Abstract
Fibrinogen-β (FBG-β), an important acute phase protein (APP), is generated by the liver as a target for inflammatory mediators. Here, we have identified FBG-β as a hepatitis C virus (HCV) core interacting protein by screening a human liver cDNA library using mammalian two-hybrid analysis. An association between FBG-β and HCV core protein was verified by confocal microscopy and coimmunoprecipitation from transfected human hepatocyte (Huh-7) cell line. HCV core or genomic RNA transfected Huh-7 cells modestly increased FBG-β protein expression when compared to the basal level in control hepatocytes. Transfection of HCV core or full-length (FL) gene into Huh-7 cells upegulated basal FBG-β promoter activity. Exogenous addition of IL-6 stimulates FBG-β promoter activity in hepatocytes. However, ectopic expression of HCV core or FL in hepatocytes inhibited IL-6 stimulated FBG-β promoter activation. Inhibition of endogenous FBG-β expression following introduction of siRNA into cells displayed a gain of function of promoter regulation by HCV core protein. Further studies suggested that HCV core gene expression in stable transfectants of Huh-7 cells resulted in a basal upregulation of FBG-β, and other APPs. However, treatment with cytokines, IL-6 or TNF-α, repressed FBG-β and other acute phase response (APR) genes. Conclusion: Our results reveal that the core/FBG-β interaction may act as a regulatory feedback, allowing repression of IL-6 stimulated APR genes. Together, these data suggested a network of interactions between HCV core and the hepatic APR genes, and may contribute to impaired innate immunity for viral persistence.
Keywords: Protein-protein interaction, mammalian two hybrid system, ectopic expression, interleukin-6, tumor necrosis factor-α
The acute-phase response (APR), an important pathophysiologic phenomenon, replaces the normal homeostatic mechanisms with new set of proteins that presumably contribute to defensive or adaptive capabilities.1–3 APR is an essential component of the innate immune system, and plays an important role in limiting hepatic tissue injury. Cytokines are the physiological mediators of APR. Cytokine-induction is characterized by changes in the concentrations of plasma proteins that are largely produced by hepatocytes.4 Fibrinogen-β (FBG), serum amyloid A (SAA), C-reactive protein (CRP), and haptoglobin (HP) are among the best defined APPs. APP genes are classified into two categories based on their cytokine responsiveness. IL-6 is the chief stimulator of the production of most acute-phase proteins.5 Type I APP genes are up-regulated by IL-1, IL-6, TNF-α, and glucocorticoids (GCs) or various combinations thereof, whereas type II genes respond to IL-6 and GCs.6 The FBG genes are classified as type II APP.7,8 Fibrinogen is expressed constitutively in the liver and in cultured hepatoma cell lines, but its production can be greatly altered during an APR by IL-6 alone or in combination with IL-1β.9–10 Neither TNF-α nor IL-1β induce expression of type II APP genes but instead frequently down-regulate their expression even in the presence of high levels of IL-6.11–12
HCV core protein has been shown to be at the crossroads among many physiological processes of liver disease progression. Our previous observations implicated that HCV core protein targets transcription factors known to be involved in the regulation of inflammatory responses and the immune system.13 We have shown that ectopic expression of HCV core protein in human hepatocytes results in an increase in the expression of IL-6, gp130, and Stat3.14 Gp130 plays a central role in cytokine action as a signal transducing receptor subunit common to all IL-6 type cytokines. Upregulation of these genes in turn may regulate activity downstream of the Stat3 signaling pathway. HCV core protein constitutively activates AP-1, which correlates with the activation of JNK and MAPKK.13 The MAPK system exerts influence on the basal expression of APPs.15 Activation of Erk1 and 2 is also part of the signaling reaction by IL-6 cytokines; because of the more prominent activation of Stat3, to achieve a high level of APP induction. HCV core protein differently regulates the JAK-STAT signaling pathway under IL-6 and IFN-γ stimuli.16 The expression of HCV core protein causes inhibition of JAK-STAT activation in the case of IL-6 treatment. HCV core-mediated modulation of JAK-STAT signaling pathway is exerted by binding to the JAK protein. Stat3/APRE-mediated transcriptional activity is suppressed by the expression of HCV core protein under IL-6 stimulation.
Here, we have investigated the role of HCV core protein in the modulation of hepatic APR genes. Our results indicated that HCV core protein associates with FBG-β, increases basal FBG-β promoter activity, and mRNA expression level. However, ectopic HCV core protein expression in hepatocytes inhibited IL-6 stimulated FBG-β promoter activity and APR mRNA expression levels.
EXPERIMENTAL PROCEDURES
HCV clones
HCV genotype 1a (GenBank accession number M62321) and clone H77 were used in this study.
Protein-fragment complementation assay (PCA)
The interaction between cellular proteins and HCV core protein was studied by PCA, as already described.17–19 PCA combines a simple cell-based cDNA-screening approach (interactions of a “bait” protein of interest with “prey” cDNA products) with specific functional assays that use the same system and provide initial validation of the cDNA products as being biologically relevant. A human liver cDNA library was inserted upstream of the 5’ end of a reporter yellow fluorescent protein (YFP) fragment (EYFP1-h-liver library). HCV core gene from genotype 1a was subcloned downstream of the 3’ end of the YFP fragment (YFP2-core191). All necessary reagents for the cloning of liver cDNA or HCV core gene into YFP fragments were kindly provided by S.W. Michnick (Universite de Montreal, Canada). Briefly, COS-7 cells were plated in 150-mm dishes 24 h before transfection. Cells were transfected (60 µg DNA /dish) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) at around 90% confluence with YFP1-cDNA library and YFP2-core. One day after transfection, cells were harvested by gentle pipetting with PBS containing 0.1mM EDTA, and cells were washed and suspended with 5% FBS. Approximately 2×107 cells were harvested and subjected to cell sorting. COS-7 cells transfected with empty vector and YFP1- and YFP2-leucine zipper expression vectors were used as negative and positive controls, respectively. Cells (4×104, 0.2% of total cell number) were sorted and approximately 70% purity of YFP-positive cells archived.
Cells and transfections
Huh-7 cells were transfected with a mammalian expression vector (pcDNA3) containing HCV core gene from genotype 1a under the control of a CMV promoter. Alternatively, cells were transfected with HCV full-length gene, mutated in the NS5B region, under the control of a CMV promoter (pCI-neo-HCV FL) using Lipofectamine 2000 (Life Technologies, Inc.). Stable cell colonies were selected using neomycin (800 µg/ml of G418). Pooled cells transfected with HCV genomic region were used in subsequent studies to avoid artifactual results from clonal variation. Parental Huh-7 cells transfected with empty vector were used in parallel as a control. Transfected Huh-7 cells were maintained in DMEM, containing 10% fetal calf serum and a lower dose of the selection antibiotic (400 µg/ml of G418).
Antibodies
Commercially available antibody to HCV core (mAB 017, Virogen, Watertown, MA), FBG-β, SAA-1, HP, CRP, and anti-HA antibody (Santa Cruz Biochemical, Santa Cruz, CA), and anti-human actin-horseradish peroxidase (Sigma, St. Louis, MO) were procured. A rabbit antiserum to HCV core protein was kindly provided by Arvind Patel (University of Glasgow, UK)
Confocal microscopy
Hepatocytes were fixed with formaldehyde (4%) for 30 minutes, and permeabilized with 0.2% (saponin-BSA) solution for 30 minutes at room temperature. Cells were extensively washed and incubated with anti-HCV core and/or anti-FBG-β, and stained with a secondary antibody conjugated with fluorochrome (Molecular Probes, Carlsbad, CA). Cell nuclei were stained with DAPI (Molecular Probes), and mounted for confocal microscopy (Olympus FV1000). Whenever necessary, the images were merged digitally to monitor colocalization in which two different colors produce a distinct color, whereas physically separate signals retain their individual colors.
In vivo co-immunoprecipitation assay
Huh-7 cells were transfected with FLAG-HCV core and/or HA-FBG-β cDNA construct using Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Empty vector DNA was used in the place of core plasmid DNA as a control. Cells were lysed 24 h after transfection with TNTG buffer (30 mM Tris, pH 8.0, 150 mM NaCl, 0.1% TritonX-100, 10% glycerol), and supplemented with a cocktail of protease inhibitors. After sonication, cell debris was removed by centrifugation. Clear cell lysates were mixed with anti-FLAG (for FLAG-Core interaction) or anti-HA antibody (for HA-FBG-β interaction) and protein-G sepharose beads for 14 h. The beads were washed with TNTG buffer, and subjected to SDS-PAGE under reducing conditions for Western blot analysis.
FBG-β promoter assay
Approximately 1800 bp of the promoter region of the FBG-β gene (−1788 to +8, taking position 1499 in Gen Bank accession number X05018 as −1) tagged with a liciferase reporter construct20 was kindly provided by Moniek P.M. deMaat, Netherland. Huh-7 cells were transfected with different doses of the plasmid DNA using Lipofectamine (Invitrogen, Carlsbad, CA). Cells were incubated in DMEM containing 0.5% fetal bovine serum after transfection. At 48 h post-transfection, cells were lysed with reporter lysis buffer (Promega, Madison, WI), and luciferase activity was determined using a luminometer (GloMax, Promega, Madison, WI) as described previously.21 Wherever necessary, siRNA to FBG-β (Santa Cruz Biotecnology, Inc.) was introduced into hepatocytes by electroporation prior to transfection of plasmid DNAs. For this, Huh-7 cells (5×105) were electroporated with 10 µM siRNA to FBG-β and seeded on a 24 well plate. Mock-electroporated cells were similarly used as a control. After 24h, cells were co-transfected with FBG-β-Luc (0.5 ug), and HCV core (250 ng). Luciferase activity was measured at 48 h post-transfection from triplicate wells. Luciferase activities are presented as an average from three independent experiments.
Real-time PCR
A quantitative real-time PCR analysis was performed for APR genes using specific primers (Table 1). Parental Huh-7 cells or stable transfectants expressing HCV core genotype 1a were treated separately with IL-6 (10 ng/ml) or TNF-α (20 ng/ml) for 6 h. RNA from cells was isolated with a kit following the supplier’s protocol (RNeasy mini kit, Qiagen). cDNA synthesis was carried out using random hexamers and Thermoscript II RNAse H reverse transcriptase (Invitrogen). mRNA expression of FBG-β, SAA-1, CRP and HP in the presence or absence of IL-6 or TNF-α was analyzed by quantitative real-time PCR using Power SYBR green PCR Master Mix (Applied Biosystems, Carlsbad, CA). RNA expression was normalized against GAPDH and the expression level was calculated using the deltadelta threshold cycle (ΔΔCT) method.
Table 1.
Primers used for real-time PCR for APR genes.
| APR gene |
Sense (5’-3’) primers | Anti-sense (5’-3’) primers |
|---|---|---|
| FBG-β | ATTAGCCAGCTTACCCAGGATGGGACCCAC | CAGTAGTATCTGCCGTTTGGATTGGCTGC |
| SAA-1 | CTGCAGAAGTGATCAGCG | ATTGTGTACCCTCTCCCC |
| CRP | ACTTCCTATGTATCCCTCAAAG | CTCATTGTCTTGTCTCTTGGT |
| HP | GCCCATCTGCCTACCATCC | GCCCCAGCCAGAAACATA |
RESULTS
Fibrinogen-β interacts with HCV core by protein fragment complementation assay (PCA)
We employed a liver cDNA library screening based on protein-fragment complementation assay (PCA) using yellow fluorescent protein (YFP) as a reporter to further gain insights into the mechanism of cellular functions of the HCV core protein (Fig. 1, panel A). FACS analysis of cells co-transfected with the expression plasmids were analyzed for positive YFP fluorescence. Cell sorting was performed for YFP fluorescent cells after a large-scale transfection. We initially identified a number of positive clones. Plasmid DNA was isolated from the clones, amplified and sequenced for confirmation of a positive interaction. Further testing for interaction revealed several clones which specifically interacted with HCV core and not with other heterologous protein baits. Approximately 4 × 104 cells (0.2% of the total cell number) were sorted and approximately 70% purity of YFP-positive cells was archived. The process was repeated twice to obtain specific interactive proteins. The clones were sequenced and analyzed by the BLAST program. Sequence analysis revealed that these isolates represented an independent overlapping cDNA with homology to FBG-β. A typical interaction of FBG-β protein with HCV core is shown from cotransfection of cells with HCV core (Fig. 1, panel B) The remaining clones identified from the PCA assay are under investigation and will be incorporated into separate studies. Among a number of other interacting clones identified from PCA, we focused on FBG-β to understand the potential functional relevance of this protein in HCV pathology.
Fig. 1. Mammalian two-hybrid analysis for protein-protein interaction.
Panel A: FACS analysis for hepatocellular protein interacting with HCV core by PCA. A human liver cDNA library was fused to fragment 1 of YFP (YFP[1]-cDNA library) and HCV core cDNA to fragment 2 (YFP2-core), in mammalian expression vectors harboring E. coli selection markers Ampicillin (Amp) and Kanamycin (Kan), respectively. COS-7 cells were cotransfected with core “bait” and human liver cDNA library “prey” fusions and positive clones indicating physical interaction between these proteins were identified by FACS. Panel B: A typical interaction of FBG-β protein with HCV core is shown from cotransfection of cells with the core and one of the identified clones (# 41) YFP fusions and detection by FACS. Dotted black line represents FBG-β clone and solid green line represent results from background control. Data were analyzed using FlowJo soft ware (Treestar). Percent of Max represents the number of events normalized according to FlowJo algorithm.
FBG-β associates with HCV core protein
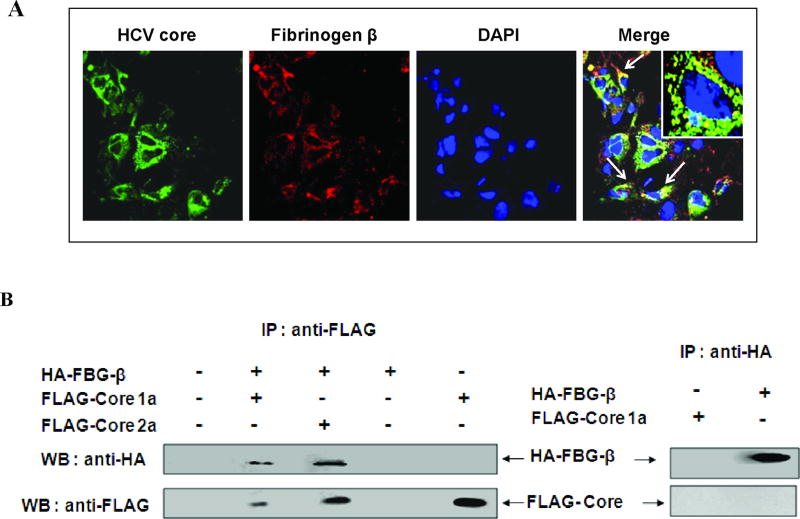
We examined Huh-7 cells stably transfected with HCV core for colocalization with FBG-β by immunofluorescence staining and confocal microscopy. Cells were fixed after 48 h of transfection and stained for immunofluorescence using antibodies to HCV core and FBG-β protein (Fig. 2, panel A). Cell nuclei were stained with DAPI. A merging of the distinct fluorochromes suggested co-localization of HCV core and FBG-β proteins outside the nucleus. Mock transfected cells were treated similarly with the antibodies as above, and did not display immunofluorescence for HCV core protein (not shown). A coimmunoprecipitation assay was performed using lysates of Huh-7 cells transiently transfected with HA-FBG-β and/or FLAG-core or empty vector as a negative control to verify the in vivo association of FBG-β and HCV core protein. Cell lysates were immunoprecipitated with a monoclonal antibody to FLAG or HA and separated by SDS-PAGE. The presence of FBG-β or HCV core protein was detected by immunoblotting with an antibody to the respective tag molecule (Fig. 2, panel B). The results from confocal microscopy and co-immunoprecipitation analysis suggested an association between FBG-β and HCV core proteins in Huh-7 cells.
Fig. 2. Association of fibrinogen-β and HCV core protein in hepatocytes.
Panel A: Co-localization of FBG-β and HCV core protein by confocal microscopy. FBG-β gene was introduced into Huh-7-HCV core stable transfectants. Cells were stained with an antibody to core tagged with Alexa green, antibody to FBG-β tagged with Alexa red, and DAPI for nuclear staining. Co-localization of merged green and red colors is shown, and the arrows point out the merged yellow color in cells. The inset in the merged immunofluorescence image shows a higher magnification of a single cell. Panel B: Co-immunoprecipitation of FBG-β and HCV core proteins. Huh-7 cells were transfected with empty vector DNA (negative control), plasmid DNAs encoding HA-FBG-β and/or FLAG-core, indicated on top of each lane. Cell lysates were immunoprecipitated with an antibody to FLAG or HA, and probed with HA or FLAG specific antibody to detect HA-FBG-β or FLAG-core protein. The presence of HA-FBG-β in transfected cells is also shown after immunoprecipitation and detection by HA specific antibody.
Ectopic expression of HCV core modestly enhanced FBG-β protein expression
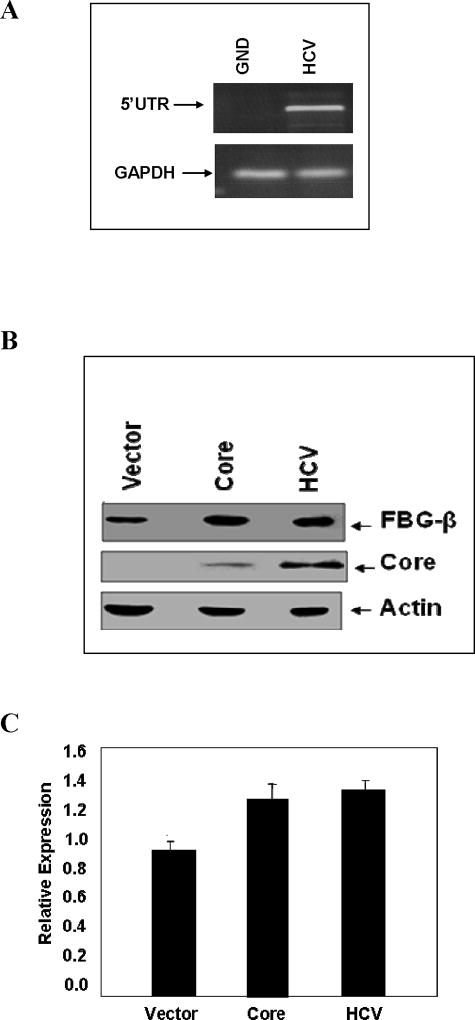
Acute-phase reactants are liver proteins whose synthesis is positively or negatively regulated during inflammation. The main mediators of this phenomenon are glucocorticoids and IL-6, a pleiotropic cytokine that also controls hematopoiesis. We determined whether ectopic expression of HCV core alters FBG-β protein expression. For this, Huh-7 cells were stably transfected with HCV core plasmid DNA, or electroporated with HCV full-length genomic RNA from genotype 1a or a NS5B polymerase defective HCV RNA (GND mutant) as a negative control. The replication of HCV genomic RNA, in comparison to the polymerase defective mutant, is shown (Fig. 3, panel A). Western blot analysis for FBG-β expression using a specific antibody is shown (Fig. 3, panel B). The relative level of FBG-β is shown by densitometric scanning after normalization against cellular actin (Fig. 3, panel C). The results suggested a modest increase in FBG-β protein expression in HCV core or genomic RNA transfected Huh-7 cells.
Fig. 3. HCV core protein expression modestly increases the basal level of FBG-β.
Panel A: Full-length RNA from HCV genotype 1a transfected cells were separately examined after 6 days by RT-PCR for the presence viral RNA using 5’UTR primers. A polymerase defective HCV RNA (GND mutant) was similarly tested as a negative control. GAPDH gene expression was used to normalize HCV RNA expression. Panel B: Huh-7 cells (control), cells transfected with HCV core plasmid DNA, or electroporated with full-length RNA from genotype 1a were lysed and subjected to Western blot analysis using an antiserum to FBG-β or HCV core protein. The blot was reprobed with an antibody to actin to ascertain the level of protein load in each lane. Positions of the protein bands are indicated by arrows on the right. Panel C: The relative level of FBG-β from experiments in panel B was estimated by densitometric scanning after normalization against actin and shown as bar diagrams. Error bars represent standard errors from three independent experiments.
HCV core attenuates IL-6 mediated FBG-β promoter activation
The acute phase response, an inflammatory process resulting from infection and/or tissue damage, is an early defense mechanism during which striking changes in protein synthesis occur mainly in the liver. IL-6 is recognized as the principal regulator of most APR genes including the three chains of FBG.10 FBG-β is considered as the rate-limiting chain for fibrinogen biosynthesis. To elucidate whether HCV core protein expression in hepatocytes modulates FBG-β at the transcriptional level, the promoter region of the human FBG-β gene linked to a luciferase reporter plasmid was used. This reporter construct was co-transfected with HCV core or FL plasmid DNA into Huh-7 cells. Cells transfected with HCV core and HCV FL displayed a ~16 fold and ~7 fold FBG-β promoter activation, respectively, as compared to basal level (Fig. 4, panel A). These results indicated that an upregulatory effect of HCV core alone or in context with other HCV proteins on human FBG-β expression at the transcriptional level.
Fig. 4. HCV core or FL transfected hepatocytes attenuate IL-6 stimulated FBG-β promoter activation.
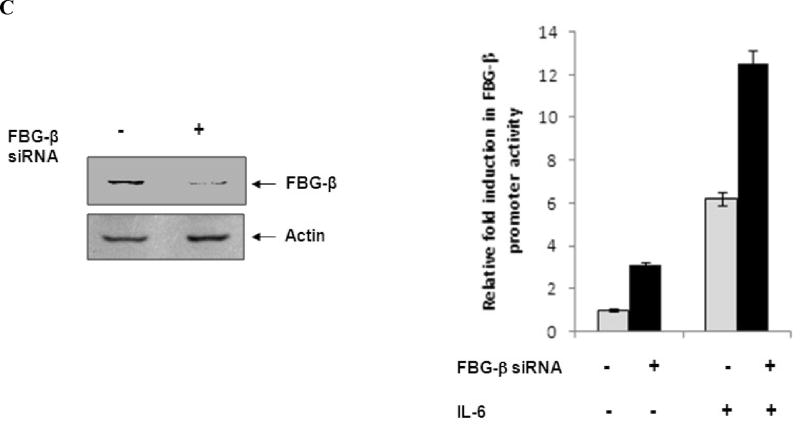
Panel A: Huh-7 cells were co-transfected with FBG-β-Luc (0.5 ug), and HCV core, FL or vector DNAs (250 ng). Luciferase activity was measured at 48 h post-transfection. Luciferase activities are presented as an average from three independent experiments with standard errors. The presence of HCV core protein in transfected cells were detected by Western blot analysis using a specific rabbit antiserum, and shown at the bottom of the bar diagram. Panel B: Transfected hepatocytes were treated with IL-6 (50 ng/ml) for 16 h before promoter assay. Fold difference of luciferase activity in the presence of IL-6 in HCV core or FL transfected hepatocytes in comparison to vector control is shown. Panel C: Huh-7 cells were electroporated with 10 µM siRNA to FBG-β in triplicate. Mock-electroporated cells were similarly used as a control. After 24 h, cells were co-transfected with FBG-β-Luc (0.5 ug), and HCV core (250 ng). Western blot analysis showing a reduction in endogenous FBG-β expression in transfected cells as compared to mock control is shown. Luciferase activity was measured at 48 h post-transfection. Transfected hepatocytes were treated with IL-6 (50 ng/ml) for 16 h before luciferase assay. Luciferase activities are presented as an average from three independent experiments with % errors.
The altered expression of many APR genes is regulated at the transcriptional level after hepatocyte stimulation by cytokines. IL-6 signaling is known to involve the activation of two independent transcription factors: Stat3 and C/EBP-β.22 We next examined the effect of exogenous IL-6 on FBG-β promoter activity in the presence of HCV core or HCV FL transfected cells. Huh-7 cells were treated with IL-6 after 48 h of transfection, and luciferase activity was measured after 16 h of cytokine treatment. IL-6 treatment enhanced the FBG-β promoter activity ~25 fold. However, in the presence of HCV core or FL, IL-6 enhanced only ~ 2 fold and ~4 fold promoter activation, respectively (Fig. 4, panel B). Therefore, we observed a significant reduction of FBG promoter activity upon IL-6 treatment in core or FL transfected cells as compared to mock transfected control. Our results indicated a significant level of attenuation of IL-6 mediated FBG-β promoter activation upon HCV core protein expression.
To further examine whether an attenuation of IL-6-stimulated FBG-β promoter activity is due to an interaction between HCV core and FBG-β, siRNA was used to knock down FBG-β in Huh-7 cells. Cells displayed ~ 70% reduction in FBG-β protein expression 72h after electroporation by Western blot analysis (Fig. 4, panel C). FBG-β knocked down cells displayed ~3 fold gain in promoter activity in the presence or absence of IL-6, suggesting that FBG-β may have a self limiting role in promoter regulation. Since knockdown of FBG-β in the presence of core protein displayed a gain in promoter function, the results suggested that HCV core/FBG-β interaction is involved in promoter modulation. An in-depth study would be required to further understand the precise mechanism for IL-6 stimulated APP promoter regulation by HCV core.
Ectopic expression of HCV core attenuates cytokine stimulated APR gene expression
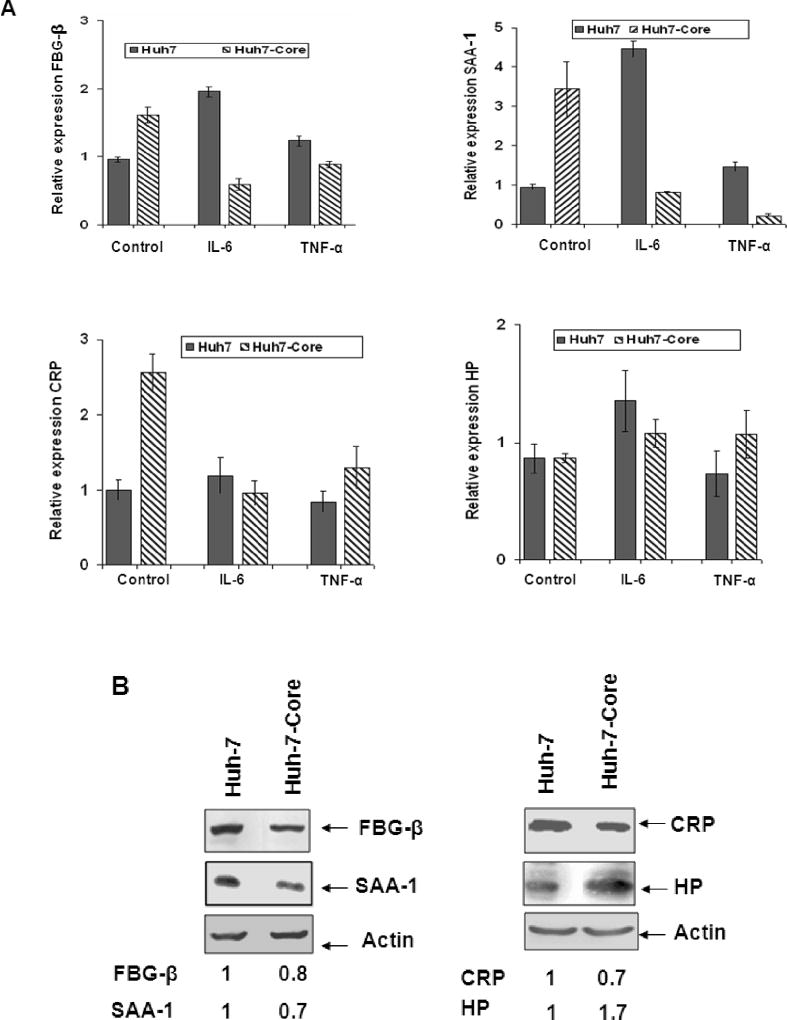
Since the presence of HCV core protein could be associated with attenuation of IL-6 mediated FBG-β promoter activation, here we examined the mRNA status of select APR genes by real-time PCR in IL-6 treated Huh-7 cells stably expressing HCV core. Cells were also treated separately with TNF-α for comparison. Stable transfectants were studied in our experiments since HCV often causes persistent infection and chronic changes of APR are considered to be detrimental rather than beneficial to the host. Mock-transfected control cells were used for comparison. Real-time PCR analysis suggested a ~2 fold increase in FBG-β mRNA expression in stable transfectants with HCV core (Fig. 5, panel A). On the other hand, transfected cells displayed ~3 fold decrease in FBG-β mRNA level upon IL-6 treatment. FBG-β regulation at the transcriptional level by TNF-α also indicated an inverse correlation in the cytokine response in HCV core treated cells. Similar observations were also made with Huh-7 cells transfected with a full-length RNA from HCV genotype 1a (data not shown).
Fig. 5. Ectopic expression of HCV core protein attenuates cytokine stimulated acute phase response.
Panel A: Real-time PCR analysis for mRNA expression of FBG-β, SAA-1, CRP, and HP was performed in control Huh-7 and HCV core stable transfectants of Huh-7 cells. Hepatocytes were treated with IL-6 (10 ng/ml) or TNF-α (20 ng/ml) for 6 h prior to RNA isolation. Standard error bars are shown from three different experiments. Panel B: Western blot analysis for FBG-β, SAA-1, CRP, and HP expression was performed using control Huh-7 and HCV core stable transfectants of Huh-7 cells. Hepatocytes were treated with IL-6 (10 ng/ml) for 6h prior to protein anlysis. The blots were run in two different sets (shown on left and right), and reprobed with an antibody to actin to ascertain the level of protein load in each lane. The relative level of specific proteins were estimated by densitometric scanning after normalization with actin, and the values are shown with that of control cells arbitrarily considered as 1 at the bottom.
We performed similar analyses for mRNA status of serum amyloid A-1 (SAA-1), C-reactive protein (CRP), and haptoglobin (HP). Results suggested an increase in SAA-1 mRNA level (~ 3.5 fold) (Fig. 5, panel A). IL-6 treatment of cells displayed ~3 fold decrease in SAA-1 mRNA level. Similarly, when cells were treated with TNF-α, HCV core stable transfectants decreased SAA-1 mRNA level (~ 4 fold). Analyses for CRP gene regulation displayed an increase in CRP mRNA level (~ 2.5 fold) (Fig. 5, panel A). IL-6 treatment did not significantly alter CRP mRNA level. TNF-α treatment also did not significantly alter CRP mRNA. Similar analyses for HP regulation did not suggest a major change at the mRNA level upon HCV core gene expression in the presence or absence of IL-6 or TNF-α.
An increase in mRNA level may not necessarily correlate with the respective protein expression level as the stability of both mRNA and protein will determine their expression status. For this, Western blot analysis was performed for the detection of the respective APPs by specific antibodies. The results suggested a reduced level of protein expression for the 3 APPs (FBG-β, SAA-1, and CRP) in IL-6 treated HCV core expressing cells (Fig. 5, panel B). On the other hand, HP-expression level increased in IL-6 treated HCV-core expressing cells as compared to parental cells. This could be due to HP protein stability in the presence of HCV core, and would warrant further investigation. We did not perform similar analysis with TNF-α treated hepatocytes, as there were modest changes of these APR genes at the mRNA level. Taken together, our results suggested that the presence of HCV core protein does not allow for an increase in FBG-β, SAA-1, and CRP gene expression at the mRNA or protein level, but attenuates their expression to different extents.
DISCUSSION
Host-pathogen crosstalk involves a high number of proteins, often with redundant functions. In the present study, we have identified FBG-β as a HCV core interacting protein by screening a human liver cDNA library. Confocal microscopy and coimmunoprecipitation analysis further suggested a physical association of the viral protein with cellular protein. Fibrinogen plays a key role in blood coagulation and thrombosis, and participates in the host defense system against infections. Cytokine stimulation regulates the expression level of APR genes, and IL-6 is a potent activator of FBG-β gene expression. However, we observed attenuation of IL-6 mediated FBG-β promoter activation in the presence of HCV core protein. A decrease in mRNA expression of FBG-β, SAA-1, and CRP in IL-6 or TNF-α stimulated core transfected cells further supported our observations that HCV core protein attenuates cytokine stimulated APR. Our observations appear to be a direct consequence of ectopic expression of the core protein, either alone or together with other viral proteins from a FL gene construct.
Human fibrinogen has been implicated in different host-pathogen interactions.23 Fibrinogen related-proteins (FREPs) can recognize a wide range of pathogens, from prokaryotes to eukaryotes, and different categories of FREPs seem to exhibit functional specialization with respect to the pathogen encountered. A growing body of evidence suggests an important role for FREPs in innate immunity. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway and may interfere with innate immunity.23,24 S. aureus produces a variety of secreted proteins which bind to the extracellular fibrinogen/C3-binding protein (Efb).25 In chronically HCV infected patients, the level of specific complement components is depleted, and C4 activity is significantly lower.26,27 Additionally, restoration of specific C4 activity occurred in subjects that responded well to interferon treatment. Our real-time PCR analysis using Huh-7 cells transfected with HCV core clearly show changes in APR gene expression similar to that seen during chronic infection of hepatocytes by HCV. We do not know whether an interaction of HCV core and FBG-β has a role on complement regulation in virus infected hepatocytes, and further studies are in progress in understanding the functional consequence.
HCV positive patients tended to have lower CRP levels in relation to IL-6 levels than HCV negative patients.28 The observed difference, especially with the stable Huh-7 cell transfectants with HCV core, could imply that liver response to IL-6 stimulation may be mitigated by the presence of HCV, and this is in agreement with our in vitro results on the negative regulatory role of HCV on APR genes, including CRP. SAA is dramatically induced in plasma during the acute phase response, and is able to displace APOA1 from HDL in vitro.29 In addition, because SAA binds to proteoglycans, SAA-containing HDL may be retained by the vascular matrix, leading to the formation of proatherogenic particles that do not participate in reverse cholesterol transport anymore. HCV infection is associated with clinically significant lower cholesterol levels (TC, LDL and HDL) when compared with normal subjects.30 Thus, the results implicate that HCV core protein may modulate HDL levels by negatively regulating APR genes.
The results with HCV core and IL-6 have broadened our view on the control of APR genes in hepatocytes. A striking effect of HCV core in Huh-7 cells is the reduced expression of certain APR genes by IL-6. The results suggest that Huh-7 cells possess an efficient signaling pathway that is utilized by HCV core protein to modulate APR gene expression. The differences in regulation of specific APR genes, such as FBG-β, in the presence or absence of HCV core by IL-6 also highlight that HCV core protein might involve a separate mode of transcriptional control mechanism that only in part include common components. Information available so far does not suggest a direct binding of HCV core protein with DNA. Knockdown of FBG-β in Huh-7 cells displayed a gain of FBG-β promoter function, suggesting that FBG-β may have a role in promoter regulation and is modulated in association with HCV core protein. An in-depth study would require in understanding the precise mechanism for APP promoter regulation by cytokine. Hepatocytes in culture are incomplete representatives of cells in vivo. However, our results are in agreement with the clinical observations from HCV infected patients.28–30 The observations also indicate an interesting molecular link between HCV core protein and the regulation of hepatic APR, and suggest that core protein could play opposing effects between cytokine stimulation14 and an anti-inflammatory response. This activity may be related to a novel persistence mechanism by which HCV is protected from innate immunity. Further understanding the complex mechanisms of HCV core dependent inhibition of APR remains to be elucidated.
Acknowledgments
Financial Support: This work was supported by grants U54-AI057160 (RR) from the NAID to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE), and DK080812 from NIDDK.
We thank S.W. Michnick for providing reagents for protein-fragment complementation assay; Moniek P.M. deMaat for the FBG-β promoter luciferase construct; and Lin Cowick for preparation of the manuscript.
List of Abbreviations
- HCV
Hepatitis C virus
- FBG-β
Fibrinogen-β
- APP
Acute phase protein
- APR
Acute phase response
- Huh-7
Human hepatocyte cell line
- HCV FL
HCV full-length genome
- IL-6
Interleukin-6
- IL-1
Interleukin-1
- TNF-α
Tumor necrosis factor-α
- SAA
Serum amyloid A
- CRP
C-reactive protein
- HP
Haptoglobin
- GC
Glucocorticoid
References
- 1.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 2.Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med. 2001;344:907–16. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 3.Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 4.Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behav & Immun. 2003;17:350–64. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- 5.Gauldie J, Richards C, Harnish D, Lansdorp P, Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc. Natl. Acad. Sci. 1987;84:7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, Fabra R, Heinrich PC. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 7.Otto JM, Grenett H, Fuller GM. The coordinated regulation of fibrinogen gene transcription by hepatocyte-stimulating factor and dexamethasone. J. Cell Biol. 1987;105:1067–1072. doi: 10.1083/jcb.105.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson-Haidaris PJ. Induction of fibrinogen biosynthesis and secretion from cultured pulmonary epithelial cells. Blood. 1997;89:873–882. [PubMed] [Google Scholar]
- 9.Zhang Z, Fuller GM. Interleukin-1β inhibits interleukin 6-mediated rat γ fibrinogen gene expression. Blood. 2000;96:3466–3472. [PubMed] [Google Scholar]
- 10.Westra J, Bijzet J, Doornbos-van der Meer B, van Rijswijk MH, Limburg PC. Differential influence of p38 mitogen activated protein kinase (MAPK) inhibition on acute phase protein synthesis in human hepatoma cell lines. Ann of the Rheum. Dis. 2006;65:929–35. doi: 10.1136/ard.2005.043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bode JG, Fischer R, Haussinger D, Graeve L, Heinrich PC, Schaper F. The Inhibitory Effect of IL-1β on IL-6-Induced α2-Macroglobulin Expression Is Due to Activation of NF-κB. J. Immunol. 2001;167:1469–1481. doi: 10.4049/jimmunol.167.3.1469. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Jones S, Hagood JS, Fuentes NL, Fuller GM. STAT3 acts as a co-activator of glucocorticoid receptor signaling. J Biol Chem. 1997;272:30607–30610. doi: 10.1074/jbc.272.49.30607. [DOI] [PubMed] [Google Scholar]
- 13.Shrivastava A, Manna SK, Ray R, Aggarwal BB. Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J. Virology. 1998;72:9722–8. doi: 10.1128/jvi.72.12.9722-9728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Basu A, Meyer K, Lai KK, Saito K, Di Bisceglie AM, Grosso LE, Ray RB, Ray R. Microarray analyses and molecular profiling of Stat3 signaling pathway induced by hepatitis C virus core protein in human hepatocytes. Virol. 2009;49:347–58. doi: 10.1016/j.virol.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Ripperger J, Fey GH, Samols D, Kordula T, Wetzler M, Van Etten RA, Baumann H. 1999. Modulation of hepatic acute phase gene expression by epidermal growth factor and src protein tyrosine kinases in murine and human hepatic cells. Hepatology. 1999;30:682–697. doi: 10.1002/hep.510300318. [DOI] [PubMed] [Google Scholar]
- 16.Hosui A, Ohkawa K, Ishida H, Sato A, Nakanishi F, Ueda K, Takehara T, Kasahara A, Sasaki Y, Hori M, Hayashi N. Hepatitis C virus core protein differently regulates the JAK-STAT signaling pathway under interleukin-6 and interferon-gamma stimuli. J Biol Chem. 2003;278:28562–71. doi: 10.1074/jbc.M210485200. [DOI] [PubMed] [Google Scholar]
- 17.Remy I, Michnick SW. A cDNA library functional screening strategy based on fluorescent protein complementation assays to identify novel components of signaling pathways. Methods. (Duluth) 2004a;32:381–388. doi: 10.1016/j.ymeth.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 18.Remy I, Michnick SW. Mapping biochemical networks with protein-fragment complementation assays. Methods in Mol Biol. 2004b;261:411–426. doi: 10.1385/1-59259-762-9:411. [DOI] [PubMed] [Google Scholar]
- 19.Banerjee A, Saito K, Meyer K, Banerjee S, Ait-Goughoulte M, Ray RB, et al. Hepatitis C virus core protein and cellular protein HAX-1 promotes 5-fluorouracil mediated hepatocyte growth inhibition. J. Virol. 2009;83:9663–9671. doi: 10.1128/JVI.00872-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verschuur M, de Jong M, Felida L, de Maat MPM, Vos HL. A Hepatocyte Nuclear Factor-3 Site in the Fibrinogen-β Promoter Is Important for Interleukin 6-induced Expression, and Its Activity Is Influenced by the Adjacent −148C/T Polymorphism. J. Biol. Chem. 2005;280:16763–16771. doi: 10.1074/jbc.M501973200. [DOI] [PubMed] [Google Scholar]
- 21.Majumder M, Ghosh AK, Steele R, Ray R, Ray RB. Hepatitis C virus NS5A physically associates with p53 and regulates p21/waf1 gene expression in a p53-dependent manner. J. Virol. 2001;75:401–1407. doi: 10.1128/JVI.75.3.1401-1407.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koj A. Initiation of acute phase response and synthesis of cytokines. Biochimica et Biophysica Acta. 1996;1317:84–94. doi: 10.1016/s0925-4439(96)00048-8. [DOI] [PubMed] [Google Scholar]
- 23.Margarit I, Bonacci S, Pietrocola G, Rindi S, Ghezzo C, Bombaci M, Nardi-Dei V, Grifantini R, Speziale P, Grandi G. Capturing host-pathogen interactions by protein microarrays: identification of novel streptococcal proteins binding to human fibronectin, fibrinogen, and C4BP. FASEB J. 2009 doi: 10.1096/fj.09-131458. (in press) [DOI] [PubMed] [Google Scholar]
- 24.Carlsson F, Sandin C, Lindahl G. 2005. Human fibrinogen bound to Streptococcus pyogenes M protein inhibits complement deposition via the classical pathway. Mol. Microbiol. 2005;56:28–39. doi: 10.1111/j.1365-2958.2005.04527.x. [DOI] [PubMed] [Google Scholar]
- 25.Lee LYL, Liang X, Höök M, Brown EL. Identification and Characterization of the C3 Binding Domain of the Staphylococcus aureus Extracellular Fibrinogen-binding Protein (Efb) J. Biol. Chem. 2004;279:50710–50716. doi: 10.1074/jbc.M408570200. [DOI] [PubMed] [Google Scholar]
- 26.Daoud MS, el-Azhary RA, Gibson LE, Lutz ME, Daoud S. Chronic hepatitis C, cryoglobulinemia, and cutaneous necrotizing vasculitis. Clinical, pathologic, and immunopathologic study of twelve patients. J. Am. Acad. Dermatol. 1996;34:219–223. doi: 10.1016/s0190-9622(96)80115-0. [DOI] [PubMed] [Google Scholar]
- 27.Dumestre-Perard C, Ponard D, Drouet C, Leroy V, Zarski JP, Dutertre N, Colomb MG. Complement C4 monitoring in the follow-up of chronic hepatitis C treatment. Clin. & Exper. Immunol. 2002;127:131–136. doi: 10.1046/j.1365-2249.2002.01729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nascimento MM, Bruchfeld A, Suliman ME, Hayashi SY, Pecoits-Filho R, Manfro RC, Pachaly MA, Renner L, Stenvinkel P, Riella MC, Lindholm B. Effect of hepatitis C serology on C-reactive protein in a cohort of Brazilian hemodialysis patients. Brazilian J Medical and Biol Res. 2005;38:783–788. doi: 10.1590/s0100-879x2005000500017. [DOI] [PubMed] [Google Scholar]
- 29.Van Lenten BJ, Wagner AC, Nayak DP, Hama S, Navab M, Fogelman AM. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation. 2001;103:2283–2288. doi: 10.1161/01.cir.103.18.2283. [DOI] [PubMed] [Google Scholar]
- 30.Siagris D, Christofidou M, Theocharis GJ, Pagoni N, Papadimitriou C, Lekkou A, Thomopoulos K, Starakis I, Tsamandas AC, Labropoulou-Karatza C. Serum lipid pattern in chronic hepatitis C: histological and virological correlations. J Viral Hepat. 2006;13:56–61. doi: 10.1111/j.1365-2893.2005.00655.x. [DOI] [PubMed] [Google Scholar]