Abstract
BACKGROUND
Associations between dairy intake and body mass index (BMI) have been inconsistently observed in epidemiological studies, and the causal relationship remains ill defined.
METHODS
We performed Mendelian randomization (MR) analysis using an established dairy intake-associated genetic polymorphism located upstream of the lactase gene (LCT-13910 C/T, rs4988235) as an instrumental variable (IV). Linear regression models were fitted to analyze associations between (a) dairy intake and BMI, (b) rs4988235 and dairy intake, and (c) rs4988235 and BMI in each study. The causal effect of dairy intake on BMI was quantified by IV estimators among 184802 participants from 25 studies.
RESULTS
Higher dairy intake was associated with higher BMI (β = 0.03 kg/m2 per serving/day; 95% CI, 0.00–0.06; P = 0.04), whereas the LCT genotype with 1 or 2 T allele was significantly associated with 0.20 (95% CI, 0.14–0.25) serving/day higher dairy intake (P = 3.15×10−12) and 0.12 (95% CI, 0.06–0.17) kg/m2 higher BMI (P = 2.11×10−5). MR analysis showed that the genetically determined higher dairy intake was significantly associated with higher BMI (β = 0.60 kg/m2 per serving/day; 95% CI, 0.27–0.92; P = 3.0×10−4).
CONCLUSIONS
The present study provides strong evidence to support a causal effect of higher dairy intake on increased BMI among adults.
The prevalence of obesity has been rapidly increasing over the world and is paralleled by a historic shift of lifestyle from traditional healthy patterns toward unhealthy patterns (1). However, the causal relationships between lifestyle factors and obesity have yet to be fully elucidated.
A body of observational epidemiologic studies investigating the association between dairy intake and weight status has reported inconsistent results. A recent systematic review of prospective cohort studies showed a negative association of dairy consumption with risk of overweight and obesity, but considerable heterogeneities existed (2), making definitive conclusions difficult. Metaanalyses of randomized controlled trials (RCTs)74 suggest that dairy consumption may not influence body weight when all the participants are analyzed, but it may reduce body weight and fat mass in the context of energy restriction (3–6). However, because of the relatively short duration of intervention (often <1 years) and the special populations often evaluated (e.g., obese individuals seeking weight loss), the effects of habitual dairy intake on body weight in general populations remain unclear.
Mendelian randomization (MR) analysis has become widely used to assess potential causal relations of environmental risk factors and diseases (7). This method is analogous to an RCT in which randomization to genotype takes place at conception (8, 9). In our recent MR analysis, we demonstrated that dairy intake was not causally related to hypertension, using an established dairy intake-associated genetic variant near the lactase gene LCT75 (10). In the current study, we performed the largest MR analysis thus far among 184802 adult participants from 25 cohorts to examine the causal relationship between habitual dairy intake and body weight in general populations.
Methods
STUDY DESIGN
The study design consisted of 2 steps. First, using cross-sectional and prospective cohort studies, we tested the dairy intake-associated LCT-13910 C/T, rs4988235 for association with diary intake and body mass index (BMI). Second, the causal effect of dairy intake on BMI was quantified with instrumental variable (IV) estimators among 184802 participants from 25 studies.
STUDY PARTICIPANTS
The study was conducted within the Mendelian Randomization of Dairy Consumption Working Group, represented here by 25 cohort studies and up to 184802 individuals (see Table 1 in the Data Supplement that accompanies the online version of this article at http://www.clinchem.org/content/vol64/issue1). Descriptions of each participating study are shown in Table 2 of the online Data Supplement. Participants from all participating studies provided written informed consent, and ethical approval was granted by local institutional review boards (see Table 3 in the online Data Supplement). Intake of dairy products was collected by self-reported questionnaire in each study; detailed information on cohort-specific data collection methods is provided in Table 4 of the online Data Supplement. Total dairy products included skim/low fat milk, whole milk, ice cream, yogurt, cottage/ricotta cheese, cream cheese, other cheese, and cream.
The primary outcome is follow-up BMI or baseline BMI, calculated as weight in kilograms divided by the square of height in meters. Height and body weight were directly measured in some studies and self-reported in others. Detailed information on the outcome measure for each study is reported in Table 5 of the online Data Supplement.
SINGLE-NUCLEOTIDE POLYMORPHISM SELECTION AND GENOTYPE PROPERTIES
The LCT-13910 C/T polymorphism (rs4988235), located upstream from the LCT gene, affects the transcription of the lactase enzyme and is associated with lactase persistence and thereby with the ability to digest lactose, the primary source of carbohydrates in milk (11). Lactase persistence is a dominantly inherited genetic trait. The TT and TC genotypes are associated with lactase persistence, and the CC genotype is associated with nonpersistence. In the present study, we chose the widely confirmed and extensively studied variant rs4988235 as the IV for dairy intake (12–14). Twenty studies used direct genotype information on rs4988235 from previously genotyped array data. Whenever rs4988235 was not genotyped directly, we used either (a) the HapMap II CEU (European) reference panel-imputed genetic information from genome-wide association studies (http://hapmap.ncbi.nlm.nih.gov/downloads/genotypes/2008-10_phaseII/) for rs4988235 or (b) genotype information from a predefined list of proxies that are in high linkage disequilibrium with rs4988235 (n = 5, r2 > 0.9). Genotyping platforms, genotype frequencies, Hardy–Weinberg equilibrium P values, and call rates (median of 98.8%) for LCT-13910 C/T are listed in Tables 1 and 6 of the online Data Supplement.
STATISTICAL ANALYSIS
A standard analysis protocol was applied to each individual study to produce comparable results. As lactase persistence is a dominantly inherited genetic trait, we examined the genetic association primarily under a dominant model (CC vs CT + TT). We also applied an additive model and recessive model (CC + CT vs TT) to examine the genetic association of LCT-13910 C/T with dairy intake and BMI. Linear regression was used to test the association of dairy intake with BMI after adjustment for age, sex, ethnicity, region, years of follow-up, and other baseline covariates (smoking status, physical activity, total energy intake, and alcohol intake), as available. Linear regression was used to test the association of LCT-13910 C/T with dairy intake or BMI after adjustment for age, sex, ethnicity, region, and total energy. Table 5 in the online Data Supplement shows the BMI outcome information.
METAANALYSIS AND BETWEEN-STUDY HETEROGENEITY
Metaanalyses were conducted using individual participant data in each study and then pooled β coefficients across studies using random-effects or fixed-effects metaanalysis. Metaanalyses were conducted in Stata version 13.0 (StataCorp, www.stata.com). All P values reported were 2-sided. We assessed between-study heterogeneity via Cochrane’s Q and I2 statistics (15–17). For the proposed cutoff of I2 > 0.25, we found nonnegligible heterogeneity between studies, in particular among the dairy–BMI associations, but also for the association between LCT-13910 C/T and dairy intake (I2 = 0.55). As a consequence, we used random-effects metaanalysis throughout. Furthermore, metaregression was used to investigate the extent to which statistical heterogeneity between results of studies could be related to 1 or more characteristics of the studies.
SE AND INFERENCE FOR THE IV ESTIMATOR
After metaanalysis, we used the IV estimator to quantify the strength of the causal association of dairy intake and BMI (18). The IV estimator was calculated as the β of the regression coefficients for LCT-13910 C/T-BMI and LCT-13910 C/T-dairy and is identical to that derived by the widely used 2-stage least-squares method (19) (see Material section in the online Data Supplement).
Results
BASELINE CHARACTERISTICS OF PARTICIPATING STUDIES
Baseline characteristics of the 184802 participants from 25 studies are shown in Table 1 here and in Table 1 of the online Data Supplement. A description of each study and additional characteristics of participants are presented in Tables 1 through 6 of the online Data Supplement. Twenty studies provided data for LCT-13910 C/T, and 5 studies provided results for the proxy single-nucleotide polymorphism (defined on the basis of r2 ≥ 0.90 with rs4988235 in individuals; see Table 6 in the online Data Supplement). Findings from χ2 tests showed that the CCHS, CGPS, FamHS, and GLACIER did not achieve Hardy–Weinberg equilibrium (see Table 6 in the online Data Supplement).
Table 1.
Baseline characteristics of participating studies.
| Studies | Sample size | Study design | Baseline year | Follow-up time, year | Age, year | BMI, kg/m2 |
|---|---|---|---|---|---|---|
| ARIC-AA | 1889 | Cohort | 1987 | 5.8 | 53.2 ± 5.7 | 29.7 ± 5.9 |
| ARIC-EA | 8233 | Cohort | 1987 | 6 | 54.3 ± 5.6 | 27.0 ± 4.7 |
| BPHRSa | 845 | Cohort | 2003 | 2.4 | 57.0 ± 8.0 | 32 ± 7.0 |
| CCHS | 8702 | Cohort | 1991–1994 | 20 | 60.0 ± 8.0 | 25 ± 4.0 |
| CGPSa | 74128 | Cohort | 2003–2011 | 5.7 | 57.0 ± 8.0 | 25.6 ± 4.0 |
| CHS | 1943 | Cohort | 1989–1990 | 8.9 | 71.1 ± 4.3 | 26.4 ± 4.2 |
| DESIR | 3468 | Cohort | 1994–1996 | 9 | 47.2 ± 9.9 | 24.6 ± 3.6 |
| DCH | 1297 | Nested cohort | 1993–1997 | 5 | 55.9 ± 4.4 | 25.2 ± 3.5 |
| Diogenes-C | 1002 | Nested case–cohort | 1993–1997 | 5 | 53.6 ± 2.60 | 25.5 ± 3.6 |
| Diogenes-W | 813 | Nested case–cohort | 1993–1997 | 5 | 53.4 ± 2.6 | 26.9 ± 4.0 |
| FamHS | 2167 | Family-based cohort | 1992 | 7.9 | 50.6 ± 10.0 | 28.8 ± 5.0 |
| GESUSa | 14751 | Cohort | 2010–2013 | 2.1 | 56.0 ± 4.0 | 26.1 ± 4.0 |
| GLACIER | 3129 | Cohort | 1991–2001 | 9.9 | 45.2 ± 6.7 | 25.1 ± 3.7 |
| GOLDNa | 818 | Cohort | — | 0 | 49.0 ± 1.06 | 28.0 ± 5.0 |
| HPFS | 7599 | Cohort | 1990 | 10 | 57.7 ± 11.8 | 25.9 ± 3.3 |
| INCHa | 647 | Cohort | 1998 | 8.7 | 63.4 ± 14.8 | 27.1 ± 4.0 |
| Inter99 | 6161 | Cohort | 1999 | 5 | 46.2 ± 7.9 | 26.3 ± 4.6 |
| MDCS | 3199 | Cohort | 1991–1996 | 16.7 | 56.3 ± 5.7 | 25.4 ± 3.7 |
| MESA | 2423 | Cohort | 1990 | 10 | 60.7 ± 9.6 | 28.2 ± 5.2 |
| NHS | 12039 | Cohort | 1990 | 10 | 57.3 ± 9.6 | 26.2 ± 5.2 |
| PREDIMED-Valencia | 940 | Cohort | 2003 | 2 | 67.0 ± 7.0 | 30.1 ± 4.2 |
| RAINE | 730 | Cohort | 2010 | 2.1 | 19.9 ± 0.3 | 24.3 ± 5.1 |
| RS | 3215 | Cohort | 1990 | 6.5 | 65.8 ± 6.8 | 26.3 ± 3.5 |
| WGHS | 23294 | Cohort | 1992 | 2 | 54.2 ± 7.1 | 25.9 ± 4.9 |
| YFS | 1370 | Cohort | 1980 | 4 | 38.1 ± 4.0 | 25.8 ± 5.0 |
Data were analyzed cross-sectionally.
ARIC-AA, ARIC (African Ancestry); ARIC-EA, ARIC (European Ancestry).
DAIRY INTAKE AND BMI
Random-effects metaanalysis was used to pool the association between dairy intake and BMI in the 176100 participants from 24 studies. We observed that high dairy intake was significantly associated with higher BMI (β = 0.03 kg/m2 per serving/day; 95% CI, 0.00–0.06; P = 0.04). The β coefficient estimates of effect sizes for the association between dairy intake and BMI ranged from −0.18 to 0.20 kg/m2 per serving/day of dairy intake, yielding an I2 for heterogeneity between studies of 79.7% (Fig. 1). Metaregression analysis showed that age significantly influenced the association of dairy consumption with BMI (P = 0.02). Stratified analysis by age showed that dairy consumption was significantly associated with higher BMI among participants >50 years (β = 0.05 kg/m2 per serving/day; 95% CI, 0.02–0.07) but tended to be associated with lower BMI among participants <50 years (β = −0.04 kg/m2 per serving/day; 95% CI, −0.11 to 0.03).
Fig. 1. Association between dairy intake and BMI among 176100 participants from 24 studies.
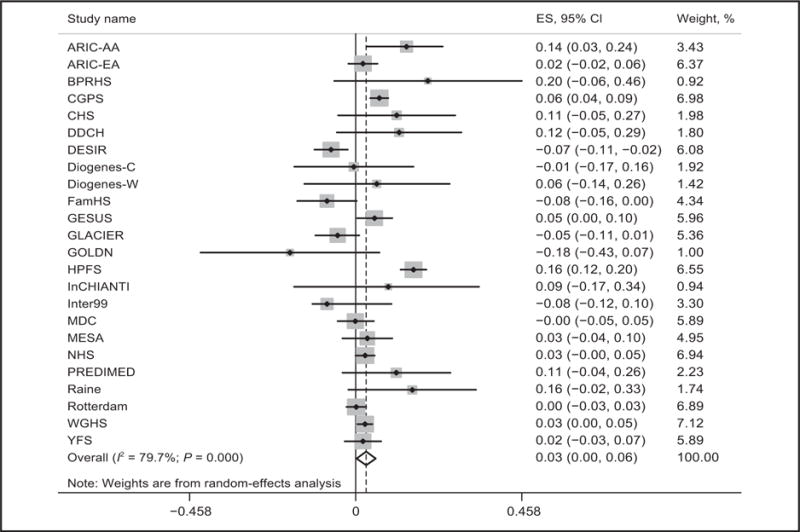
Linear regression was used to test the association of dairy intake (serving/day) with BMI(kg/m2) after adjustment of sex, ethnicity, region, years of follow-up, and other baseline covariates if available (age, smoking status, physical activity, total energy intake, and alcohol intake) in each study. We pooled β coefficients across 24 studies using random-effects metaanalysis because of the heterogeneity between studies (I2 = 79.7%; P < 0.001). ARIC-AA, ARIC (African Ancestry); ARIC-EA, ARIC (European Ancestry); PREDIMED, PREDIMED-Valencia; ES, effect size.
GENETIC ASSOCIATION OF LCT-13910 C/T WITH DAIRY INTAKE AND BMI
Random-effects metaanalysis, with a dominant model, was used to pool the genetic association of the LCT-13910 C/T with dairy intake in the 176100 participants from 24 studies (I2 = 83.0%). We found that the LCT-13910 C/T CT+TT genotype was significantly associated with 0.20 more dairy servings per day (β = 0.20 serving/day; 95% CI, 0.14–0.25; P = 3.15×10−12). We also pooled the genetic association with BMI in the 184802 participants from 25 studies using fixed-effects metaanalysis (I2 = 14.4%; P = 0.258) and found that the LCT-13910 C/T CT + TT genotype was significantly associated with 0.12 higher BMI unit (kg/m2) (β = 0.12; 95% CI, 0.06 – 0.17; P = 2.11×10−5) (Fig. 2).
Fig. 2. Genetic association and estimated causality between dairy intake and BMI in dominant model.
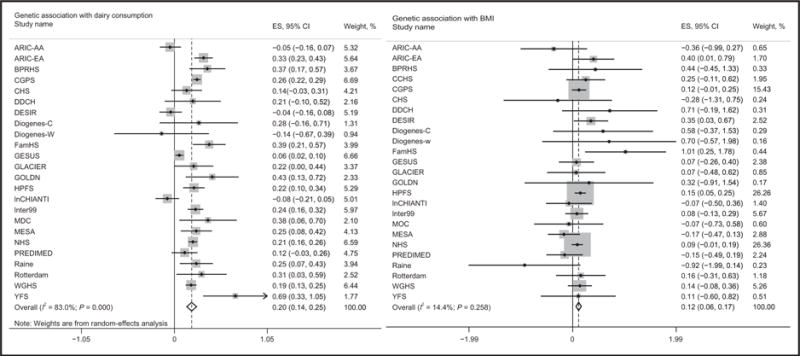
The LCT-13910 C/T located upstream of the lactase (LCT) gene was selected as IV. The TT and TC genotypes are associated with lactase persistence, and the CC genotype is associated with nonpersistence. Random-effects metaanalysis was used to pool the genetic association with dairy intake in 176100 participants from 24 studies because of the heterogeneity between studies (I2 = 83.0%). Fixed-effects metaanalysis was used to pool the genetic association with BMI association in 184802 participants from 25 studies (I2 =14.4%). PREDIMED, PREDIMED-Valencia; ES, effect size.
In sensitivity analyses, we found similarly significant genetic associations of LCT-13910 C/T with dairy intake (β = 0.09 serving/day; 95% CI, 0.06–0.12; P = 4.24×10−9), and genetic association with BMI (β = 0.09 serving/day; 95% CI, 0.04–0.14; P = 0.0003) under the additive model (see Fig. 1 in the online Data Supplement). Significant genetic associations were also observed under the recessive model (see Table 7 in the online Data Supplement).
IV ESTIMATED CAUSALITY BETWEEN DAIRY INTAKE AND BMI
After we pooled estimated effect sizes from each study using metaanalysis, we used the IV estimators to quantify the strength of the causal association of dairy intake and BMI, with the LCT-13910 C/T used as an IV. The MR estimate was computed from the ratio of the coefficient of the association between the LCT-13910 C/T and BMI to that of the association between the LCT-13910 C/T and dairy intake. This IV estimate reflects the potential causal effect of dairy intake on BMI. Fig. 3 presents the observational association of dairy intake with BMI, as well as the IV estimated causal effect of dairy intake on BMI. The pooled results show that genetically higher dairy intake was significantly associated with higher BMI (β = 0.60 kg/m2 per 1 serving/day; 95% CI, 0.27–0.92; P = 3.0×10−4) under the dominant model. We also found evidence of significant causal association between dairy intake and BMI under the additive model (β = 1.00 kg/m2; 95% CI, 0.30–1.69; P = 4.0×10−3) and the recessive model (β = 1.59 kg/m2; 95% CI, 0.28–2.91; P = 0.009) (see Fig. 1 and Table 7 in the online Data Supplement). We did not observe significant differences in observational results or IV estimated results (P = 0.63).
Fig. 3. MR triangulation for BMI.
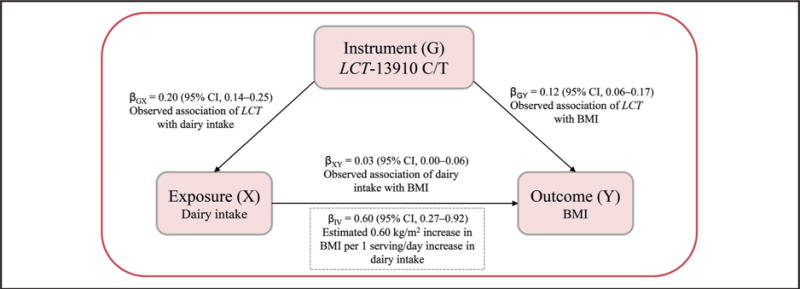
The IV estimator was used to quantify the strength of the causal association of dairy intake with BMI using LCT-13910 C/T as IV.
We further conducted stratified analyses of genetic association with dairy intake and BMI and estimated causality by age, BMI, follow-up years, sample size, study design, and ethnic group. Table 2 presents the genetic associations and the IV estimate for the association of dairy intake with BMI from overall and subgroup analyses. Genetic associations of the LCT-13910 C/T with BMI and a causal effect of dairy intake on BMI were observed only among those of European ancestry and in studies with mean age ≥50 years, BMI ≥25 kg/m2, follow-up time ≥5 years, and sample size ≥1000.
Table 2.
Stratified analyses of estimated causality between dairy intake and BMI in dominant model.
| Dairy (outcome), serving/day
|
BMI (outcome), kg/m2
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Studies SNP (IV)
|
Studies SNP (IV)
|
Estimated causality
|
||||||
| Subgroup | No. | β (95% CI) | P value | No. | β (95% CI) | P value | β (95% CI) | P value |
| Age, years | ||||||||
|
| ||||||||
| ≥50 | 19 | 0.19 (0.12–0.26) | <0.001 | 19 | 0.12 (0.05–0.19) | <0.001 | 0.64 (0.22–1.07) | <0.001 |
|
| ||||||||
| <50 | 5 | 0.35 (0.17–0.53) | <0.001 | 6 | 0.06 (−0.13 to 0.24) | 0.513 | 0.16 (−0.38 to 0.69) | 0.371 |
|
| ||||||||
| BMI, kg/m2 | ||||||||
|
| ||||||||
| ≥25 | 19 | 0.20 (0.14–0.27) | <0.001 | 19 | 0.11 (0.06–0.17) | 0.001 | 0.55 (0.23–0.88) | <0.001 |
|
| ||||||||
| <25 | 5 | 0.21 (0.15–0.27) | <0.001 | 6 | 0.15 (−0.17 to 0.47) | 0.147 | 0.68 (−0.83 to 2.20) | 0.269 |
|
| ||||||||
| Follow-up, years | ||||||||
|
| ||||||||
| ≥5 | 13 | 0.20 (0.13–0.27) | <0.001 | 14 | 0.11 (0.03–0.19) | 0.001 | 0.55 (0.09–1.00) | <0.001 |
|
| ||||||||
| <5 | 11 | 0.24 (0.13–0.36) | <0.001 | 11 | 0.13 (−0.01 to 0.26) | 0.112 | 0.51 (−0.07 to 1.10) | 0.074 |
|
| ||||||||
| Sample size | ||||||||
|
| ||||||||
| ≥1000 | 20 | 0.23 (0.17–0.29) | <0.001 | 21 | 0.12 (0.05–0.18) | <0.001 | 0.51 (0.19–0.83) | <0.001 |
|
| ||||||||
| <1000 | 4 | 0.21 (0.15–0.27) | <0.001 | 4 | 0.04 (−0.35 to 0.43) | 0.912 | 0.18 (−1.63 to 2.00) | 0.615 |
|
| ||||||||
| Ethnic group | ||||||||
|
| ||||||||
| European | 19 | 0.21 (0.15–0.28) | <0.001 | 20 | 0.13 (0.08–0.18) | <0.001 | 0.62 (0.30–0.93) | <0.001 |
|
| ||||||||
| Non-European | 5 | 0.22 (0.04–0.41) | <0.001 | 5 | −0.18 (−0.42 to 0.07) | 0.611 | −0.79 (−2.07 to 0.49) | 0.662 |
|
| ||||||||
| Study design | ||||||||
|
| ||||||||
| Cohort | 19 | 0.20 (0.17–0.22) | <0.001 | 20 | 0.12 (0.06–0.17) | <0.001 | 0.60 (0.32–0.88) | <0.001 |
|
| ||||||||
| Cross-sectional | 5 | 0.1 (0.13–0.19) | <0.001 | 5 | 0.11 (−0.01 to 0.22) | 0.231 | 0.69 (−0.04 to 1.41) | 0.06 |
|
| ||||||||
| HWE | ||||||||
|
| ||||||||
| ≤0.05 | 20 | 0.23 (0.16–0.28) | <0.001 | 21 | 0.12 (0.07–0.19) | <0.001 | 0.52 (0.18–0.82) | <0.001 |
|
| ||||||||
| >0.05 | 4 | 0.21 (0.16–0.28) | <0.001 | 4 | 0.05 (−0.34 to 0.45) | 0.753 | 0.19 (−1.57 to 1.76) | 0.714 |
SNP, single-nucleotide polymorphism; HWE, Hardy–Weinberg equilibrium.
Linear regression was used to test the association of MCM6 variant rs4988235 with dairy intake or BMI after adjustment of age, sex, ethnicity, region, total energy, and principal component for population stratification, as appropriate.
We pooled β coefficients across studies using random-effect metaanalysis because of the heterogeneity between studies (I2 > 50%; P < 0.001).
We used the IV estimators to quantify the strength of the causal association of dairy intake and BMI in each study. The IV estimator that is identical to that derived by the widely used 2-stage least-squares method will be calculated as the β of the regression coefficients MCM6 rs4988235-BMI and MCM6 rs4988235-dairy.
Discussion
In thus far the largest MR analysis study, including 184802 adults from 25 cohorts, our results support a causal relation between higher dairy intake and increased BMI in the absence of caloric restriction.
A number of recent prospective cohort studies exploring the association between intake of dairy foods and weight status have generated inconsistent results (12, 20–22). Evidence from a systematic review showed that much confusion remains about this relationship (2). Possible reasons for such apparent inconsistencies in findings include varying sample sizes, residual and unmeasured confounding, and reverse causality because of changes in outcome and factors over time. In addition, 1 metaanalysis of RCTs showed that ad libitum dairy interventions in long-term trials might increase body weight (5). However, several RCTs with energy restriction reported opposing effects (5). Differences in dose of dairy, foods included as diary, mode of administration, duration of intervention, energy restriction, or baseline differences in dairy intake or body weight between the trials may be prone to bias, undermining their validity. Furthermore, the types of dairy products may also partly account for this apparent discrepancy. A limited number of studies have examined the impact of type of dairy product on body composition. In 1 metaanalysis study, both whole fat and low fat dairy food intervention significantly increased body weight (4). In addition, fermented milk products such as yogurt have shown beneficial effects in the control of body weight (23), whereas cheese intake has exhibited a positive association with obesity (24). Taken together, previous evidence makes definitive conclusions on the relation between dairy consumption and adiposity difficult.
Recently, genetic analysis has become widely used to infer causality of environmental factors on human disease (7). The MR analysis is a method that uses genetic variants that are robustly associated with such modifiable factors to generate more reliable evidence regarding which interventions should produce health benefits (25). The MR analysis is not prone to confounding or reverse causation. It is well known that alleles that are randomly assigned at meiosis are independent of nongenetic confounding and are unmodified by health conditions. Therefore, MR is analogous to an RCT and can be used to support the hypothesis that the association of dairy intake with BMI is causal. Recently, a genetic analysis from the 1982 Pelotas (Brazil) Birth Cohort (26) did not support a causal relationship between high dairy intake and increased BMI. However, this study was limited by small sample size (n = 2808) and low power to derive valid conclusions. In our well-powered study, we individually analyzed 184802 individuals from 25 cohorts and provide strong evidence that high dairy intake was causally associated with higher BMI. Results from our MR and multivariable analyses were highly consistent, both confirming higher BMI in those eating more dairy products.
The potential mechanisms underlying the impact of dairy intake on the regulation of body weight have not been clearly elucidated. The most postulated mechanism is that the hormone estrone found in dairy products may promote increases in body weight (20, 27). In addition, intake of dairy foods is associated with higher plasma insulin-like growth factor I, which may contribute to weight gain (28). Furthermore, previous studies suggested that extra dairy intake in ad libitum dietary interventions may lead to increased energy intake, which may result in weight gain, offsetting the otherwise potential protective effect of the dairy intervention (5). In contrast, in most energy-restricted trials, energy intakes were better controlled. Thus, the potential benefits of dairy on body weight could be interpreted as the effect of the substitution of dairy products for certain other foods (5). Therefore, total energy intake needs to be considered when assessing the role of dairy intake in weight control (3–6). In addition, metaanalysis of RCTs showed that dairy consumption increased lean (muscle) mass and decreased body fat (3). Increased protein intake from dairy products may promote maintenance of lean mass (6). Thus, in the present study, it is possible that the higher BMI related to high dairy intake could be mainly because of increased lean muscle mass. Future research is needed to further illustrate potential mechanisms of dairy products on body weight and composition in the context of energy restriction.
Our study has several strengths. First, to the best of our knowledge, this is thus far the largest MR analysis on the causality of high dairy intake on BMI. The large sample size allowed us to assess the consistency of associations across multiple studies and to gain sufficient power for conclusive estimation of causal effect. Second, the MR design used in our genetic analyses should have largely prevented potentially distorting influences. Third, the lactose-tolerance variant is a well-established genetic marker for dairy intake, with solid biological basis and, therefore, a valid IV for dairy intake (10, 13). Lastly, most of the studies included were homogeneous, and we performed analysis individually in each study. Therefore, the effect of population stratification on the instrumental results should be minimal.
Potential limitations with the MR approach include the possibility of pleiotropy and population stratification. Pleiotropy refers to a situation in which a gene affects ≥2 apparently unrelated phenotypic traits; we could not exclude the possibility of pleiotropic effects of the LCT genotype. However, to our knowledge, no pleiotropic effect has been reported. Furthermore, the associations of rs4988235 with lactase persistence and milk intake vary across populations. We adjusted for only geographical region and ethnicity in the statistical models. Hence, bias from population stratification is deemed likely (29). Finally, although many important covariates were adjusted in our models, some residual and unmeasured confounding might remain.
Conclusions
In summary, the present study suggests a causal effect of higher dairy intake on increased BMI; our results also emphasize that total energy intake needs to be considered when assessing the role of dairy intake in weight control.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions. The authors thank the study participants, the staff from the Rotterdam Study and the participating general practitioners and pharmacists. The authors also acknowledge the funding agencies supporting the Northern Sweden Diet Database and the Västerbotten Intervention Project, including the Swedish Research Council. The authors thank Ville Aalto, Irina Lisinen, and Mika Helminen for their expert technical assistance in the statistical analyses. The PREDIMED-Valencia study also acknowledges the collaborative support provided by the Real Colegio Complutense at Harvard University. The authors thank the Raine Study participants and their families, as well as the Raine Study research staff for cohort coordination and data collection. The authors thank the NH&MRC for their long-term funding to the study during the past 25 years and also the following institutes for providing funding for Core Management of the Raine Study: The University of Western Australia (UWA), Curtin University, the Raine Medical Research Foundation, the UWA Faculty of Medicine, Dentistry and Health Sciences, the Telethon Kids Institute, the Women’s and Infants’ Research Foundation (King Edward Memorial Hospital), and Edith Cowan University. The authors thank the Western Australian DNA Bank (National Health and Medical Research Council of Australia National Enabling Facility) for assistance. The authors also thank the Raine Study participants for their ongoing participation in the study, the Raine Study Team for study coordination and data collection, the UWA Centre for Science for use of the facility and the Sleep Study Technicians. The D.E.S.I.R. Study Group: INSERM CESP U1018: B. Balkau, P. Ducimetière, E. Eschwège; INSERM U367: F. Alhenc-Gelas; CHU d’Angers: A. Girault; Bichat Hospital: F. Fumeron, M. Marre, R. Roussel; CHU de Rennes: F. Bonnet; CNRS UMR8090, Lille: S. Cauchi P. Froguel; Centres d’Examens de Santé: Alençon, Angers, Blois, Caen, Chartres, Chateauroux, Cholet, Le Mans, Orléans, Tours; Institute de Recherche Médecine Générale: J. Cogneau; general practitioners of the region; Institute inter-Regional pour la Santé: C. Born, E. Caces, N. Copin, J.G. Moreau, O. Lantieri, F. Rakotozafy, J. Tichet, S. Vol.
Footnotes
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest: For complete author disclosures, see the online version of this paper at http://www.clinchem.org/content/vol64/issue1.
Nonstandard abbreviations: RCT, randomized controlled trial; MR, Mendelian randomization; BMI, body mass index; IV, instrumental variable.
Human Genes: LCT, lactase; MCM6, minichromosome maintenance complex component 6.
References
- 1.Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:871S–3S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- 2.Zou Y, Bao Q, Kumar S, Hu M, Wang GY, Dai G. Four waves of hepatocyte proliferation linked with three waves of hepatic fat accumulation during partial hepatectomy-induced liver regeneration. PLoS One. 2012;7:e30675. doi: 10.1371/journal.pone.0030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abargouei AS, Janghorbani M, Salehi-Marzijarani M, Esmaillzadeh A. Effect of dairy consumption on weight and body composition in adults: a systematic review and meta-analysis of randomized controlled clinical trials. Int J Obes (Lond) 2012;36:1485–93. doi: 10.1038/ijo.2011.269. [DOI] [PubMed] [Google Scholar]
- 4.Benatar JR, Sidhu K, Stewart RA. Effects of high and low fat dairy food on cardio-metabolic risk factors: a meta-analysis of randomized studies. PLoS One. 2013;8:e76480. doi: 10.1371/journal.pone.0076480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen M, Pan A, Malik VS, Hu FB. Effects of dairy intake on body weight and fat: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96:735–47. doi: 10.3945/ajcn.112.037119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booth AO, Huggins CE, Wattanapenpaiboon N, Nowson CA. Effect of increasing dietary calcium through supplements and dairy food on body weight and body composition: a meta-analysis of randomised controlled trials. Br J Nutr. 2015;114:1013–25. doi: 10.1017/S0007114515001518. [DOI] [PubMed] [Google Scholar]
- 7.Nelson CP, Hamby SE, Saleheen D, Hopewell JC, Zeng L, Assimes TL, et al. Genetically determined height and coronary artery disease. N Engl J Med. 2015;372:1608–18. doi: 10.1056/NEJMoa1404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lawlor DA, Harbord RM, Sterne JAC, Timpson N, Smith GD. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 9.Didelez V, Sheehan N. Mendelian randomization as an instrumental variable approach to causal inference. Stat Methods Med Res. 2007;16:309–30. doi: 10.1177/0962280206077743. [DOI] [PubMed] [Google Scholar]
- 10.Ding M, Huang T, Bergholdt HK, Nordestgaard BG, Ellervik C, Qi L, et al. for the Mendelian Randomization of Dairy Consumption Working Group Dairy consumption, systolic blood pressure, and risk of hypertension: Mendelian randomization study. BMJ. 2017;356:j1000. doi: 10.1136/bmj.j1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enattah NS, Sahi T, Savilahti E, Terwilliger JD, Peltonen L, Jarvela I. Identification of a variant associated with adult-type hypolactasia. Nat Genet. 2002;30:233–7. doi: 10.1038/ng826. [DOI] [PubMed] [Google Scholar]
- 12.Bergholdt HK, Nordestgaard BG, Ellervik C. Milk intake is not associated with low risk of diabetes or overweight-obesity: a Mendelian randomization study in 97,811 Danish individuals. Am J Clin Nutr. 2015;102:487–96. doi: 10.3945/ajcn.114.105049. [DOI] [PubMed] [Google Scholar]
- 13.Bergholdt HK, Nordestgaard BG, Varbo A, Ellervik C. Milk intake is not associated with ischaemic heart disease in observational or Mendelian randomization analyses in 98,529 Danish adults. Int J Epidemiol. 2015;44:587–603. doi: 10.1093/ije/dyv109. [DOI] [PubMed] [Google Scholar]
- 14.Travis RC, Appleby PN, Siddiq A, Allen NE, Kaaks R, Canzian F, et al. Genetic variation in the lactase gene, dairy product intake and risk for prostate cancer in the European prospective investigation into cancer and nutrition. Int J Cancer. 2013;132:1901–10. doi: 10.1002/ijc.27836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–6. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 18.Wald A. The fitting of straight lines if both variables are subject to error. Ann Math Stat. 1940;11:284–300. [Google Scholar]
- 19.Palmer TM, Sterne JA, Harbord RM, Lawlor DA, Sheehan NA, Meng S, et al. Instrumental variable estimation of causal risk ratios and causal odds ratios in Mendelian randomization analyses. Am J Epidemiol. 2011;173:1392–403. doi: 10.1093/aje/kwr026. [DOI] [PubMed] [Google Scholar]
- 20.Berkey CS, Rockett HR, Willett WC, Colditz GA. Milk, dairy fat, dietary calcium, and weight gain: a longitudinal study of adolescents. Arch Pediatr Adolesc Med. 2005;159:543–50. doi: 10.1001/archpedi.159.6.543. [DOI] [PubMed] [Google Scholar]
- 21.Wang H, Troy LM, Rogers GT, Fox CS, McKeown NM, Meigs JB, Jacques PF. Longitudinal association between dairy consumption and changes of body weight and waist circumference: the Framingham heart study. Int J Obes (Lond) 2014;38:299–305. doi: 10.1038/ijo.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panahi S, Tremblay A. The potential role of yogurt in weight management and prevention of type 2 diabetes. J Am Coll Nutr. 2016:1–15. doi: 10.1080/07315724.2015.1102103. [DOI] [PubMed] [Google Scholar]
- 24.Snijder MB, van der Heijden AA, van Dam RM, Stehouwer CD, Hiddink GJ, Nijpels G, et al. Is higher dairy consumption associated with lower body weight and fewer metabolic disturbances? The Hoorn study. Am J Clin Nutr. 2007;85:989–95. doi: 10.1093/ajcn/85.4.989. [DOI] [PubMed] [Google Scholar]
- 25.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet. 2014;23:R89–98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartwig FP, Horta BL, Smith GD, de Mola CL, Victora CG. Association of lactase persistence genotype with milk consumption, obesity and blood pressure: a Mendelian randomization study in the 1982 Pelotas (Brazil) birth cohort, with a systematic review and meta-analysis. Int J Epidemiol. 2016;45:1573–87. doi: 10.1093/ije/dyw074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remesar X, Tang V, Ferrer E, Torregrosa C, Virgili J, Masanes RM, et al. Estrone in food: a factor influencing the development of obesity? Eur J Nutr. 1999;38:247–53. doi: 10.1007/s003940050068. [DOI] [PubMed] [Google Scholar]
- 28.Ma J, Giovannucci E, Pollak M, Chan JM, Gaziano JM, Willett W, Stampfer MJ. Milk intake, circulating levels of insulin-like growth factor-I, and risk of colorectal cancer in men. J Natl Cancer Inst. 2001;93:1330–6. doi: 10.1093/jnci/93.17.1330. [DOI] [PubMed] [Google Scholar]
- 29.Smith GD, Lawlor DA, Harbord R, Timpson N, Day I, Ebrahim S. Clustered environments and randomized genes: a fundamental distinction between conventional and genetic epidemiology. Plos Med. 2007;4:1985–92. doi: 10.1371/journal.pmed.0040352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


