Why was the cohort set up?
Indigenous Australians fare worse than other Australians on almost every measure of physical and mental health. Compared with non-Indigenous Australians, Indigenous people are more likely to have chronic disease such as obesity, diabetes, kidney disease, heart disease and circulatory problems,1 occurring at an earlier age, leading to premature mortality and influencing the lower life expectancies in Indigenous people being on average 10 years less than those of non-Indigenous people.2 Babies born to Indigenous women weigh on average almost 200 g less than those born to non-Indigenous women and are twice as likely to be low birthweight (LBW) (< 2500 g).3 In 1987, the Indigenous LBW rate (13%) in the Northern Territory (NT) was approximately double that of non- Indigenous births. Casual observation suggested that there were high numbers of fetal growth-restricted babies (FGR) delivered to Indigenous women, but difficulties in obtaining reliable gestational ages (GA) and conducting long-term follow-up meant the prevalence and outcomes of these Indigenous FGR babies were unknown. These factors led to the commencement of a prospective Aboriginal Birth Cohort study (ABC) to study the antecedents, prevalence and 10-year outcomes of FGR.
Subsequent reports in the late 1980s, linking LBW/FGR to chronic adult diseases, led to the study being extended to a life course study. The study’s aim is to investigate the hypothesis that the high rates of chronic non-communicable diseases seen in this population were the result of processes that began in utero and continued after birth (Developmental Origins of Health and Disease hypothesis).
Who is in the cohort?
Babies were eligible for enrolment if they were a singleton born between January 1987 and March 1990 to a mother self-identified as either Aboriginal or Torres State Islander, referred collectively as Indigenous, in the Delivery Suite Register at the Royal Darwin Hospital (RDH). Mothers were approached by an Aboriginal research assistant and invited to enrol themselves and their babies in the study. RDH is the place of delivery for 98% of Indigenous mothers within the local Darwin Health Region of 120 000 km2 and also functions as the tertiary referral hospital for a sparsely populated (0.2 people /km2), vast area (2 million km2 ) of northern Australia.
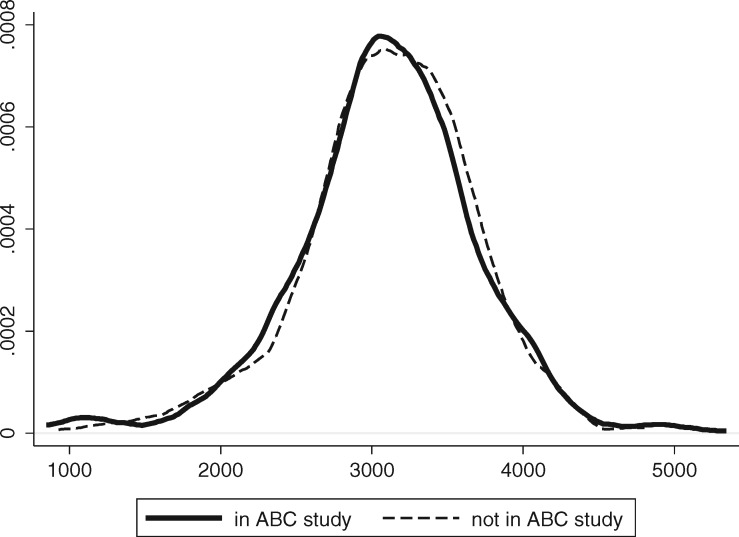
Of the 1238 babies eligible for enrolment, 686 were recruited to the study. Recruitment was dependent on the availability of the neonatal paediatrician (S.S.) and the ability to locate the mothers.4 Babies were not randomly selected, but there were no significant differences in the mean birthweight or the sex ratio between those recruited and those not recruited (see Figure 1). However, the rates of LBW were significantly higher in those recruited (Table 1).
Figure 1.
Birth weight frequencies of those “in study” and those “not in study”.
Table 1.
Characteristics of those recruited and not recruited to the Aboriginal Birth Cohort
| Total births | Recruited | Not recruited | |
|---|---|---|---|
| Number | 1238 | 686 | 552 |
| Birthweight in grams mean (DS) | 3042 (635) | 3020 (668) | 3068 (592) |
| Male % (n) | 52 (643) | 55 (352) | 45 (291) |
| LBW % (n) | 15.8 (195) | 17.8* (122) | 13.2* (73) |
| Gestational age in weeks mean (SD) | N/A | 38.7 (1.95) | N/A |
| FGR % (n) | N/A | 24 (167) | N/A |
| Preterm % (n) | N/A | 10 (63) | N/A |
N/A, not available.
P-value < 0.05.
How often have they been followed up?
Although born in Darwin, participants now reside in over 40 urban and remote communities across the NT. Between 1991 and 1994, a preliminary follow-up at mean age 5 years of a subset of participants living in the Darwin Health Region was conducted at RDH,, with only anthropometry collected (this wave has not been detailed in Table 3). Based on this experience, subsequent face-to-face comprehensive health assessments of the full cohort were conducted at their place of residence. This has occurred on three occasions: Wave 2 between 1998 and 2001 (mean age 11.4 years), Wave 3 between 2006 and 2008 (mean age 18.2 years) and Wave 4 between 2013 and 2015 (mean age 25.4 years). Excellent follow-up rates have been achieved across the waves (see Table 2).
Table 3.
List of procedures for Aboriginal Birth Cohort
| Wave 1 Birth |
Wave 2 Childhood | Wave 3 Adolescence | Wave 4 Young adult | ||
|---|---|---|---|---|---|
| (1987–90) |
(1998–2001) | (2006–08) | (2013–15) | ||
| Baby | Mother | ||||
| Socioeconomic status | |||||
| Marital status, education, employment | ✓ | ✓ | ✓ | ✓ | |
| Car ownership, household size, children | ✓ | ✓ | ✓ | ✓ | |
| Language spoken | ✓ | ✓ | ✓ | ✓ | |
| Anthropometric | |||||
| Height | ✓ | ✓ | ✓ | ✓ | ✓ |
| Weight | ✓ | ✓ | ✓ | ✓ | ✓ |
| Head circumference | ✓ | ✓ | ✓ | ✓ | |
| Mid upper arm and waist circumferences | ✓ | ✓ | ✓ | ||
| Hip circumference | ✓ | ✓ | |||
| Body composition via skin calipers | ✓ | ||||
| Body composition via bio-impedance scale | ✓ | ✓ | |||
| Metabolic and cardiovascular | |||||
| Blood pressure | ✓ | ✓ | ✓ | ||
| Fasting plasma glucose and insulin | ✓ | ✓ | ✓ | ||
| Haemoglobin A1c | ✓ | ✓ | |||
| Fasting total cholesterol, HDL-C and triglycerides | ✓ | ✓ | ✓ | ||
| Apolipoprotein A1, apolipoprotein B and lipoprotein(a) | ✓ | ✓ | ✓ | ||
| Carotid intima-media thickness | ✓ | ✓ | |||
| Digital pulse volume | ✓ | ✓ | |||
| Kidney function | |||||
| Random urinary albumin/creatinine ratio | ✓ | ✓ | ✓ | ||
| Random urinalysis | ✓ | ✓ | |||
| Estimated glomerular filtration rate | ✓ | ✓ | ✓ | ||
| Renal size and morphology | ✓ | ✓ | ✓ | ||
| Inflammatory | |||||
| Impetigo, scabies, acanthosis nigricans and fungal infection | ✓ | ✓ | ✓ | ||
| Full blood count | ✓ | ✓ | ✓ | ||
| C-reactive protein | ✓ | ✓ | |||
| Serum asymmetrical and symmetrical dimethylarginine | ✓ | ✓ | |||
| Interleukin-6, interleukin-10 | ✓ | ||||
| Respiratory | |||||
| Lung function via spirometry | ✓ | ✓ | |||
| Additional biomarkers | |||||
| Serum folate | ✓ | ✓ | ✓ | ||
| Iron, transferrin, ferritin | ✓ | ✓ | |||
| Thyroid function tests | ✓ | ✓ | |||
| Liver function test | ✓ | ||||
| Hepatitis B serology | ✓ | ✓ | |||
| Random urinary iodine concentration | ✓ | ✓ | |||
| Random urine cotinine level | ✓ | ✓ | |||
| Lifestyle | |||||
| Tobacco and cannabis smoking, alcohol consumption | ✓ | ✓ | ✓ | ✓ | |
| Exercise level | ✓ | ✓ | ✓ | ||
| Grip strength | ✓ | ✓ | |||
| Chair stands | ✓ | ||||
| Balance | ✓ | ✓ | |||
| Dietary intake | ✓ | ||||
| Emotional and stress | |||||
| Emotional status | ✓ | ✓ | |||
| Major life events | ✓ | ||||
| Heart rate variability | ✓ | ✓ | |||
| Hair and fingernail cortisol | ✓ | ||||
| Dental examination | ✓ | ||||
| Cognitive function | ✓ | ✓ | |||
LDL-C, high-density lipoprotein cholesterol.
Table 2.
Aboriginal Birth Cohort follow-up rates across the waves
| Years | Target population | Mean age (years) | Number | Cumulative deaths | Number examined | % Living examineda |
|---|---|---|---|---|---|---|
| Wave | ||||||
| 1987–90 | All Indigenous births RDH | Birth | 1238 | 0 | 686 | Not applicable |
| Wave 1 Birth | ||||||
| 1991–94 | Darwin Health Region | 5.0 | 570 | 15 | 335 | 58.7% |
| Wave 1b | ||||||
| 1998–2001 | Whole cohort | 11.4 | 686 | 18 | 570 | 85.3% |
| Wave 2 Childhood | ||||||
| 2006–08 | Whole cohort | 18.2 | 686 | 27 | 467 | 71.0% |
| Wave 3 Adolescence | ||||||
| 2014–16 | Whole cohort | 25.4 | 686 | 39 | 459 | 70.9% |
| Wave 4 Young adult |
Defined as number examined divided by (686 minus known deaths).
In addition to the number directly examined, further participants have been located but not examined in each follow-up (Wave 2: n = 63, Wave 3: n = 81, Wave 4: n = 80). Non-assessment was due to logistic reasons relating to inclement weather, mobility of participants or (single participants) living in very remote locations and residing interstate, with refusals accounting for 1, 11 and 22, respectively.5 At commencement of Wave 4 there were 39 known deaths, including 10 neonatal deaths, 10 traumatic deaths and 7 suicides. Of the traumatic deaths, 4 were non-accidental. There were 24 participants seen in Wave 3 that were not seen in Wave 2, and 14 participants seen in Wave 4 that were not seen in either Wave 2 or Wave 3. Overall, 11.1% (76) participants have not been seen since recruitment.
What has been measured?
Wave 1: Recruitment (1987-1990)
Within 4 days of birth, the one neonatal peadiatrician (S.S.) assisted by an Aboriginal research assistant interviewed the Indigenous mothers and collected self-reported information about home location, smoking, alcohol consumption, wearing shoes and cooking indoors. The ability to read an English newspaper was directly tested. Details of medical and obstetric history, antenatal attendance, mode of delivery, placenta weight, birth measurements and Apgar scores were extracted from clinical records. Accurate gestational ages were not readily available and therefore postnatal clinical estimation of gestational age by a previously validated method6 was performed by the neonatal paediatrician (S.S.).7 Maternal weight and height were measured within 10 days of birth.
Follow-up waves
Despite the varied and remote field settings, scientific rigour was scrupulously maintained for all data collected. Measured biomedical data were directly collected via standardized procedures, with questionnaires completed via face-to-face interaction with the participants. Data collected included personal demographic characteristics, detailed anthropometry, physical health status, specialized ultrasound measures and biomedical assays. Community-level measures were obtained from administrative data sets. Table 3 lists data collected for each of the waves. In Wave 3, data collection was expanded beyond anthropometry and chronic disease biomarkers collected in Wave 2, to include other measures such as emotional status, substance use, cognitive function, oral health and non-invasive ultrasound measures of thyroid and kidney volume and carotid intima-media artery thickness (cIMT).4,8 In Wave 4 the major additions included details of nutritional intake, inflammation, stress markers and major life events.
What has it found? Key findings and publications
Gestational ageing, birth outcomes and maternal risk factors
Only 6.5% of mothers knew their last menstrual period reliably and only 8% had an early dating ultrasound (< 14 weeks). Using a contemporary Australian reference, 25% of routine births had FGR and 7.7% were preterm.9 A third of mothers were < 20 years of age, > 50% smoked throughout pregnancy and had limited antenatal care. The prevalence of maternal morbidity was high. Risk factors for FGR were maternal smoking, undernutrition and age < 20 years.10
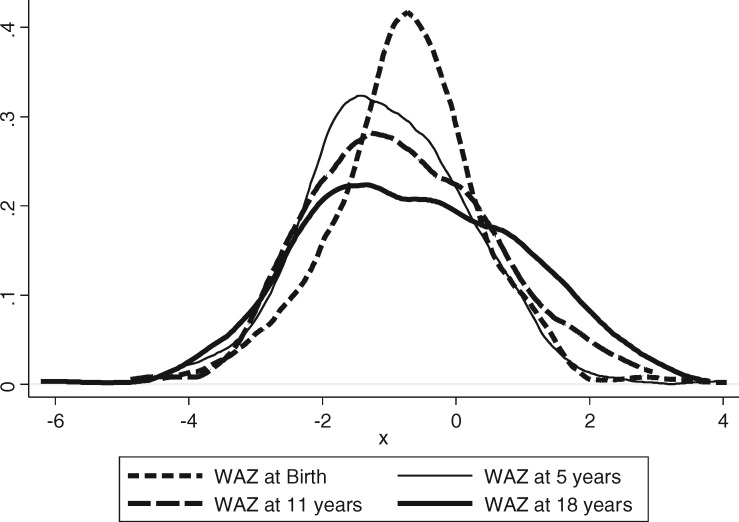
Growth
Using international Centers of Disease Control growth curves 2000, mean weight-for-age z-scores (WAZ) at birth, 11 years and 18 of age were all below the median, with the distributions of WAZ shifted to the left (Figure 2). After birth, excess of underweight (WAZ < -2) persisted but proportions of overweight (WAZ > 2) gradually increased with time. This is clearly seen in the flattening of curves in the Figure 2.
Figure 2.
Frequencies of weight-for-age at birth, age 5, 11 and 18 years.
At 11, 18 and 25 years, participants who had been FGR at birth were smaller, lighter, and had smaller head circumferences compared with those without FGR. The high-risk combination for chronic disease, of FGR with later obesity, was rare in this cohort at 18 years of age.11 Although undernutrition is still predominant at 25 years, the rates of overweight are rising.
Relationships between birthweight and biomarkers of chronic disease
In cross-sectional analyses, current weight and not birthweight has been the predominant determinant of biomarkers of chronic disease. At 11 years, other than a small negative effect of birthweight on systolic blood pressure in post-pubertal boys, all other measured biomarkers showed positive relationships to current child weight.12 Similarly at 18 years, there were no inverse relationships between birthweight and insulin and glucose concentrations,13 and a path analysis showed contemporaneous body mass index (BMI) was the strongest predictor of blood pressure with only an indirect effect of birthweight mediated through later BMI.14
Strategicopportunities
In a strategic move, the study was used as a vehicle to collect data for two studies investigating topical health problems. This required awareness of wider national health priorities, and the ABC provided the platform to examine these issues in this hard-to-reach, marginalized population. Due to financial and logistic constraints, the National Iodine Nutritional Survey did not include the NT; however the Wave 3 follow-up demonstrated iodine deficiency in the participants.15 These results supported the decision for a national approach of mandatory fortification of iodized salt in bread. Wave 4 results are poised to show the impact this national intervention has on iodine levels in this population. The Wave 3 follow-up also showed significantly higher rates of adverse oral health outcomes compared with age-matched participants in the National Adult Oral Health Survey16 which under-represented the Indigenous population. Additionally, the ABC participants were among the first in Australia to routinely receive hepatitis B vaccinations at birth. Currently the longevity of the vaccine is being monitored to inform the need and timing of a booster dose. A list of publications is available from the Aboriginal Birth Cohort web site [http://www.lifecoursemenzies.net.au].
Engagement of communities involved
The study has clear commitment to engaging with Indigenous communities, recruiting community members as assistants and building Indigenous capacity, encouraging and facilitating formal research training (Certificate 2 in Research Methods). An Indigenous reference group is attached to the study. Due to the difficulty in providing individual feedback except in limited circumstances, feedback is aimed at the communities. Updates are published in community newsletters and in the national Aboriginal and Islander Health Worker Journal and provided to local community groups.
What are the main strengths and weaknesses?
his cohort is the longest-running and largest Indigenous birth cohort in Australia, and one of the few in the world focusing on indigenous populations in English-speaking countries.17 A primary strength of this study is the high follow-up rates of a birth cohort in a contemporary Indigenous population which maintains many traditional connections with the land and with ceremony associated with death and other life events.
The core strength of the study is availability of reliable gestational age assessment. GA was assessed postnatally by a single neonatal paediatrician (S.S.) in this population where only 6.5% recalled their last menstrual period (LMP) and 8% had early dating ultrasounds. In addition, detailed antenatal and birth characteristics (including birthweight, length and head circumference) were collected using standard protocols.
A major strength of the study is the breadth of data obtained via face-to-face health checks using standardized methods by a core group of trained researchers, despite multiple locations. These measures of body composition and physical health have evolved as the cohort has aged to include arterial function, dental examinations and ultrasound measures of thyroid, kidney and cIMT, emotional and lifestyle status and more social factors.
The results of the health checks provide important data on the health of the population at specific ages. Data from Wave 3 provided comprehensive health information in adolescence. This is an age where concerns about health, particularly mental health, are high but people do not engage with health services and health information remains sparse.
The information obtained at each stage informs policy and practice. Salient changes over the years include the decision to make dating ultrasounds available at the place of residence to improve gestational age assessment. Ultrasounds at < 20 weeks are now available for over 70% of aboriginal mothers living in remote areas.18 Wave 2 showed differences between children living in remote compared with urban areas,19 which provided impetus to the development of separate health policies targeting the different groups. The low prevalence of chronic disease markers at adolescence suggests that there is still a window of opportunity beyond childhood to target interventions aimed at reducing the high burden of chronic disease in this high risk population.
The generalization of findings applies to the Indigenous people living in similar rural and remote locations in northern areas of Australia. Findings would also apply to other transitional populations with nutritional profiles with high rates of underweight and increasing overweight. The study highlights the fact that despite logistic challenges relating to geography, accessibility and cultural issues, it is possible to collect standardized biomedical measures and achieve excellent retention rates. Experiences in maintaining and following this cohort are generalizable to other difficult-to-reach populations.
The relatively small cohort size is a limitation. Recruitment was dependent on the availability of the recruiting investigator and the ability to find the Indigenous mothers in the hospital grounds within 4 days of delivery, and these factors contributed to the limited sample size taking over 2 years to collect. The Indigenous population of Australia is predominantly urban;20 however, urban dwellers were relatively under-represented at recruitment and have proved more difficult to locate at follow-up.
The small amount of socioeconomic data collected at recruitment and in the early follow-ups is a shortcoming. Standard measures of socioeconomic status used in the general population do not adequately differentiate a range within this population. Data linkage with available data through government departments of school attendance and educational attainment, incarcerations of immediate family, child care and domestic violence in the home, and community fruit and vegetable intake are planned.
If this life course study was organized today, there would be some procedural changes. The initial cohort would be larger and not restricted to Indigenous babies. There are limited Australian childhood/adolescent data available and it would have been advantageous to have a contemporary non-Indigenous birth cohort for comparative purposes. A cohort of non-Indigenous people born in Darwin in the same time period as the Indigenous cohort has now been recruited (Top End Cohort).21
At the time of recruitment, extra neonatal anthropometric measurements such as waist and mid-arm circumference and skinfold measurements would be collected, and ethical approval would be sought for cord blood and placental sample storage for future measures of genetic markers and predictors of chronic disease yet to be identified. Frequent follow-ups within the first years of life, with a focus on early growth trajectories, would have occurred. Some of these data are available through community growth records and this collection is in process.
Funding remains a challenge but as the momentum of the cohort has grown, funding has improved and advantageous national and international collaborations have occurred.
Can I get hold of data? Where can I find out more?
All data are stored confidentially and arenot freely available in the public domain, but specific proposals for collaboration are welcomed. Collaborations are established through formal agreement with the steering committee.
Aboriginal Birth Cohort Update in a nutshell
Between 1987 and 1990, 686 Indigenous babies born in Darwin, Australia, were recruited, making this the longest-running prospective birth cohort of Indigenous Australians.
Initial aims of the study were to examine nutritional and developmental outcomes of FGR till 10 years of age. Publication of Barker’s hypothesis led to the extension of time frame to cover the life course, to study the developmental origins of health and disease.
Face-to-face comprehensive assessments have occurred at Wave 2 (mean age 11.4 years: 85% of living participants), Wave 3 (mean age 18.2 years: 71% of living participants) and Wave 4 (mean age 25.4 years: 71% of living participants).
At each follow-up, the same core data were collected and have been reinforced over time by expanding detail and depth of assessments. At Wave 2, anthropometry, puberty stage, biomarkers and renal ultrasounds were collected. Wave 3 saw expansion to include oral health, lifestyle and emotional status, iodine status with thyroid ultrasound, hepatitis B immunization and cognitive and novel cardiovascular measures. At Wave 4, additional measures of nutritional intake, stress and inflammatory markers were collected.
The major strengths of the study are the availability of reliable gestational age, the direct standardized collection of comprehensive health data and excellent retention rates.
Collaborations are welcome and details of how to access data are provided.
Funding
This work was supported by the National Health and Medical Research Council of Australia (Project Grant APP1046391).
Conflict of interest: None declared.
Acknowledgments
The authors are indebted to the dedicated Life Course Research team who located participants and collected the data over many years. We thank the young adults belonging to the ABC for their co-operation and all the individuals who helped in the urban and remote locations.
References
- 1. Australian Bureau of Statistics. Australian Aboriginal and Torres Strait Islander Health Survey: First Results, Australia, 2012-13. Catalogue no 4727.0.55.001. Canberra: ABS, 2013. [Google Scholar]
- 2. Australian Institute of Health and Welfare. Mortality and Life Expectancy of Indigenous Australians: 2008 to 2012. Cat. no. IHW 140. Canberra: AIHW, 2014. [Google Scholar]
- 3. Australian Institute of Health and Welfare. Birthweight of Babies Born to Indigenous Mothers. Cat. no. IHW 138. Canberra: AIHW, 2014. [Google Scholar]
- 4. Sayers SM, Mackerras D, Singh G, Bucens I, Flynn K, Reid A.. An Australian Aboriginal birth cohort: a unique resource for a life course study of an Indigenous population. A study protocol. BMC Intl Health Hum Rights 2003;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lawrance M, Sayers SM, Singh GR.. Challenges and strategies for cohort retention and data collection in an indigenous population: Australian Aboriginal Birth Cohort. BMC Med Res Methodol 2014;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sayers S, Powers J.. An evaluation of three methods used to assess the gestational age of Aboriginal neonates. J Paediatr Child Health 1992;28:312–17. [DOI] [PubMed] [Google Scholar]
- 7. Dubowitz LM, Dubowitz V, Goldberg C.. Clinical assessment of gestational age in the newborn infant. J Pediatr 1970;77:1–10. [DOI] [PubMed] [Google Scholar]
- 8. Sayers S, Singh G, Mackerras D. et al. Australian Aboriginal Birth Cohort study: follow-up processes at 20 years. BMC Int Health Hum Rights 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sayers SM, Powers JR.. Birth size of Australian Aboriginal babies. Med J Aust 1993;159:586–91. [DOI] [PubMed] [Google Scholar]
- 10. Sayers S, Powers J.. Risk factors for Aboriginal low birthweight, intrauterine growth retardation and preterm birth in the Darwin Health Region. Aust N Z J Public Health 1997;21:524–30. [DOI] [PubMed] [Google Scholar]
- 11. Sayers S, Mott S, Singh G.. Fetal growth restriction and 18‐year growth and nutritional status: Aboriginal birth cohort 1987–2007. Am J Hum Biol 2011;23:417–19. [DOI] [PubMed] [Google Scholar]
- 12. Sayers S, Singh G, Mott S, McDonnell J, Hoy W.. Relationships between birthweight and biomarkers of chronic disease in childhood: Aboriginal Birth Cohort Study 1987–2001. Paediatr Perinat Epidemiol 2009;23:548–56. [DOI] [PubMed] [Google Scholar]
- 13. Sayers SM, Mott SA, Mann KD, Pearce MS, Singh GR.. Birthweight and fasting glucose and insulin levels: results from the Aboriginal Birth Cohort Study. Med J Aust 2013;199:112–16. [DOI] [PubMed] [Google Scholar]
- 14. Mann KD, Pearce MS, Sayers SM, Singh GR.. Pathways between birthweight and later body size in predicting blood pressure: Australian Aboriginal Cohort Study 1987–2007. J Hypertens 2015;33:933–39. [DOI] [PubMed] [Google Scholar]
- 15. Mackerras DE, Singh G, Eastman CJ.. Iodine status of Aboriginal teenagers in the Darwin region before mandatory iodine fortification of bread. Med J Aust 2011;194:126–30. [DOI] [PubMed] [Google Scholar]
- 16. Jamieson LM, Sayers SM, Roberts-Thomson KF.. Clinical oral health outcomes in young Australian Aboriginal adults compared with national-level counterparts. Med J Aust 2010;192:558–61. [DOI] [PubMed] [Google Scholar]
- 17. McNamara BJ, Gubhaju L, Chamberlain C, Stanley F, Eades SJ.. Early life influences on cardio-metabolic disease risk in aboriginal populations—what is the evidence? A systematic review of longitudinal and case-control studies. Int J Epidemiol 2012;41:1661–82. [DOI] [PubMed] [Google Scholar]
- 18. F T. Northern Territory Midwives’ Collection. Mothers and Babies 2010. Darwin, NT: Department of Health, 2013. [Google Scholar]
- 19. Mackerras DE, Reid A, Sayers SM, Singh GR, Bucens IK, Flynn KA.. Growth and morbidity in children in the Aboriginal Birth Cohort Study: the urban-remote differential. Med J Aust 2003;178:56–60. [DOI] [PubMed] [Google Scholar]
- 20. Australian Bureau of Statistics. Australian Social Trends 2008. Cat. No. 4102.0. Canberra: ABS, 2008. [Google Scholar]
- 21. Davison B, Cunningham T, Singh G.. Engaging adolescents and young adults in a longitudinal health study: experience from the Top End cohort. Aust N Z J Public Health 2011;35:86–87. [DOI] [PubMed] [Google Scholar]