Abstract
Background
Lead and cadmium exposures have markedly declined in the USA following the implementation of large-scale public health policies and could have contributed to the unexplained decline in cardiovascular mortality in US adults. We evaluated the potential contribution of lead and cadmium exposure reductions to explain decreasing cardiovascular mortality trends occurring in the USA from 1988–94 to 1999–2004.
Methods
Prospective study in 15 421 adults ≥40 years old who had participated in the National Health and Nutrition Examination Survey 1988–94 or 1999–2004. We estimated the amount of change in cardiovascular mortality over time that can be independently attributed to the intermediate pathway of changes in blood lead and urine cadmium concentrations.
Results
There was a 42.0% decrease in blood lead and a 31.0% decrease in urine cadmium concentrations. The cardiovascular mortality rate ratio [95% confidence intervals (CIs)] associated with a doubling of metal levels was 1.19 (1.07, 1.31) for blood lead and 1.20 (1.09, 1.32) for urine cadmium. The absolute reduction in cardiovascular deaths comparing 1999–2004 to 1988–94 was 230.7 deaths/100 000 person-years, in models adjusted for traditional cardiovascular risk factors. Among these avoided deaths, 52.0 (95% CI 8.4, 96.7) and 19.4 (4.3, 36.4) deaths/100 000 person-years were attributable to changes in lead and cadmium, respectively.
Conclusions
Environmental declines in lead and cadmium exposures were associated with reductions in cardiovascular mortality in US adults. Given the fact that lead and cadmium remain associated with cardiovascular disease at relatively low levels of exposure, prevention strategies that further minimize exposure to lead and cadmium may be needed.
Keywords: lead, cadmium, cardiovascular mortality, trends, NHANES
Key Messages
Blood lead and urine cadmium have been associated with a broad range of cardiovascular endpoints in multiple epidemiologic studies. However, the contribution of lead and cadmium changes over time to cardiovascular mortality trends has not been formally investigated.
Our findings suggest that reducing lead and cadmium exposures may be an overlooked public health achievement by preventing a substantial amount of cardiovascular deaths in the USA.
Since both metals remain associated with cardiovascular disease at relatively low levels of exposure, primary prevention strategies minimizing avoidable lead and cadmium exposures could further contribute to the prevention and control of cardiovascular disease in general populations.
Introduction
Rates of cardiovascular disease mortality, especially coronary heart disease, have markedly declined in the USA since the 1970 s.1 Primary prevention is based on the premise that reduction in established risk factor levels will translate into subsequent reductions in disease incidence and mortality. Accordingly, models based on aggregated data have estimated that between 25% and 50% of the US mortality trend is explained by primary prevention strategies.2 These analyses, however, do not consider that the reduction in cardiovascular mortality may be explained by changes in non-traditional risk factors including environmental determinants of cardiovascular risk.
Lead and cadmium exposures have declined in the USA following the implementation of large-scale public health policies such as tobacco control, air-pollution reduction, hazardous-waste remediation, renovation of drinking-water infrastructures and banning of lead in gasoline.3,4 Both blood lead and urine cadmium have been associated with a broad range of cardiovascular endpoints in multiple epidemiologic studies. Considering the consistency of the associations across populations, the evidence of a dose–response relationship across studies5,6 and biological plausibility from experimental findings,7–10 lead and cadmium have been proposed as cardiovascular disease risk factors.5,6,11 Little is known, however, about their potential contribution to the decline in cardiovascular mortality in US adults.
The objective of this study was to evaluate whether population changes in the distribution of blood lead and urine cadmium levels explain changes in cardiovascular mortality over time in representative samples of the US general population, after accounting for changes in established traditional cardiovascular risk factors. We implemented a causal inference mediation approach12 to estimate how much of the decline in cardiovascular mortality rates between 1988–94 and 1999–2004 can be independently attributed to changes in lead and cadmium exposures as measured based on established biomarkers of metal internal dose.
Methods
Study population
The National Health and Nutrition Examination Survey (NHANES) uses a complex multistage sampling design to obtain representative samples of the non-institutionalized US population.13 We used data from NHANES III (1988–94), which were collected in two phases (1988–91 and 1991–94), and from NHANES 1999–2004, which were collected in three phases (1999–2000, 2001–02 and 2003–04). We included 21 418 adults aged 40 and older. Supplementary Figure 1 (available as Supplementary Data at IJE online) shows the flow chart of participant exclusions by study period, which left 15 421 participants for this analysis.
Blood lead and urine cadmium
Blood lead and urine cadmium concentrations were measured at the Environmental Health Laboratory (Atlanta, GA, USA), Centers for Disease Control and Prevention, National Center for Environmental Health (NCEH) (see Supplementary Methods, available as Supplementary Data at IJE online). Whole-blood lead concentrations were measured by a Perkin-Elmer model 5000 graphite furnace atomic absorption (GFAA) spectrophotometer with deuterium background correction or a Perkin-Elmer model 5100 GFAA spectrophotometer with Zeeman effect background correction in NHANES 1988–94; by an atomic absorption spectrometer with Zeeman background correction (SIMAA 6000 model; PerkinElmer, Norwalk, CT, USA) in 1999–2002; and by an inductively coupled plasma-mass (ICP-MS) spectrometer (PerkinElmer/SCIEX model 500; PerkinElmer, Shelton, CT, USA) in 2003–04.
In NHANES 1988–94, urine cadmium was measured in duplicate by GFAA spectrometry (model 3030; PerkinElmer, Norwalk, CT, USA) with Zeeman background correction, and the average of the two measurements was reported.14 In NHANES 1999–2004, urine cadmium was measured only in a random one-third subsample of the study population by ICP-MS spectrometry (ELAN, PerkinElmer) in 1999–2002 and by ICP–dynamic reaction cell (DRC)-MS (ELAN DRC, PerkinElmer) in 2003–04. The remaining two-thirds of 1999–2004 NHANES participants who were not selected for urine cadmium measurements (N = 4852) had cadmium concentrations missing completely at random and we imputed their cadmium concentrations as the median of each participant’s posterior cadmium distribution generated from a Markov Chain Monte Carlo with Gibbs sampling under a prediction model based on urine cadmium determinants. The imputation method has been described in detail elsewhere.15 The median (interquartile range) of measured and imputed urine cadmium levels in NHANES 1999–2004 were 0.40 (0.21, 0.71) and 0.38 (0.22, 0.61) μg/L, respectively. Creatinine-corrected urine cadmium concentrations were reported in micrograms cadmium per gram creatinine.
Cardiovascular risk factors
Information on age, sex, race/ethnicity, smoking status, physical activity, and medical history and medication use was based on self-report. Height, weight, and systolic and diastolic blood pressure were measured at the physical examination. We used a combination of laboratory, examination and interview data to define smoking status, leisure-time physical inactivity, obesity, hypertension, diabetes, high total cholesterol and low HDL cholesterol according to standard criteria (see Supplementary Methods, available as Supplementary Data at IJE online).
Mortality follow-up
NHANES 1988–94 and 1999–2004 participants were followed for mortality through 31 December 2006. Vital status and cause of death were determined by probabilistic matching between NHANES records and death certificates from the National Death Index (NDI) based on identifying data elements.13
Cause of death was determined using the underlying cause listed on death certificates and was coded using the International Classification of Diseases, 10th Revision (ICD-10).16 The study endpoints were cardiovascular disease (ICD-10 codes I00–I78) and coronary heart disease (ICD-10 codes I20–I25) mortality. Follow-up time for each individual was calculated as the difference between the age at the date of the NHANES examination and the age at the date of death, age on 31 December 2006 or age 90 years, whichever occurred first. Follow-up was censored at age 90 years because of the high mortality after this age and the low number of participants who were contributing person-time experience. The length of available follow-up differed by NHANES period (maximum follow-up of 19 years for 1988–94 participants and 8 years for 1999–2004). In order to make the length of follow-up comparable for both survey waves, we censored the follow-up of NHANES 1988–94 as of 31 December 1996.
Statistical methods
Due to the different selection probabilities of NHANES participants, all analyses were weighted to the underlying US adult population aged 40 or older. Statistical methods for the estimation of summary trends, association between lead and cadmium with cardiovascular outcomes, and subgroup analyses can be found in the Supplementary Methods (available as Supplementary Data at IJE online). The objective of our mediation analysis was to evaluate whether the effect of survey period on mortality (effect of time on mortality) can be explained (i.e. mediated) by temporal changes in established biomarkers of exposure to lead and cadmium (i.e. mediators), after accounting (i.e. adjusting) for cardiovascular risk factors (such as age or other traditional cardiovascular risk factors). More details regarding our conceptual mediation model and corresponding directed acyclic graph (DAG) can be found in the Supplementary Methods (available as Supplementary Data at IJE online). Thus, we studied the contribution of changes in blood lead and urine cadmium concentrations between NHANES 1988–94 and 1999–2004 to the corresponding absolute changes in cardiovascular and coronary heart disease mortality rates (i.e. changes in mortality mediated by lead and cadmium) in two alternative ways. First, we used the traditional ‘difference in coefficients’ approach.17 We estimated the absolute change in mortality rates comparing 1999–2004 to 1988–94 from the coefficient associated with survey period indicator (NHANES III vs NHANES 1999–2004) in two nested Aalen additive hazard models with individual age-at-death data. Both models included the same set of confounders, but one model adjusted for log-transformed metal concentrations and the other one did not. We then calculated the effects mediated through lead and cadmium as the difference in the mortality rate changes across surveys (in absolute and relative terms) estimated from both models.
Second, we used the ‘product of coefficients’ method in causal mediation analysis.12 We fitted linear regression models for log-transformed lead and cadmium concentrations by survey period indicator and confounders (mediator models), as well as the complete Aalen additive hazard model for mortality by survey period indicator, log-transformed lead and cadmium, and confounders (outcome model). We then calculated the effects mediated through lead and cadmium as the product of the mean change in log-metal concentrations between surveys estimated from the mediator models and the absolute change in mortality rates associated with log-transformed metal concentrations in the outcome model. The difference and product methods are identical for continuous outcomes and are expected to give similar results when using Aalen additive hazard models in survival settings, provided that the association of lead and cadmium with mortality is homogeneous in both survey periods.18
We used a semi-parametric specification of the additive hazard models, which only allowed the baseline hazard to be time-dependent. All additive hazard and linear regression models were fitted with three increasing levels of adjustment. The first models adjusted for age (as time scale in Aalen models and as restricted cubic splines of baseline age in linear models), sex and race. To account for changes in traditional cardiovascular risk factors over time, the second models further included baseline smoking status, physical inactivity, obesity, hypertension, diabetes, high total cholesterol, low HDL cholesterol and lipid-lowering medication. Finally, blood lead and urine cadmium were moderately correlated (r spearman = 0.27). Thus, the third models mutually adjusted log-transformed lead and cadmium concentrations for each other.
Mediated effects were expressed as the absolute decline in mortality rates from 1988–94 to 1999–2004 attributable to reductions in lead and cadmium, as well as the percentage of the adjusted mortality decline across surveys explained by these metals, with 95% confidence intervals (CIs) derived by simulation from the estimated model coefficients and covariance matrices.12
Results
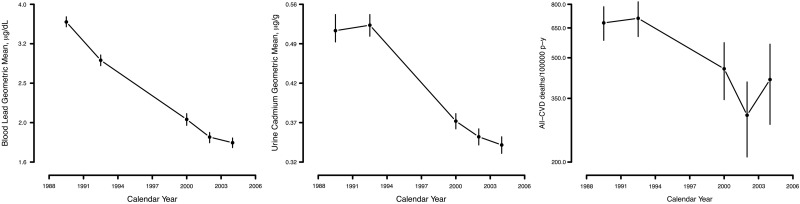
The age-, sex- and race-adjusted cardiovascular mortality rate was 691.9 deaths per 100 000 person-years in 1988–94 and 392.7 deaths per 100 000 person-years in 1999–2004 (see Supplementary Table 1, available as Supplementary Data at IJE online). Reductions in cardiovascular and coronary heart disease mortality were observed overall and in almost all subgroups investigated, including sex and smoking status categories (Figure 1; see Supplementary Table 1, available as Supplementary Data at IJE online). The age-, sex- and race-adjusted geometric means of blood lead and urine cadmium were 3.2 µg/dL and 0.51 µg/g, respectively, in 1988–94 and 1.9 µg/dL and 0.36 µg/g, respectively, in 1999–2004 (see Supplementary Table 2, available as Supplementary Data at IJE online). The risk factor-adjusted rate ratios (95% CI) associated with a doubling of blood lead and urine cadmium concentrations were 1.19 (1.07, 1.31) and 1.20 (1.09, 1.32), respectively, for cardiovascular mortality and 1.24 (1.10, 1.41) and 1.19 (1.02, 1.38), respectively, for coronary heart disease mortality (Table 1). The association of blood lead and urine cadmium with cardiovascular mortality endpoints was similar across survey periods. Never smokers showed stronger associations of lead and coronary heart disease mortality compared with ever smokers (Table 1).
Figure 1.
Age-, sex- and race-adjusted geometric mean blood lead and urine cadmium concentrations and cardiovascular disease (CVD) mortality rates across 1988–2004 National Health and Nutrition Examination Survey phases. Vertical bars show 95% confidence intervals based on 15 000 bootstrap re-samples.
Table 1.
Rate ratios for cardiovascular and coronary heart disease mortality associated with a two-fold increase in baseline blood lead and urine cadmium concentrationsa
| Cardiovascular disease |
Coronary heart disease |
|||
|---|---|---|---|---|
| Mortality rate ratio (95% CI) | P for interaction | Mortality rate ratio (95% CI) | P for interaction | |
| Blood lead | ||||
| Overall | 1.19 (1.07, 1.31) | 1.24 (1.10, 1.41) | ||
| Sex | 0.07 | 0.11 | ||
| Men | 1.09 (0.95, 1.25) | 1.14 (0.98, 1.33) | ||
| Women | 1.31 (1.13, 1.52) | 1.44 (1.14, 1.82) | ||
| Smoking | 0.07 | 0.003 | ||
| Never | 1.35 (1.14, 1.59) | 1.63 (1.32, 2.01) | ||
| Ever | 1.10 (0.96, 1.25) | 1.10 (0.95, 1.28) | ||
| Survey | 0.47 | 0.77 | ||
| 1988–94 | 1.17 (1.04, 1.31) | 1.25 (1.10, 1.42) | ||
| 1999–2004 | 1.26 (1.05, 1.50) | 1.20 (0.88, 1.62) | ||
| Urine cadmium | ||||
| Overall | 1.20 (1.09, 1.32) | 1.19 (1.02, 1.38) | ||
| Sex | 0.62 | 0.83 | ||
| Men | 1.22 (1.08, 1.38) | 1.20 (1.03, 1.40) | ||
| Women | 1.17 (1.01, 1.35) | 1.16 (0.90, 1.51) | ||
| Smoking | 0.31 | 0.89 | ||
| Never | 1.14 (1.00, 1.30) | 1.17 (0.90, 1.52) | ||
| Ever | 1.24 (1.11, 1.39) | 1.20 (1.02, 1.40) | ||
| Survey | 0.09 | 0.32 | ||
| 1988–94 | 1.17 (1.06, 1.29) | 1.17 (1.00, 1.36) | ||
| 1999–2004 | 1.43 (1.16, 1.78) | 1.43 (0.97, 2.10) | ||
aModels were adjusted for age, sex, race, smoking status, physical inactivity, obesity, hypertension, diabetes, high total cholesterol, low HDL cholesterol, lipid-lowering medication and survey period. Lead models were further adjusted for log-transformed cadmium concentrations and cadmium models for log-transformed lead concentrations.
The age-, sex-, race- and risk-factor-adjusted difference in cardiovascular mortality rates between 1999–2004 and 1988–94 was –230.7 (–328.2, –133.1) per 100 000 person-years (Table 2). Further adjusting for blood lead (without cadmium) explained 25.4% of the reduction in cardiovascular disease mortality rates, whereas further adjusting for urine cadmium (without lead) explained 11.2%. Simultaneously adjusting for both blood lead and urine cadmium explained 32.0% of the reduction in cardiovascular disease mortality rates from 1988–94 to 1999–2004. A similar pattern was observed for coronary heart disease mortality (Table 2). In absolute terms, of the 230.7 deaths per 100 000 person-years avoided in 1999–2004 compared with 1988–94 in age-, sex-, race- and risk-factor-adjusted analyses, we estimated that 51.9 (95% CI 9.0, 97.1) and 19.2 (95% CI 4.5, 36.2) deaths per 100 000 person-years were independently attributable to adjusted changes in lead and cadmium, respectively (Tables 3 and 4).
Table 2.
Absolute change in cardiovascular and coronary heart disease mortality rates (per 100 000 person-years) comparing National Health and Nutrition Examination Surveys 1999–2004 to 1988–94a before and after adjustment for blood lead and urine cadmium
| Cardiovascular disease |
Coronary heart disease |
|||
|---|---|---|---|---|
| Level of adjustment | Absolute change in mortality rate comparing 1999–2004 to 1988–94 (95% CI) | Difference in change (%)b | Absolute change in mortality rate comparing 1999–2004 to 1988–94 (95% CI) | Difference in change (%)b |
| Adjusted for age, sex and race | –269.1 (–363.0, –175.4) | 0 (reference) | –208.2 (–275.7, –140.7) | 0 (reference) |
| Further adjusted for log-lead | –187.9 (–287.1, –88.8) | –81.2 (30.2) | –153.9 (–222.7, –85.2) | –54.3 (26.1) |
| Further adjusted for log-cadmium | –217.5 (–310.0, –125.0) | –51.6 (19.2) | –177.1 (–243.1, –111.1) | –31.1 (14.9) |
| Adjusted for age, sex, race and risk factorsc | –230.7 (–328.2, –133.1) | 0 (reference) | –185.8 (–252.8, –118.7) | 0 (reference) |
| Further adjusted for log-lead | –172.1 (–274.8, –69.4) | –58.6 (25.4) | –150.5 (–219.7, –81.4) | –35.3 (19.0) |
| Further adjusted for log-cadmium | –204.9 (–301.5, –108.2) | –25.8 (11.2) | –171.2 (–237.0, –105.4) | –14.6 (7.9) |
| Adjusted for age, sex, race, risk factorsc and log-cadmium | –204.9 (–301.5, –108.2) | 0 (reference) | –171.2 (–237.0, –105.4) | 0 (reference) |
| Further adjusted for log-lead | –156.9 (–259.6, –54.1) | –48.0 (23.4) | –142.1 (–211.2, –72.9) | –29.1 (17.0) |
| Adjusted for age, sex, race, risk factorsc and log-lead | –172.1 (–274.8, –69.4) | 0 (reference) | –150.5 (–219.7, –81.4) | 0 (reference) |
| Further adjusted for log-cadmium | –156.9 (–259.6, –54.1) | –15.2 (8.8) | –142.1 (–211.2, –72.9) | –8.4 (5.6) |
aAbsolute change in mortality rate by survey period before and after adjustment for blood lead and urine cadmium was estimated from Aalen additive hazard models with age at follow-up as the time scale, survey period indicators and progressive degree of adjustments.
bMediated effect by blood lead and cadmium was calculated using the ‘difference of coefficient method’ as the absolute change in mortality rates between surveys in the reference model minus that absolute change in the model further adjusted for the log-transformed metal concentration, expressed both in absolute terms (difference in change) and relative to the change in the reference model.
cBaseline cardiovascular risk factors included smoking status, physical inactivity, obesity, hypertension, diabetes, high total cholesterol, low HDL cholesterol and lipid-lowering medication.
Table 3.
Adjusted mean changes in blood lead and urine cadmium concentrations comparing National Health and Nutrition Examination Surveys 1999–2004 to 1988–94a
| Blood lead (μg/dL) |
Urine cadmium (μg/g) |
|||
|---|---|---|---|---|
| Level of adjustment | Log-transformed mean change (95% CI) | Geometric mean ratio (95% CI) | Log-transformed mean change (95% CI) | Geometric mean ratio (95% CI) |
| Adjusted for age, sex and race | –0.54 (–0.60, –0.48) | 0.58 (0.55, 0.62) | –0.37 (–0.42, –0.31) | 0.69 (0.66, 0.73) |
| Adjusted for age, sex, race and smoking statusb | –0.52 (–0.57, –0.46) | 0.60 (0.56, 0.63) | –0.32 (–0.37, –0.27) | 0.72 (0.69, 0.76) |
| Adjusted for age, sex, race, smoking status and the other log-metal | –0.48 (–0.54, –0.42) | 0.62 (0.58, 0.65) | –0.23 (–0.29, –0.17) | 0.80 (0.75, 0.84) |
aObtained from linear regression models of log-transformed lead and cadmium concentrations by survey period indicators and progressive degrees of adjustment.
bFurther adjustment for other baseline cardiovascular risk factors (physical inactivity, obesity, hypertension, diabetes, high total cholesterol, low HDL cholesterol and lipid-lowering medication) did not materially affect the results.
Table 4.
Absolute change in cardiovascular and coronary heart disease mortality rates (per 100 000 person-years) between National Health and Nutrition Examination Surveys 1988–94 and 1999–2004 attributable to changes in blood lead and urine cadmium concentrations (‘mediated effect’) using the ‘product of coefficients method’
| Cardiovascular disease |
Coronary heart disease |
|||||
|---|---|---|---|---|---|---|
| Mediated effect (95% CI)b |
Mediated effect (95% CI)b |
|||||
| Level of adjustment | Absolute change in mortality rate per log-metal unit increase (95% CI)a | Difference in change | Percentage of adjusted change | Absolute change in mortality rate per log-metal unit increase (95% CI)a | Difference in change | Percentage of adjusted change |
| Adjusted for age, sex and race | ||||||
| Log-lead | 161.0 (73.0, 247.7) | –87.3 (–136.5, –39.1) | 32.4 (14.6, 54.0) | 107.5 (45.4, 168.8) | –58.2 (–92.9, –24.3) | 27.9 (12.0, 46.5) |
| Log-cadmium | 157.1 (93.3, 220.0) | –57.5 (–83.5, –33.5) | 21.5 (12.1, 34.2) | 94.6 (46.0, 142.5) | –34.7 (–54.0, –16.7) | 16.7 (8.0, 27.2) |
| Adjusted for age, sex, race and risk factorsc | ||||||
| Log-lead | 124.0 (31.9, 214.8) | –64.3 (–113.3, –16.3) | 28.1 (7.4, 54.0) | 74.5 (8.1, 139.9) | –38.6 (–73.8, –4.2) | 20.8 (2.5, 41.3) |
| Log-cadmium | 97.0 (29.5, 163.6) | –31.2 (–54.4, –9.5) | 13.7 (4.1, 26.7) | 54.6 (3.2, 105.3) | –17.6 (–35.0, –1.0) | 9.5 (0.6, 19.4) |
| Adjusted for age, sex, race, risk factorsc and log-metal | ||||||
| Log-lead | 108.0 (17.3, 197.5) | –52.0 (–96.7, –8.4) | 26.2 (4.1, 55.3) | 65.6 (0.4, 129.8) | –31.6 (–63.4, –0.3) | 18.7 (0.2, 40.1) |
| Log-cadmium | 85.2 (19.0, 150.6) | –19.4 (–36.4, –4.3) | 12.3 (2.4, 29.5) | 47.4 (–3.1, 97.2) | –10.8 (–23.4, 0.7) | 7.4 (–0.5, 17.7) |
aAbsolute changes in mortality rates (per 100 000 person-years) associated with one-unit increase in log-transformed blood lead or urine cadmium concentrations were obtained from Aalen additive hazard models of age-at-death data by survey period indicators, log-transformed baseline metal concentrations and progressive degree of adjustments.
bEffects mediated through lead and cadmium were estimated with the ‘product of coefficients method’ that multiplies the coefficient for the mean change in log-transformed metal concentrations between surveys (Table 3) by the absolute change in mortality rates associated with one-unit increase in log-transformed metal concentrations (first column of this table) expressed in absolute terms (difference in change reflecting the number of avoided deaths per 100 000 person-years) and relative to the adjusted changes in mortality rates between surveys before adding log-metal concentrations to the model (reference model from Table 2). The 95% confidence intervals (CIs) were derived by simulation from the estimated model coefficients and covariance matrices.
cBaseline cardiovascular risk factors included smoking status, physical inactivity, obesity, hypertension, diabetes, high total cholesterol, low HDL cholesterol and lipid-lowering medication.
We conducted sensitivity analysis by adding additional adjustments for variables that can act as a proxy of socio-economical status (low education, familiar poverty index ratio) and exposure to tobacco smoke (cotinine and pack-years of cumulative active smoking), with consistent findings (Supplementary Table 3, available as Supplementary Data at IJE online). After additional sensitivity analyses that included adjustment for systolic blood pressure (continuous) and blood-pressure-lowering medication instead of hypertension status, and total and HDL cholesterol levels (continuous) instead of high total cholesterol and low HDL cholesterol categories, the results remained essentially unchanged (data not shown). The reduction in cardiovascular mortality attributed to lead was markedly stronger in women and in never smokers (see Supplementary Tables 4 and 5, available as Supplementary Data at IJE online).
Discussion
Using individual data from NHANES, cardiovascular mortality rates decreased by 43% from 1988–94 to 1999–2004. After accounting for traditional risk factors, 32% of the cardiovascular mortality avoided in the USA comparing 1999–2004 to 1988–94 was attributed to overall declines in lead and cadmium exposures. Although a large proportion of the reduction in cardiovascular disease mortality remained unexplained, these findings support the importance of lead and cadmium in explaining the marked reduction in cardiovascular disease observed in the USA in the last decades.
Lead exposure, one of the most important environmental problems of the twentieth century, has declined markedly since the 1970 s, after the US government banned the use of lead in gasoline, paint and the soldering of cans. Lead exposure, however, still remains substantial because of widespread soil contamination, persistence of past uses (house paint and plumbing), continuing industrial uses (primarily for batteries) and presence in tobacco and tobacco smoke.4 The general population is exposed through ambient air, dust, drinking water, alcohol, and certain foods and tobacco smoke.19,20 Blood lead is an established biomarker of recent exposure, although it also shows a slow component with a half-life of 5–20 years that reflects endogenous exposure from bone lead redistribution.21
Cadmium is a by-product from mining and smelting. Its industrial production started in the 1930 s.22–24 The use of cadmium in consumer products (e.g. plastics, pigments, batteries)22–24 and cadmium content in fertilizers has resulted in soil contamination. Soil contamination of cadmium is problematic because vegetables and grains bioconcentrate cadmium,22 providing a major pathway for exposure through diet and tobacco. Ambient air and dust can also contribute to cadmium exposure, particularly in urban areas and in the vicinity of industrial sources and waste sites.25 Cadmium progressively accumulates in the kidney (half of body burden), liver, pancreas and the central nervous system.22 In urine, cadmium reflects kidney cadmium contents and, with a half-life of 15–30 years, is an established biomarker of cumulative body burden.
NHANES has conducted biomonitoring of lead and cadmium exposure in representative samples of the non-institutionalized US population since 1976.13 These national data have documented the marked reduction in exposure to lead and cadmium over the last decades3,4 showing the positive impact of large-scale public health policies. NHANES II (1976 to 1980), which coincided with the banning of lead in gasoline, documented the tight connection between changes in lead concentrations in ambient air and blood lead concentrations.26,27 Recent declines in cadmium exposure in the US population have been partly attributed to declines in smoking prevalence and intensity and in exposure to second-hand smoke, benefiting both ever and never smokers.3
Experimental and epidemiological studies provide strong evidence to infer a causal role for lead and cadmium in atherosclerosis.9,10,28 Potential mechanisms include disruption of redox balance,7–9,29–32 epigenetic33 and endocrine34 pathways. Epidemiologic studies from different geographical locations and time periods have reported positive associations of lead and cadmium with atherosclerotic disease,5,6 including cardiovascular mortality and the prevalence and incidence of endpoints such as myocardial infarction and coronary heart disease,35–40 stroke,36,39,41,42 heart failure,41–43 peripheral arterial disease,4,15,44–47 and carotid atherosclerosis.28,48,49 Meta-analysis and systematic reviews of observational studies add to the body of evidence supporting the role of lead and cadmium as cardiovascular risk factors.5,6
Whereas randomized clinical trials evaluating the cardiovascular effect of lead and cadmium exposures are obviously not available, a double-blind placebo-controlled randomized trial of patients with previously diagnosed myocardial infarction found a reduction in a composite cardiovascular endpoint in participants who received chelation therapy with ethylene diamine tetra acetic acid (EDTA) compared with placebo.50 Lead and cadmium are chelated by EDTA and their removal provides a potential explanation for these results.51 Unfortunately, lead and cadmium levels were not measured in that trial. Future studies investigating the effects of chelation to prevent cardiovascular disease should include metal measurements and incident endpoints.52
Many environmental, dietary and lifestyle factors have changed in the last decades. For instance, air-pollution levels have declined substantially in the USA.53 The contribution of changes in air pollution to declining blood lead and urine cadmium biomarkers in our study population is unknown. If that contribution is important, declining trends in blood lead and urine cadmium could potentially act as a proxy for declines in other air pollutants. Although we cannot discard that our associations for lead and cadmium could be related to reductions in air-pollution exposure in the last decades, multiple sources contribute to lead and cadmium exposure beyond air pollution.
In addition to environmental and lifestyle factors, there have been multiple advances in treatment and secondary prevention of cardiovascular disease in the USA, including generalized use of cardioprotective medications such as β-blockers, cardiac interventions and advanced acute support care. In our data, we lacked information on prognostic factors and cardiovascular interventions, and we could not incorporate into the analysis changes in secondary prevention in the USA that has likely resulted in a decrease in cardiac deaths. Furthermore, since we did not have longitudinal data in lead or cadmium exposure in the same subjects, we cannot discard that some of the period effects that we attribute to lead or cadmium can be caused by changes in unmeasured primary or secondary prevention factors.
Other limitations should also be considered in the interpretation of our findings. Mortality outcomes were obtained from death certificates, with potential miscoding of the cause of death. Changes in death certificates coding over time could affect observed trends in cardiovascular mortality. However, methods for matching NHANES participants with the NDI have been validated13 and developed with the goal of tracking mortality changes over time. In addition, we did not have data on incident events or on duration of disease. Studies evaluating incident events are needed to fully evaluate the impact of decreasing lead and cadmium exposures on cardiovascular burden.
As in other epidemiologic studies, we cannot discard residual confounding. We adjusted for potential confounders using models with increasing degree of adjustment. The causal interpretation of mediated effects under all the degrees of adjustment is imperfect, since adjusting for demographic factors only leaves room for substantial residual confounding, whereas full adjustment may introduce inter-related causal pathways that could compromise the identifiability of mediation effects.54 Lead and cadmium are prevalent metals with common sources of exposure including air pollution and smoking.21–23 However, the estimated contribution of lead and cadmium to cardiovascular mortality trends were largely independent of each other, as the sum of their contributions in separate models was similar to their joint contribution in a single model.55 In addition, the difference of coefficients and the product of coefficients methods showed consistent results, as expected.z
Our analysis has important strengths, including the relatively large sample size, the availability of detailed information on relevant cardiovascular risk factors, the standardization of the study protocol, the extensive laboratory quality control and the representativeness of the study sample. Importantly, blood lead and urine cadmium were measured in the same laboratory under strict quality-control measures with the goal of tracking concentrations over time.
The declines in lead and cadmium exposure occurring in the last decades in the USA were associated with large reductions in cardiovascular mortality. These findings support that reducing lead and cadmium exposures may have potentially resulted in an overlooked public health achievement. The general population, however, remains exposed to both lead and cadmium, and both metals remain associated with cardiovascular disease at relatively low levels of exposure.5,6 Preventive strategies to enable additional reductions in exposure to lead and cadmium could further improve cardiovascular health in the population.
Supplementary Data
Supplementary data are available at IJE online.
Supplementary Material
Acknowledgements
This work was supported by the Strategic Action for Research in Health Sciences (CP12/03080; PI15/00071), CIBERESP and CIBEROBN. The Strategic Action for Research in Health Sciences, CIBEROBN and CIBERESP are initiatives from the Carlos the third National Health Institutes in Madrid and the Spanish Ministry of Economy and Competitiveness and are co-funded with European Funds for Regional Development (FEDER). A.N.-A. was supported by the National Institute of Environmental Health Sciences grant P30ES009089. The findings and conclusions in this article are those of the authors and not necessarily those of the Centers for Disease Control and Prevention.
Conflict of interest: The authors have no conflicts of interest to declare.
References
- 1. Cooper R, Cutler J, Desvigne-Nickens P. et al. Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States: findings of the National Conference on Cardiovascular Disease Prevention. Circulation 2000;102:3137–47. [DOI] [PubMed] [Google Scholar]
- 2. Roger VL. Cardiovascular diseases in populations: secular trends and contemporary challenges—Geoffrey Rose lecture, European Society of Cardiology meeting 2014. Eur Heart J 2015;36:2142–46. [DOI] [PubMed] [Google Scholar]
- 3. Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Perspect 2012;120:204–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med 2005;165:2155–61. [DOI] [PubMed] [Google Scholar]
- 5. Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease—a systematic review. Environ Health Perspect 2007;115:472–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep 2013;15:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almenara CCP, Broseghini-Filho GB, Vescovi MVA. et al. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS One 2013;8:e68418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Angeli JK, Cruz Pereira CA, de Oliveira Faria T, Stefanon I, Padilha AS, Vassallo DV. Cadmium exposure induces vascular injury due to endothelial oxidative stress: the role of local angiotensin II and COX-2. Free Radic Biol Med 2013;65:838–48. [DOI] [PubMed] [Google Scholar]
- 9. Knoflach M, Messner B, Shen YH. et al. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J 2011;75:2491–95. [DOI] [PubMed] [Google Scholar]
- 10. Messner B, Bernhard D. Cadmium and cardiovascular diseases: cell biology, pathophysiology, and epidemiological relevance. Biometals 2010;23:811–22. [DOI] [PubMed] [Google Scholar]
- 11. Cosselman KE, Navas-Acien A, Kaufman JD. Environmental factors in cardiovascular disease. Nat Rev Cardiol 2015;12:627–42. [DOI] [PubMed] [Google Scholar]
- 12. Lange T, Hansen JV. Direct and indirect effects in a survival context. Epidemiology 2011;22:575–81. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Diseases Control and Prevention (CDC), National Centers for Health Statistics (NCHS). NHANES—National Health and Nutrition Examination Survey. http://www.cdc.gov/nchs/nhanes.htm (15 April 2016, date last accessed). [Google Scholar]
- 14. Paschal DC, Burt V, Caudill SP. et al. Exposure of the U.S. population aged 6 years and older to cadmium: 1988–1994. Arch Environ Contam Toxicol 2000;38:377–83. [DOI] [PubMed] [Google Scholar]
- 15. Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US national health and nutrition examination survey. Am J Epidemiol 2010;172:671–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. World Health Organization (WHO). International Classification of Diseases (ICD-10). http://www.who.int/classifications/icd/en/ (15 April 2016, date last accessed). [Google Scholar]
- 17. Jiang Z, VanderWeele TJ. When is the difference method conservative for assessing mediation? Am J Epidemiol 2015;182:105–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. VanderWeele TJ. Causal mediation analysis with survival data. Epidemiology 2011;22:582–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hense HW, Filipiak B, Novak L, Stoeppler M. Nonoccupational determinants of blood lead concentrations in a general population. Int J Epidemiol 1992;21:753–62. [DOI] [PubMed] [Google Scholar]
- 20. Apostolou A, Garcia-Esquinas E, Fadrowski JJ, McLain P, Weaver VM, Navas-Acien A. Secondhand tobacco smoke: a source of lead exposure in US children and adolescents. Am J Public Health 2012;102:714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agency for Toxic Substances and Disease Registry (ATSDR)—Toxicological Profile: Lead. 2007. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=96&tid=22 (15 April 2016, date last accessed). [PubMed]
- 22. Agency for Toxic Substances and Disease Registry (ATSDR)—Toxicological Profile: Cadmium. 2012. http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=48&tid=15 (15 April 2016, date last accessed). [PubMed]
- 23. Beryllium, cadmium, mercury, and exposures in the glass manufacturing industry. Working Group views and expert opinions, Lyon, 9–16 February 1993. IARC Monogr Eval Carcinog Risks Hum 1993;58:1–415. [PMC free article] [PubMed] [Google Scholar]
- 24. U.S. Geological Survey. Minerals Information: Cadmium. http://minerals.usgs.gov/minerals/pubs/commodity/cadmium/ (15 April 2016, date last accessed).
- 25. Hogervorst J, Plusquin M, Vangronsveld J. et al. House dust as possible route of environmental exposure to cadmium and lead in the adult general population. Environ Res 2007;103:30–37. [DOI] [PubMed] [Google Scholar]
- 26. Pirkle JL. The decline in blood lead levels in the United States. JAMA: American Medical Association 1994;272:284. [PubMed] [Google Scholar]
- 27. U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards. Lead | AirTrends | Air & Radiation | EPA. http://www3.epa.gov/airtrends/lead.html (15 April 2016, date last accessed).
- 28. Messner B, Knoflach M, Seubert A. et al. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol 2009;29:1392–98. [DOI] [PubMed] [Google Scholar]
- 29. Valko M, Morris H, Cronin MTD. Metals, toxicity and oxidative stress. Curr Med Chem 2005;12:1161–1208. [DOI] [PubMed] [Google Scholar]
- 30. Jin T, Lu J, Nordberg M. Toxicokinetics and biochemistry of cadmium with special emphasis on the role of metallothionein. Neurotoxicology 1998;19:529–35. [PubMed] [Google Scholar]
- 31. Vaziri ND. Mechanisms of lead-induced hypertension and cardiovascular disease. Am J Physiol Heart Circ Physiol 2008;295:H454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kamal M, Fathy MM, Taher E, Hasan M, Tolba M. Assessment of the role of paraoxonase gene polymorphism (Q192R) and paraoxonase activity in the susceptibility to atherosclerosis among lead-exposed workers. Ann Saudi Med 2011;31:481–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ruiz-Hernandez A, Kuo C-C, Rentero-Garrido P. et al. Environmental chemicals and DNA methylation in adults: a systematic review of the epidemiologic evidence. Clin Epigenetics 2015;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Iavicoli I, Fontana L, Bergamaschi A. The effects of metals as endocrine disruptors. J Toxicol Environ Health B Crit Rev 2009;12:206–23. [DOI] [PubMed] [Google Scholar]
- 35. Everett CJ, Frithsen IL. Association of urinary cadmium and myocardial infarction. Environ Res 2008;106:284–86. [DOI] [PubMed] [Google Scholar]
- 36. Lee M-S, Park SK, Hu H, Lee S. Cadmium exposure and cardiovascular disease in the 2005 Korea National Health and Nutrition Examination Survey. Environ Res 2011;111:171–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gustavsson P, Plato N, Hallqvist J. et al. A population-based case-referent study of myocardial infarction and occupational exposure to motor exhaust, other combustion products, organic solvents, lead, and dynamite. Stockholm Heart Epidemiology Program (SHEEP) Study Group. Epidemiology 2001;12:222–28. [DOI] [PubMed] [Google Scholar]
- 38. Kosmala W, Kuliczkowski W, Jolda-Mydlowska B, Przewlocka-Kosmala M, Kucharski W, Antonowicz-Juchniewicz J. Relation between heavy metals and left ventricular diastolic function in patients with coronary artery disease. Toxicol Mech Methods 2004;14:177–82. [DOI] [PubMed] [Google Scholar]
- 39. Pocock SJ, Shaper AG, Ashby D, Delves HT, Clayton BE. The relationship between blood lead, blood pressure, stroke, and heart attacks in middle-aged British men. Environ Health Perspect 1988;78:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kromhout D. Blood lead and coronary heart disease risk among elderly men in Zutphen, the Netherlands. Environ Health Perspect 1988;78:43–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tellez-Plaza M, Guallar E, Howard BV. et al. Cadmium exposure and incident cardiovascular disease. Epidemiology 2013;24:421–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peters JL, Perlstein TS, Perry MJ, McNeely E, Weuve J. Cadmium exposure in association with history of stroke and heart failure. Environ Res 2010;110:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borné Y, Barregard L, Persson M, Hedblad B, Fagerberg B, Engström G. Cadmium exposure and incidence of heart failure and atrial fibrillation: a population-based prospective cohort study. BMJ Open 2015;5:e007366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Navas-Acien A, Silbergeld EK, Sharrett R, Calderon-Aranda E, Selvin E, Guallar E. Metals in urine and peripheral arterial disease. Environ Health Perspect 2005;113:164–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tellez-Plaza M, Guallar E, Fabsitz RR. et al. Cadmium exposure and incident peripheral arterial disease. Circ Cardiovasc Qual Outcomes 2013;6:626–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pan TC, Horng CJ, Lin SR, Lin TH, Huang CW. Simultaneous determination of Zn, Cd, Pb, and Cu in urine of patients with blackfoot disease using anodic stripping voltammetry. Biol Trace Elem Res 1993;38:233–41. [DOI] [PubMed] [Google Scholar]
- 47. Fagerberg B, Bergström G, Borén J, Barregard L. Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: a cohort and an experimental study. BMJ Open 2013;3:e002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fagerberg B, Barregard L, Sallsten G. et al. Cadmium exposure and atherosclerotic carotid plaques—results from the Malmö diet and Cancer study. Environ Res 2015;136:67–74. [DOI] [PubMed] [Google Scholar]
- 49. Fagerberg B, Bergström G, Borén J, Barregard L. Cadmium exposure is accompanied by increased prevalence and future growth of atherosclerotic plaques in 64-year-old women. J Intern Med 2012;272:601–10. [DOI] [PubMed] [Google Scholar]
- 50. Lamas GA, Goertz C, Boineau R. et al. Effect of disodium EDTA chelation regimen on cardiovascular events in patients with previous myocardial infarction: the TACT randomized trial. JAMA 2013;309:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ouyang P, Gottlieb SH, Culotta VL, Navas-Acien A. EDTA chelation therapy to reduce cardiovascular events in persons with diabetes. Curr Cardiol Rep 2015;17:96. [DOI] [PubMed] [Google Scholar]
- 52. Lamas GA, Navas-Acien A, Mark DB, Lee KL. Heavy metals, cardiovascular disease, and the unexpected benefits of edetate chelation therapy. J Am Coll Cardiol 2016;67:2411–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. US Environmental Protection Agency, Office of Air Quality Planning and Standards. AirTrends | Air & Radiation | EPA. https://www3.epa.gov/airtrends/ (15 April 2016, date last accessed). [Google Scholar]
- 54. Lange T, Rasmussen M, Thygesen LC. Assessing natural direct and indirect effects through multiple pathways. Am J Epidemiol 2014;179:513–18. [DOI] [PubMed] [Google Scholar]
- 55. VanderWeele TJ, Vansteelandt S. Mediation analysis with multiple mediators. Epidemiol Method 2014;2:95–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.