Abstract
Aims
It is unclear whether the potential benefits of physical activity differ according to level of cardiorespiratory fitness (CRF) or strength. The aim of this study was to determine whether the association between physical activity and mortality is moderated by CRF and grip strength sufficiently to inform health promotion strategies.
Methods and results
498 135 participants (54.7% women) from the UK Biobank were included (CRF data available in 67 702 participants). Exposure variables were grip strength, CRF, and physical activity. All-cause mortality and cardiovascular disease (CVD) events were the outcomes. 8591 died over median 4.9 years [IQR 4.3–5.5] follow-up. There was a significant interaction between total physical activity and grip strength (P < 0.0001) whereby the higher hazard of mortality associated with lower physical activity was greatest among participants in the lowest tertile for grip strength (hazard ratio, HR:1.11 [95% CI 1.09–1.14]) and lowest among those in the highest grip strength tertile (HR:1.04 [1.01–1.08]). The interaction with CRF did not reach statistical significance but the pattern was similar. The association between physical activity and mortality was larger among those in the lowest tertile of CRF (HR:1.13 [1.02–1.26]) than those in the highest (HR:1.03 [0.91–1.16]). The pattern for CVD events was similar.
Conclusions
These data provide novel evidence that strength, and possibly CRF, moderate the association between physical activity and mortality. The association between physical activity and mortality is strongest in those with the lowest strength (which is easily measured), and the lowest CRF, suggesting that these sub-groups could benefit most from interventions to increase physical activity.
Keywords: Mortality, Cardiovascular disease, Physical activity, Fitness, Strength, UK Biobank
Introduction
The independent, protective associations of physical activity, cardiorespiratory fitness (CRF), and strength with mortality are well established, with studies generally reporting stronger associations with mortality for CRF and strength than for physical activity.1–3 Increasing physical activity is a major mechanism by which CRF and strength can be increased;4–6 thus the association between physical activity and mortality is partially mediated by its effect on CRF and strength and is partially independent of them.7 However, around half of the variation in CRF and strength is inherited8,9 and it is unclear whether the association between physical activity and mortality is moderated by a person's underlying level of CRF and strength. There are limited data suggesting that low levels of physical activity are associated with increased hazard of mortality in men and women with low CRF, but that the association between physical activity and mortality may be weaker in those with high CRF. Data are completely lacking on whether the association between physical activity and mortality varies by strength.
Understanding the interactions between physical activity, CRF, and strength has implications for health promotion strategies. Currently, physical activity interventions are generally targeted at everyone in the least active sub-group of the population.10 This is based on the overall curvilinear relationship between level of physical activity and all-cause mortality which suggests that the incremental benefit is greatest among those with the lowest baseline level of physical activity.4,11 However, this approach assumes that the association between physical activity and mortality is consistent, irrespective of CRF and strength or other characteristics. If the relationship between physical activity and mortality does differ according to strength and CRF, information on grip strength—which is easily measured—and CRF could help to define better the sub-group of the population who could benefit most from physical activity interventions. The purpose of this study was therefore to determine whether the associations of physical activity with mortality and cardiovascular disease (CVD) events were influenced by CRF and grip strength among adults enrolled in UK Biobank, a very large, prospective, population-based cohort study.
Methods
Study design
Between April 2007 and December 2010, UK Biobank recruited 502 682 participants (5.5% response rate), aged 40–69 years from the general population.12 Participants attended 1 of 22 assessment centres across England, Wales, and Scotland13 where they completed a touch-screen questionnaire, had physical measurements taken and provided biological samples, as described in detail elsewhere.13,14 In this prospective, population-based study, all-cause mortality, and incidence of CVD were the outcomes; CRF, grip strength, and total physical activity were the exposure variables; and socio-demographic factors (age, sex, ethnicity, area socioeconomic deprivation index), smoking status, body mass index (BMI), diagnosis of diabetes, cancer, heart disease (stroke, high blood pressure angina, and heart attack) and longstanding illness were covariates. Our sample was restricted to the 498 135 participants who had valid data on mortality status at follow-up and baseline data for at least one of the following: grip strength, physical activity, and CRF. Physical activity and grip strength data were available for 498 135 and 495 786 participants, respectively. 77 961 participants, recruited from August 2009, underwent a CRF test and valid CRF data were obtained in 67 702 participants.
Procedures
Date of death was obtained from death certificates held by the National Health Service (NHS) Information Centre for participants from England and Wales and the NHS Central Register Scotland for participants from Scotland. Hospital admissions were identified via record linkage to Health Episode Statistics records for England and Wales and to the Scottish Morbidity Records for Scotland. Incident cardiovascular vascular disease was defined as an ICD 10 code of I21, I21.4 and I21.9, recorded on a death certificate or hospital admission (see Supplementary material online for details). Grip strength was assessed using a Jamar J00105 hydraulic hand dynamometer and the mean of the right hand and left hand values, expressed as kg, was used in the analysis. Cardiorespiratory fitness was assessed using a 6-min incremental ramp cycle ergometer test with workload calculated according to age, height, weight, resting heart rate, and sex. Heart rate was monitored pre-exercise, throughout activity and during recovery via a four-lead ECG. The work rate at maximal heart rate was estimated by extrapolating the pre-exercise heart rate (i.e. at work rate zero Watts) and the heart rate and work rate at the end of the test, to the age-predicted maximal heart rate (208–0.7 × age15 assuming a linear relationship.16 Maximal oxygen uptake (i.e. at maximal heart rate) was estimated from the regression equation for the relationship between work rate and oxygen uptake (oxygen uptake (in mL kg−1 min−1) = 7 + (10.8 × work rate (W))/body mass (kg))17 and then expressed in terms of maximal METs (where 1 MET ≡ 3.5 mL kg−1 min−1). Physical activity was based on self-report, using the IPAQ short form,18 and total physical activity was computed as the sum of walking, moderate and vigorous activity, measured as metabolic equivalents (MET-h/week). Total time spent in sedentary behaviours were derived from the sum of self-reported time spent driving, using computer and watching television. Area-based socioeconomic status was derived from postcode of residence, using the Townsend score.19 Age was calculated from dates of birth and baseline assessment. Smoking status was categorized into never, former, and current smoking. Medical history (physician diagnosis of depression, stroke, angina, heart attack, hypertension, cancer, diabetes, or long-standing illness) was collected from the self-completed, baseline assessment questionnaire. Alcohol intake frequency was collected using a self-reported lifestyle questionnaire. Height and body weight were measured by trained nurses during the initial assessment centre visit. Body mass index was calculated as (weight/height2) and the WHO criteria to classify BMI into: underweight <18.5, normal weight 18.5–24.9, overweight 25.0–29.9, and obese ≥30.0 kg m−2. Body composition (body fat and fat-free mass) was measured using bio-impedance by trained nurses. Further details of these measurements can be found in the UK Biobank online protocol (http://www.ukbiobank.ac.uk) and our Supplementary material online.
Statistical analyses
The relationship between grip strength, CRF, and physical activity was explored using Pearson correlation coefficients. The associations of grip strength, CRF, and total physical activity with all-cause mortality and CVD events were investigated using Cox-proportional hazard models. Two approaches were used. Firstly, separate associations of grip strength, CRF, and physical activity with mortality/CVD events were calculated. Grip strength, CRF, and physical activity were treated as continuous variables and hazard ratios (HRs) were calculated per 1 SD difference in fitness, grip strength, and total PA, using age- and sex-specific z-scores. To enable comparability with other reports in the literature, HRs were also calculated for a 5 kg difference in grip strength, 1-MET difference in CRF, and 5-MET-h/week difference in total physical activity. Secondly, joint associations between physical activity, CRF, and mortality/CVD events and physical activity, grip strength, and mortality/CVD events were calculated. Age- and sex-specific quintiles for physical activity and age- and sex-specific tertile for grip strength and CRF were derived and HRs were calculated, with the referent category comprising individuals who were in both the highest quintile of physical activity and the strongest/fittest tertile. To investigate whether CRF and/or grip strength moderated the association between physical activity and mortality/CVD events, we also tested the statistical significance of interaction terms and conducted sub-group analyses where appropriate.
For each of the approaches described above, we ran four incremental models that included an increasing number of covariates: ‘model 0’ included age, sex; ‘model 1’ included age, sex, ethnicity (white, black, South Asian, Chinese, and other), smoking status (current, former, and never), BMI category (underweight, normal weight, overweight, and obese), deprivation index and alcohol intake as covariates; ‘model 2’ also adjusted for depression, stroke, angina, heart attack, hypertension, cancer, diabetes, or long-standing illness; and ‘model 3’ also adjusted for total sedentary time per day, as well as total physical activity per day and grip strength as appropriate. Finally, ‘model 4’ was equivalent to ‘model 2’ but participants with a history of cancer, stroke, angina, or myocardial infarction at baseline were excluded from the analysis. Cardiorespiratory fitness was not included as a covariate in the models for physical activity or grip strength because data on CRF were only available for ∼14% (n = 67 702) of the UK Biobank cohort. The proportional hazard assumption was checked by tests based on Schoenfeld residuals. All analyses were performed using STATA 14 statistical software (StataCorp LP).
Ethical approval
The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee and all participants provided written informed consent to participate in the UK Biobank study. The study protocol is available online (http://www.ukbiobank.ac.uk/).
Results
8591 (1.7%) participants died over a median follow-up period of 4.9 years [IQR 4.3–5.5] and 2.1 years for CVD [IQR 1.4–2.8]. Over the follow-up period, 8591 participants died and there were 3433 CVD events, of which 2787 were fatal.
The main characteristics of the participants by quintiles of total physical activity, tertile of CRF, and grip strength are summarized in Supplementary material online, Tables S1–S3, respectively. The correlation between grip strength and CRF was moderate (r = 0.415, P < 0.0001), but total physical activity only correlated weakly with both CRF (r = 0.111, P < 0.0001) and grip strength (r = 0.088, P < 0.0001). Excluding people with diabetes, cancer, longstanding illness, and heart-related disease in a sensitivity analysis did not alter these correlations (Supplementary material online, Table S4).
Table 1 shows the HRs for all-cause mortality for physical activity, grip strength, and CRF. Higher levels of activity, strength, and CRF were associated with lower mortality, with stronger associations observed for grip strength and CRF than for physical activity. These associations were slightly attenuated, but remained statistically significant, after adjustment for potential confounders. Similar findings were observed when CVD events was the outcome (Supplementary material online, Table S5).
Table 1.
Cox-proportional hazard model of the association between physical activity, grip strength, and cardiorespiratory fitness and all-cause mortality
| n | n deaths | HR per 1 SD decrease | P | HR per 5 MET-h/week decrease | P | |
|---|---|---|---|---|---|---|
| Physical activity | ||||||
| Model 0 | 498 135 | 8588 | 1.15 (1.12;1.18) | <0.0001 | 1.04 (1.03;1.05) | <0.0001 |
| Model 1 | 479 495 | 8207 | 1.13 (1.10;1.15) | <0.0001 | 1.03 (1.03;1.04) | <0.0001 |
| Model 2 | 462 422 | 7809 | 1.09 (1.06;1.12) | <0.0001 | 1.03 (1.02;1.03) | <0.0001 |
| Model 3 | 461 919 | 7784 | 1.07 (1.05;1.10) | <0.0001 | 1.02 (1.01;1.03) | <0.0001 |
| Model 4 | 384 794 | 4208 | 1.05 (1.02;1.09) | <0.0001 | 1.01 (1.00;1.02) | <0.0001 |
| HR per 1 SD decrease | HR per 5 kg decrease | |||||
| Grip strength | ||||||
| Model 0 | 495 786 | 8491 | 1.35 (1.32;1.38) | <0.0001 | 1.21 (1.20;1.23) | <0.0001 |
| Model 1 | 478 968 | 8181 | 1.29 (1.26;1.31) | <0.0001 | 1.18 (1.16;1.20) | <0.0001 |
| Model 2 | 461 919 | 7784 | 1.19 (1.16;1.22) | <0.0001 | 1.12 (1.11;1.14) | <0.0001 |
| Model 3 | 461 919 | 7784 | 1.19 (1.16;1.21) | <0.0001 | 1.12 (1.10;1.14) | <0.0001 |
| Model 4 | 384 794 | 4208 | 1.18 (1.14;1.21) | <0.0001 | 1.12 (1.09;1.14) | <0.0001 |
| HR per 1 SD decrease | HR per 1-MET decrease | |||||
| Cardiorespiratory fitness | ||||||
| Model 0 | 67 702 | 597 | 1.29 (1.18;1.41) | <0.0001 | 1.11 (1.07;1.15) | <0.0001 |
| Model 1 | 64 637 | 577 | 1.29 (1.17;1.42) | <0.0001 | 1.11 (1.07;1.15) | <0.0001 |
| Model 2 | 62 507 | 549 | 1.23 (1.11;1.35) | <0.0001 | 1.09 (1.04;1.13) | <0.0001 |
| Model 3 | 61 859 | 543 | 1.19 (1.08;1.32) | <0.0001 | 1.07 (1.03;1.12) | <0.0001 |
| Model 4 | 52 266 | 319 | 1.22 (1.07;1.39) | <0.0001 | 1.08 (1.03;1.14) | <0.0001 |
Data presented as hazard ratio (95%CI). A 1 SD change in fitness, grip strength, and total physical activity is equivalent to 2.3 METs, 6.2 and 2845 MET-h/week for women, and 2.7 METs, 8.9 kg, and 3379 MET-h/week for men, respectively.
Model 0 was adjusted for age and sex.
Model 1 was adjusted for sex, age, ethnicity, smoking status, deprivation index, BMI, and alcohol intake.
Model 2 was adjusted for Model 0 plus depression, stroke, angina, heart attack, hypertension, cancer, diabetes, or long-standing illness.
Model 3 for grip strength was adjusted for Model 1 plus total sedentary time and total physical activity.
Model 3 for fitness was adjusted for Model 1 plus total sedentary time, total physical activity, and grip strength.
Model 3 for total physical activity was adjusted for Model 1 plus total sedentary time, and grip strength.
Model 4 was adjusted as Model 2 but individuals with cancer, stroke, angina, and heart attack were excluded.
SD, standard deviation; HR, hazard ratio; MET, basal metabolic equivalent.
Table 2 shows the association between physical activity and mortality, stratified by grip strength and CRF tertile, with those with the highest activity as the reference group within in strength/CRF tertile. Figure 1 displays these data with the most active and most fit/strong individuals as reference group. There was a statistically significant interaction between physical activity and grip strength with mortality with the HR per quintile change in physical activity being almost three times as great in those low compared with high grip strength. A similar pattern was observed for CRF, but the interaction was not statistically significant. Figure 2 and Supplementary material online, Table S6 show the same data for CVD events. Here a significant increase in CVD events with decreasing physical activity was only observed in those in the lowest tertile for grip strength or CRF. There was a statistically significant interaction between physical activity and grip strength with CVD events.
Table 2.
Cox-proportional hazard models of the association between physical activity and all-cause mortality by tertile of cardiorespiratory fitness and grip strength
| All | Total number | Number of deaths | Quintiles of physical activity |
Hazard ratio per one quintile change in physical activitya | P-trend | P-interaction | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q5 (highest) | Q4 | Q3 | Q2 | Q1 (lowest) | ||||||
| Cardiorespiratory fitnessb | ||||||||||
| Lowest | 20 227 | 224 | 1.00 (reference) | 1.26 (0.78;2.03) | 1.31 (0.83;2.08) | 1.54 (0.96;2.47) | 1.63 (1.04;2.54) | 1.13 (1.02;1.26) | 0.025 | 0.579 |
| Middle | 20 733 | 176 | 1.00 (reference) | 0.97 (0.60;1.57) | 0.86 (0.52;1.43) | 1.25 (0.79;1.96) | 1.55 (0.98;2.47) | 1.11 (1.01;1.22) | 0.022 | |
| Highest | 21 059 | 147 | 1.00 (reference) | 1.01 (0.56;1.80) | 0.95 (0.58;1.55) | 0.98 (0.61;1.56) | 1.22 (0.75;1.97) | 1.03 (0.91;1.16) | 0.633 | |
| Grip strengthb | ||||||||||
| Lowest | 161 007 | 3473 | 1.00 (reference) | 1.06 (0.94;1.21) | 1.11 (0.98;1.26) | 1.30 (1.15;1.45) | 1.51 (1.35;1.68) | 1.11 (1.09;1.14) | <0.0001 | <0.0001 |
| Middle | 157 296 | 2438 | 1.00 (reference) | 0.95 (0.83;1.08) | 0.97 (0.85;1.10) | 1.05 (0.93;1.20) | 1.27 (1.12;1.44) | 1.06 (1.03;1.09) | <0.0001 | |
| Highest | 143 616 | 1873 | 1.00 (reference) | 0.88 (0.76;1.02) | 0.98 (0.85;1.13) | 0.97 (0.84;1.12) | 1.19 (1.04;1.37) | 1.04 (1.01;1.08) | 0.007 | |
Data presented as adjusted HR (95%CI).
aHazard ratios are presented per quintile decrease in PA by fitness and grip strength strata.
bAnalyses were adjusted for age, sex, ethnicity, deprivation index, BMI, smoking status, total sedentary time, alcohol intake, depression, stroke, angina, heart attack, hypertension, cancer, diabetes, or long-standing illness.
Figure 1.
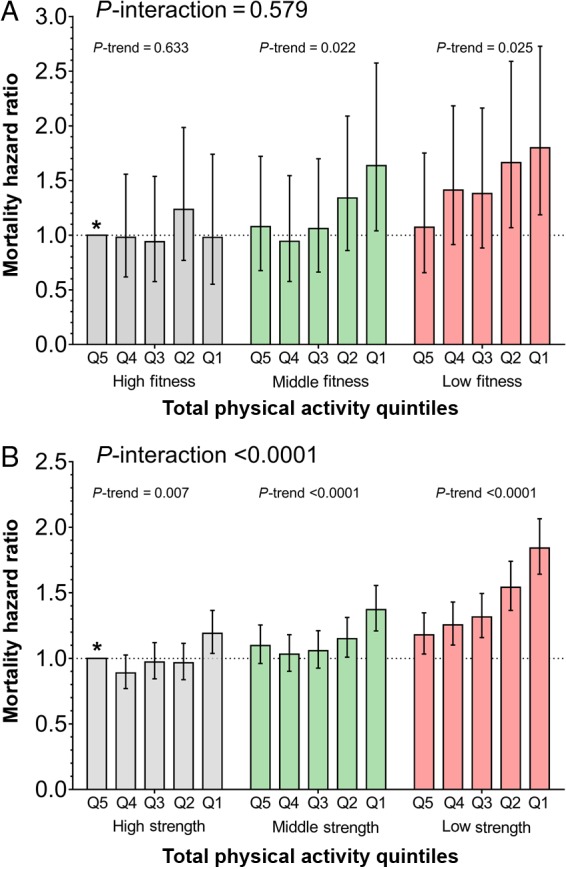
Association between all-cause mortality and physical activity within cardiorespiratory fitness (A) and grip strength (B) strata. Data presented as hazard ratio (95% CI), adjusted for age, sex, ethnicity, deprivation index, BMI, smoking status, total sedentary time, alcohol intake, depression, diabetes, hypertension, cancer diagnosis, stroke, angina, heart attack, and long-standing illness. Q5 represents highly active individuals and Q1 highly inactive individuals. Individuals in the highest quintile of physical activity (Q5) and the highest tertile for cardiorespiratory fitness or grip strength were used as the reference group (*). P-interaction describes the interaction between physical activity and cardiorespiratory fitness or grip strength with all-cause mortality; P-trend describes the association between physical activity with all-cause mortality within tertiles for cardiorespiratory fitness or grip strength.
Figure 2.
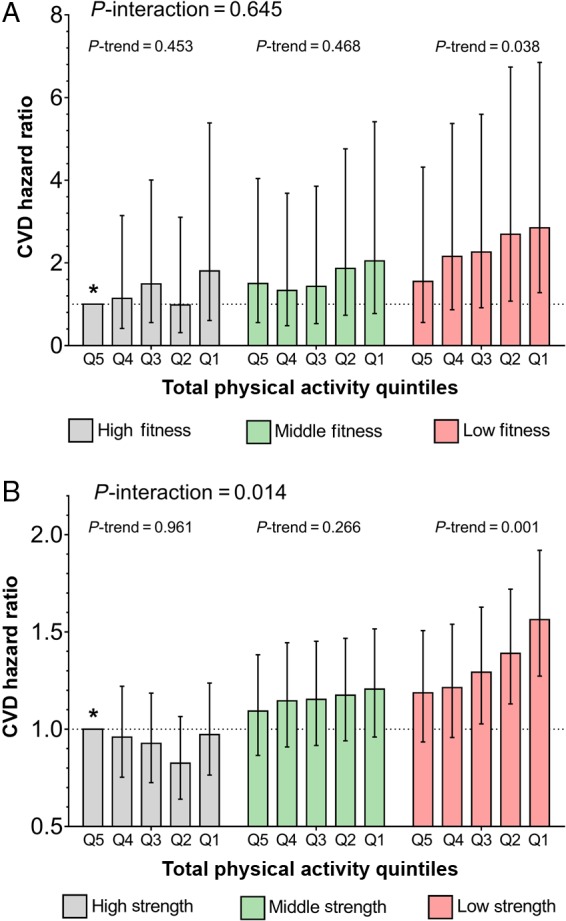
Association between cardiovascular disease events and physical activity within cardiorespiratory fitness (A) and grip strength (B) strata. Data presented as hazard ratio (95% CI), adjusted for age, sex, ethnicity, deprivation index, BMI, smoking status, total sedentary time, alcohol intake, depression, diabetes, hypertension, cancer diagnosis, stroke, angina, heart attack, and long-standing illness. Q5 represents highly active individuals and Q1 highly inactive individuals. Individuals in the highest quintile of physical activity (Q5) and the highest tertile for cardiorespiratory fitness or grip strength were used as the reference group (*). P-interaction describes the interaction between physical activity and cardiorespiratory fitness or grip strength with CVD events; P-trend describes the association between physical activity with CVD events within tertiles for cardiorespiratory fitness or grip strength.
When the sensitivity analyses were performed following exclusion of all participants with a history of cancer, stroke, angina, or heart attack from the analysis, the findings were not substantially altered (Table 1; Supplementary material online, Table S5, model 4). Similarly, when first year follow-up events were removed from the analysis, the results were not altered.
Discussion
The main finding of this study is that the association of physical activity with mortality and CVD events is moderated by grip strength and possibly CRF. Grip strength and CRF were moderately correlated with each other, but both had weak correlations with self-reported physical activity: all three factors were associated with mortality, independent of each other, and potential confounders. The associations between grip strength (HR of 1.35 per SD difference) and CRF (HR 1.29) with mortality were of similar magnitude and were both stronger than the association physical activity (HR 1.15) and mortality in the group overall. However, although physical activity was inversely associated with all-cause mortality in all levels of grip strength and those with low and middle CRF, the association was strongest in those with the lowest strength, and possibly lowest CRF, suggesting that these sub-groups would potentially benefit most from interventions aimed at increasing physical activity. For CVD events, significant increases in risk with decreasing physical activity were only evident in those with the lowest strength or lowest CRF. The evidence is stronger in relation to grip strength which could be easily measured in clinical practice and, therefore, may be a useful method of identifying a high risk group and targeting interventions accordingly.
Our finding of inverse associations between of CRF,2 grip strength,20 and total physical activity3 and all-cause mortality is in agreement with previous studies. A meta-analysis conducted on 102 980 study participants reported that 1-MET lower CRF was associated with a 13% increased hazard of all-cause mortality.2 These results are similar to our findings, where we show that 1-MET lower CRF is associated with an HR of 1.11 for all-cause mortality. In our study, adjusting for a history of disease and excluding participants with pre-existing CVD-related diseases or prior cancer, attenuated the HR for a 1-MET reduction in CRF by about a third to 1.08. A similar association was observed for grip strength, where a 5-kg lower grip strength was associated with an HR of 1.22 for all-cause mortality. A comparable result was reported in a recent study conducted on the 139 691 participants in the PURE study,20 where a 5 kg lower grip strength was associated with an HR of 1.16 for all-cause mortality. As was observed for CRF, our HR for all-cause mortality per 5-kg reduction in grip strength was attenuated by about a third to 1.12 HR after adjusting the model for disease history or by excluding participants with pre-existing CVD-related diseases or cancer. Thus, low CRF and grip strength were both significantly associated with higher mortality hazard, independent of pre-existing disease, and our results appear to have good external validity.
Blair et al.1 reported an inverse additive association of physical activity and CRF in relation to all-cause mortality among 35 519 participants from the Aerobic Centre Longitudinal Study. Mortality was highest among the unfit, inactive group, and lowest among the fit, highly active group. However, when these analyses were adjusted for confounders, the association disappeared,1 possibly due to insufficient statistical power. Our study has substantially greater statistical power due to its much larger size. The trends demonstrated in Figure 1 clearly illustrate that for those who have low CRF or grip strength, there is a strong, dose-related relationship between physical activity and all-cause mortality whereas among those with high CRF or grip strength, the association is much weaker. These findings are relevant for public health policies. These currently encourage increased physical activity among all those who are physically inactive; however, based on our results, tailoring physical activity interventions at individuals who have low grip strength or low CRF it may have a greater impact on reducing disease or mortality outcomes for these individuals who have a ‘low fitness profile’ (i.e. physically inactive plus low strength or low CRF). There is evidence from randomised controlled trials that inactive individuals who undergo lifestyle intervention, including increased physical activity, reduce their risk of all-cause, and cardiovascular mortality.21 However, it is unknown whether individuals who have a ‘low fitness profile’, could actually benefit even more by increasing their PA. Thus this needs to be further investigated using appropriate intervention trials. Regarding the applicability of CRF or grip strength on clinical settings; we know that CRF is not easy to measure in routine clinical practice, however, grip strength requires little training, is simple and cheap to administer, and has high reproducibility.22 Therefore, it could easily be administered as a screening tool in routine clinical practice to identify individuals for whom increasing physical activity would be particularly beneficial.22
Hand-grip strength is highly correlated with leg strength, and thus provides a valid index of overall limb muscle strength throughout the age range.23 There is some evidence to suggest that resistance training interventions—which improve strength—can increase in glycolytic capacity and up-regulate insulin action and capacity for glucose utilization in muscle.24 Randomized trials have also shown that resistance exercise training can improve glucose regulation, lipid levels, and reduce adiposity and type 2 diabetes risk,25 all well-known risk factors for mortality. Thus, the association between grip strength and mortality is mechanistically plausible. Similarly, animal model studies have demonstrated that selective breeding low cardiorespiratory fitness leads to an adverse cardio-metabolic risk profile26 and reduced life-expectancy27 in rats, implying a causal relationship between CRF and mortality.
Strengths and limitations
The UK Biobank provided an opportunity to test our research question in a very large, prospective cohort and the main outcome used in this study was collected using NHS death records. Additionally, strength and CRP were objectively assessed using validated methods, trained staff, and standard-operating procedures. Hand-grip strength is highly correlated with leg strength and provides a valid index of overall limb muscle strength throughout the age range.23 Although the response rate to UK Biobank was only 5.5%, the UK Biobank cohort is representative of the general population with respect to age, sex, ethnicity, and deprivation within the age range recruited, although it is not representative in other regards.12 While this limits the ability to generalize prevalence rates, estimates of the magnitude of associations regarding disease or mortality risk in the current study will not be affected by this and will therefore be generalizable.12,28 Reverse causality is possible in any observational study; however, when all participants with existing disease were removed from the analysis the effect and direction of the association remained significant. Although existing disease and comorbidities present before the UK Biobank measurement day were self-reported, these self-reported records were based on disease that had been medically diagnosed. Data for atrial fibrillation and heart failure were not explicitly reported as separate conditions in the dataset at baseline, but these conditions are likely to have been captured in the ‘long-standing illness’ variable that we adjusted for in our models. Thus, any potential confounding effects of these conditions would have been largely captured in our statistical adjustments. Endpoint determination for CVD events was based only on ICD-10 codes and was not subject to further scientific adjudication. Physical activity was measured by self-report using a validated questionnaire. Thus misreporting of activity levels may have attenuated the association between physical activity and mortality compared with objective physical activity measurement.29 However, this is unlikely to have substantially confounded the differential influence of physical activity on mortality risk across the grip strength and CRF groups, unless the extent misreporting of physical activity was systematically higher in the high CRF and high grip strength groups, where the effects of physical activity on mortality were least strong. Cardiorespiratory fitness data were only available in ∼14% of participants, although this sub-group was representative of the wider cohort (see Supplementary material online, Tables S1 and S2). However, the absence of a significant interaction between CRF and physical activity with mortality—despite similar trends to those for the grip strength and physical activity with mortality—may reflect a lack of statistical power to detect effect in this smaller sample. As dietary intake data were only available for around half of the cohort (n = 211 066), we did not include this as covariate in our models. Alcohol intake was available for the entire cohort and we did adjust for this; however, we cannot exclude the possibility that other that dietary factors could have influence our findings.
In conclusion, insufficient physical activity is a preventable contributor to the global burden of morbidity and mortality. However, to maximize potential public health gains population-level interventions to encourage physical activity in the whole population could be complemented by specific interventions targeted at the sub-group of the population who are at highest risk. Cardiorespiratory fitness and strength are both predictors of all-cause mortality and CVD events independent of physical activity. Our results suggest that the ability of physical activity to reduce hazard of death or CVD events may be greatest among those with low baseline strength and fitness. Current guidelines advocate targeting physical activity interventions merely on the basis of current levels of physical activity. Our findings suggest that targeting on the basis of strength, and possibly fitness, could greatly improve our ability to identify those individuals who could benefit most, thereby increasing the clinical and cost effectiveness of physical activity interventions. These conclusions require testing in the context of a future randomized controlled trial.
Supplementary material
Supplementary material is available at European Heart Journal online.
Authors’ contributions
C.C.-M., Y.F., J.M.R.G. performed statistical analysis; J.P.P., N.S., J.M.R.G. handled funding and supervision; D.F.M., J.P.P. acquired the data; C.C.-M., J.P.P., N.S., J.M.R.G. conceived and designed the research; C.C.-M., J.P.P., N.S., J.M.R.G. drafted the manuscript; C.C.-M., D.M.L., J.A., S.M., Y.F., U.E.N., D.F.M., J.P.P., N.S., J.M.R.G. made critical revision of the manuscript for key intellectual content.
Funding
The UK Biobank was supported by the Wellcome Trust, Medical Research Council, Department of Health, Scottish Government, and the Northwest Regional Development Agency. It has also had funding from the Welsh Assembly Government and the British Heart Foundation. The research was designed, conducted, analysed, and interpreted by the authors entirely independently of the funding sources.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exercise 2001;33:S379–S399. [DOI] [PubMed] [Google Scholar]
- 2. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women a meta-analysis. J Am Med Assoc 2009;301:2024–2035. [DOI] [PubMed] [Google Scholar]
- 3. Cooper R, Kuh D, Hardy R, Team FAS, Team HAS. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. Br Med J 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee D-c, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol 2010;24:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure – a randomized controlled trial. J Am Med Assoc 2007;297:2081–2091. [DOI] [PubMed] [Google Scholar]
- 6. Duscha BD, Slentz CA, Johnson JL, Houmard JA, Bensimhon DR, Knetzger KJ, Kraus WE. Effects of exercise training amount and intensity on peak oxygen consumption in middle-age men and women at risk for cardiovascular disease. Chest 2005;128:2788–2793. [DOI] [PubMed] [Google Scholar]
- 7. Williams PT. Usefulness of cardiorespiratory fitness to predict coronary heart disease risk independent of physical activity. Am J Cardiol 2010;106:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functional. Genetic Epidemiol 2002;23:110–122. [DOI] [PubMed] [Google Scholar]
- 9. Bouchard C. Genomic predictors of trainability. Exp Physiol 2012;97:347–352. [DOI] [PubMed] [Google Scholar]
- 10. WHO. Global recommendations on physical activity for health. World Health Organization; 2010. [PubMed] [Google Scholar]
- 11. Blair SN, Kohl HW, Barlow CE, Paffenbarger RS, Gibbons LW, Macera CA. Changes in physical-fitness and all-cause mortality – a prospective-study of healthy and unhealthy men. J Am Med Assoc 1995;273:1093–1098. [PubMed] [Google Scholar]
- 12. Collins R. What makes UK Biobank special? Lancet 2012;379:1173–1174. [DOI] [PubMed] [Google Scholar]
- 13. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmer LJ. UK Biobank: bank on it. Lancet 2007;369:1980–1982. [DOI] [PubMed] [Google Scholar]
- 15. Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol 2001;37:153–156. [DOI] [PubMed] [Google Scholar]
- 16. Medicine ACoS. Guidelines for Exercise Testing and Prescription. 9th ed Baltimore: Wolters Kluwer Health/Lippinoctt, Williams & Wilkins; 2014. [Google Scholar]
- 17. Swain DP. Energy cost calculations for exercise prescription – an update. Sports Med 2000;30:17–22. [DOI] [PubMed] [Google Scholar]
- 18. Guo W, Bradbury KE, Reeves GK, Key TJ. Physical activity in relation to body size and composition in women in UK Biobank. Ann Epidemiol 2015;25:406–413. [DOI] [PubMed] [Google Scholar]
- 19. Townsend P, Phillimore M, Beattie A. Health and Deprivation: Inequality and the North. London: Croom Helm; 1988. [Google Scholar]
- 20. Leong DP, Teo KK, Rangarajan S. Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. The Lancet 2015;386:266–273. [DOI] [PubMed] [Google Scholar]
- 21. Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, Yang W, Zhang B, Shuai Y, Hong J, Engelgau MM, Li H, Roglic G, Hu Y, Bennett PH. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diab Endocrinol 2014;2:474–480. [DOI] [PubMed] [Google Scholar]
- 22. Roberts HC, Denison HJ, Martin HJ, Patel HP, Syddall H, Cooper C, Sayer AA. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011;40:423–429. [DOI] [PubMed] [Google Scholar]
- 23. Bohannon RW, Magasi SR, Bubela DJ, Wang Y-C, Gershon RC. Grip and knee extension muscle strength reflect a common construct among adults. Muscle Nerve 2012;46:555–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holten MK, Zacho M, Gaster M, Juel C, Wojtaszewski JFP, Dela F. Strength training increases insulin-mediated glucose uptake, GLUT4 content, and insulin signaling in skeletal muscle in patients with type 2 diabetes. Diabetes 2004;53:294–305. [DOI] [PubMed] [Google Scholar]
- 25. Cornelissen VA, Fagard RH, Coeckelberghs E, Vanhees L. Impact of resistance training on blood pressure and other cardiovascular risk factors a meta-analysis of randomized, controlled trials. Hypertension 2011;58:950–958. [DOI] [PubMed] [Google Scholar]
- 26. Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 2005;307:418–420. [DOI] [PubMed] [Google Scholar]
- 27. Koch LG, Kemi OJ, Qi N, Leng SX, Bijma P, Gilligan LJ, Wilkinson JE, Wisloff H, Hoydal MA, Rolim N, Abadir PM, van Grevenhof EM, Smith GL, Burant CF, Ellingsen O, Britton SL, Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res 2011;109:1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Manolio TA, Collins R. Enhancing the feasibility of large cohort studies. J Am Med Assoc 2010;304:2290–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Celis-Morales CA, Perez-Bravo F, Ibanez L, Salas C, Bailey ME, Gill JM. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS ONE 2012;7:e36345. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


