E, Syrigos K. EGFR gene deregulation mechanisms in lung adenocarcinoma: A molecular review. Pathol Res Pract. 2016;8:672–677. doi: 10.1016/j.prp.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 4.Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH. ZD1839 (Iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res. 2002;20:5749–5754. [PubMed] [Google Scholar]
- 5.Steuer CE, Khuri FR, Ramalingam SS. The next generation of epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of lung cancer. Cancer. 2015;8:E1–6. doi: 10.1002/cncr.29139. [DOI] [PubMed] [Google Scholar]
- 6.Chung C. Tyrosine kinase inhibitors for epidermal growth factor receptor gene mutation-positive non-small cell lung cancers: an update for recent advances in therapeutics. J Oncol Pharm Pract. 2016;3:461–476. doi: 10.1177/1078155215577810. [DOI] [PubMed] [Google Scholar]
- 7.Greig SL. Osimertinib: First Global Approval. Drugs. 2016;2:263–273. doi: 10.1007/s40265-015-0533-4. [DOI] [PubMed] [Google Scholar]
- 8.Tan WL, Jain A, Takano A, Newell EW, Iyer NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL, Tan DSW. Novel therapeutic targets on the horizon for lung cancer. Lancet Oncol. 2016;8:e347–362. doi: 10.1016/S1470-2045(16)30123-1. [DOI] [PubMed] [Google Scholar]
- 9.Reck M, Popat S, Reinmuth N, De Ruysscher D, Kerr KM, Peters S, and ESMO Guidelines Working Group Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii27–39. doi: 10.1093/annonc/mdu199. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Guo H, Zhao S, Wang Y, Yang M, Yu J, Yan Y. Efficacy of epidermal growth factor receptor inhibitors in combination with chemotherapy in advanced non-small cell lung cancer: A meta-analysis of randomized controlled trials. Oncotarget. 2016;7:39823–39833. doi: 10.18632/oncotarget.9503. http://doi.org/10.18632/oncotarget.9503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thatcher N, Chang A, Parikh P, Rodrigues Pereira J, Ciuleanu T, von Pawel J, Thongprasert S, Tan EH, Pemberton K, Archer V, Carroll K. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer) Lancet. 2005;9496:1527–1537. doi: 10.1016/S0140-6736(05)67625-8. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, Lu S, Zhang L, Hu C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;8:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 13.Kim ES, Hirsh V, Mok T, Socinski MA, Gervais R, Wu YL, Li LY, Watkins CL, Sellers MV, Lowe ES, Sun Y, Liao ML, Osterlind K, et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet. 2008;9652:1809–1818. doi: 10.1016/S0140-6736(08)61758-4. [DOI] [PubMed] [Google Scholar]
- 14.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, Nishiwaki Y, Ohe Y, Yang JJ, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;10:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 15.Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;25:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 16.Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;2:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 17.Han JY, Park K, Kim SW, Lee DH, Kim HY, Kim HT, Ahn MJ, Yun T, Ahn JS, Suh C, Lee JS, Yoon SJ, Han JH, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;10:1122–1128. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 18.Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, Heo DS, Crino L, Tan EH, et al. Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol. 2012;5:528–538. doi: 10.1016/S1470-2045(12)70087-6. [DOI] [PubMed] [Google Scholar]
- 19.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, Xu CR, Massey D, Kim M, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;2:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 20.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, Porta R, Cobo M, Garrido P, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;3:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;3:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Chichester, UK: 2008. [Google Scholar]
- 23.Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health. 1988;9:123–60. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 24.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;27:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 25.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, Lu S, Cheng Y, Han B, Chen L, Huang C, Qin S, Zhu Y, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;9:1883–1889. doi: 10.1093/annonc/mdv270. [DOI] [PubMed] [Google Scholar]
- 26.Melosky B, Hirsh V. Management of Common Toxicities in Metastatic NSCLC Related to Anti-Lung Cancer Therapies with EGFR-TKIs. Front Oncol. 2014;4:238. doi: 10.3389/fonc.2014.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, Shi Y, Kim SW, Laskin J, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;5:577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 28.Haaland B, Tan PS, de Castro G, Lopes G. Meta-analysis of first-line therapies in advanced non-mall-cell lung cancer harboring EGFR-activating mutations. J Thorac Oncol. 2014;6:805–811. doi: 10.1097/JTO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haspinger ER, Agustoni F, Torri V, Gelsomino F, Platania M, Zilembo N, Gallucci R, Garassino MC, Cinquini M. Is there evidence for different effects among EGFR-TKIs? Systematic review and meta-analysis of EGFR tyrosine kinase inhibitors (TKIs) versus chemotherapy as first-line treatment for patients harboring EGFR mutations. Crit Rev Oncol Hematol. 2015;2:213–227. doi: 10.1016/j.critrevonc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Greenhalgh J, Dwan K, Boland A, Bates V, Vecchio F, Dundar Y, Jain P, Green JA. First-line treatment of advanced epidermal growth factor receptor (EGFR) mutation positive non-squamous non-small cell lung cancer. Cochrane Database Syst Rev. 2016;5:CD010383. doi: 10.1002/14651858.CD010383.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. Clinical Pharmacokinetics and Pharmacodynamics of Afatinib. Clin Pharmacokinet. 2016. [DOI] [PMC free article] [PubMed]
- 32.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;1:74–79. doi: 10.1016/j.lungcan.2015.01.026. [DOI] [PubMed] [Google Scholar]