Abstract
Objective
Subjective cognitive decline (SCD), or self-reported cognitive decline despite normal neuropsychological test performance, is a risk factor for objective cognitive decline and Alzheimer’s disease (AD). While brain mechanisms contributing to SCD are not well defined, studies show associations with vascular risk factors and altered cerebral blood flow (CBF), raising the hypothesis that those with SCD might be experiencing vascular dysregulation, or a disruption in the normal relationship between CBF and cognition. We examined whether the association between CBF and verbal memory performance differs between those with SCD (SCD+) and those without SCD (SCD−).
Methods
Linear mixed effect models were employed to investigate whether the voxel-wise relationship between arterial spin labeling (ASL) MRI-measured CBF and verbal memory performance was modified by SCD among a group of 70 cognitively normal older adults (35 SCD+, 35 SCD−; mean age=72) matched on age, gender, and symptoms of depression.
Results
Results indicated that the SCD− group exhibited positive associations between verbal memory and CBF within the posterior cingulate cortex, middle temporal gyrus, and inferior frontal gyrus, whereas the SCD+ group displayed negative associations between verbal memory and CBF within the posterior cingulate cortex, middle temporal gyrus, hippocampus, fusiform gyrus, and inferior frontal gyrus.
Conclusions
Findings suggest that while higher CBF is supportive of memory function in those without SCD, higher CBF may no longer support memory function in those presenting with SCD, perhaps reflecting neurovascular dysregulation.
Mesh terms (6): Alzheimer’s disease, aging, cognitive symptoms, dementia, perfusion, brain vascular disorder, age related memory disorders, functional MRI
Introduction
Aging is associated with cognitive decline, yet less is known about the neurobiological basis of this change (Salthouse, 2011). Identifying risk factors and mechanisms of age-related cognitive decline is among the greatest challenges to improving the health of older adults. The brain relies on proper functioning of the vascular neural network for the maintenance of cognitive function. This network includes the neurovascular unit (endothelial cells, pericytes, glia and neurons) and the upstream arteries and arterioles that feed into the microcirculation of the brain (Lo & Rosenberg, 2009; Zhang et al., 2012). Research suggests that dysfunction within the vascular neural network can lead to neuronal injury and degeneration (Lo & Rosenberg, 2009; Zlokovic, 2010). Cerebral blood flow (CBF), or the rate of delivery of arterial blood to the capillary bed of a particular mass of tissue, is a functional measure of the vascular neural network and has been implicated in both normal aging and Alzheimer’s disease (AD)-related cognitive decline (Bertsch et al., 2009; Hays, Zlatar, & Wierenga, 2016; Heo et al., 2010). CBF has demonstrated reliable correlations with cognition across the lifespan in both normal and pathologic aging (Bangen et al., 2012; Bertsch et al., 2009; Okonkwo et al., 2014; Wierenga et al., 2012). Furthermore, CBF measurement has been shown to distinguish between normal controls and those with AD, identify those at risk for mild cognitive impairment (MCI) and AD, and predict conversion to MCI and AD, suggesting its usefulness as a preclinical marker of cognitive decline (Hays et al., 2016; Wierenga, Hays, & Zlatar, 2014a).
Subjective cognitive decline (SCD), or self-reported cognitive decline despite normal neuropsychological test performance, has been identified as a risk factor for objective cognitive decline (Glodzik-Sobanska et al., 2007; Lista et al., 2015; Reisberg, Shulman, Torossian, Leng, & Zhu, 2010; Y. Sun, Yang, Lin, & Han, 2015; L. Wang et al., 2004). Although our understanding of SCD is in its infancy, some speculate that SCD represents an early stage of cognitive decline, with very subtle cognitive deterioration undetectable by current neuropsychological testing (Reisberg & Gauthier, 2008), while others suggest that SCD results from early neuronal dysfunction together with compensatory mechanisms that preserve cognitive functions (Y. Sun et al., 2015). There is also evidence that SCD is strongly related to depression and other affective symptoms, highlighting the importance of controlling and/or adjusting for psychiatric symptoms when investigating SCD in the context of cognitive decline (Schmand, Jonker, Geerlings, & Lindeboom, 1997; Y. Sun et al., 2015). Studies suggest that those with SCD are not only at a greater risk for future cognitive decline but that decline occurs at an accelerated rate, compared to those without SCD (Reisberg et al., 2010). Moreover, those with SCD are more likely to convert to MCI and AD (Glodzik-Sobanska et al., 2007; Lista et al., 2015; Y. Sun et al., 2015), as demonstrated by findings from a recent meta-analysis indicating adults with SDC are twice as likely to develop dementia compared to those without SCD, with annual conversion rates of 2.3% in adults with SCD compared to 1% in those without SCD (Mitchell, Beaumont, Ferguson, Yadegarfar, & Stubbs, 2014). Individuals with SCD also exhibit pathologic markers of AD, such as amyloid-beta and tau protein deposition (Barnes, Schneider, Boyle, Bienias, & Bennett, 2006), regional brain atrophy (Cherbuin, Sargent-Cox, Easteal, Sachdev, & Anstey, 2015; Stewart et al., 2011), and altered brain function (Y. Sun et al., 2015), with evidence of both higher and lower levels of regional cerebral perfusion and metabolism compared to those without SCD. For example, Hohman and colleagues found that high levels of cognitive complaints were associated with higher positron emission tomography (PET)-measured CBF in the insula, inferior parietal cortex, lingual gyrus, fusiform gyrus, and the cerebellum (Hohman, Beason-Held, Lamar, & Resnick, 2011). Similarly, another study showed that those with cognitive complaints demonstrated higher arterial spin labeling (ASL)-measured CBF along midline default mode network regions compared to those without cognitive complaints (Wang et al., 2013). Conversely, Mosconi and colleagues found that those with subjective memory complaints showed lower 2-[18F]fluoro-2-deoxy-D-glucose (FDG)-PET-measured glucose metabolism in parietotemporal and parahippocampal regions compared to those without memory complaints while another study found higher FDG-PET-measured glucose metabolism in the medial temporal lobe and lower glucose metabolism in the precuneus among a similar group of older adults with memory complaints (Mosconi et al., 2008; Scheef et al., 2012). Although cerebral perfusion and glucose metabolism are thought to be tightly linked (Roy & Sherrington, 1890; Verfaillie et al., 2015), it appears that SCD-related PET and ASL studies have produced conflicting results, likely due to differences in sample characteristics (e.g., operational definitions of SCD or normal control) or methodology (e.g., imaging modality limitations, statistical or experimental control of confounding variables). As such, it may be important to extend these prior studies using cutting-edge methodologies within well-characterized samples to further clarify the relationship between SCD and cerebral perfusion. Overall, current evidence suggests that those with SCD may exhibit altered CBF in regions typically associated with memory function, aging and AD-risk. This notion is consistent with the vascular theory of AD, which holds that vascular damage contributes to the development of AD. Notably, SCD has also been independently associated with vascular risk factors (Paradise, Glozier, Naismith, Davenport, & Hickie, 2011).
Taken together, evidence showing that SCD is associated with alterations in cerebral perfusion and future cognitive decline suggests that those with SCD may be experiencing vascular dysregulation, or a disruption in the normal relationship between CBF and cognition, yet no study to date has examined whether SCD modifies the relationship between CBF and cognition. The exploration of this moderating effect combined with the utilization of non-invasive neuroimaging techniques among a well-characterized sample of cognitively normal older adults might help elucidate the underlying mechanisms of SCD and enable early intervention strategies aimed at preventing cognitive decline and AD. The current study used ASL magnetic resonance imaging (MRI) to determine if the relationship between CBF and memory function was modified by SCD. Based on previous reports, we hypothesized that SCD status would have a direct association with CBF in areas associated with aging and AD-risk (hippocampus, parahippocampal gyrus, posterior cingulate, precuneus). We also hypothesized that the association of resting CBF and cognitive function (memory) would be moderated by SCD status (SCD+, SCD−), whereby those in the SCD+ group would display an inverse relationship, suggesting evidence of vascular dysregulation and supporting its role in cognitive decline and AD-risk.
Methods
Participants
See Table 1 for participant demographic and cognitive characteristics. Participants were community-dwelling older adult volunteers between the ages of 65 and 88 enrolled in a longitudinal study of aging and/or other ongoing research studies at the VA San Diego Healthcare System (VASDHS) and the University of California San Diego (UCSD). Of the 80 participants with available data who met inclusion/exclusion criteria, 35 reported SCD (SCD+) and were matched to 35 controls who reported no SCD (SCD−), based on age, sex, and Geriatric Depression Scale (GDS) score using the nearest neighbor matching method (Stuart, 2010).
Table 1.
Participant demographic and cognitive characteristics.
| SCD− (N=35) | SCD+ (N=35) | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mean | SD | Mean | SD | Stat | df | p | ||
|
|
||||||||
| Age* | 73 | 6.25 | 72.54 | 5.07 | t= 0.34 | 65.25 | 0.738 | |
| Gender | 22 female | -- | 24 female | -- | χ2= 0.25 | 68 | 0.802 | |
| GDS (30-item) | 2.02 | 2.31 | 2.89 | 2.26 | t= −1.57 | 68 | 0.122 | |
| Education | 16.57 | 2.44 | 16.2 | 2.27 | t= 0.66 | 68 | 0.512 | |
| ApoE genotype | 12 ε4 | 34.3% | 14 ε4 | 40% | χ2= 0.35 | 1 | 0.624 | |
| Family history of dementia | 15 | 42.9% | 18 | 51.4% | χ2= 0.52 | 1 | 0.632 | |
| DRS Total Score | 140.46 | 3.29 | 140.60 | 2.83 | t= −0.20 | 68 | 0.846 | |
| Whole Brain rCBF* | 71.75 | 14.82 | 72.10 | 11.68 | t= −0.11 | 64.49 | 0.913 | |
| SMRS (transformed) | 0.37 | 1.54 | −1.64 | .90 | -- | -- | -- | |
| VM Composite Total Z-score | 0.09 | 0.83 | −0.09 | 0.83 | t= 0.93 | 68 | 0.354 | |
| VM Composite Raw Scores: | ||||||||
| WMS-R LM Immediate Recall | 31.09 | 5.48 | 30.14 | 6.65 | t= 0.65 | 68 | 0.520 | |
| WMS-R LM Delayed Recall | 28.06 | 6.70 | 27.60 | 7.20 | t= 0.28 | 68 | 0.784 | |
| CVLT-2 List 1–5 Total | 51.40 | 10.46 | 49.05 | 10.00 | t= 0.96 | 68 | 0.342 | |
| CVLT-2 SD Free Recall | 10.91 | 3.07 | 10.20 | 3.17 | t= 0.96 | 68 | 0.342 | |
| CVLT-2 LD Free Recall | 11.74 | 3.09 | 11.00 | 2.96 | t= 1.03 | 68 | 0.317 | |
ApoE = Apolipoprotein E; DRS = Mattis Dementia Rating Scale; rCBF = Resting Cerebral Blood Flow; GDS = Geriatric depression scale; SMRS = Subjective memory rating scale; VM = Verbal Memory; WMS-R = Wechsler Memory Scale-Revised; LM = Logical Memory; CVLT-2 = California Verbal Learning Test-2; SD = Short Delay; LD = Long Delay; df = degrees of freedom for independent samples T-test. B represents the standardized coefficient.
Denotes equal variances not assumed (df reported are adjusted for unequal variances).
Normal cognitive function was determined based on a comprehensive neuropsychological test battery. Participants were excluded if they met the empirically-derived criteria for mild cognitive impairment developed by Jak and colleagues (Jak et al., 2009). Potential participants were also excluded if they had a history of dementia, severe head injury, uncontrolled hypertension, had a DSM-IV diagnosis of learning disability, attention deficit disorder, mood disorder, or substance abuse, or if they reported a significant level of depressive symptoms on the 30-item GDS (i.e., GDS≥10). Participants were also excluded if they had contraindications to MRI scanning, or if they were taking prescription psychoactive medications. All data was collected in accordance with UCSD and VA institutional research standards for human research and the Helinski Declaration.
Testing Sequence
All participants completed a comprehensive neuropsychological battery to determine normal cognitive functioning. Self-report measures, including the Subjective Memory Rating Scale (SMRS), were administered directly after the completion of cognitive testing. All participants were tested in one of three similar testing suites at UCSD and most were tested in the morning hours, though specific times varied. Participants also underwent an fMRI scan at the same location within 90 days of completing cognitive testing.
Verbal Memory Composite
A verbal memory composite score was created using trials 1–5, short delay free-recall, and long delay free-recall raw scores from the California Verbal Learning Test 2 (CVLT-2), measuring word list learning, and the Logical Memory immediate and delayed recall subtests of the Wechsler Memory Scale-Revised (WMS-R), measuring story recall. These tests were selected based on results from a principal component analysis previously reported by our group on a similar sample of older adults (Wierenga et al., 2012). Verbal memory composite scores were derived by averaging the z-scores for each of the tests within the composite for the entire sample.
SCD assessment
The SMRS is a 5-item questionnaire asking: “In the past year, do you think: (1) Your ability to remember the names of people you have just met has changed 2) Your ability to remember the faces of people you have just met has changed (3) Your ability to remember the names of close friends or relatives has changed (4) Your ability to remember appointments correctly has changed? (5) Your ability to judge the passage of time and guessing the time of day without looking at a clock or the sun has changed.” Response categories for each item were: 1=definitely improved, 2=slightly improved, 3=no change, 4=slightly deteriorated, and 5=definitely deteriorated. SCD+ was operationally defined by normal cognitive function based on current neuropsychological performance and the presence of scores of “4=slightly deteriorated” and/or “5=definitely deteriorated” and the absence of any scores of “1=definitely improved” or “2=slightly improved”. SCD− was operationally defined by normal cognitive function and the presence of scores of “1=definitely improved”, “2=slightly improved”, and/or “3=no change” and the absence of scores of “4=slightly deteriorated” or “5=definitely deteriorated.” Therefore, those in the SCD− group reported only improvements and stability in memory, while those in the SCD+ group reported deterioration and no improvements. For ease of interpretation, total scores on the SMRS were centered around “3=no change” so that positive total scores represented subjective improvements and negative total scores represented subjective declines. Although this measure is focused on memory changes, we use the term “subjective cognitive decline” rather than “subjective memory decline” as is recommended by the Subjective Cognitive Decline Initiative working group (Jessen et al., 2014). Among a sample of 1,883 dementia-free older adults, the SMRS demonstrated a Cronbach alpha coefficient of 0.6. Exploratory factor analysis found one common factor with an Eigen value greater than 1 and factor loadings from 0.4 to 0.5 for the five items. Furthermore, the SMRS demonstrated adequate face validity among this same sample (L. Wang et al., 2004). In the current sample, the SMRS demonstrated a Cronbach alpha coefficient of 0.68.
Apolipoprotein E genotyping
Genotyping was performed by the ADCS Biomarker Core at UCSD using real time PCR Restriction Fragment Length Polymorphism analysis. Genomic DNA was collected from participants using buccal swab and extracted using Qiamp DNA blood mini kit (Qiagen) followed by PCR amplification (Wierenga, et al., 2012).
MRI acquisition
Imaging data were acquired on a GE Discovery MR750 3T whole-body system with a body transmit coil and an 8-channel receive-only head coil at the University of California San Diego Center for Functional MRI. The structural brain sequence consisted of a high-resolution T1-weighted Fast Spoiled Gradient Recall (3D FSPGR) scan: 172 1 mm contiguous sagittal slices, FOV=25cm, TR=8ms, TE=3.1ms, flip angle=12, T1=600ms, 256×192 matrix, Bandwidth=31.25kHZ, frequency direction=S-I, NEX=1, scan time=8 min 13 seconds. Resting CBF was acquired with the Multiphase Pseudocontinuous ASL (MPPCASL) sequence, which is optimized for robust CBF quantification (Jung, et al., 2010): tagging duration=2sec, TI=3.6sec, TR=4.2sec, TE=minimum, reps=64, FOV=22×22 cm, 20 5mm axial slices with a single shot spiral acquisition, collecting 8 cycles where each cycle consists of 8 images acquired with unique phase offsets, acquisition time=4:46 minutes. A spiral scan with a long TR (4000ms) and short TE (3.4ms) was also acquired to obtain an estimate of the equilibrium magnetization of cerebral spinal fluid, which is used to convert the perfusion signal into calibrated CBF units (mL blood/100g tissue/min). Finally, a minimum contrast image was acquired to adjust for transmit and receive coil inhomogeneities. Two field map scans were also acquired and used for off-line field map correction to help correct for signal bunching and dropouts in the frontal/medial temporal lobes.
MRI pre-processing
Image processing was performed with Analysis of Functional NeuroImages (AFNI, afni.nimh.nih.gov) (Cox, 1996), FMRIB Software Library (FSL, Oxford, United Kingdom), and locally created Matlab scripts. Field map correction was applied to the ASL time series prior to co-registration to the middle time point to minimize the effects of participant motion. For each participant, a mean ASL image was formed from the average difference of the control and tag images using surround subtraction to create an uncorrected perfusion time series, and slice timing delays were accounted for, making the inversion time (TI2) slice specific (Liu and Wong, 2005). This mean ASL image was then converted to absolute units of CBF (mL/100 g tissue/min) using an estimate of the equilibrium magnetization of CSF as a reference signal (Chalela, et al., 2000). This procedure resulted in a calibrated perfusion value for each voxel. Skull stripping of the high-resolution T1-weighted image was performed using AFNI’s 3dSkullStrip. Tissue segmentation was performed using FSL’s Automated Segmentation Tool algorithm to define CSF, gray matter (GM) and white matter (WM) regions. The high-resolution T1-weighted image and partial volume segmentations were registered to ASL space, and partial volume segmentations were down-sampled to the resolution of the ASL data. To correct the CBF measures for partial volume effects and ensure that CBF values were not influenced by known decreased perfusion in white matter or increased volume of CSF (Parkes, et al., 2004), we used the method previously reported by Johnson and colleagues (Johnson, et al., 2005). These calculations assume that CSF has 0 CBF, and that CBF in GM is 2.5 times greater than that in WM. The following formula was used to compute partial volume corrected CBF signal intensities: CBFcorr=CBFuncorr/(GM+0.4*WM). CBFcorr and CBFuncorr are corrected and uncorrected CBF values, respectively. GM and WM are gray matter and white matter partial volume fractions, respectively. Information from the high resolution structural image and the FSL FAST was used to determine the tissue content of each perfusion voxel. A 4.0 mm full-width, half-maximum Gaussian filter was applied to the CBFcorr data. Voxels with negative intensities were replaced with zero (Brown, et al., 2003) and GM voxels were thresholded at 0.9 probability. CBFcorr data were registered to the MNI-152 atlas using FMRIB’s Non-linear Image Registration Tool (FNIRT), part of FSL (http://fsl.fmrib.ox.ac.uk/fsl/) and resampled to a 3×3×3 mm resolution grid.
Statistical analyses
T-tests were used to compare groups on age, years of education, GDS score, SMRS score, whole brain resting CBF and cognitive variables. Chi-square tests were used to compare groups on sex, APOE status, and family history of dementia. A voxel-wise linear mixed-effects (LME) regression model was conducted in R with resting CBF as the dependent variable and with independent variables: 1) verbal memory composite score, 2) SCD status (SCD+ vs. SCD−), and 3) the interaction term between verbal memory composite score and SCD status. In addition to matching groups on age, GDS score, and sex, statistical analyses also adjusted for these same variables to further decrease the chance of confounding (Pearce, 2016). The LME yielded statistical maps displaying the brain regions for which there were significant main effects of SCD status on CBF and the interactive effects of SCD status and verbal memory composite on CBF. Significance was determined by applying cluster-size correction derived from Monte-Carlo simulations (via AFNI’s 3dClustSim) to guard against false positives on data initially thresholded at a value of p<0.025 (uncorrected). Based on these simulations, it was determined that a cluster size of 28 contiguous voxels (756 mm3) ensured an overall p<0.025 (corrected). To characterize the direction and magnitude of interactive and main effects, post-hoc regression analyses were carried out using the mean CBF extracted from each significant cluster. Post-hoc analyses were conducted in IBM SPSS Statistics, version 22 (bootstrapped with 1,000 samples) and were conducted only to characterize the significant LME interaction terms.
Results
Groups did not differ significantly on age, APOE status, family history of dementia, years of education, sex, DRS score, GDS score, whole-brain resting CBF, the verbal memory composite score, nor on any of the cognitive tests that comprised the verbal memory composite score (see Table 1).
Main effect of SCD on CBF
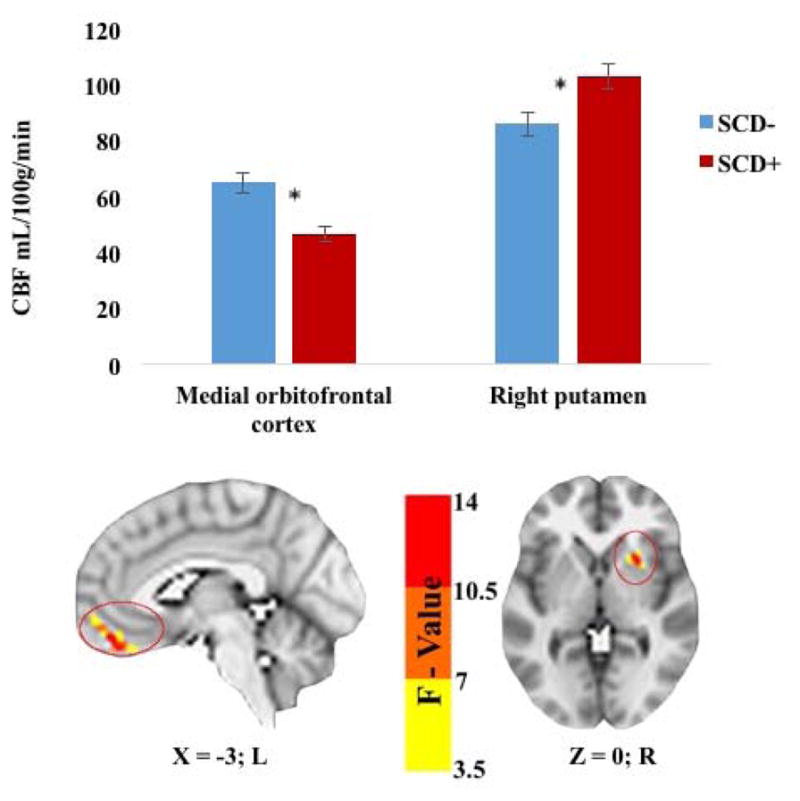
Significant main effects of SCD on CBF were found in two clusters within the medial orbitofrontal cortex and the right putamen. Cluster locations with coordinates and corresponding Beta values by group are listed on Table 2. To characterize the direction and magnitude of the main effects of SCD on CBF, mean CBF was extracted from the two main effect clusters, with evidence of both positive and negative associations between SCD status and CBF. Compared to the SCD− group, those in the SCD+ group demonstrated lower CBF in the medial orbitofrontal cortex and higher CBF in the right putamen (see Figure 2).
Table 2.
Main effect of SCD status on CBF.
| SCD Status × CBF Cluster Locations
| |||||||
|---|---|---|---|---|---|---|---|
| Mean Cerebral Blood Flow In: | Voxels | X | Y | Z | Max F-value | Beta | p |
| Bi OFC | 55 | 3 | −42 | −24 | 13.4 | −0.50 | 0.001 |
| R Putamen | 33 | −24 | −12 | −3 | 13.2 | 0.36 | 0.002 |
CBF = Cerebral blood flow; OFC = Orbital frontal cortex; Bi = Bilateral; R = Right; X, Y, and Z coordinates represent the peak F-value in MNI space. Beta values represent the standardized partial regression coefficients, with higher absolute values representing larger effect sizes.
Figure 2. Main effect of memory function on CBF.
SCD= Subjective cognitive decline; CBF = Cerebral blood flow; R = right. * Denotes significance at p<0.025.
Main effect of memory function on CBF
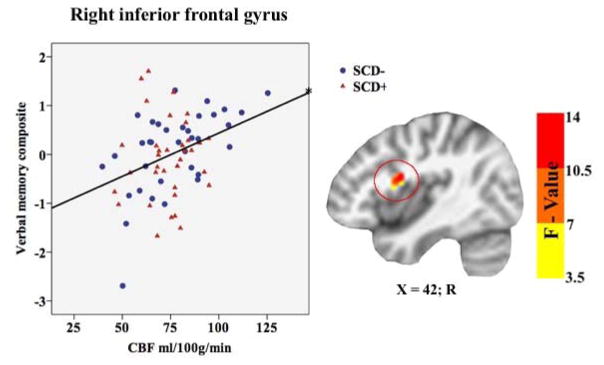
A significant main effect of memory function on CBF was found in one cluster within the right inferior frontal gyrus. Cluster location with coordinates and corresponding Beta values by group are listed on Table 3. To characterize the direction and magnitude of the main effect of memory function on CBF, mean CBF was extracted from the main effect cluster, showing that memory function was positively associated with CBF in both groups (SCD+/−) within the right inferior frontal gyrus (see Figure 3).
Table 3.
Main effect of verbal memory function on CBF.
| Verbal Memory Composite × CBF Cluster Locations | |||||||
|---|---|---|---|---|---|---|---|
| Mean Cerebral Blood Flow In: | Voxels | X | Y | Z | Max F-value | Beta | p |
| R IFG | 39 | −42 | −3 | 18 | 15.7 | 0.30 | 0.016 |
CBF = Cerebral blood flow; R = Right; X, Y, and Z coordinates represent the peak F-value in MNI space; IFG = Inferior frontal gyrus. Beta value represents the standardized partial regression coefficient with higher absolute values representing larger effect sizes.
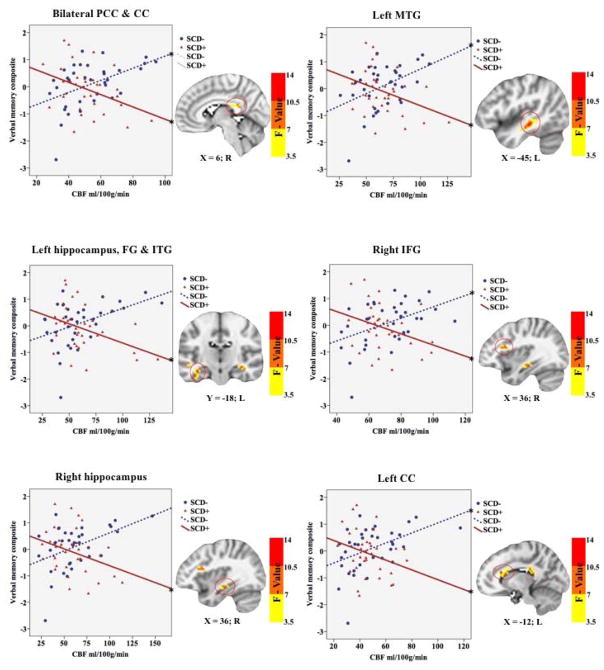
Figure 3. Interactive effects of SCD status and verbal memory on CBF.
SCD = Subjective cognitive decline; CBF = Cerebral blood flow; PCC = Posterior cingulate cortex; CC = Corpus callosum; MTG = Middle temporal gyrus; FG = Fusiform Gyrus; ITG = Inferior temporal gyrus; IFG = Inferior frontal gyrus; L = left; R = right. * Denotes significance at p<0.025.
Interactive effects of SCD status and verbal memory on CBF
Significant interactions between SCD status and verbal memory composite scores on CBF were found in six clusters within the right hippocampus, inferior frontal gyrus, left middle temporal gyrus, left hippocampus and fusiform gyrus and inferior temporal gyrus, body of the corpus callosum, and bilateral posterior cingulate cortex and splenium of the corpus callosum. Cluster locations with coordinates and corresponding Beta values by group are listed on Table 4. To characterize the direction and magnitude of the interaction effects mean CBF was extracted from the six significant clusters, demonstrating a consistent pattern of positive associations between CBF and verbal memory functions for those in the SCD− group, and negative associations between CBF and verbal memory functions for those in the SCD+ group (see Figure 4). Higher CBF was associated with better verbal memory function for those in the SCD− group within bilateral posterior cingulate cortices, left middle temporal gyrus, right inferior temporal gyrus, and the corpus callosum. In contrast, higher CBF was associated with worse verbal memory performance within the left middle temporal gyrus, bilateral hippocampi, bilateral posterior cingulate cortices, left fusiform gyrus, left inferior temporal gyrus, and right inferior frontal gyrus in the SCD+ group (see Figure 4).
Table 4.
Interactive effects of SCD status and verbal memory on CBF.
| SCD status × Verbal Memory Composite Cluster Locations | SCD− | SCD+ | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Mean Cerebral Blood Flow In: | Voxels | X | Y | Z | Max F-value | Beta | p | Beta | p |
| B PCC & CC | 92 | −18 | −45 | 12 | 13.3 | 0.27 | 0.020 | −0.33 | 0.006 |
| L MTG | 63 | −45 | −27 | −9 | 11.9 | 0.21 | 0.035 | −0.38 | 0.001 |
| L Hc, FG & ITG | 36 | −36 | −15 | −18 | 12.6 | 0.14 | 0.237 | −0.38 | 0.004 |
| R IFG | 30 | 30 | 18 | 18 | 10.3 | 0.30 | 0.007 | −0.27 | 0.013 |
| R Hc | 28 | 27 | −15 | −12 | 9.4 | 0.22 | 0.077 | −0.30 | 0.003 |
| L CC | 28 | −12 | 24 | 18 | 11.9 | 0.29 | 0.014 | −0.25 | 0.004 |
CBF = Cerebral blood flow; B = Bilateral; L = Left; R = Right; X, Y, and Z coordinates represent the peak F-value in MNI space; PCC = Posterior cingulate cortex; CC = Corpus callosum; MTG = Middle temporal gyrus; Hc = Hippocampus; FG = Fusiform Gyrus; ITG = Inferior Temporal Gyrus; IFG = Inferior Frontal Gyrus. Beta values represent the standardized partial regression coefficients, with higher absolute values representing larger effect sizes.
Discussion
Results showed that older adults with SCD demonstrated lower CBF in the orbitofrontal cortex and higher CBF in the putamen, compared to those without SCD. Furthermore, our sample of cognitively normal older adults demonstrated an overall positive association between memory function and CBF that was modified by SCD, such that those presenting without SCD showed positive associations between memory functions and CBF within frontal, temporal, and parietal regions, whereas those presenting with SCD showed negative associations between memory function and CBF within frontal, temporal, and parietal regions. Our results showing that those with SCD demonstrate both higher and lower regional CBF, compared to those without SCD, are consistent with other SCD-related perfusion studies, further supporting the notion that regionally specific perfusion differences exist between these groups in areas that have been implicated in normal aging and AD-risk (Dai et al., 2009; Hays et al., 2016; Meltzer et al., 2000).
This is the first study, to our knowledge, to show that SCD modifies the relationship between voxel-wise CBF and memory function. These findings suggest that the normal beneficial effects of higher CBF on cognition may be disrupted among those with SCD, as higher CBF no longer appears to support better cognitive functioning in this group within regions associated with normal aging and AD-risk (Frederiksen, 2013; Grasby et al., 1993; Hays et al., 2016; Wierenga et al., 2014a). Although the underlying mechanisms associated with the observed differences between our groups are still unknown, other SCD-related studies showing higher activation, perfusion, and nodal efficiency among those with SCD in similar regions, have suggested that compensatory mechanisms could explain these differences (Erk et al., 2011; Z. Sun, 2015; Z. Wang, 2014). Similarly, the observance of higher CBF among those at risk for cognitive decline or AD has been interpreted as recruitment of early compensatory mechanisms, thought to reflect efforts to maintain adequate brain oxygenation in the face of vascular aging or damage. The notion of CBF-related compensation among those at risk for AD supports the vascular theory of AD, which posits that vascular damage plays a role in the pathogenesis of AD (Wierenga, Hays, & Zlatar, 2014b). This theory is further supported by longitudinal and cross-sectional studies showing that those in the preclinical phases of AD tend to show more areas of hyperperfusion early in the disease process, followed by more areas of hypoperfusion, thought to represent increasing heterogeneity of capillary flow patterns with disease progression (Ostergaard et al., 2013; Wierenga et al., 2014a). Taken together, this evidence suggests that the modifying effect of SCD on the relationship between CBF and cognition may reflect vascular dysregulation among those with SCD, and that the perfusion alterations observed in this group may reflect attempts to compensate for these vascular changes.
The results of this current study are similar to those of a recent study of AD-risk, which showed that APOE status modifies the relationship between CBF and cognition, such that those carrying the e4 allele, a known risk factor for AD, showed inverse relationships between CBF and memory function, while non-carriers displayed positive relationships (Zlatar et al., 2016), adding to an accumulation of evidence suggesting that SCD may reflect an early preclinical stage of AD (Glodzik-Sobanska et al., 2007; Z. Sun, 2015). We found no differences in APOE status or family history of dementia between those with and without SCD in our sample, indicating that SCD might represent an independent risk factor for cognitive decline. The fact that group differences in the relationship between CBF and cognition were found despite normal cognitive function lends further support to the notion that SCD+ is distinct from normal aging (i.e., SCD−). It is also possible that current neuropsychological testing is not sensitive to the early subtle cognitive decline associated with SCD (Z. Sun, 2015). Results suggest that vascular dysregulation is occurring in those with SCD, even in the absence of clinical symptoms, further supporting its role as a preclinical marker of risk for cognitive decline.
The accurate assessment of SCD is a significant challenge facing the field. To address the lack of standardized and well-validated assessment tools for SCD, the Subjective Cognitive Decline Initiative (SCD-I) Working Group was established in 2014 with the goal of developing a conceptual framework and research criteria for SCD (Jessen et al., 2014). They recommended “well-constructed, easy-to-administer items with adequate reliability across diverse samples of older adults” (Rabin et al., 2015). Given that such a measure has yet to be developed or validated, the working group offered preliminary recommendations for instrument selection: 1. Select measures with appropriate demographic characterization. 2. Select measures with adequate content coverage for the target population. 3. Consider issues of psychometric adequacy (Rabin et al., 2015). Despite the psychometric limitations of the SMRS, its use represents an improvement over much of the SCD literature, which consists of studies utilizing only one or two questions to determine SCD. Furthermore, the five questions on the SMRS, which ask about changes in memory for names, faces, appointments and the ability to judge time over the last year, provide better clinical insight to specific subjective experiences of cognitive decline over a specified period, compared to questions which ask about cognitive difficulties in general without reference to decline over time. Future SCD-related research would benefit from the development and widespread use of a more comprehensive consensus measure of SCD that has been shown to be valid and reliable.
In conclusion, this evidence supports the notion that those with SCD may be experiencing dysregulation within the vascular neural network. Elucidating the early vascular changes that accompany risk for cognitive decline and AD could lead to the identification of vasoprotective treatments with the potential to delay or prevent the onset of cognitive decline and AD. Although future research is needed to determine whether vascular dysregulation in those with SCD reflects normal or pathologic processes, the current results support SCD as a valid construct to detect those who might be at risk for cognitive decline and AD. Integrating SCD with other known markers of cognitive decline and AD could lead to earlier and more accurate identification of those who would likely benefit from treatments aimed at preventing cognitive decline.
Strengths and limitations
As mentioned above, this study was limited by the challenge faced by all studies of SCD, namely that of accurate assessment of subjective cognitive decline. We chose to use the SMRS because it offers several advantages over other available measures. However, the SMRS consists of only five self-report questions, from which we wholly defined our groups. Moreover, most of the questions on the SMRS are memory-related, reducing the likelihood of capturing those with SCD in non-memory cognitive domains, and the Cronbach alpha coefficient of 0.68 is not particularly high, although it is still within the range of acceptability for reliability (Loewenthal, 2001). Nevertheless, the proportion of participants classified as having SCD was in line with prevalence rates in other studies (Jonker, Geerlings, & Schmand, 2000; Mitchell, 2008). It is also possible that the timing of SCD assessment in this study may have influenced the way in which participants responded to this questionnaire, as we administered the SMRS directly after objective cognitive testing. However, it is important to note that the SMRS asks about changes in specific abilities (memory for names, faces, appointments and the ability to judge time) that were not tested within the cognitive battery. Furthermore, the SMRS asks participants to report changes in these specific abilities over the last year (rather than changes over weeks or months), further reducing the likelihood that the objective cognitive testing influenced responding on this questionnaire. Nonetheless, future studies should consider evaluating SCD and objective cognitive functioning on separate days. Overall, these limitations further highlight the need for a comprehensive consensus measure of SCD, together with standardized administration instructions, including recommendations for the sequencing of SCD assessment in relation to objective cognitive testing.
Despite a well-characterized and relatively large sample, the use of a cross-sectional design restricted our ability to draw causal conclusions and limited our ability to determine whether the effects of SCD on CBF and the relationship between CBF and cognition represent normal or pathologic processes. It is also important to note that our sample had relatively high levels of education, and although this demographic factor was not associated with SCD status, its limited range may reduce the generalizability of these findings. Although groups (SCD+/SCD−) did not differ significantly in the mean time interval between cognitive testing and fMRI scanning (23 and 17 days, respectively), decreasing the time interval between cognitive testing and fMRI may improve accuracy of brain-behavior associations. Although only linear relationships were explored in the current study, future studies should consider exploring non-linear relationships between CBF and cognitive performance. Future investigations should also include longitudinal designs with larger, more diverse samples to replicate and extend the current findings. Furthermore, the inclusion of additional markers of AD, such as CSF biomarkers would help better characterize those with SCD.
The strengths of the current study include the use of non-invasive ASL MRI to measure CBF, the availability of several cognitive test performances to characterize cognitive status, and the use of voxel-wise linear mixed effects models which allowed us to examine the associations between SCD, verbal memory performance, and CBF across the entire brain. Despite these strengths, ASL methods are limited by low spatial resolution, transit time effects, and low signal-to-noise ratio (SNR) in deep white matter. Lastly, the current study represents an improvement over other studies of SCD, in our inclusion of a well-characterized, well-controlled sample of older adults. Many other studies of SCD have failed to exclude or control for depressive symptoms and other psychiatric disorders, making it difficult to determine whether observed results were due to SCD. The current study matched participants on age, sex, and GDS, in addition to statistically correcting for these same variables within the analyses.
Conclusions
This study found that the relationship between CBF and cognition is disrupted in cognitively normal older adults with SCD, compared to those without SCD. Whereas higher CBF supports verbal memory functions in those without SCD, it appears that higher CBF is no longer supportive of verbal memory function among those with SCD. The current findings suggest that those with SCD may be experiencing vascular dysregulation and support SCD as a marker of risk for cognitive decline and AD. Future longitudinal studies should examine changes in perfusion and cognition over time among those with SCD to determine whether the moderating effect of SCD on the relationship between CBF and cognition represents normal age-related or pathologic processes and to further characterize the role of SCD in the trajectory from normal to pathologic aging.
Figure 1. Main effects of SCD status and CBF.
SCD= Subjective cognitive decline; CBF = Cerebral blood flow; L = left; R = right. * Denotes significance at p<0.025. Error bars represent standard error.
Acknowledgments
This work was supported by VA CSR&D Merit Award 5I01CX000565 (to C.E.W.); K23 AG049906 (to Z.Z.Z.); and National Science Foundation Graduate Research Fellowship Program 2015207525 (to C.C.H). There are no conflicts of interest to disclose.
References
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, Bondi MW. Assessment of Alzheimer’s disease risk with functional magnetic resonance imaging: an arterial spin labeling study. Journal of Alzheimer’s Disease: JAD. 2012;31(Suppl 3):S59–74. doi: 10.3233/JAD-2012-120292. https://doi.org/10.3233/JAD-2012-120292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Schneider JA, Boyle PA, Bienias JL, Bennett DA. Memory complaints are related to Alzheimer disease pathology in older persons. Neurology. 2006;67(9):1581–1585. doi: 10.1212/01.wnl.0000242734.16663.09. https://doi.org/10.1212/01.wnl.0000242734.16663.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Hermes M, Walter C, Khan R, Naumann E. Resting cerebral blood flow, attention, and aging. Brain Research. 2009;1267:77–88. doi: 10.1016/j.brainres.2009.02.053. https://doi.org/10.1016/j.brainres.2009.02.053. [DOI] [PubMed] [Google Scholar]
- Cherbuin N, Sargent-Cox K, Easteal S, Sachdev P, Anstey KJ. Hippocampal Atrophy Is Associated with Subjective Memory Decline: The PATH Through Life Study. The American Journal of Geriatric Psychiatry. 2015;23(5):446–455. doi: 10.1016/j.jagp.2014.07.009. https://doi.org/10.1016/j.jagp.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Mild Cognitive Impairment and Alzheimer Disease: Patterns of Altered Cerebral Blood Flow at MR Imaging. Radiology. 2009;250(3):856–866. doi: 10.1148/radiol.2503080751. https://doi.org/10.1148/radiol.2503080751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Spottke A, Meisen A, Wagner M, Walter H, Jessen F. Evidence of neuronal compensation during episodic memory in subjective memory impairment. Archives of General Psychiatry. 2011;68(8):845–852. doi: 10.1001/archgenpsychiatry.2011.80. https://doi.org/10.1001/archgenpsychiatry.2011.80. [DOI] [PubMed] [Google Scholar]
- Frederiksen KS. Corpus callosum in aging and dementia. Danish Medical Journal. 2013;60(10):B4721. [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, … de Leon MJ. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dementia and Geriatric Cognitive Disorders. 2007;24(3):177–184. doi: 10.1159/000105604. https://doi.org/10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RSJ, Dolan RJ. Functional mapping of brain areas implicated in auditory—verbal memory function. Brain. 1993;116(1):1–20. doi: 10.1093/brain/116.1.1. https://doi.org/10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Hays CC, Zlatar ZZ, Wierenga CE. The Utility of Cerebral Blood Flow as a Biomarker of Preclinical Alzheimer’s Disease. Cellular and Molecular Neurobiology. 2016;36(2):167–179. doi: 10.1007/s10571-015-0261-z. https://doi.org/10.1007/s10571-015-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo S, Prakash RS, Voss MW, Erickson KI, Ouyang C, Sutton BP, Kramer AF. Resting hippocampal blood flow, spatial memory and aging. Brain Research. 2010;1315:119–127. doi: 10.1016/j.brainres.2009.12.020. https://doi.org/10.1016/j.brainres.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohman TJ, Beason-Held LL, Lamar M, Resnick SM. Subjective Cognitive Complaints and Longitudinal Changes in Memory and Brain Function. Neuropsychology. 2011;25(1):125–130. doi: 10.1037/a0020859. https://doi.org/10.1037/a0020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jak AJ, Bondi MW, Delano-Wood L, Wierenga C, Corey-Bloom J, Salmon DP, Delis DC. Quantification of five neuropsychological approaches to defining mild cognitive impairment. The American Journal of Geriatric Psychiatry: Official Journal of the American Association for Geriatric Psychiatry. 2009;17(5):368–375. doi: 10.1097/JGP.0b013e31819431d5. https://doi.org/10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, … Wagner M. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s & Dementia. 2014;10(6):844–852. doi: 10.1016/j.jalz.2014.01.001. https://doi.org/10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker C, Geerlings MI, Schmand B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. International Journal of Geriatric Psychiatry. 2000;15(11):983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Lista S, Molinuevo JL, Cavedo E, Rami L, Amouyel P, Teipel SJ, … Hampel H. Evolving Evidence for the Value of Neuroimaging Methods and Biological Markers in Subjects Categorized with Subjective Cognitive Decline. Journal of Alzheimer’s Disease. 2015;48(s1):S171–S191. doi: 10.3233/JAD-150202. https://doi.org/10.3233/JAD-150202. [DOI] [PubMed] [Google Scholar]
- Lo EH, Rosenberg GA. The Neurovascular Unit in Health and Disease Introduction. Stroke. 2009;40(3 suppl 1):S2–S3. doi: 10.1161/STROKEAHA.108.534404. https://doi.org/10.1161/STROKEAHA.108.534404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenthal KM. An Introduction to Psychological Tests and Scales. Psychology Press; 2001. [Google Scholar]
- Meltzer CC, Cantwell MN, Greer PJ, Ben-Eliezer D, Smith G, Frank G, … Price JC. Does Cerebral Blood Flow Decline in Healthy Aging? A PET Study with Partial-Volume Correction. Journal of Nuclear Medicine. 2000;41(11):1842–1848. [PubMed] [Google Scholar]
- Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. International Journal of Geriatric Psychiatry. 2008;23(11):1191–1202. doi: 10.1002/gps.2053. https://doi.org/10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatrica Scandinavica. 2014;130(6):439–451. doi: 10.1111/acps.12336. https://doi.org/10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, … de Leon MJ. Hypometabolism and Altered Cerebrospinal Fluid Markers in Normal Apolipoprotein E E4 Carriers with Subjective Memory Complaints. Biological Psychiatry. 2008;63(6):609–618. doi: 10.1016/j.biopsych.2007.05.030. https://doi.org/10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo OC, Xu G, Oh JM, Dowling NM, Carlsson CM, Gallagher CL, … Johnson SC. Cerebral Blood Flow is Diminished in Asymptomatic Middle-Aged Adults with Maternal History of Alzheimer’s Disease. Cerebral Cortex (New York, NY: 1991) 2014;24(4):978–988. doi: 10.1093/cercor/bhs381. https://doi.org/10.1093/cercor/bhs381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard L, Aamand R, Gutierrez-Jimenez E, Ho YC, Blicher JU, Madsen SM, … West MJ. The capillary dysfunction hypothesis of Alzheimer’s disease. Neurobiol Aging. 2013;34(4):1018–31. doi: 10.1016/j.neurobiolaging.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry. 2011;11:108. doi: 10.1186/1471-244X-11-108. https://doi.org/10.1186/1471-244X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. https://doi.org/10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, … Sikkes SAM. Subjective Cognitive Decline in Older Adults: An Overview of Self-Report Measures Used Across 19 International Research Studies. Journal of Alzheimer’s Disease: JAD. 2015;48(0 1):S63–S86. doi: 10.3233/JAD-150154. https://doi.org/10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisberg B, Gauthier S. Current evidence for subjective cognitive impairment (SCI) as the pre-mild cognitive impairment (MCI) stage of subsequently manifest Alzheimer’s disease. International Psychogeriatrics / IPA. 2008;20(1):1–16. doi: 10.1017/S1041610207006412. https://doi.org/10.1017/S1041610207006412. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2010;6(1):11–24. doi: 10.1016/j.jalz.2009.10.002. https://doi.org/10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy CS, Sherrington CS. On the Regulation of the Blood-supply of the Brain. The Journal of Physiology. 1890;11(1–2):85–158. 17. doi: 10.1113/jphysiol.1890.sp000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. Neuroanatomical substrates of age-related cognitive decline. Psychological Bulletin. 2011;137(5):753–784. doi: 10.1037/a0023262. https://doi.org/10.1037/a0023262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kolsch H, … Jessen F. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology. 2012;79(13):1332–1339. doi: 10.1212/WNL.0b013e31826c1a8d. https://doi.org/10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- Schmand B, Jonker C, Geerlings MI, Lindeboom J. Subjective memory complaints in the elderly: depressive symptoms and future dementia. The British Journal of Psychiatry. 1997;171(4):373–376. doi: 10.1192/bjp.171.4.373. https://doi.org/10.1192/bjp.171.4.373. [DOI] [PubMed] [Google Scholar]
- Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, Dufouil C. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. The British Journal of Psychiatry. 2011;198(3):199–205. doi: 10.1192/bjp.bp.110.078683. https://doi.org/10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical Science: A Review Journal of the Institute of Mathematical Statistics. 2010;25(1):1–21. doi: 10.1214/09-STS313. https://doi.org/10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yang FC, Lin CP, Han Y. Biochemical and Neuroimaging Studies in Subjective Cognitive Decline: Progress and Perspectives. CNS Neuroscience & Therapeutics. 2015;21(10):768–775. doi: 10.1111/cns.12395. https://doi.org/10.1111/cns.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z. Aging, Arterial Stiffness, and Hypertension. Hypertension. 2015;65(2):252–256. doi: 10.1161/HYPERTENSIONAHA.114.03617. https://doi.org/10.1161/HYPERTENSIONAHA.114.03617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie SCJ, Adriaanse SM, Binnewijzend MAA, Benedictus MR, Ossenkoppele R, Wattjes MP, … Barkhof F. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin? European Radiology. 2015;25(10):3050–3059. doi: 10.1007/s00330-015-3696-1. https://doi.org/10.1007/s00330-015-3696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, Larson EB. Subjective memory deterioration and future dementia in people aged 65 and older. Journal of the American Geriatrics Society. 2004;52(12):2045–2051. doi: 10.1111/j.1532-5415.2004.52568.x. https://doi.org/10.1111/j.1532-5415.2004.52568.x. [DOI] [PubMed] [Google Scholar]
- Wang, West J, Risacher S, McDonald B, Tallman E, Ghetti B, … Saykin A. Characterization of regional cerebral blood flow in mild cognitive impairment and older adults with cognitive complaints. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2013;9(4):P71. https://doi.org/10.1016/j.jalz.2013.05.114. [Google Scholar]
- Wang Z. Characterizing early Alzheimer’s disease and disease progression using hippocampal volume and arterial spin labeling perfusion MRI. Journal of Alzheimer’s Disease: JAD. 2014;42(Suppl 4):S495–502. doi: 10.3233/JAD-141419. https://doi.org/10.3233/JAD-141419. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Dev SI, Shin DD, Clark LR, Bangen KJ, Jak AJ, … Bondi MW. Effect of mild cognitive impairment and APOE genotype on resting cerebral blood flow and its association with cognition. Journal of Cerebral Blood Flow & Metabolism. 2012;32(8):1589–1599. doi: 10.1038/jcbfm.2012.58. https://doi.org/10.1038/jcbfm.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, Zlatar ZZ. Cerebral blood flow measured by arterial spin labeling MRI as a preclinical marker of Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 2014a;42(Suppl 4):S411–419. doi: 10.3233/JAD-141467. https://doi.org/10.3233/JAD-141467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CE, Hays CC, Zlatar ZZ. Cerebral Blood Flow Measured by Arterial Spin Labeling MRI as a Preclinical Marker of Alzheimer’s Disease. Journal of Alzheimer’s Disease: JAD. 2014b doi: 10.3233/JAD-141467. https://doi.org/10.3233/JAD-141467. [DOI] [PMC free article] [PubMed]
- Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network—a new paradigm in stroke pathophysiology. Nature Reviews Neurology. 2012;8(12):711–716. doi: 10.1038/nrneurol.2012.210. https://doi.org/10.1038/nrneurol.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlatar ZZ, Bischoff-Grethe A, Hays CC, Liu TT, Meloy MJ, Rissman RA, … Wierenga CE. Higher Brain Perfusion May Not Support Memory Functions in Cognitively Normal Carriers of the ApoE ε4 Allele Compared to Non-Carriers. Frontiers in Aging Neuroscience. 2016:151. doi: 10.3389/fnagi.2016.00151. https://doi.org/10.3389/fnagi.2016.00151. [DOI] [PMC free article] [PubMed]
- Zlokovic BV. Neurodegeneration and the neurovascular unit. Nature Medicine. 2010;16(12):1370–1371. doi: 10.1038/nm1210-1370. https://doi.org/10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]