Abstract
Background: When vaccinations with vaccinia against smallpox and Bacillus Calmette-Guérin (BCG) against tuberculosis were phased out in some high-income countries around 1980, the impact on overall mortality was not examined. Recent studies from low-income countries have suggested that these vaccines are associated with mortality reductions, not explained by specific disease protection. We examined whether vaccinia and BCG administered in childhood were associated with long-term mortality reductions in a high-income population.
Methods: In this case-cohort study, we followed 47 622 schoolchildren from Copenhagen, Denmark, born 1965 to 1976, from their first health examination to 2010. This cohort experienced the phase-out of vaccinia and BCG vaccination programmes.
Results: A sub-cohort of 5 316 individuals (699 excluded) was followed for 164 450 person-years (0.2% were lost to follow-up), and 401 deaths due to natural causes (841 deaths in total) occurred in the full cohort. Compared with individuals who had not received vaccinia or BCG, those who had received both vaccinia and BCG had an adjusted hazard ratio (aHR) of 0.54 [95% confidence interval (CI): 0.36–0.81] for mortality due to natural causes of death; those who only received BCG had an aHR of 0.58 (95% CI: 0.39–0.85). Vaccinia and BCG were not associated with any protection against deaths by accidents, suicide or murder, the combined aHR being 0.94 (95% CI: 0.62–1.42).
Conclusions: Vaccinia and BCG vaccinations were associated with better long-term survival, which was not explained by specific protection. Vaccines with beneficial non-specific effects may reduce overall mortality even after the target diseases are eradicated.
Keywords: BCG vaccine, heterologous immunity, mortality, non-specific effects of vaccines, smallpox vaccine, vaccinia
Introduction
The last case of smallpox was diagnosed in 1977 and, in 1980, vaccinia vaccination was stopped globally. In the same period, several high-income countries phased out the Bacillus Calmette-Guérin vaccine (BCG) against tuberculosis since tuberculosis was no longer a major health problem. No investigations, however, examined whether there were effects on overall morbidity or mortality levels from stopping these vaccinations.
Strong indications of non-specific effects (NSE) of vaccines were discovered in Guinea-Bissau 25 years ago.1 Numerous studies have now found that live attenuated vaccines including vaccinia, BCG, measles and oral polio vaccine (OPV) reduce mortality more than expected from specific prevention; hence, these vaccines have beneficial NSE, presumably through immune training.2,3 In 2014, the World Health Organization (WHO) recommended further research on NSE of vaccines.4 If vaccines have beneficial NSE, it may have negative public health consequences to stop a vaccination programme. This possibility requires urgent testing because the world is about to eradicate polio, measles and rubella.5,6 If eradicated, the live vaccines against these diseases will be scaled down, stopped or replaced with inactivated vaccines. Studies of NSE have primarily examined children, but studies in adults also found that vaccinia and BCG vaccinations were associated with lower mortality.7–9
In Denmark, individuals born between 1965 and 1976 experienced a phase-out of the compulsory vaccinia vaccination given before entering school and of the free of charge BCG vaccination offered in the first grades of school (Supplementary Text 1, available as Supplementary data at IJE online). With information on vaccinia and BCG vaccination from the frequent school health examinations and with follow-up to the mid life of the individuals using Danish national registers, we tested whether vaccinia and BCG vaccinations are associated with lower mortality due to natural causes of death in Denmark.
Methods
Design, setting and study population
This case-cohort study is based on 47 622 schoolchildren registered in the Copenhagen School Health Record Register (CSHRR),10 born 1965–76. This cohort grew up when vaccinia (compulsory before school entry) and BCG (recommended at school entry) were phased out (Supplementary Text 1). This type of study does not require ethical approval by the Danish Central Scientific Ethics Committee. Data access permission was granted by the Danish Protection Agency (ref: 2015–41–3976).
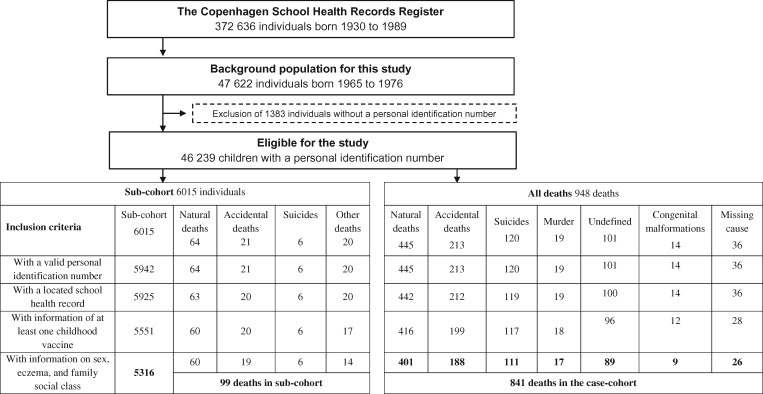
From the CSHRR we selected a 10% random sample of children within strata of sex and year of birth and also all children born on the first day of every month. Hence, the sub-cohort covered 13% of the overall population. The sub-cohort was to be used in different studies investigating different health outcomes. When the failure proportion is <1%, then a sub-cohort of 10% or larger has been shown to be highly efficient.11 Information on vaccinations, health and social conditions was computerized from the original records as described and analysed in previous studies on NSE.12–14 We restricted the analyses to individuals with a valid personal identification number and full information on vaccinations and identified confounders (Figure 1).
Figure 1.
Flow chart of the study population. Cause of death categories defined in Supplementary Table 1. The high ratio of accidents to natural deaths may be because the cohort is only followed up during the first half of their lives.
Exposure and co-variables
Children, regardless of whether they were cases or in the sub-cohort, were regarded as unvaccinated if this was indicated or alternatively if they lacked information on a particular vaccine but had information on other early childhood vaccinations in their health record. We excluded children with no information of any received childhood vaccination. Vaccinia and BCG vacccinations were analysed in four groups (no vaccinia or BCG; vaccinia only; BCG only; both BCG and vaccinia) and as any of these vaccines versus none of them. The available additional variables were sex, eczema (no information; yes), family social class based on parental occupation and/or education at school entry of the child [I (highest); II; III; IV; V; unclassified), number of siblings (none; 1; 2; 3+), day-care before school entry, birth by caesarean section and immigration status.
Outcomes
In 1968, all residents in Denmark and those born thereafter were assigned a personal identification number by law, which enables linkage of registers. We linked the CSHRR to the Danish Civil Registration System15 to assess vital status and to the Danish Register of Causes of Death16 to assess causes of deaths. Causes of death are coded according to the WHO's International Classification of Diseases (ICD) version 8 (1970–93) and version 10 (from 1994).16
The main outcome was natural deaths, with exclusion of smallpox and tuberculosis deaths (vaccine-preventable causes) and congenital malformations, as these could have affected both vaccination status and mortality (Supplementary Table 1, available as Supplementary data at IJE online). We hypothesized that vaccination status was associated with natural deaths, but not with accidental deaths, suicides or murders (Supplementary Table 1). The latter three causes were used as control outcomes to ensure that there were no general associations between vaccination status and any cause of death. Alcohol or drug addiction was often listed as a secondary cause of death. We therefore conducted an analysis of a possible association between vaccination status and deaths related to addiction as an additional control analysis (Supplementary text 2, available as Supplementary data at IJE online).
Statistical analysis
Adjusted binomial regression was used to calculate risk ratios for vaccination with vaccinia and BCG, respectively, according to background factors. Hazard ratios (HRs) for mortality were estimated using the Cox proportional hazards model with robust variance estimation to account for the case-cohort design.17 Age was the time scale and analyses were stratified by year of birth. Children entered the study at their first school health examination (earliest 1971). If the date of first school health examination was missing (53 children in sub-cohort), the child was given the median entry-age for all children.
Children changed vaccination status on the date of their registered vaccinations. Children with missing vaccination dates but confirmed vaccination (262 children in sub-cohort) entered the study at their last health examination. Natural deaths not in the sub-cohort were included 1 day before the individual’s death using Prentice’s method for case-cohort studies, where cases outside the sub-cohort add to the numerator but do not add person-time,18 which has been shown to produce reliable estimates.17 End of follow-up was death from natural causes or censoring (emigration, non-natural cause of death, loss-to-follow-up or 25 February 2010), whichever occurred first. The proportional hazards assumption was investigated by testing if the HR differed by age (<20/20–30/>30 years). Sex, eczema and family social class were assumed necessary co-variables to adjust for confounding using a causal diagram.
We investigated whether sex, eczema, and family social class were effect modifiers. We also investigated heterogeneity of effects by age intervals [<20/20–30/>30 years], age at vaccination [0–3/3–6/6–9/>9 years), time since vaccination [0–10/10–20/20–30/>30 years] and birth cohort. Birth cohorts were divided into 1965–68 (vaccinia and BCG coverage >80%), 1969–72 (coverage 20–80%) and 1973–76 (coverage <20%). We assessed the HRs for major disease categories of natural causes of death.
As an alternative way of testing the robustness of our estimates, we used stabilized inverse probability of treatment weighting (IPTW) via exact matching to estimate the average treatment effect on the treated. We applied method C by Månsson et al. for case-cohort studies.19 Another sensitivity analysis was conducted by restricting the study population to only children born in the highest family social class, for a more homogeneous study population. In a third sensitivity analysis, we analysed the association between vaccination status and death due to natural causes only within the sub-cohort, ignoring the cases outside the sub-cohort.
All statistical analyses were conducted using Stata 13.1 (StataCorp LP, College Station, TX). We used R (a language and environment for statistical computing: R Foundation for Statistical Computing, Vienna) for data visualization.
Results
The sub-cohort consisted of 5316 children after excluding 699 due to missing health records, exclusion of children with no childhood vaccinations or missing information on sex, eczema or family social class (Figure 1). Children with missing information were less vaccinated with vaccinia and BCG, more were males, less had eczema and they had a lower social class (Supplementary Table 2, available as Supplementary data at IJE online). The sub-cohort was followed for 164 450 person-years with a median of 32.1 person-years, and it included 99 deaths (60 being due to natural causes); 386 (7.3%) emigrated and 11 (0.2%) were lost to follow-up.
In the background cohort, 948 deaths occurred. Health records with full information about vaccination and necessary co-variables were obtained for 841 deaths (Figure 1); 401 deaths had natural causes (47.7%), 188 were accidents (22.4%), 111 suicides (13.2%), 17 murders (2.0%), 89 were classified as undefined (10.6%), 26 were missing (3.1%), and 9 were due to congenital malformations (Supplementary Table 3, available as Supplementary data at IJE online) (1.1%). There were no smallpox or tuberculosis deaths.
Vaccination coverage decreased in individuals born from 1965 to 1976 (Supplementary Figure 1, available as Supplementary data at IJE online). Vaccinia was given at 1 to 8 years of age and was rapidly phased out from 1976 to 1977 (Supplementary Figure 2, available as Supplementary data at IJE online). BCG was mostly given at school entry and in the spring (Supplementary Figure 3, available as Supplementary data at IJE online); few children were BCG vaccinated in 1978 (Supplementary Figure 3).
Vaccinia coverage was higher among girls than boys (58.6% versus 55.0%, P-value = 0.007) but a difference was not apparent for BCG (Table 1). Eczema was a contraindication for vaccinia, and children with reported eczema had a lower prevalence of vaccinia and BCG compared with those without eczema (Table 1). Social class V and unclassified tended to be less vaccinated compared with social class I, but the remaining groups showed no obvious trends (Table 1). The distribution of family social class among the BCG-vaccinated followed the general change throughout the discontinuation period (Supplementary Table 4).
Table 1.
Distribution of exposure variables and vaccination status at the last school health examination among the sub-cohort
| Vaccinia at the last school health examinationa |
BCG at the last school health examinationa |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Yes | RRb | Joint chi square test, P-value | No | Yes | RRb | Joint chi square test, P-value | ||
| n = 2298 | n = 3018 | (95% CI) | n = 1227 | n = 4089 | (95% CI) | ||||
| Sex | |||||||||
| Females | 42.4% (1090) | 58.6% (1544) | 1 (ref) | 22.9% (604) | 77.1% (2030) | 1 (ref) | |||
| Males | 45.0% (1208) | 55.0% (1474) | 0.98 (0.96–1.00) | 23.2% (623) | 76.7% (2059) | 0.99 (0.97–1.02) | |||
| Eczema | |||||||||
| Not reported | 42.2% (2113) | 57.8% (2889) | 1 (ref) | 22.5% (1127) | 77.5% (3875) | 1 (ref) | |||
| Yes | 58.9% (185) | 41.1% (129) | 0.89 (0.82–0.96) | 31.9% (100) | 68.2% (214) | 0.92 (0.86–0.99) | |||
| Family social class | |||||||||
| I | 51.4% (196) | 48.6% (185) | 1 (ref) | 0.01 | 29.4% (112) | 70.6% (269) | 1 (ref) | 0.002 | |
| II | 46.9% (317) | 53.1% (359) | 1.01 (0.95–1.07) | 20.4% (138) | 79.6% (538) | 1.04 (0.98–1.11) | |||
| III | 41.4% (368) | 58.6% (520) | 1.01 (0.96–1.06) | 22.1% (196) | 77.9% (692) | 1.00 (0.95–1.06) | |||
| IV | 40.7% (760) | 59.3% (1108) | 1.02 (0.97–1.07) | 21.8% (408) | 78.2% (1460) | 1.01 (0.96–1.07) | |||
| V | 40.9% (478) | 59.1% (691) | 0.98 (0.93–1.03) | 24.0% (280) | 76.1% (889) | 0.97 (0.91–1.02) | |||
| Unclassified | 53.6% (179) | 46.4% (155) | 0.91 (0.84–0.99) | 27.8% (93) | 72.2% (241) | 0.94 (0.87–1.01) | |||
aIn this table, the distribution of vaccination status follows the status at the last health examination. In the Cox proportional hazards analyses, individuals are allowed to change vaccination status (except as in Supplementary Table 7).
bRisk ratios (RR) are calculated with binomial regression and adjusted for other variables in the table as well as birth cohort from 1965–68, 1969–72 and 1973–76.
The mortality rate was higher among males than females (aHR = 1.63 (95% CI: 1.32–2.01)), and individuals with a lower social class tended to have higher mortality rates. Eczema was not associated with higher mortality (Supplementary Table 5, available as Supplementary data at IJE online).
Vaccinia and BCG vaccination and death due to natural causes
More than 50% of follow-up time came from individuals who had received both vaccinia and BCG, and this combination was associated with lower mortality, the HR adjusted for year of birth, age, sex, eczema and family social class (aHR) being 0.54 (95% CI: 0.36–0.81) compared with individuals who had not received these vaccines (Table 2). Around 25% of follow-up time came from individuals who had received only BCG, and they had an aHR of 0.58 (95% CI: 0.39–0.85) compared with non-vaccinia/non-BCG-vaccinated individuals. Individuals with vaccinia only contributed 8% of the follow-up time and had an aHR of 0.75 (95% CI: 0.47–1.22). Adjusting for all co-variables, the estimates remained virtually unchanged (Supplementary Table 6). Consistent with these results, the alternative analyses using the IPTW approach showed beneficial effect estimates for both vaccinia and BCG (Supplementary Table 7, available as Supplementary data at IJE online). Conducting the analysis only for the sub-cohort revealed similar estimates for having received both vaccinia and BCG (Supplementary Table 8, available as Supplementary data at IJE online). Results were similar when the analysis was restricted to individuals from the highest family social class (Supplementary Table 9, available as Supplementary data at IJE online).
Table 2.
Associations between vaccination status and deaths due to natural causes, overall and by sex
| Natural deathsan = 401 (60 in sub-cohort) | Sub-cohort person-years (164 450) | Sub-cohort according to latest vaccine status (n) | Crude age- adjusted hazard ratiob (95% CI) | Adjusted hazard ratioc (95% CI) | ||
|---|---|---|---|---|---|---|
| Vaccinia and BCG | ||||||
| None | 53 (8) | 24 414 | 840 | 1 (ref) | 1 (ref) | |
| Only vaccinia | 44 (6) | 13 558 | 387 | 0.72 (0.45–1.17) | 0.75 (0.47–1.22) | |
| Only BCG | 65 (15) | 40 861 | 1458 | 0.58 (0.39–0.85) | 0.58 (0.39–0.85) | |
| Both vaccinia and BCG | 239 (31) | 85 618 | 2631 | 0.51 (0.34–0.76) | 0.54 (0.36–0.81) | |
| Vaccinia and/or BCG | 348 (52) | 140 036 | 4476 | 0.56 (0.39–0.79) | 0.57 (0.40–0.81) | |
| Females, vaccinia and BCG | ||||||
| None | 16 (2) | 11 797 | 406 | 1 (ref) | 1 (ref) | |
| Only vaccinia | 20 (4) | 6877 | 198 | 1.03 (0.50–2.12) | 1.05 (0.51–2.15) | |
| Only BCG | 25 (9) | 19 162 | 684 | 0.77 (0.40–1.49) | 0.79 (0.41–1.52) | |
| Both vaccinia and BCG | 89 (14) | 43 881 | 1346 | 0.60 (0.33–1.09) | 0.62 (0.34–1.14) | |
| Vaccinia and/or BCG | 134 (27) | 69 920 | 2228 | 0.68 (0.39–1.20) | 0.71 (0.40–1.24) | |
| Males, vaccinia and BCG | ||||||
| None | 37 (6) | 12 617 | 434 | 1 (ref) | 1 (ref) | |
| Only vaccinia | 24 (2) | 6680 | 189 | 0.60 (0.33–1.09) | 0.61 (0.34–1.11) | |
| Only BCG | 40 (6) | 21 699 | 774 | 0.49 (0.30–0.78) | 0.49 (0.30–0.78) | |
| Both vaccinia and BCG | 150 (17) | 41 738 | 1285 | 0.49 (0.30–0.78) | 0.50 (0.31–0.80) | |
| Vaccinia and/or BCG | 214 (25) | 70 116 | 2248 | 0.51 (0.34–0.77) | 0.52 (0.34–0.78) | |
| Test of interaction between vaccinia and/or BCG versus none and sex, P-value | 0.37 | 0.35 | ||||
aVaccinia- and BCG-vaccinated individuals were on average born earlier than the non-vaccinated individuals and were therefore older and experienced higher mortality rates towards the end of follow-up. All hazard ratios adjust for age by stratifying for year of birth.
bStratified by year of birth.
cStratified by year of birth and adjusted for sex, eczema and family social class.
For infectious diseases, cardiovascular diseases, neurological diseases, major autoimmune diseases and other diseases, the aHR was approximately 0.5 for individuals having received vaccinia and/or BCG compared with not having received vaccinia or BCG, and the aHR was approximately 1 for cancers (Table 3).
Table 3.
Associations between vaccination status and major disease categories
| Vaccinia and/or BCG versus neither vaccinia nor BCG | Deaths, unvaccinated (sub-cohort) | Deaths, vaccinated (sub-cohort) | Crude age-adjusted hazard ratioa (95% CI), vaccinia and/or BCG versus none | Adjusted hazard ratiob (95% CI), vaccinia and/or BCG versus none |
|---|---|---|---|---|
| All causes of natural deaths | 53 (8) | 348 (52) | 0.56 (0.39–0.79) | 0.57 (0.40–0.81) |
| Cancers | 13 (1) | 120 (18) | 1.02 (0.54–1.92) | 1.02 (0.54–1.92) |
| Cardiovascular diseases | 11 (2) | 51 (6) | 0.36 (0.19–0.70) | 0.37 (0.19–0.72) |
| Infectious diseases | 5 (2) | 28 (4) | 0.46 (0.15–1.42) | 0.49 (0.16–1.53) |
| Neurological diseases | 4 (0) | 14 (3) | 0.22 (0.05–0.92) | 0.23 (0.06–0.93) |
| Major autoimmune diseases | 2 (1) | 12 (1) | 0.30 (0.05–1.64) | 0.39 (0.07–1.99) |
| Other diseases | 18 (2) | 123 (20) | 0.51 (0.27–0.96) | 0.53 (0.28–0.98) |
aStratified by year of birth.
bStratified by year of birth and adjusted for sex, eczema and family social class.
Cancers: ICD-8 (149.0, 151.9, 153.1, 155.0, 158.0, 160.2, 163.1, 170.3, 170.7, 170.8, 170.9, 171.1, 172.7, 180.0, 186.0, 191.0, 192.5, 202.2, 204.0, 205.0, 205.1, 207.0), ICD-10 (C02.9, C06.9, C11.9, C13.9, C15.9, C16.9, C18.0, C18.6, C18.7, C18.9, C20.9, C22.1, C22.2, C23.9, C25.9, C34.9, C43.8, C43.9, C49.9, C50.8, C50.9, C51.9, C53.8, C53.9, C55.9, C56.9, C62.9, C70.0, C71.2, C71.9, C74.0, C74.9, C80.9, C81.9, C83.5, C85.1, C85.9, C91.0, C92.0, C92.1, C92.3, D43.2, D48.9). Cardiovascular diseases: ICD-8 (424.1, 427.2, 428.0, 430.0, 430.9, 433.9, 441.0), ICD-10 (I21.0, I21.3, I21.9, I25.1, I25.9, I26.9, I31.9, I38.9, I42.0, I42.1, I45.6, I46.0, I46.9, I47.1, I51.7, I60.7, I60.9, I61.0, I61.3, I61.5, I61.9, I62.0, I63.3, I63.9, I64.9, I70.9, I72.9, I82.9). Infectious diseases: ICD-8 (036.1, 066.0, 320.1, 464.0, 481.0) ICD-10 (A36.8, A40.0, A41.2, B19.9, B20.3, B20.6, B20.8, B23.8, B24.9, G00.1, G03.0, I30.1, I80.2, J04.0, J13.9, J15.0, J18.9, J42.9, K85.9, O86.0). Neurological diseases: ICD-8 (330.3, 330.9, 340.0, 345.9, 347.9), ICD-10 (G35.9, G40.9, G71.0, G93.4, R54.9). Major autoimmune diseases: ICD-8 (563.0, 734.1), ICD-10 (D82.9, D86.0, E10.0, E10.7, E14.0, E14.1, I05.9, I10.9, M32.9). Other diseases: ICD-8 (079.7, 225.2, 225.3, 227.0, 273.0,,279.0, 303.8, 571.0, 582.0, 631.4), ICD-10 (D32.9, D35.2, D35.3, D61.9, D72.1, E11.9, E27.1, E28.2, E66.9, E84.0, E84.1, E84.8, E88.0, F10.1, F10.2, F14.1, F19.1, F19.2, F20.0, F20.9, J44.9, J45.9, K70.0, K70.1, K70.3, K70.4, K70.9, K72.9, K74.6, K76.7, K80.8, K86.0, N18.9, N19.9, O95.9, R98.9, R99.0, R99.9). Of these, 079.7, 149.0, 186.0, 303.8 and 428.0 were not identified as ICD-8 codes. We assumed they were mistakenly entered as ICD-9 codes.
Potential effect modification
The effect of vaccinia and/or BCG was the same by sex (P-value for interaction = 0.35) (Table 2). Vaccinia and/or BCG versus none was associated with lower mortality for individuals with and without eczema, the beneficial effect being stronger for individuals with eczema [aHR 0.24 (95% CI: 0.09–0.61)] than individuals without eczema [aHR 0.64 (95% CI: 0.44–0.93)] (P-value for interaction = 0.05) (Supplementary Table 10, available as Supplementary data at IJE online). Social class did not modify the association (Supplementary Table 10).
Individuals who had received both vaccinia and BCG had similar beneficial effects with increasing age (Table 4). The age at BCG vaccination was associated with mortality from natural causes (Supplementary Table 11, available as Supplementary data at IJE online). Time since vaccination did not influence the association (Supplementary Table 11). The beneficial effects of vaccinia and BCG may have been strongest for the birth cohorts 1965–68 and 1973–76 (Table 4), but tests of different effect estimates all gave P-values over 0.1. When disregarding the last 4 birth years of the discontinuation period, where potential selection biases for BCG vaccination would have had the largest effect, combined vaccinia and BCG vaccination was still associated with an aHR of 0.59 (CI 95%: 0.36–0.96) (Supplementary Table 12, available as Supplementary data at IJE online). The IPTW analysis suggested that the lower effect in the 1969–72-birth cohort was not due to BCG, but vaccinia had no beneficial effect in this birth cohort (Supplementary Table 7).
Table 4.
Associations between vaccination status and deaths due to natural causes, stratified by age group and birth cohort
| Natural deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Natural deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Natural deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Test for interaction, P-value | ||
|---|---|---|---|---|---|---|---|---|
| Up to 20 years old | Age 20 to 30 years | 30 years and above (max 45 years) | ||||||
| Vaccinia and BCG | ||||||||
| None | 9 (3) | 1 (ref) | 25 (3) | 1 (ref) | 19 (2) | 1 (ref) | ||
| Only vaccinia | 8 (2) | 1.73 (0.60–5.04) | 7 (2) | 0.42 (0.17–1.05) | 29 (2) | 0.85 (0.43–1.66) | 0.13 | |
| Only BCG | 8 (2) | 0.55 (0.22–1.37) | 31 (4) | 0.42 (0.22–0.79) | 36 (9) | 0.76 (0.43–1.35) | 0.38 | |
| Both vaccinia and BCG | 25 (5) | 0.87 (0.32–2.35) | 52 (7) | 0.43 (0.22–0.84) | 162 (19) | 0.59 (0.33–1.08) | 0.50 | |
| Vaccinia and/or BCG | 41 (9) | 0.72 (0.33–1.58) | 80 (13) | 0.42 (0.24–0.74) | 227 (30) | 0.68 (0.40–1.17) | 0.38 | |
|
1965–68 |
1969–72 |
1973–76 |
||||||
| Vaccinia and BCG | ||||||||
| None | 11 (4) | 1 (ref) | 13 (0) | 1 (ref) | 29 (4) | 1 (ref) | ||
| Only vaccinia | 32 (3) | 0.51 (0.24–1.09) | 11 (3) | 1.14 (0.50–2.62) | 1 (0) | 0.48 (0.06–3.61) | 0.35 | |
| Only BCG | 14 (3) | 0.56 (0.23–1.32) | 34 (9) | 0.80 (0.41–1.54) | 17 (3) | 0.45 (0.24–0.85) | 0.47 | |
| Both vaccinia and BCG | 187 (20) | 0.35 (0.18–0.68) | 49 (19) | 0.97 (0.50–1.87) | 3 (1) | 0.51 (0.15–1.67) | 0.10 | |
| Vaccinia and/or BCG | 233 (26) | 0.38 (0.19–0.74) | 94 (22) | 0.91 (0.49–1.66) | 21 (4) | 0.46 (0.25–0.83) | 0.12 | |
aStratified by year of birth and adjusted for sex, eczema and family social class.
BCG and vaccinia vaccination and non-natural and addiction related causes of death
Deaths due to accidents, suicides and murders were not associated with vaccinia and BCG vaccinations, the combined aHR being 0.94 (95% CI: 0.62–1.42) (Table 5). Furthermore, vaccinia- and BCG-vaccinated individuals had the same risk of dying from alcohol or drug-related causes of deaths as vaccinia- and BCG-unvaccinated individuals, the aHR being 1.18 (CI 95%: 0.68–2.06) (Supplementary Text 2, available as Supplementary data at IJE online). Most undefined deaths were related to alcohol or drugs and ‘undefined and missing causes of death’ were therefore not linked to vaccination status, the combined aHR being 0.91 (CI 95%: 0.42–1.97) (Supplementary Table 13, available as Supplementary data at IJE online).
Table 5.
Associations between vaccination status and deaths due to accidents, suicide or murder
| Vaccinia and BCG | Sub-cohort person-years (164 450) | Sub-cohort according to latest vaccine status (n) | Accidents |
Suicides |
Murders |
Combined |
||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | Deaths (sub-cohort) | Adjusted hazard ratioa (95% CI) | |||
| None | 24 414 | 840 | 20 (1) | 1 (ref) | 13 (0) | 1 (ref) | 2 (0) | 1 (ref) | 35 (1) | 1 (ref) |
| Only vaccinia | 13 558 | 387 | 21 (1) | 1.31 (0.63–2.76) | 7 (1) | 0.69 (0.27–1.74) | 2 (0) | 1.23 (0.09–17.43) | 30 (2) | 1.08 (0.61–1.92) |
| Only BCG | 40 861 | 1458 | 41 (6) | 1.03 (0.60–1.78) | 18 (1) | 0.63 (0.29–1.38) | 3 (0) | 0.99 (0.07–14.09) | 62 (7) | 0.87 (0.55–1.38) |
| Both vaccinia and BCG | 85 618 | 2631 | 106 (11) | 1.04 (0.55–1.99) | 73 (4) | 0.99 (0.51–1.92) | 10 (1) | 0.88 (0.08–9.73) | 189 (16) | 1.02 (0.64–1.65) |
| Vaccinia and/or BCG | 140 036 | 4476 | 168 (18) | 1.06 (0.63–1.79) | 98 (6) | 0.76 (0.40–1.44) | 15 (1) | 0.97 (0.08–12.25) | 281 (25) | 0.94 (0.62–1.42) |
aStratified by year of birth and adjusted for sex, eczema and family social class.
Discussion
Vaccinia and BCG combined and BCG independently were associated with lower mortality due to natural causes compared with not receiving these vaccines. Vaccinia without BCG showed a similar tendency but this subgroup had limited follow-up time. Vaccinations were not associated with deaths due to accidents, suicide, murder or the misuse of alcohol or drugs.
Strengths and weaknesses
Information about vaccination status was collected systematically from standardized vaccination cards at school health examinations. The personal identification number ensured virtually complete follow-up to death or emigration.15 The prospective design precludes any effect of recall bias. A few BCG-vaccinated children may have been misclassified as ‘not vaccinated’ because late BCG vaccination after school entry might not have been noted (Supplementary Text 1). Parents could have forgotten the vaccination cards, resulting in a misclassification of both vaccinia and BCG status. Such misclassifications would lead to underestimation of any effect. Eczema was a known contraindication for vaccinia, but it did not explain why vaccinia and BCG were associated with a reduced risk of natural death.
The suddenness with which vaccinia was stopped could have resulted in a vaccinated group including primarily early vaccinia-vaccinated children. However, the association between vaccinia and mortality did not differ by age of vaccination. Vaccinia vaccinations for the 1969–72 birth cohorts were not associated with any benefit. We have been unable to identify changes in vaccinia administration practices during this period, but it remains possible that the strain of vaccinia or the vaccination technique may have varied. Because BCG vaccination, in contrast to vaccinia vaccination, was gradually phased out, it could be speculated that the uptake of vaccine was more dependent on external factors; and, particularly if the more healthy and privileged children were vaccinated preferentially during this period, it could create a spurious beneficial effect of the BCG vaccine for survival. However, there were no indications that there was a change in the social composition during the phase-out years (Supplementary Table 4), and excluding the last years, where such biases would be expected to be strongest, did not change the conclusions (Supplementary Table 12).
The finding that neither vaccinia nor BCG were linked with the control outcomes or deaths due to alcohol or drug addiction in adult life, supports that the observed protective effects of vaccinia and BCG are the result of biology rather than confounding by parental influence on health related behaviours. An alternative explanation is that frail individuals were less likely to be vaccinated (Supplementary Text 1) and had a higher risk of dying subsequently (frailty bias20), which would result in the strengthening of the protective association between vaccination and death due to natural causes. By excluding individuals with no information about receiving any childhood vaccination in their health record, we intended to exclude very frail children from the reference group. Also to test for frailty bias, we restricted our analysis to children born in the highest family social class and, reassuringly, we observed the same beneficial association of vaccinia and BCG vaccination and mortality due to natural causes of death (Supplementary Table 9). Nevertheless, it remains possible that there were other unknown factors associated with not receiving vaccination and with natural death that we did not identify, and the potential impact of vaccinia and BCG on mortality should be examined in other populations.
Consistency with previous studies
In 1810, when vaccinia vaccination became compulsory in Copenhagen (coverage above 70%), an extraordinary reduction in mortality was observed even though registered smallpox deaths had been low for a decade.21 Already at that time some physicians reported that vaccinia had NSE.22 Similarly, BCG was associated with a remarkable reduction in mortality when first introduced in northern Sweden in the 1920s.23 Recently, two randomized trials among low-birthweight-infants showed that BCG given at birth provided a strong non-specific benefit and that it reduced neonatal mortality by more than 40% compared with delayed BCG vaccination.24,25 In a recent observational study from Spain among children aged less than 15 years, BCG was associated with fewer hospital admissions for respiratory infections and sepsis.26 Interestingly, whereas most studies of overall mortality effects of live vaccines in low-income countries have suggested that females benefit most from these vaccines, we did not observe different effect estimates between males and females, which is in agreement with recent Danish studies of the beneficial non-specific effects of the live vaccines MMR and OPV.27,28
Only a few previous studies have evaluated vaccinia and BCG and mortality among adults.7–9 A prospective 8-year study of 542 malignant melanoma patients showed that BCG and vaccinia collectively were associated with an HR of 0.41 (95% CI: 0.25–0.69) and vaccinia alone was associated with an HR of 0.55 (95% CI: 0.34–0.89) for all-cause mortality.9 In our study we found no association to death due to cancers, but malignant melanoma deaths were only eight of the 133 cancer deaths in the study. Two prospective studies conducted in Guinea-Bissau collected information on vaccinations through reading vaccinia and BCG scars. With follow-up periods of 4 and 3 years, respectively, both studies found lower mortality among individuals with vaccinia scars compared with individuals without any scar, the HRs being 0.60 (95% CI: 0.41–0.87)7 and 0.22 (95% CI: 0.08–0.61)8 respectively.
Potential mechanisms
Lifelong immunological memory has been shown for vaccinia29 and BCG.30 Other studies have found that vaccinia and BCG may prevent unrelated diseases.9,12–14,31,32 These NSE may be due to cross-reactivity of T and B memory cells with other pathogens or due to training of the innate immune system.33 For example, BCG stimulates monocyte responses to non-related pathogens, resulting in increased phagocytosis, reactive oxygen species production, intracellular killing, pattern-recognizing receptor expression and pro-inflammatory cytokine production.33 These effects can be long-lasting, possibly due to functional reprogramming of monocyte precursors in the bone marrow.33
In contrast to intramuscular and hypodermal administration, the mode of vaccine administration by skin scarification has been shown in animal studies to have important immune-stimulating properties.34 It is possible that the particular modes of administrating vaccinia by disruption of the skin and BCG intradermally contribute to reprogramming of the immune system and the reduced risk of death, but there are likely other mechanisms involved as well. The immunological training explaining non-specific effects of vaccines in childhood is only beginning to be studied2,33 and there has been virtually no immunological study of how vaccines may affect the long-term immune profile.
Conclusion
Vaccinia and BCG administered during childhood were associated with lower mortality from natural causes during almost 40 years of follow-up. These observations are consistent with a growing body of evidence documenting that live attenuated vaccines induce beneficial immune training. For instance, randomized trials of BCG, measles vaccine and oral polio vaccine in low-income countries with high mortality have shown that these vaccines are associated with beneficial non-specific effects on childhood mortality. Vaccinia and BCG in Denmark may similarly have long-term beneficial NSE and reduce adult mortality due to natural causes of death.
The long-term effects of vaccines have not been tested in randomized trials, so it cannot be excluded that some of the beneficial effect in the present study could be due to unmeasured confounding. Still, there are clearly reasons not only to examine these observations elsewhere but also to study the possible mechanisms through which vaccinia and BCG may improve general health. Future studies should explore how morbidity is affected by these vaccines and which diseases are most affected. Vaccinia is unlikely to be reintroduced, but BCG could be used more widely. Though live attenuated vaccines may cause side effects, if we understand their non-specific effects, they can be further investigated immunologically and in the end it may be possible to generate these effects in other ways.
Global health programmes aim to eradicate polio and measles and the corresponding vaccines will be scaled down, stopped or replaced by inactivated vaccines. Since BCG and vaccinia may have been associated with long-term lower mortality, other vaccines should undergo thorough evaluations for potential non-specific health effects before they are terminated.
Dataset and statistical code can be shared upon request to the corresponding author but dataset requires approval by authorities.
Supplementary Data
Supplementary data are available at IJE online.
Funding
This work was supported by an unrestricted Faculty of Health Sciences scholarship from the University of Southern Denmark for A.R. The establishment of the sub-cohort and initial work initiated by M.V. was supported by an unrestricted PhD grant from the Danish Graduate School in Public Health Science and University of Copenhagen, Lundbeck Foundation [R34-A3862]; Dagmar Marshalls Foundation. This work was partially supported by funding from the European Research Council under the European Union’s Seventh Framework Programme to J.L.B. (FP/2007–2013)/ERC [Grant Agreement no. 281419, childgrowth2cancer]. CVIVA is funded by the Danish National Research Foundation [DNRF108]. The Bandim Health Project receives support from the Danish International Development Agency (DANIDA). P.A. held a research professorship grant from the Novo Nordisk Foundation. The funding agencies had no role in the study design, data collection, data analysis, data interpretation nor in the preparation, review or approval of the manuscript.
Supplementary Material
Acknowledgements
The authors wish to thank Professor Thorkild I. A. Sørensen, from the Institute of Preventive Medicine at Copenhagen University Hospital, for access to data from the Copenhagen School Health Record Register, which has been built in collaboration between the Institute of Preventive Medicine and the Copenhagen City Archives.
Conflict of interest: None.
Author contributions
Study concept and design: all authors. Literature search: A.R., M.V. Acquisition of data: M.V.,S.S., J.L.B., Adam R. Definition of outcomes: L.K.H., A.R. Analysis and interpretation: all authors. Statistical analyses: A.R., H.R. Drafting of the manuscript: A.R. Critical revision of the manuscript for important intellectual content: M.V., S.S., L.K.H., H.R., Adam R., J.L.B., C.S.B., P.A.
Key messages
Studying the cohort of Copenhagen schoolchildren who experienced the phase-out of both BCG and smallpox vaccinations in the 1970s, we found that having received both of these vaccines was associated with a 46% (19–64%) reduction in the hazard rate for death from natural causes.
BCG and smallpox vaccinations were not associated with deaths due to non-natural causes (accidents, suicides and murders).
Live attenuated vaccines, such as BCG and the smallpox vaccine, may have long-term beneficial non-specific effects on mortality.
Caution should be observed before phasing out live attenuated vaccines after eradication of the disease, as is currently planned for oral polio vaccine and possibly for measles vaccine.
References
- 1. Aaby P, Jensen H, Samb B et al. . Differences in female-male mortality after high-titre measles vaccine and association with subsequent vaccination with diphtheria-tetanus-pertussis and inactivated poliovirus: reanalysis of West African studies. Lancet 2003;361:2183–88. [DOI] [PubMed] [Google Scholar]
- 2. Benn CS, Netea MG, Selin LK, Aaby P. A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 2013;34:431–39. [DOI] [PubMed] [Google Scholar]
- 3. Aaby P, Kollmann TR, Benn CS. Nonspecific effects of neonatal and infant vaccination: public-health, immunological and conceptual challenges. Nat Immunol 2014;15:895–99. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Weekly Epidemiological Record. No. 21. Geneva: WHO, 2014. [Google Scholar]
- 5. American Red Cross, United Nation Foundation, U.S. Centers for Disease Control and Prevention, UNICEF, the World Health Organization. Global Measles and Rubella. Geneva: WHO, 2012. [Google Scholar]
- 6. World Health Organization, Rotary International, U.S. Centers for Disease Control and Prevention, UNICEF. Polio Eradication and Endgame. Geneva: WHO, 2013. [Google Scholar]
- 7. Aaby P, Gustafson P, Roth A et al. . Vaccinia scars associated with better survival for adults. An observational study from Guinea-Bissau. Vaccine 2006;24:5718–25. [DOI] [PubMed] [Google Scholar]
- 8. Jensen ML, Dave S, Schim van der Loeff M et al. . Vaccinia scars associated with improved survival among adults in rural Guinea-Bissau. PLoS One 2006;1:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kölmel KF, Grange JM, Krone B et al. . Prior immunisation of patients with malignant melanoma with vaccinia or BCG is associated with better survival. An European Organization for Research and Treatment of Cancer cohort study on 542 patients. Eur J Cancer 2005;41:118–25. [DOI] [PubMed] [Google Scholar]
- 10. Baker JL, Sørensen TIA. The Copenhagen School Health Records Register. Scand J Public Health 2011;39(Suppl 7):87–90. [DOI] [PubMed] [Google Scholar]
- 11. Cai J, Zeng D. Sample size/power calculation for case-cohort studies. Biometrics 2004;60:1015–24. [DOI] [PubMed] [Google Scholar]
- 12. Sorup S, Villumsen M, Ravn H et al. . Smallpox vaccination and all-cause infectious disease hospitalization: a Danish register-based cohort study. Int J Epidemiol 2011;40:955–63. [DOI] [PubMed] [Google Scholar]
- 13. Villumsen M, Sorup S, Jess T et al. . Risk of lymphoma and leukaemia after bacille Calmette-Guérin and smallpox vaccination: a Danish case-cohort study. Vaccine 2009;27:6950–58. [DOI] [PubMed] [Google Scholar]
- 14. Villumsen M, Jess T, Sorup S et al. . Risk of inflammatory bowel disease following Bacille Calmette-Guérin and smallpox vaccination: a population-based Danish case-cohort study. Inflamm Bowel Dis 2013;19:1717–24. [DOI] [PubMed] [Google Scholar]
- 15. Pedersen CB. The Danish Civil Registration System. Scand J Public Health 2011;39(Suppl 7):22–25. [DOI] [PubMed] [Google Scholar]
- 16. Helweg-Larsen K. The Danish Register of Causes of Death. Scand J Public Health 2011;39(Suppl 7):26–29. [DOI] [PubMed] [Google Scholar]
- 17. Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol 1999;52:1165–72. [DOI] [PubMed] [Google Scholar]
- 18. Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika 1986;73:1–11. [Google Scholar]
- 19. Månsson R, Joffe MM, Sun W, Hennessy S. On the estimation and use of propensity scores in case-control and case-cohort studies. Am J Epidemiol 2007;166:332–39. [DOI] [PubMed] [Google Scholar]
- 20. Farrington CP, Firth MJ, Moulton LH et al. . Epidemiological studies of the non-specific effects of vaccines: II - methodological issues in the design and analysis of cohort studies. Trop Med Int Health 2009;14:977–85. [DOI] [PubMed] [Google Scholar]
- 21. Mercer AJ. Smallpox and epidemiological-demographic change in Europe: The role of vaccination. Popul Stud (Camb) 1985;39:287–307. [DOI] [PubMed] [Google Scholar]
- 22. Mayr A. Taking advantage of the positive side-effects of smallpox vaccination. J Vet Med B Infect Dis Vet Public Health 2004;51:199–201. [DOI] [PubMed] [Google Scholar]
- 23. Naeslund C. Expérience de vaccination par le BCG dans la province du Norrbotten (Suède). [Experience of BCG vaccination in the province of Norrbotten (Sweden).] Rev Tuberc 1931;Mar 25:617–36. [Google Scholar]
- 24. Aaby P, Roth A, Ravn H et al. . Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period? J Infect Dis 2011;204:245–52. [DOI] [PubMed] [Google Scholar]
- 25. Biering-Sørensen S, Aaby P, Napirna BM et al. . Small randomized trial among low-birth-weight children receiving bacillus Calmette-Guérin vaccination at first health center contact. Pediatr Infect Dis J 2012;31:306–08. [DOI] [PubMed] [Google Scholar]
- 26. López MJ de C, Pardo-Seco JJ, Martinón-Torres F. Nonspecific (heterologous) protection of neonatal BCG vaccination against hospitalization due to respiratory infection and sepsis. Clin Infect Dis 2015;60:1611–19. [DOI] [PubMed] [Google Scholar]
- 27. Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P, Ravn H. Live vaccine against measles, mumps, and rubella and the risk of hospital admissions for nontargeted infections. JAMA 2014;311:826–35. [DOI] [PubMed] [Google Scholar]
- 28. Sørup S, Stensballe LG, Krause TG, Aaby P, Benn CS, Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis 2016;3:ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taub DD, Ershler WB, Janowski M et al. . Immunity from smallpox vaccine persists for decades: a longitudinal study. Am J Med 2008;121:1058–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aronson NE, Santosham M, Comstock GW et al. . Long-term efficacy of BCG vaccine in American Indians and Alaska Natives. JAMA 2004;291:2086. [DOI] [PubMed] [Google Scholar]
- 31. Steenhuis TJ, Van Aalderen WMC, Bloksma N et al. . Bacille-Calmette-Guerin vaccination and the development of allergic disease in children: a randomized, prospective, single-blind study. Clin Exp Allergy 2008;38:79–85. [DOI] [PubMed] [Google Scholar]
- 32. Gilat T, Hacohen D, Lilos P, Langman MJS. Childhood factors in ulcerative colitis and Crohn’s disease: an international cooperative study. Scand J Gastroenterol 1987;22:1009–24. [DOI] [PubMed] [Google Scholar]
- 33. Kleinnijenhuis J, van Crevel R, Netea MG. Trained immunity: consequences for the heterologous effects of BCG vaccination. Trans R Soc Trop Med Hyg 2015;109:29–35. [DOI] [PubMed] [Google Scholar]
- 34. Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med 2010;16:224–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.