ABSTRACT
Background: An increased number of podocyte-derived extracellular vesicles (pEVs) may reflect podocyte injury in renal disease. Elevated glomerular pressure and other insults may injure podocytes, yet it remains unclear whether the numbers of pEVs are altered in hypertensive patients. We tested the hypothesis that urinary pEV levels would be elevated in patients with renovascular hypertension (RVH) compared with essential hypertension (EH) or healthy volunteers (HVs).
Methods: We prospectively enrolled patients with EH (n = 30) or RVH (n = 31) to study renal blood flow (RBF) and cortical perfusion using multidetector computed tomography under controlled condition (regulated sodium intake and renin—angiotensin blockade). After isolation from urine samples, pEVs (nephrin and podocalyxin positive) were characterized by flow cytometry. Fourteen RVH patients were studied again 3 months after stenting or continued medical therapy. HVs (n = 15) served as controls.
Results: The fraction of pEV among urinary EVs was elevated in RVH compared with HVs and EH (11.4 ± 6.4, 6.8 ± 3.4 and 6.3 ± 3.7%, respectively; P < 0.001) and remained unchanged after 3 additional months of therapy and after controlling for clinical parameters. However, eGFR- and age-adjusted pEV levels did not correlate with any clinical or renal parameters.
Conclusions: In hypertensive patients under controlled conditions, urinary pEV levels are elevated in patients with RVH and low eGFR compared with patients with EH and relatively preserved renal function. These pEVs may reflect podocyte injury secondary to kidney damage, and their levels might represent a novel therapeutic target.
Keywords: CKD, extracellular vesicles, hypertension, podocyte, renovascular hypertension
INTRODUCTION
Podocytes are critical for maintenance of the glomerular filtration barrier, and their injury is associated with increased protein leakage and decreased GFR [1, 2]. Hypertensive conditions may damage podocytes, with podocyturia predicting clinical outcomes [3]. For example, loss of podocytes in preeclampsia may lead to a disruption of the glomerular filtration barrier and, ultimately, proteinuria [4]. Both animal and human studies illustrate that changes to podocyte morphology followed by progressive podocyte loss are often associated with albuminuria [5, 6]. Importantly, early detection of podocyte injury can lead to early intervention to prevent kidney disease progression [7].
Extracellular vesicles (EVs) are a heterogeneous group of spherical structures bounded by a lipid bilayer and carrying a cargo of proteins and nucleic acids, and include exosomes, microvesicles, and apoptotic bodies that are somewhat difficult to distinguish [8–11]. EVs may serve for intercellular communication and are often released into the extracellular space by cells that are damaged or stressed by a hostile microenvironment, such as hypoxia [12, 13]. Recent studies suggest that urinary podocyte-derived EVs (pEVs) may serve as biomarkers of podocyte-specific injury, as their levels increase at an early stage of glomerular injury in diabetic nephropathy and kidney disease [6]. Hence, increased urinary levels of pEVs may herald podocyte stress and predict subsequent podocyte loss [6, 14].
Prolonged uncontrolled hypertension may induce vascular remodeling, as well as obliteration and collapse of glomerular structures. Renovascular hypertension (RVH) is characterized by decreases in renal blood flow (RBF), perfusion [15–17], and function compared with matched patients with essential hypertension (EH) [18, 19], suggesting post-stenotic kidney damage, which might be secondary to hypoxia [20]. Notably, ischemic injury to stenotic kidneys (STK) and non-STK exposure to high arterial pressure might both induce podocyte damage that precedes overt glomerular injury. However, it is yet unclear whether pEV levels are altered in the early stages of ischemic or hypertensive kidney disease.
We hypothesized that pEV numbers would be elevated in the urine of patients with RVH compared with EH with a comparable severity of hypertension, owing to post-stenotic kidney injury. To address this, we compared the numbers of urinary pEVs obtained from patients with RVH, EH and healthy volunteers (HVs) and assessed the relationship between pEVs and clinical and biochemical parameters in the same patients.
MATERIALS AND METHODS
Patients
The study was approved by the institutional review board, was Health Insurance Portability and Accountability Act compliant and written informed consent was obtained from each patient. We prospectively recruited 71 hypertensive patients ≥18 years of age with EH (n = 40) or RVH (n = 31) and with serum creatinine <2.5 mg/dL to participate in studies [21, 22] between January 2008 and September 2012. Similar to the Cardiovascular Outcomes with Renal Atherosclerotic Lesions (CORAL) study [23], renal artery stenosis was defined as cross-sectional luminal obstruction >60% as per computed tomography (CT) or magnetic resonance angiography or Doppler velocities ≥300 cm/s. Exclusion criteria included diabetes requiring medications, recent cardiovascular events (myocardial infarction, stroke, congestive heart failure within 6 months), pregnancy and kidney transplant. The 3-day inpatient protocol in the clinical research unit included regulated dietary sodium intake (150 mEq/day), an isocaloric diet and CT scanning on Day 3. All urine samples were collected on Day 1 before the imaging study. Antihypertensive medications were continued; for uniformity, all patients were treated with agents that block the renin-angiotensin system [angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs)]. Normotensive HVs [systolic blood pressure (SBP) <130 mmHg and diastolic blood pressure (DBP) <80 mmHg] were prospectively enrolled through the Mayo Biobank to serve as control subjects.
RVH patients subsequently underwent renal stenting or continued their medical therapy, according to clinical indications and management decisions (including resistant hypertension, progressive decline in kidney function or episodes of circulatory congestion). Fourteen RVH patients (eight medically treated and six stented following standard procedures) returned for repeat measurements 3 months after the initial protocol.
Systemic and renal hemodynamics and function
We calculated eGFR using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation [24]. Blood pressure was measured by automated oscillometric recording (Omron, Kyoto, Japan), averaging three measurements at 5, 7 and 9 min after a 5-min rest. Blood samples for plasma renin activity were obtained from the inferior vena cava and the right and left renal vein of all hypertensive patients, as previously described [19]. Urine samples were collected for 24 h in all hypertensive patients, and spot urine samples were obtained from HVs. Samples were stored at −80°C until measurement.
To measure RBF, a 5F pigtail Cobra catheter (Cook, Inc., Bloomington, IN, USA) was placed in the right atrium for central venous injection of contrast for flow studies using a dual-source 64-slice multidetector computed tomography (MDCT) scanner (SOMATOM Definition, Siemens Medical Solution, Forchheim, Germany), as described previously [25]. Four tomographic slices (each 5-mm thick) localized in the hilum region were acquired after a bolus injection of iopamidol-370 or iohexol-350 (0.5 mL/kg, ∼10 mL/s) using a power injector during coached respiratory suspension, and reconstructed using a B40f kernel. Fifteen minutes later, a kidney volume study (5-mm-thick slices) was performed in the helical mode after a second similar contrast injection to determine right and left cortical and medullary regional volumes.
MDCT images were reconstructed and displayed with the Analyze software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN, USA) to delineate cortical and medullary regions of interest (ROIs) in each kidney. Cortical and medullary time-attenuation data were then plotted and fitted by an extended gamma-variate model to derive curve-fitting parameters to obtain measures of cortical perfusion and RBF, as described previously [26–28]. Renal volumes were measured with previously validated statistical volume estimation with Analyze, which involves sampling of randomly distributed points over the identified ROIs. On each CT section, the cortex, medulla and renal contours were differentiated by the substantial cortical enhancement during the vascular phase and their volumes calculated from the number of sampled points [25]. RBF was subsequently calculated as the sum of cortical and medullary blood flows obtained from each cortex and medulla as the product of its perfusion and volume [29].
EV isolation and analysis
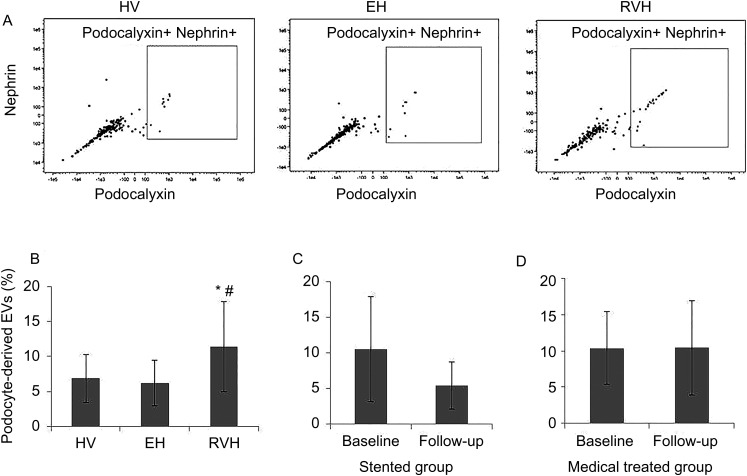
EVs were isolated from whole urine using Total Exosome Isolation reagent (Invitrogen, Waltham, MA, USA) according to the manufacturer's guidelines. Urine samples (1000 µL) were centrifuged at 2000 g for 30 min at 4°C to remove cells and debris. Supernatants (800 µL) were mixed with 1 volume of the Total Exosome Isolation reagent and incubated for 1 h at room temperature. After incubation, samples were centrifuged at 10 000 g for 1 h at 4°C. Pelleted exosomes were resuspended in PBS. Isolated EVs were stained for 20 min at 37°C with 5 µM Vybrant DiO (Molecular Probes, Eugene, OR, USA) cell-labeling solution. Labeled EVs were washed with Total Exosome Isolation reagent and stained with cell-specific antibodies. Podocalyxin (1:20; eBioscience) and nephrin (1:250; Bioss) antibodies were used to specifically identify pEVs. To exclude endothelial, mesangial, parietal or other cells, we also stained EVs using CD31 (1:20; eBioscience), platelet-derived growth factor (PDGF)-β receptor (1:5; Abcam) and claudin (1:10; R&D Systems). EVs were quantified using a FlowSight (Amnis, Seattle, WA, USA) flow cytometer, as previously described [30, 31], by acquiring at least 50 000 DiO-positive events. Vybrant DiO was used to identify events as EVs [32]. The flow-gating strategy included positive gate for DiO events and negative gates for CD31, PDGF-β and claudin. This was followed by either a double- or single-positive gate for both nephrin and podocalyxin (Figure 1A). The level of EVs was expressed as a percentage (double positive events of podocyte markers/negative events of other markers of all DiO events).
FIGURE 1.
(A) Representative flow cytometry scatter plots of EVs in HVs, EH and RVH patients. (B) After adjustment for eGFR and age, urinary levels of podocyte-derived EVs were higher in RVH compared with EH or HVs. *P = 0.01 versus HVs, #P = 0.01 versus EH. Changes in individual podocyte-derived EVs from baseline to 3 months follow-up were not statistically significant in either (C) stented or (D) medically treated patients with RVH.
Statistical analysis
Data were analyzed using the JMP software package version 10.0 (SAS Institute, Cary, NC, USA). Normally distributed data were expressed as mean ± SD and non-normally distributed data as median (range). Comparisons among the HV, EH and RVH groups were performed using ANOVA followed by an unpaired two-tailed t-test (or the Wilcoxon rank-sum test for skewed data) and correlation coefficients using least-square fit. To control for different variables among groups, we used analysis of covariance (ANCOVA). After checking the assumption of the ANCOVA, we selected eGFR and age as covariates. Within-group comparisons were performed with the Wilcoxon signed-rank test. For correlation analysis of single kidney variables, STKs of RVH were compiled with left kidneys of EH, whereas the contralateral kidneys (CLKs) in RVH were compiled with the right kidney of EH. Partial correlation analysis was used to adjust for the eGFR and age simultaneously. Statistical significance for all tests was judged at P < 0.05.
RESULTS
Demographic data
Table 1 summarizes the characteristics of patients included in this study. EH patients were younger than those in the two other groups. The mean arterial pressure (MAP) of EH and RVH was higher than in HVs. RVH eGFR was lower than in EH and HVs. Urinary albumin of RVH was higher than in EH and HVs. RBF and cortical perfusion of RVH were lower than in EH.
Table 1.
Clinical characteristics of HVs and patients with EH or RVH
| HV (n = 15) | EH (n = 30) | RVH (n = 31) | P-value | P-valuea | P-valueb | P-valuec | |
|---|---|---|---|---|---|---|---|
| Age (years) | 72.0 ± 5.7 | 56.3 ± 16.3 | 68.3 ± 8.3 | <0.001 | <0.001 | 0.58 | 0.002 |
| Men, n (%) | 5 (33) | 12 (40) | 21 (67) | 0.03 | 0.02 | 0.001 | 0.66 |
| Antihypertensive drugs (n) | – | 2.6 ± 1.1 | 3.1 ± 1.2 | 0.093 | |||
| Taking ARB or ACEi (%) | 100 | 100 | |||||
| Serum creatinine (mg/dL) | 0.90 ± 0.17 | 0.89 ± 0.28 | 1.38 ± 0.40 | <0.001 | <0.001 | 0.006 | 0.93 |
| eGFR (mL/min/1.73 m2) | 70 (57–84) | 84 (62–95) | 49 (35–65) | <0.001 | <0.001 | 0.001 | 0.06 |
| Mean arterial pressure (mmHg) | 86.6 ± 5.1 | 94.8 ± 11.0 | 93.6 ± 11.4 | 0.04 | 0.61 | 0.02 | 0.01 |
| Body mass index (kg/m2) | 25.4 ± 4.4 | 30.0 ± 8.3 | 28.0 ± 4.2 | 0.06 | |||
| Plasma renin activity (ng/mL/h) | 8.27 ± 7.42 | 11.32 ± 2.04 | 0.20 | ||||
| Urinary microalbumin (µg/mL) | 3.9 (2.2–11.3) | 5.0 (4.9–5.1) | 6.1 (5.0–14.6) | 0.02 | 0.02 | 0.03 | 0.32 |
| RBF of kidney (mL/min) | 389.7 ± 160.6 | 270.8 ± 124.4 | 0.003 | ||||
| Cortical perfusion (mL/min/mL) | 3.71 ± 1.17 | 2.48 ± 0.62 | <0.001 |
Values given as mean ± SD or median (range). ARB, angiotensin receptor blocker; ACEi, angiotensin-converting enzyme inhibitor; eGFR, estimated glomerular filtration rate; RBF, renal blood flow.
aP-value RVH versus EH.
bP-value RVH versus HVs.
cP-value EH versus HVs.
Urinary levels of pEVs are elevated in RVH
Urinary levels of podocalyxin and nephrin double-positive pEVs were elevated in RVH compared with EH and HV subjects (P < 0.001 and P = 0.01, respectively). After eGFR and age adjustment, the levels of pEVs of RVH remained higher than in the other groups (Figure 1). There was no effect of sex on pEV levels (P = 0.30). The levels of single-positive pEVs (both nephrin and podocalyxin) showed similar significant differences among the groups (data not shown).
Correlation of pEV levels with clinical and renal parameters
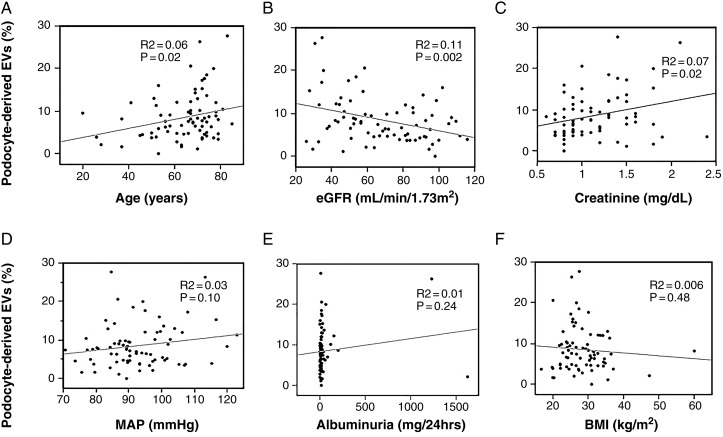
Among all participants, pEV levels correlated inversely with eGFR and directly with serum creatinine and age, but not with MAP, albuminuria or BMI (Figure 2).
FIGURE 2.
Nonadjusted correlation analysis among all participants. Podocyte-derived EV fractions correlated directly with age and creatinine levels and inversely with eGFR.
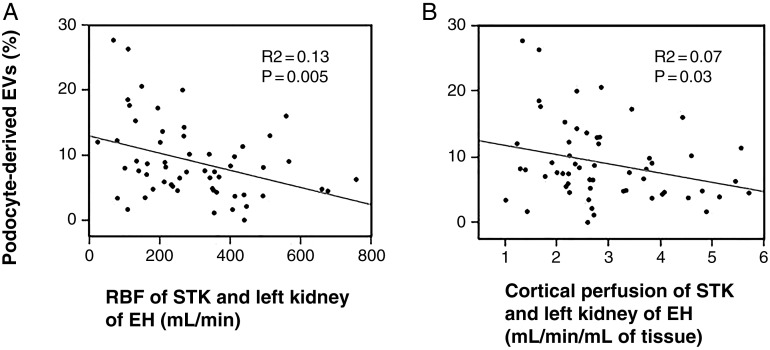
In hypertensive patients, pEVs showed inverse correlations with RBF and cortical perfusion of the STK of RVH patients and the left kidney in EH (Figure 3). Interestingly, within this normal range, albuminuria did not correlate with SBP, age, eGFR, RBF or cortical perfusion. After controlling for eGFR and age, no correlation was apparent between podocyte EVs and these clinical parameters (data not shown).
FIGURE 3.
Nonadjusted correlations in hypertensive patients. Podocyte-derived EV level correlated inversely with RBF (A) and cortical perfusion (B) of the stenotic kidney or left kidney of hypertensive patients.
Treatment did not affect pEV levels in RVH
Stenting significantly decreased both systolic and diastolic blood pressure at follow-up (from 146.15 ± 19.8 to 137.0 ± 16.4 mmHg, P = 0.03; and from 71.1 ± 11.1 to 67.5 ± 11.6 mmHg, P = 0.02, respectively). There was no significant change in blood pressure in medically treated RVH patients (data not shown). When classified based on subsequent treatment option, pEV levels in stented RVH patients (12.3 ± 6.8%, n = 18) were not different (P = 0.38) from those in the medically treated group (10.1 ± 5.9%, n = 13). When measurements were repeated in subgroups of RVD patients 3 months after stenting (n = 6) or with continued medical therapy (n = 8), pEV levels remained unchanged compared with baseline levels (Figure 1).
Urinary level of CD31+ EVs
Urinary levels of CD31+ EV in RVH were lower than in EH or HV subjects after covariate adjustment (85.5 ± 5.3, 90.5 ± 3.8 and 90.2 ± 4.6%; P = 0.001 and P = 0.004, respectively), and unaffected by stenting (83.2 ± 5.7 versus 85.8 ± 6.7%, P = 0.345). Urinary CD31+ EVs also remained stable in the medically treated RVH group (87.8 ± 1.9 versus 84.5 ± 7.1, P = 0.401). All data were expressed as a percentage (CD31+/negative events of other markers of all DiO events).
DISCUSSION
Our study demonstrates that urinary fractions of podocyte-derived EVs, assessed using flow cytometry targeting podocalyxin and nephrin, are elevated in patients with RVH compared with EH and HVs. Since pEVs are considered to indicate podocyte injury [6, 14, 33, 34], we interpret these results as indicating that RVH patients have greater podocyte injury compared with EH with similar levels of blood pressure, possibly secondary to intrarenal damage beyond stenosis. The significant correlations of pEV levels, but not albuminuria, with renal function again support pEVs as markers of renal damage in hypertension. Taken together, our findings are consistent with podocyte damage as a component of kidney injury beyond a stenotic lesion in human RVH.
EVs might be released from different cell types along the nephron, such as (proximal or distal) tubular cells and podocytes [6, 14, 34–38]. Because analysis of urinary EVs may provide a noninvasive window into the physiological and pathological state of the kidney, this approach is attractive as a potential site-specific renal ‘biopsy’. In addition to indicating a stress response, EVs carry a wide range of proteins and genetic information, such as micro-RNA [39], that might afford insight into disease course and prognosis. Alas, in the absence of standardized protocols for isolation and analysis of EVs [10], and given the heterogeneity of many EV preparations [11], the application of this tool is challenging. We also found lower urinary levels of CD31+ EVs in RVH than in the other groups, potentially reflecting microvascular and endothelial cell loss in the stenotic kidney. However, many common endothelial cell markers are expressed on a broad range of cell types [40], and this hypothesis-generating finding warrants further studies.
Podocyte-derived EVs can be identified by several markers, including Wilm's tumor-1 or nephrin [34]. In this study we used stringent criteria of double-positivity for podocalyxin and nephrin. Podocalyxin is a marker successfully used for identification of urinary podocytes, but it is not entirely podocyte specific, as it may also be expressed on nonrenal as well as glomerular endothelial and parietal epithelial cells. Hence an additional marker is recommended to define urinary podocytes [41]. On the other hand, nephrin is highly podocyte specific, but may be downregulated in proteinuric kidney disease [42]. Therefore, we used double-positivity for podocalyxin and nephrin expression in flow cytometry analysis. Notably, we observed similar differences among the groups based on single positivity of podocalyxin or nephrin, indicating the robustness of the pEV changes in RVH.
The fraction of pEVs in patients with EH was not different from that in HVs, suggesting relative preservation of podocytes in EH with preserved renal function. Hypertensive glomerular lesions are conventionally characterized by mesangial proliferation, matrix accumulation and glomerulosclerosis, and few studies have explored podocyte damage in hypertension [43, 44]. Nagase et al. [43] reported that podocyte injury in hypertension precedes glomerulosclerosis and is reversed by aldosterone blockade. We do not have direct histological evaluation of podocytes in EH in this study, but urinary microalbumin levels and eGFR of this group were within the normal range and blood pressure was relatively well controlled. Moreover, all EH patients were treated with ACEis or ARBs, which might protect podocytes [43, 45]. In concert with the unchanged levels of pEVs in EH, these notions argue against severe podocyte lesions in this group.
Atherosclerotic renal artery stenosis produces partial lumen occlusion in RVH, eventually lowering kidney perfusion and RBF [46]. Stenotic lesions not only induce hypoxia in the kidney, but also activate the renin–angiotensin–aldosterone system and induce oxidative stress, inflammation and microvascular rarefaction [16, 19], which might accelerate hypertension, podocyte damage and progressive renal dysfunction [22, 47]. Interestingly, in hypertensive patients, urinary pEV levels correlated inversely with STK RBF and renal cortical perfusion, but these relationships disappeared after adjustment for eGFR, possibly due to the close association of RBI and GFR. Presumably complex pathophysiologic mechanisms related to a decrease in renal function underlie the loss of urinary pEVs in RVH. Kalani et al. [34] reported that in diabetic patients, podocyte-derived exosomes negatively correlate with eGFR, and found an association with albuminuria and proteinuria. In our study, albuminuria did not show any association with other clinical parameters or with pEVs, possibly because the albuminuria of our study groups was within a normal range. Thus, urinary pEV levels in hypertension might provide an early marker of kidney injury independent of albuminuria.
Technical issues involving EV isolation are challenging. We used a commercial isolation kit, which avoids laborious ultracentrifugation and the need for large urine volumes, and is also suitable for extraction of RNA and DNA [33, 48–51], which might make it practical in the clinical setting. However, the cost of isolation kits is not negligible, especially when applied to a large number of samples [35]. Additionally, this method may cause coprecipitation of the most abundant soluble proteins, such as albumin and Tamm–Horsfall glycoprotein. Although total protein levels of our samples were nearly normal, and serum protein does not affect EV purity with the commercial kit [52], the effect of slightly greater proteinuria in RVH on EV numbers is uncertain. In addition, centrifugation and discarding the supernatant are necessary steps to isolate EVs, but might result in loss of EVs from the samples. Given that quantification of the number of EVs shows moderate reproducibility [52, 53], we expressed pEV as a fraction of total EV. Indeed, longitudinal measurement of pEV levels in treated RVH patients demonstrated the reproducibility of our approach.
Recent randomized clinical trials show that revascularization adds little additional benefit with respect to recovery of kidney function [23]. The failure of revascularization in the RVH group to affect pEV levels is consistent with our previous study [22], showing no decrease in renal vein levels of inflammatory markers in the stented kidney. Although the number of stented patients was small, our findings underscore the concept that revascularization alone does not suffice to restore kidney structure and function.
Our study has some limitations. First, our cohort is relatively small, thus follow-up data and large studies are needed to confirm our results. Second, we could not compare directly pEVs with podocyte histology, and our inferences of podocyte damage in RVH are indirect. Furthermore, given that urine samples derive from both kidneys, it is difficult to ascribe pEV levels specifically to the stenotic kidney. While the precise podocyte stress cannot be determined in this study, the comparable blood pressure volumes in RVH and EH patients argue against hypertensive renal injury as the predominant cause of elevated urinary pEV levels in RVH. Third, the present study cannot link higher pEV counts to progression of kidney disease, which would require longitudinal studies. Indeed, our findings should be tested in other cohorts. Finally, the urine samples from the HVs were obtained in untimed collection. Although useful to assess proteinuria [54], their compatibility to timed urine collection is limited.
In conclusion, we observed elevated urinary levels of podocyte-derived EVs in RVH patients compared with EH and HVs, which might be related to intrarenal injury distal to renal artery stenosis. EVs might be promising markers of podocyte and early glomerular damage in hypertension. Future studies are needed to evaluate and validate the role of podocyte-derived EVs in hypertension and other diseases and their utility for monitoring success of therapy.
ACKNOWLEDGEMENTS
This study was partly supported by NIH grants DK100081, DK73608, HL123160, DK104273 and DK102325.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54: 687–697 [DOI] [PubMed] [Google Scholar]
- 2. Kriz W, Shirato I, Nagata M et al. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 2013; 304: F333–F347 [DOI] [PubMed] [Google Scholar]
- 3. Garovic VD, Wagner SJ, Turner ST et al. Urinary podocyte excretion as a marker for preeclampsia. Am J Obstet Gynecol 2007; 196: 320 e321–327 [DOI] [PubMed] [Google Scholar]
- 4. Craici IM, Wagner SJ, Bailey KR et al. Podocyturia predates proteinuria and clinical features of preeclampsia: longitudinal prospective study. Hypertension 2013; 61: 1289–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meyer TW, Bennett PH, Nelson RG. Podocyte number predicts long-term urinary albumin excretion in Pima Indians with type II diabetes and microalbuminuria. Diabetologia 1999; 42: 1341–1344 [DOI] [PubMed] [Google Scholar]
- 6. Burger D, Thibodeau JF, Holterman CE et al. Urinary podocyte microparticles identify prealbuminuric diabetic glomerular injury. J Am Soc Nephrol 2014; 25: 1401–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viberti G, Mogensen CE, Groop LC et al. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 1994; 271: 275–279 [PubMed] [Google Scholar]
- 8. Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct 2006; 35: 277–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Regev-Rudzki N, Wilson DW, Carvalho TG et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 2013; 153: 1120–1133 [DOI] [PubMed] [Google Scholar]
- 10. van der Pol E, Boing AN, Harrison P et al. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev 2012; 64: 676–705 [DOI] [PubMed] [Google Scholar]
- 11. Witwer KW, Buzas EI, Bemis LT et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013; 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayers L, Nieuwland R, Kohler M et al. Dynamic microvesicle release and clearance within the cardiovascular system: triggers and mechanisms. Clin Sci (Lond) 2015; 129: 915–931 [DOI] [PubMed] [Google Scholar]
- 13. Milane L, Singh A, Mattheolabakis G et al. Exosome mediated communication within the tumor microenvironment. J Control Release 2015; 219: 278–294 [DOI] [PubMed] [Google Scholar]
- 14. Zhou H, Kajiyama H, Tsuji T et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. Am J Physiol Renal Physiol 2013; 305: F553–F559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gloviczki ML, Lerman LO, Textor SC. Blood oxygen level-dependent (BOLD) MRI in renovascular hypertension. Curr Hypertens Rep 2011; 13: 370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lerman LO, Nath KA, Rodriguez-Porcel M et al. Increased oxidative stress in experimental renovascular hypertension. Hypertension 2001; 37: 541–546 [DOI] [PubMed] [Google Scholar]
- 17. Lerman LO, Taler SJ, Textor SC et al. Computed tomography-derived intrarenal blood flow in renovascular and essential hypertension. Kidney Int 1996; 49: 846–854 [DOI] [PubMed] [Google Scholar]
- 18. Khangura KK, Eirin A, Kane GC et al. Cardiac function in renovascular hypertensive patients with and without renal dysfunction. Am J Hypertens 2014; 27: 445–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eirin A, Gloviczki ML, Tang H et al. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J 2013; 34: 540–548a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gloviczki ML, Keddis MT, Garovic VD et al. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol 2013; 8: 546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gloviczki ML, Glockner JF, Crane JA et al. Blood oxygen level-dependent magnetic resonance imaging identifies cortical hypoxia in severe renovascular disease. Hypertension 2011; 58: 1066–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Saad A, Herrmann SM, Crane J et al. Stent revascularization restores cortical blood flow and reverses tissue hypoxia in atherosclerotic renal artery stenosis but fails to reverse inflammatory pathways or glomerular filtration rate. Circ Cardiovasc Interv 2013; 6: 428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cooper CJ, Murphy TP, Cutlip DE et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med 2014; 370: 13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daghini E, Juillard L, Haas JA et al. Comparison of mathematic models for assessment of glomerular filtration rate with electron-beam CT in pigs. Radiology 2007; 242: 417–424 [DOI] [PubMed] [Google Scholar]
- 26. Krier JD, Ritman EL, Bajzer Z et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol 2001; 281: F630–F638 [DOI] [PubMed] [Google Scholar]
- 27. Chade AR, Rodriguez-Porcel M, Grande JP et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation 2002; 106: 1165–1171 [DOI] [PubMed] [Google Scholar]
- 28. Daghini E, Primak AN, Chade AR et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology 2007; 243: 405–412 [DOI] [PubMed] [Google Scholar]
- 29. Lerman LO, Bell MR, Lahera V et al. Quantification of global and regional renal blood flow with electron beam computed tomography. Am J Hypertens 1994; 7: 829–837 [DOI] [PubMed] [Google Scholar]
- 30. Jayachandran M, Lugo G, Heiling H et al. Extracellular vesicles in urine of women with but not without kidney stones manifest patterns similar to men: a case control study. Biol Sex Differ 2015; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turco AE, Lam W, Rule AD et al. Specific renal parenchymal-derived urinary extracellular vesicles identify age-associated structural changes in living donor kidneys. J Extracell Vesicles 2016; 5: 29642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rivera J, Cordero RJ, Nakouzi AS et al. Bacillus anthracis produces membrane-derived vesicles containing biologically active toxins. Proc Natl Acad Sci USA 2010; 107: 19002–19007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zubiri I, Posada-Ayala M, Sanz-Maroto A et al. Diabetic nephropathy induces changes in the proteome of human urinary exosomes as revealed by label-free comparative analysis. J Proteomics 2014; 96: 92–102 [DOI] [PubMed] [Google Scholar]
- 34. Kalani A, Mohan A, Godbole MM et al. Wilm's tumor-1 protein levels in urinary exosomes from diabetic patients with or without proteinuria. PLoS One 2013; 8: e60177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Musante L, Tataruch DE, Holthofer H. Use and isolation of urinary exosomes as biomarkers for diabetic nephropathy. Front Endocrinol (Lausanne) 2014; 5: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dimuccio V, Ranghino A, Pratico Barbato L et al. Urinary CD133+ extracellular vesicles are decreased in kidney transplanted patients with slow graft function and vascular damage. PLoS One 2014; 9: e104490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Moon PG, Lee JE, You S et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics 2011; 11: 2459–2475 [DOI] [PubMed] [Google Scholar]
- 38. Hara M, Yanagihara T, Kihara I et al. Apical cell membranes are shed into urine from injured podocytes: a novel phenomenon of podocyte injury. J Am Soc Nephrol 2005; 16: 408–416 [DOI] [PubMed] [Google Scholar]
- 39. Miranda KC, Bond DT, McKee M et al. Nucleic acids within urinary exosomes/microvesicles are potential biomarkers for renal disease. Kidney Int 2010; 78: 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Middleton J, Americh L, Gayon R et al. A comparative study of endothelial cell markers expressed in chronically inflamed human tissues: MECA-79, Duffy antigen receptor for chemokines, von Willebrand factor, CD31, CD34, CD105 and CD146. J Pathol 2005; 206: 260–268 [DOI] [PubMed] [Google Scholar]
- 41. Skoberne A, Konieczny A, Schiffer M. Glomerular epithelial cells in the urine: what has to be done to make them worthwhile? Am J Physiol Renal Physiol 2009; 296: F230–F241 [DOI] [PubMed] [Google Scholar]
- 42. Garovic VD, Wagner SJ, Petrovic LM et al. Glomerular expression of nephrin and synaptopodin, but not podocin, is decreased in kidney sections from women with preeclampsia. Nephrol Dial Transplant 2007; 22: 1136–1143 [DOI] [PubMed] [Google Scholar]
- 43. Nagase M, Shibata S, Yoshida S et al. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 2006; 47: 1084–1093 [DOI] [PubMed] [Google Scholar]
- 44. Kretzler M, Koeppen-Hagemann I, Kriz W. Podocyte damage is a critical step in the development of glomerulosclerosis in the uninephrectomised-desoxycorticosterone hypertensive rat. Virchows Arch 1994; 425: 181–193 [DOI] [PubMed] [Google Scholar]
- 45. Matsusaka T, Asano T, Niimura F et al. Angiotensin receptor blocker protection against podocyte-induced sclerosis is podocyte angiotensin II type 1 receptor-independent. Hypertension 2010; 55: 967–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kwon SH, Lerman LO. Atherosclerotic renal artery stenosis: current status. Adv Chronic Kidney Dis 2015; 22: 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Textor SC, Lerman LO. Paradigm shifts in atherosclerotic renovascular disease: where are we now? J Am Soc Nephrol 2015; 26: 2074–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rekker K, Saare M, Roost AM et al. Comparison of serum exosome isolation methods for microRNA profiling. Clin Biochem 2014; 47: 135–138 [DOI] [PubMed] [Google Scholar]
- 49. Zeringer E, Li M, Barta T et al. Methods for the extraction and RNA profiling of exosomes. World J Methodol 2013; 3: 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schageman J, Zeringer E, Li M et al. The complete exosome workflow solution: from isolation to characterization of RNA cargo. Biomed Res Int 2013; 2013: 253957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li M, Rai AJ, Joel DeCastro G et al. An optimized procedure for exosome isolation and analysis using serum samples: application to cancer biomarker discovery. Methods 2015; 87: 26–30 [DOI] [PubMed] [Google Scholar]
- 52. Caradec J, Kharmate G, Hosseini-Beheshti E et al. Reproducibility and efficiency of serum-derived exosome extraction methods. Clin Biochem 2014; 47: 1286–1292 [DOI] [PubMed] [Google Scholar]
- 53. Mitchell PJ, Welton J, Staffurth J et al. Can urinary exosomes act as treatment response markers in prostate cancer? J Transl Med 2009; 7: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(2 Suppl 1): S1–S266 [PubMed] [Google Scholar]