Abstract
Study Objectives:
The carbonic anhydrase inhibitor acetazolamide (AZT) modulates blood pressure at high altitude and reduces sleep-disordered breathing in patients with obstructive sleep apnea (OSA). We aimed to investigate the treatment effect of AZT and in combination with continuous positive airway pressure (CPAP) on blood pressure in patients with hypertension and OSA.
Methods:
In a prospective, randomized, three-way crossover study, 13 male patients with hypertension and moderate to severe OSA (age 64 ± 7 years, body mass index 29 ± 4 kg/m2, and mean apnea-hypopnea index 37 ± 23 events/h) received AZT, CPAP, or AZT plus CPAP for 2-week periods. Antihypertensive medication was washed out. Office and 24-hour blood pressure, arterial stiffness, polygraphic sleep study data, and blood chemistry were compared.
Results:
AZT alone and AZT plus CPAP, but not CPAP alone, reduced office mean arterial pressure compared to baseline (−7 [95% CI −11 to −4], −7 [95% CI −11 to −4] and −1 [95% CI −5 to 4] mmHg, respectively; repeated- measures analysis of variance (RM-ANOVA; P = .015). Aortic systolic pressure and augmentation index, assessed by radial artery oscillatory tonometry, were unaffected by CPAP but decreased after AZT and AZT plus CPAP (RM-ANOVA P = .030 and .031, respectively). The apnea-hypopnea index was significantly reduced in all three treatment arms, most prominently by AZT plus CPAP (RM-ANOVA P = .003). The reduction of venous bicarbonate concentration following AZT was correlated with the change of apnea-hypopnea index (r = 0.66, P = .013).
Conclusions:
AZT reduced blood pressure, vascular stiffness, and sleep-disordered breathing in patients with OSA and comorbid hypertension. Carbonic anhydrase inhibition may constitute a potential target for drug therapy in patients with sleep apnea and comorbid hypertension.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Identifier: NCT02220803; Title: A Short Term Open, Randomized Cross-over Trial Exploring the Effect of Carbonic Anhydrase Inhibition by Acetazolamide on Sleep Apnea Associated Hypertension and Vascular Dysfunction; URL: https://clinicaltrials.gov/ct2/show/NCT02220803 and Registry: EU Clinical Trials Register; EudraCT Number: 2013-004866-33; Title: A short term open, randomized cross over trial exploring the effect of carbonic anhydrase inhibition by acetazolamide on sleep apnea associated hypertension; URL: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2013-004866-33
Citation:
Eskandari D, Zou D, Grote L, Hoff E, Hedner J. Acetazolamide reduces blood pressure and sleep-disordered breathing in patients with hypertension and obstructive sleep apnea: a randomized controlled trial. J Clin Sleep Med. 2018;14(3):309–317.
Keywords: blood pressure, carbonic anhydrase, hypertension, obstructive sleep apnea, pharmacotherapy, randomized controlled trial, treatment
BRIEF SUMMARY
Current Knowledge/Study Rationale: A pharmacological therapy of obstructive sleep apnea (OSA) is lacking. The effectiveness of the carbonic anhydrase inhibitor acetazolamide to reduce blood pressure in patients with hypertension and OSA has not been systematically investigated.
Study Impact: In this randomized crossover study, we found that acetazolamide alone or in combination with continuous positive airway pressure (CPAP) significantly reduced blood pressure and vascular stiffness compared to CPAP in patients with moderate to severe OSA who had withdrawn antihypertensive medication. In addition, acetazolamide decreased the apnea-hypopnea index by 42% and this reduction was associated with a decrease of bicarbonate concentration. Our findings suggest that carbonic anhydrase related mechanisms may be involved in blood pressure regulation and sleep-disordered breathing in patients with OSA and comorbid hypertension.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common disorder associated with excessive daytime sleepiness as well as cardiovascular and metabolic disease.1 Comorbid systemic hypertension (HT) is particularly frequent2 and recent guidelines rank OSA as the single most common cause of secondary HT.3 Continuous positive airway pressure (CPAP), the primary choice of treatment in OSA, is associated with limited adherence4 and has only a moderate effect on elevated blood pressure (BP)5 or for the prevention of secondary cardiovascular events in patients with OSA, most of whom were hypertensive.6 Conventional antihypertensive medication reduces BP in patients with HT and sleep apnea but has little effect on existing OSA severity.7–9 Hence, add-on CPAP treatment is needed to effectively treat sleep-disordered breathing in these patients.
The ubiquitous enzyme carbonic anhydrase (CA) facilitates production, transport, and elimination of carbon dioxide (CO2) via reversible catalysis of the hydration of CO2 into bicarbonate (HCO3−).10 CA thereby plays a fundamental role for the extent of tissue acidification and maintenance of blood gas stability. We have previously reported that higher CA activity in whole blood was associated with apnea severity in patients with OSA.11 Pharmacological inhibition of CA, by acetazolamide (AZT) or other CA inhibitory drugs, induced a reduction of OSA that may be attributed to respiratory stimulation following tissue acidification.12–15 Moreover, several studies demonstrated that CA activity influences vascular tone in a manner suggesting that CA may be involved in BP modulation.16–18 We recently demonstrated an association between CA activity and BP in patients with OSA11 and the CA inhibitor zonisamide reduced BP in a subgroup of patients with OSA and comorbid HT.15 In addition, AZT was found to prevent BP elevation in both healthy subjects19 and patients with OSA20,21 during high-altitude sojourns. Hence, it is possible that the mechanisms related to CA activity directly may contribute to BP regulation in patients with OSA and HT.
To our knowledge the use of AZT, alone or in combination with CPAP, to reduce BP in patients with HT and OSA has not been systematically explored. In the current study, we investigated the treatment effect of AZT, CPAP, and AZT plus CPAP on BP and sleep apnea in patients with moderate to severe OSA and comorbid HT (after antihypertensive medication withdrawn). The adherence-adjusted treatment efficacy of CPAP and AZT was also compared.15,22
METHODS
The study was presented in accordance with the CONSORT criteria.23 The protocol was approved by the Ethics Committee at the University of Gothenburg (977-13) and was registered at ClinicalTrials.gov (NCT02220803) as well as European Clinical Trials Database (EudraCT: 2013-004866-33). A written informed consent was obtained from each study participant prior to study entry.
Study Design
This was a single-center, open, randomized, crossover trial. Following screening (Visit 1), antihypertensive medications were withdrawn. Patients received, in randomized order, AZT, CPAP, or a combination of AZT and CPAP during three 2-week periods separated by 2-week washout periods (baseline assessments at Visits 2, 4, and 6 and treatment assessments at Visits 3, 5, and 7). Variables recorded in conjunction with each study visit included office and 24-hour BP, arterial stiffness assessed by a noninvasive arteriograph system, overnight sleep polygraphy, and anthropometrics. Blood samples including HCO3− were obtained at each visit and questionnaires (Epworth Sleepiness Scale [ESS]24 and Functional Outcomes of Sleep Questionnaire [FOSQ]25) were collected. AZT was titrated to a maximum of 750 mg/d and maintained in the 500–750 mg interval in accordance with individual clinical tolerability. CPAP was administered using the default setting of the autoregulated algorithm in the 5–15 cm H2O pressure range. Side effects were continuously monitored.
Study Participants
A total of 14 male patients with OSA, treated systemic HT, and planned for CPAP treatment at the Department of Sleep Medicine, Sahlgrenska University Hospital were recruited for study participation. The inclusion criteria were male sex, age 18–75 years, ongoing antihypertensive medication (minimum one medication), an apnea-hypopnea index (AHI) of > 15 events/h, a body mass index ≤ 35 kg/m2 and an ESS score > 6. Exclusion criteria were hypersensitivity to ongoing medication and/ or with other sulfonamides, seizure disorders, unstable cardiovascular, pulmonary, or gastrointestinal disease, impaired renal or hepatic function, depression, or any form of abuse. Patients with documented severe nocturnal hypoxemia (> 10 episodes with an oxygen desaturation [SpO2], below 50%), with a sleepiness-related potential occupational hazard or therapy-resistant hypertension were excluded. One patient was excluded due to excessive blood pressure (systolic blood pressure [SBP] > 165 mmHg and diastolic blood pressure [DBP] > 95 mmHg) during the washout period.
Study Procedures
The randomization list was open and generated by an independent monitor, blinded to the study protocol, at the University Hospital Clinical Research Center. Medications were washed out 14 days prior to randomization. Supine office SBP and DBP and heart rate (HR) were determined in accordance with recommended guidelines.26 Twenty-four–hour BP assessments were performed on the days prior to the study visits. Mean arterial pressure (MAP) was calculated as one-third of the SBP plus two-thirds of the DBP. The radial artery oscillatory tonometry was applied in the morning following the sleep study to assess central hemodynamics and arterial stiffness.27 The overnight ambulatory polygraphic recording was performed at the patient's home preceding each study visit. Habitual sleep time was calculated as the mean of the lights-off to lights-on time recorded during the six study visits.
Blood Pressure Assessment
Office SBP/DBP was assessed in the supine position after 10 minutes of rest at each study site visit. An appropriately sized cuff was placed on the right arm at the heart level and blood pressure was determined to the nearest 1 mmHg. The mean of three automated sequential recordings (Omron M6, Omron Healthcare, Kyoto, Japan) was used to express value entries. HR was expressed as beats per minute. Twenty-four– hour BP assessment (Watch BP03 device, Microlife, Widnau, Switzerland) was performed on the days prior to the study visits. Automatic assessments, with the cuff placed on the non-dominant arm, were made every 15 minutes during daytime (9:00 am – 10:00 pm) and every 20 minutes during the night (10:00 pm – 9:00 am).
Vascular Function Assessment
Arterial stiffness and central hemodynamic parameters were assessed by oscillometric tonometry (Arteriograph, TensioMed, Hungary) at the radial artery. All measurements were recorded during fasting conditions between 8:00 am – 12:00 am in a tempered room and in a semirecumbent position after 10 minutes of rest.
Sleep Study
The polygraphic sleep recording (Embletta X10, Embla, Colorado, United States) montage consisted of a nasal cannula, thorax and abdominal respiratory effort belts, and a finger pulse oximeter. Sleep and respiratory events were manually scored by one experienced technician (blinded to the protocol and otherwise not involved in any study procedure) according to the American Academy of Sleep Medicine criteria.28 In detail, apnea events were scored if there was a ≥ 90% decrease in the amplitude of airflow. Hypopnea events were scored if there was a ≥ 50% reduction in airflow associated with a ≥ 3% oxygen saturation (SpO2) drop. Minimum event duration of 10 seconds was required. AHI was calculated as the total number of apnea and hypopnea events divided by analysis time (lights offto lights on during the recording session). Oxygen desaturation index (ODI) was calculated as the total number of events with a ≥ 3% reduction of SpO2 divided by analysis time. Mean nocturnal minimum SpO2 was calculated from pulse oximetry during the analysis period.
Drug Titration and Adherence Assessment
Acetazolamide (Diamox, Mercury Pharmaceuticals Ltd, London, United Kingdom) was titrated starting with 250 mg b.i.d. (morning and 2 hours prior to bedtime) for 3 consecutive days and titrated to 250 mg t.i.d. during the remaining 2 study weeks. Adherence was assessed by tablet count. CPAP was administered using autotitrating devices (S9TM, ResMed Ltd, Sydney, Australia) in accordance with established clinical routines. CPAP adherence was assessed at the end of each treatment period by readouts from the built-in memory card of the device and reported in terms of mean hours of use (averaged to a nightly mean for the respective treatment period). Side effects and safety were continuously monitored throughout the entire study period. Safety measures were assessed during each study visit, and patients were advised to contact the study center in case adverse events/side effects occurred between study visits.
Sample Size Estimation and Efficacy Variables
The sample size calculation was based on a previous study examining the effects of CPAP and a CA inhibitory drug on BP.15 In detail, we assumed the difference in the relative reduction (treatment minus baseline) of office MAP between AZT and CPAP would exceed 8 mmHg, with a standard deviation of 10 mmHg. With a power of 80% for detecting a group difference (a two-sided 5% significance level), 14 patients should be included in this crossover study. The primary efficacy variable was the change in office BP during the AZT, CPAP and AZT plus CPAP treatment regimens. Secondary objectives included 24-hour BP, daytime vascular function, sleep-disordered breathing (AHI, ODI as well as mean and minimum overnight SpO2, venous blood gases, and subjective measures of daytime sleepiness and sleep quality (ESS and FOSQ).
In a subsequent post hoc analysis, the change in AHI and ODI in the AZT group was adjusted for mean study drug adherence (%). The effect of CPAP on sleep apnea was corrected for adherence with therapy (h/night) by habitual subjective sleep length in order to reflect the overall apnea exposure during the treatment.
Statistics
Statistical analysis was conducted using SPSS version 22 (IBM Corp., Armonk, New York, United States). Data were analyzed as per protocol. The Shapiro-Wilk test was used to assess normality of data. The pre-post treatment effect was assessed by paired t test. Between-groups comparison at baseline was analyzed using analysis of variance (ANOVA). Analysis of difference between interventions over time was conducted using repeated-measures ANOVA (RM-ANOVA) with Bonferroni adjustment for post hoc analysis. Spearman correlation was used to assess the associations between variables. Data are presented as mean ± standard deviation or mean and 95% confidence interval (CI). A value of P < .05 was considered statistically significant.
RESULTS
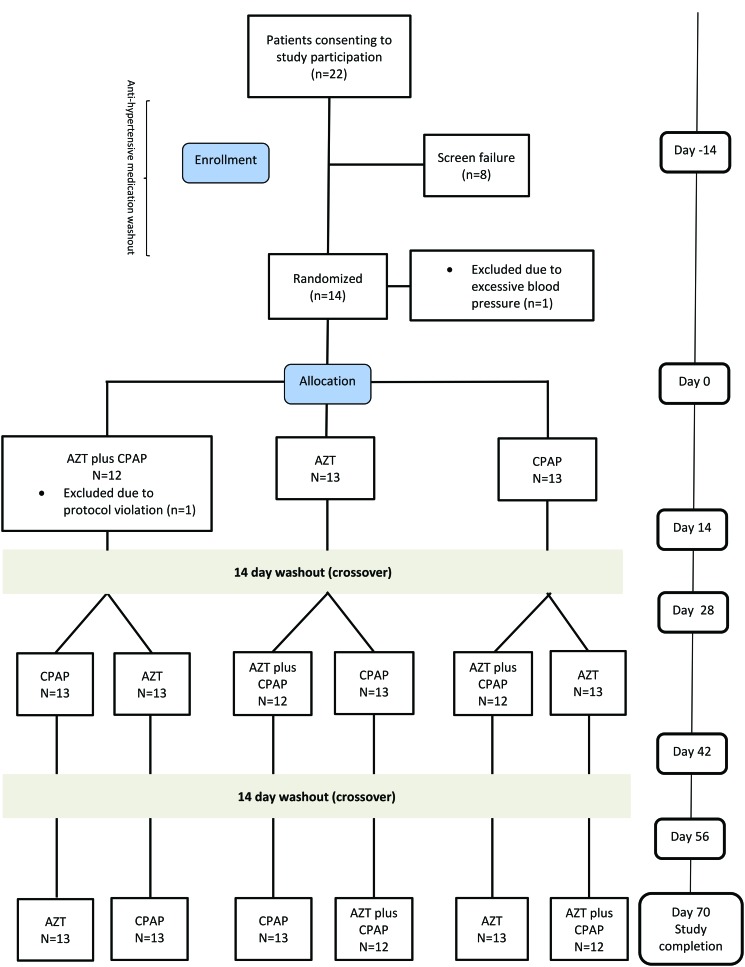
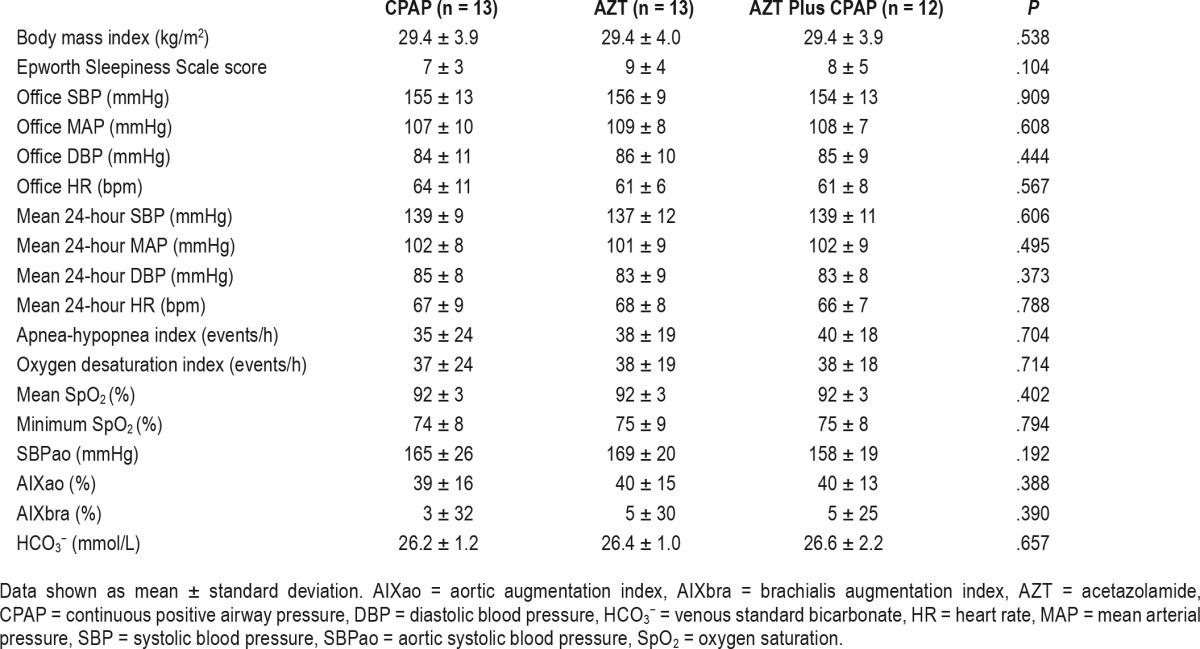
Thirteen male subjects were included in the final analysis (age 64 ± 7 years, body mass index 29 ± 4 kg/m2 and mean AHI 37 ± 23 events/h; Figure 1). The mean values on prescribed antihypertensive medication prior to wash-out period were office SBP/DBP: 146 ± 14/82 ± 9 mmHg and HR: 65 ± 12 bpm. For information regarding antihypertensive medication and comorbidities prior to the study see Table S1 in the supplemental material. The mean study drug daily doses during the AZT and AZT plus CPAP period were 659 ± 91 and 658 ± 91 mg, respectively. Mean CPAP usage was 4.8 ± 2.2 and 5.0 ± 2.0 h/night and the mean pressure was 7.7 ± 1.8 and 7.7 ± 1.8 cm H2O for the CPAP and AZT plus CPAP periods, respectively. The mean reported habitual sleep time was 7.3 ± 1.2 h/night. One patient from the AZT plus CPAP treatment period was excluded due to poor study adherence. The three baseline assessments did not differ with respect to anthropometrics, office and 24-hour BP, hemodynamic characteristics, or sleep-disordered breathing (Table 1).
Figure 1. Study flow chart.
AZT = acetazolamide, CPAP = continuous positive airway pressure.
Table 1.
Patient characteristics at baseline following washout of medication during the three study conditions.
Primary Outcome Analysis
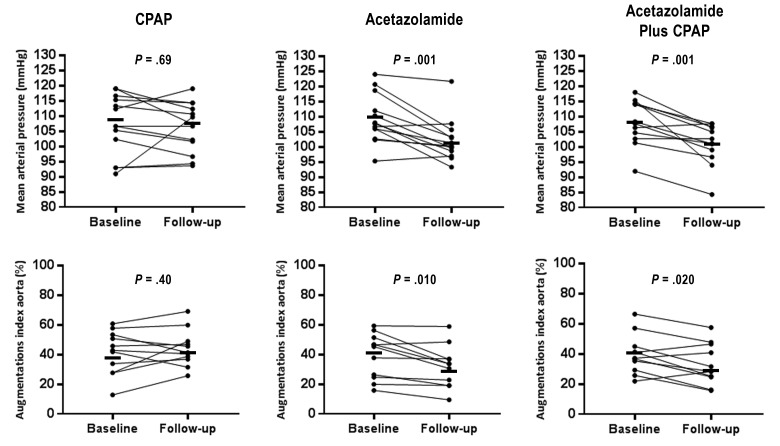
Compared to baseline, AZT plus CPAP, AZT but not CPAP treatment significantly reduced office MAP (−7 [95% CI −11 to −4], −7 [95% CI −11 to −4] and −1 [95% CI −5 to 4] mmHg, P = .001, .001 and .69, respectively; Figure 2). Both AZT and AZT plus CPAP treatment reduced office SBP and MAP whereas it remained unchanged after CPAP therapy (RMANOVA P = .024 and .015, respectively; Table 2). There was a statistical trend toward a difference in office DBP among the three interventions (P = .053). Change of office HR did not differ among the groups. The 24-hour SBP, MAP, DBP, and HR did not differ between the three interventions although there was a trend for 24-hour MAP and DBP (RE-ANOVA P = .088 and .092 respectively). There was no difference in the nighttime and daytime 24-hour BP with regard to therapy given. Overall office BP after AZT, CPAP, or AZT plus CPAP was similar to that of the screening visit (before antihypertensive medication washout). The office SBP after CPAP therapy was numerically higher than AZT, AZT plus CPAP, and screening periods but this difference did not reach statistical significance (ANOVA P = .073; Table S2 in the supplemental material).
Figure 2. Hemodynamic changes following CPAP, acetazolamide, and acetazolamide plus CPAP treatments.
Individual data of office mean arterial pressure (top panels) and aortic augmentation index (bottom panels) at baseline and follow-up in the CPAP, acetazolamide, and acetazolamide plus CPAP groups. Mean values at baseline and follow-up are indicated by the solid black bars. Significance values refer to comparison within groups. CPAP = continuous positive airway pressure.
Table 2.
Treatment effects of three interventions.
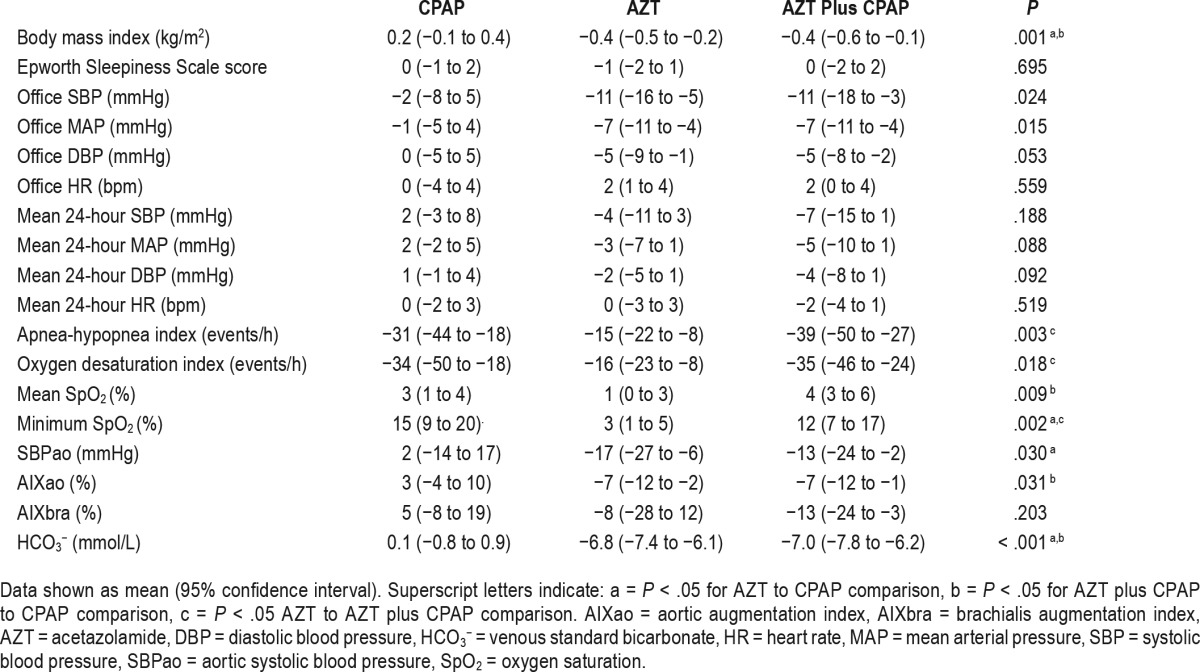
Hemodynamic and Arterial Stiffness Parameters
Compared to baseline, AZT plus CPAP, AZT but not CPAP treatment significantly reduced aortic SBP and aortic augmentation index (Table 2 and Figure 2). Brachialis augmentation index reduced after AZT plus CPAP treatment (P = .020). There was a statistical difference among treatments in aortic SBP and aortic augmentation index but not brachialis augmentation index (RM-ANOVA P = .030, .031 and .203, respectively).
Sleep-Disordered Breathing Parameters
CPAP, AZT, and AZT plus CPAP all significantly reduced AHI and ODI and improved nighttime oxygenation (all P < .05; Table 2). There was a significant difference in AHI, ODI, mean SpO2, and minimum SpO2 among treatments (RM-ANOVA P = .003, .018, .009, and .002, respectively). The resolution of AHI and ODI was more pronounced with AZT plus CPAP compared with AZT (post hoc P = .011 and .030, respectively). Compared to baseline, AZT reduced AHI by 42% (95% CI, 26 to 59) and ODI by 46% (95% CI, 29 to 63). Both mean and minimum SpO2 were increased after AZT (1% [95% CI, 0 to 3] and 3% [95% CI, 1 to 5], P = .009 and .011, respectively).
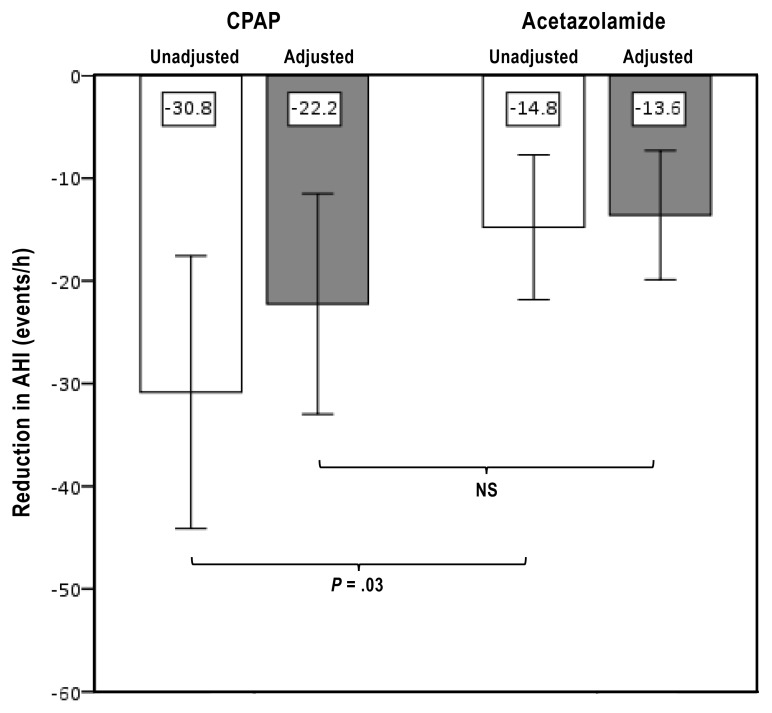
The overall therapeutic effect in sleep apnea was adjusted for adherence to therapy and treatment efficacy in a post hoc analysis. The time with effective positive pressure treatment in the CPAP group corresponded to 66 ± 27% of reported habitual sleep time. The adherence to pharmacological therapy was 95 ± 7% and 95 ± 8% in the AZT and AZT plus CPAP group, respectively. After adjustment of adherence, there was no significant difference between the AZT and CPAP treatment periods in terms of net AHI reduction (Figure 3).
Figure 3. Reduction in AHI.
Mean unadjusted (white bars) and adherence-adjusted (gray bars) reduction in CPAP and acetazolamide groups. Shown is mean change of reduction in AHI and 95% confidence interval. AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, NS = nonsignificant.
Interaction Between Bicarbonate, Hemodynamic Variables and OSA After AZT
AZT alone or in combination with CPAP reduced HCO3−, pH, and base excess concentration (all P < .01; Table 2 and Table S3 in the supplemental material). The reduction of HCO3− concentration, following AZT, was correlated with the reduction of AHI (Spearman correlation, r = 0.66 P = .013; Figure 4). There was no association between the change of OSA severity and any hemodynamic variable studied after AZT.
Figure 4. Spearman correlation between change in venous standard bicarbonate concentration and change in AHI.
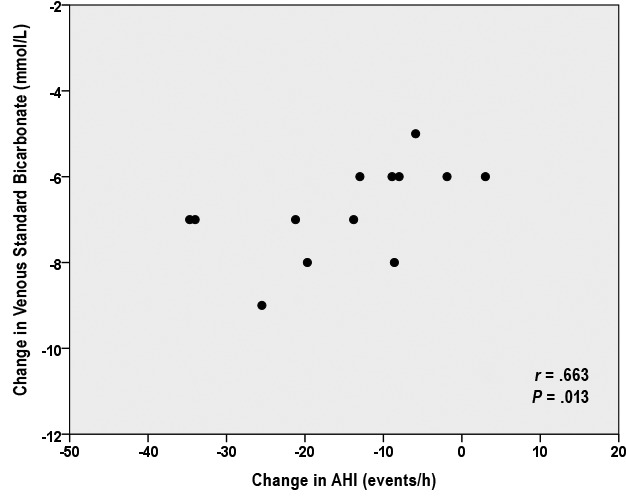
AHI = apnea-hypopnea index.
Subjective Outcome Measures, Side Effects and Safety
The ESS and FOSQ scores remained unchanged in all treatment groups. The most frequently reported adverse events were paresthesia (46%, 33%, and zero) and dyspepsia (31%, 25%, and zero) during the AZT, AZT plus CPAP, and CPAP-alone treatment, respectively (Table S4 in the supplemental material). The intensity of side effects was mild and in part controlled by dose reduction if applicable. There was no early study termination due to side effects.
DISCUSSION
In this prospective randomized crossover study, compared to CPAP, 2-week treatment of AZT or AZT plus CPAP reduced office SBP/MAP and improved arterial stiffness (reduction of aortic augmentation index) in patients with OSA and established hypertension. Unlike other antihypertensive medications,7 the CA inhibitor AZT reduced AHI by 42% and the improvement was associated with a reduction of HCO3−, a surrogate marker of CA activity. In line with previous findings,11,15 our study supports the notion that altered CA activity may constitute a potential mechanism for BP regulation in OSA. This randomized controlled trial also suggests that a combined pharmacological CA inhibition and mechanical therapy may induce cardiovascular effects that potentially provide benefits on top of AHI reduction by CPAP in patients with HT and OSA.
Epidemiological and clinical data suggest a strong association between OSA and arterial HT, in particular therapy-resistant HT.29–31 However, results from randomized controlled studies in patients with HT and OSA suggested that the BP reduction effect of CPAP may be limited.8,9,32,33 However, some34,35 but not all studies7,36 reported AHI reduction after antihypertensive treatment in patients with HT and OSA, but the effectiveness seems modest. Although limited in size, our study is the first to show that clinically relevant reduction of both BP and AHI may be achieved by pharmacological CA inhibition. In our study we found a potent reduction not only of blood pressure but also arterial vascular stiffness after AZT in patients with OSA. A similar BP lowering effect was previously shown in patients with HT and OSA after treatment with the CA inhibitor zonisamide.15 The exact mechanism behind the hypotensive effect of CA inhibition by AZT in OSA remains unclear but several explanations may be considered. First, CA inhibition may cause tissue hypercapnia and a subsequent compensatory vasodilatory response aiming to reduce CO2 levels.37,38 Second, the CA inhibitor AZT exhibits weak diuretic properties, possibly reflected by a slight body weight reduction in the current study, which in part may have contributed to the lowering of BP.39 Third, a previous experimental study reported a rapid vasodilation after AZT in a forearm flow model.40 A modulation of membranous calcium-activated potassium channels as well as altered nitric oxide metabolism was proposed as possible mechanisms behind this vasodila-tory response.16,17,41 These findings indicate that BP reduction by AZT suggests that CA mechanisms may contribute to BP regulation in OSA.
The treatment effect of AZT on OSA in our study supports previous findings in uncontrolled studies12–14 as well as a controlled study at high altitude.21 However, the current study specifically addressed patients with OSA and unmedicated HT. Several therapeutic mechanisms of AZT in OSA have been proposed. The CA inhibitor AZT is known to induce metabolic acidosis following a reduction of renal tubular hydrogen ion secretion along with an increased secretion of sodium, potassium, HCO3−, and water.12 In addition, reduced conversion of CO2 into HCO3− leads to increased tissue and blood CO2 concentration compatible with increased ventilatory drive.42 Indeed, AZT has been shown to reduce loop gain in OSA.14 It may be speculated that a strong ventilatory response to arousal, in association with high CA activity, may cause instability of the control of breathing during sleep. In fact, the extent of HCO3− reduction following AZT in our study was found to be linearly related to the degree of improvement of OSA. Another mechanism relates to the fact that several CA inhibitors such as AZT exhibit diuretic properties and may thereby reduce the extracellular fluid volume, which would result in a reduction of caudal to rostral fluid displacement during sleep and horizontal body positioning. This effect may have contributed to a relative reduction of upper airway collapsibility in OSA.35,43 It is worth noticing that the reduction of OSA by AZT in the current controlled trial was comparable to that obtained by adherence-adjusted CPAP therapy, suggesting that pharmacological therapy may be feasible in patients with OSA intolerant to CPAP.
The current study had several specific strengths. The prospective crossover design with different treatment conditions provides a powerful way to ascertain whether AZT acts via OSA improvement alone or via other mechanisms. Furthermore, the study contained a washout of antihypertensive medication that enabled us to preclude any influence of medication on the intervention. Vascular function (eg, arterial stiffness) was assessed. Study limitations included the small sample size, an open-label design, and the rather short treatment period of 2 weeks that may have led to an underestimation of the actual treatment effect of CPAP on respiration and BP. Polysomnography was not performed in the study; hence, the effect of AZT on sleep in unknown. Finally, neither female subjects nor patients with HT without OSA were studied in the current protocol.
CONCLUSIONS
This is, to our knowledge, the first study with a controlled design to compare the effect of AZT and CPAP, alone or in combination, on BP and respiratory disturbance in patients with HT and OSA. CA inhibition by AZT led to a reduction of BP and vascular stiffness, which was superior to that observed after CPAP. Conversely, CPAP was more effective than AZT in terms of OSA elimination, but this difference was reduced after adjustment for adherence to therapy. Our findings suggest that CA activity influences BP regulation and sleep-disordered breathing in patients with OSA. Larger placebo-controlled clinical trials addressing both effect and long-term outcome are warranted to fully explore the potential of CA-inhibitory drug therapies in OSA.
DISCLOSURE STATEMENT
All authors have read and approved the manuscript. Work for this study was performed at the Center for Sleep and Vigilance Disorders, Department of Internal Medicine and Clinical Nutrition, Sahlgrenska Academy, University of Gothenburg, Gothenburg, Sweden. The study was supported by Swedish Heart and Lung Foundation as well as research and education grants from the Sahlgrenska University hospital. Dr. Grote and Dr. Hedner have an approved and a pending patent related to inhibition of carbonic anhydrase for the treatment of sleep apnea. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Ann-Christin Lundquist, RN, for excellent assistance of the study and Prof. Gunnar Nyberg for generous support with study equipment.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AZT
acetazolamide
- BP
blood pressure
- CA
carbonic anhydrase
- CI
confidence interval
- CO2
carbon dioxide
- CPAP
continuous positive airway pressure
- DBP
diastolic blood pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- HCO3−
bicarbonate
- HR
heart rate
- HT
hypertension
- MAP
mean arterial pressure
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- RM-ANOVA
repeated-measures ANOVA
- SBP
systolic blood pressure
- SpO2
oxygen saturation
REFERENCES
- 1.McNicholas WT, Bonsignore MR Management Committee of EU COST ACTION B26. Sleep apnoea as an independent risk factor for cardiovascular disease: current evidence, basic mechanisms and research priorities. Eur Respir J. 2007;29(1):156–178. doi: 10.1183/09031936.00027406. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard P, Palta M, et al. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 4.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32(9):1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 6.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med. 2016;375(10):919–931. doi: 10.1056/NEJMoa1606599. [DOI] [PubMed] [Google Scholar]
- 7.Kraiczi H, Hedner J, Peker Y, Grote L. Comparison of atenolol, amlodipine, enalapril, hydrochlorothiazide, and losartan for antihypertensive treatment in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1423–1428. doi: 10.1164/ajrccm.161.5.9909024. [DOI] [PubMed] [Google Scholar]
- 8.Pepin JL, Tamisier R, Barone-Rochette G, Launois SH, Levy P, Baguet JP. Comparison of continuous positive airway pressure and valsartan in hypertensive patients with sleep apnea. Am J Respir Crit Care Med. 2010;182(7):954–960. doi: 10.1164/rccm.200912-1803OC. [DOI] [PubMed] [Google Scholar]
- 9.Thunstrom E, Manhem K, Rosengren A, Peker Y. Blood pressure response to losartan and continuous positive airway pressure in hypertension and obstructive sleep apnea. Am J Respir Crit Care Med. 2016;193(3):310–320. doi: 10.1164/rccm.201505-0998OC. [DOI] [PubMed] [Google Scholar]
- 10.Maren TH. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- 11.Wang T, Eskandari D, Zou D, Grote L, Hedner J. Increased carbonic anhydrase activity is associated with sleep apnea severity and related hypoxemia. Sleep. 2015;38(7):1067–1073. doi: 10.5665/sleep.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tojima H, Kunitomo F, Kimura H, Tatsumi K, Kuriyama T, Honda Y. Effects of acetazolamide in patients with the sleep apnoea syndrome. Thorax. 1988;43(2):113–119. doi: 10.1136/thx.43.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whyte KF, Gould GA, Airlie MA, Shapiro CM, Douglas NJ. Role of protriptyline and acetazolamide in the sleep apnea/hypopnea syndrome. Sleep. 1988;11(5):463–472. doi: 10.1093/sleep/11.5.463. [DOI] [PubMed] [Google Scholar]
- 14.Edwards BA, Sands SA, Eckert DJ, et al. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol. 2012;590(5):1199–1211. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eskandari D, Zou D, Karimi M, Stenlof K, Grote L, Hedner J. Zonisamide reduces obstructive sleep apnoea: a randomised placebo-controlled study. Eur Respir J. 2014;44(1):140–149. doi: 10.1183/09031936.00158413. [DOI] [PubMed] [Google Scholar]
- 16.Pickkers P, Hughes AD, Russel FG, Thien T, Smits P. In vivo evidence for K(Ca) channel opening properties of acetazolamide in the human vasculature. Br J Pharmacol. 2001;132(2):443–450. doi: 10.1038/sj.bjp.0703825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aamand R, Dalsgaard T, Jensen FB, Simonsen U, Roepstorff A, Fago A. Generation of nitric oxide from nitrite by carbonic anhydrase: a possible link between metabolic activity and vasodilation. Am J Physiol Heart Circ Physiol. 2009;297(6):H2068–H2074. doi: 10.1152/ajpheart.00525.2009. [DOI] [PubMed] [Google Scholar]
- 18.Pichon A, Connes P, Quidu P, et al. Acetazolamide and chronic hypoxia: effects on haemorheology and pulmonary haemodynamics. Eur Respir J. 2012;40(6):1401–1409. doi: 10.1183/09031936.00216011. [DOI] [PubMed] [Google Scholar]
- 19.Parati G, Revera M, Giuliano A, et al. Effects of acetazolamide on central blood pressure, peripheral blood pressure, and arterial distensibility at acute high altitude exposure. Eur Heart J. 2013;34(10):759–766. doi: 10.1093/eurheartj/ehs140. [DOI] [PubMed] [Google Scholar]
- 20.Latshang TD, Nussbaumer-Ochsner Y, Henn RM, et al. Effect of acetazolamide and autoCPAP therapy on breathing disturbances among patients with obstructive sleep apnea syndrome who travel to altitude: a randomized controlled trial. JAMA. 2012;308(22):2390–2398. doi: 10.1001/jama.2012.94847. [DOI] [PubMed] [Google Scholar]
- 21.Nussbaumer-Ochsner Y, Latshang TD, Ulrich S, Kohler M, Thurnheer R, Bloch KE. Patients with obstructive sleep apnea syndrome benefit from acetazolamide during an altitude sojourn: a randomized, placebo-controlled, double-blind trial. Chest. 2012;141(1):131–138. doi: 10.1378/chest.11-0375. [DOI] [PubMed] [Google Scholar]
- 22.Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16(5):921–927. doi: 10.1183/09031936.00.16592100. [DOI] [PubMed] [Google Scholar]
- 23.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 25.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 26.World Health Organization ISoHWG. 2003 World Health Organization (WHO)/ International Society of Hypertension (ISH) statement on management of hypertension. J Hypertens. 2003;21(11):1983–1992. doi: 10.1097/00004872-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Baulmann J, Schillings U, Rickert S, et al. A new oscillometric method for assessment of arterial stiffness: comparison with tonometric and piezo-electronic methods. J Hypertens. 2008;26(3):523–528. doi: 10.1097/HJH.0b013e3282f314f7. [DOI] [PubMed] [Google Scholar]
- 28.Iber C, Ancoli-Israel S, Chesson AL, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 30.Tkacova R, McNicholas WT, Javorsky M, et al. Nocturnal intermittent hypoxia predicts prevalent hypertension in the European Sleep Apnoea Database cohort study. Eur Respir J. 2014;44(4):931–941. doi: 10.1183/09031936.00225113. [DOI] [PubMed] [Google Scholar]
- 31.Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug-resistant hypertension. J Hypertens. 2001;19(12):2271–2277. doi: 10.1097/00004872-200112000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Robinson GV, Smith DM, Langford BA, Davies RJ, Stradling JR. Continuous positive airway pressure does not reduce blood pressure in nonsleepy hypertensive OSA patients. Eur Respir J. 2006;27(6):1229–1235. doi: 10.1183/09031936.06.00062805. [DOI] [PubMed] [Google Scholar]
- 33.Campos-Rodriguez F, Grilo-Reina A, Perez-Ronchel J, et al. Effect of continuous positive airway pressure on ambulatory BP in patients with sleep apnea and hypertension: a placebo-controlled trial. Chest. 2006;129(6):1459–1467. doi: 10.1378/chest.129.6.1459. [DOI] [PubMed] [Google Scholar]
- 34.Grote L, Wutkewicz K, Knaack L, Ploch T, Hedner J, Peter JH. Association between blood pressure reduction with antihypertensive treatment and sleep apnea activity. Am J Hypertens. 2000;13(12):1280–1287. doi: 10.1016/s0895-7061(00)01207-3. [DOI] [PubMed] [Google Scholar]
- 35.Kasai T, Bradley TD, Friedman O, Logan AG. Effect of intensified diuretic therapy on overnight rostral fluid shift and obstructive sleep apnoea in patients with uncontrolled hypertension. J Hypertens. 2014;32(3):673–680. doi: 10.1097/HJH.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 36.Planes C, Foucher A, Leroy M, et al. Effect of celiprolol treatment in hypertensive patients with sleep apnea. Sleep. 1999;22(4):507–513. doi: 10.1093/sleep/22.4.507. [DOI] [PubMed] [Google Scholar]
- 37.Kontos HA, Richardson DW, Patterson JL., Jr Effects of hypercapnia on human forearm blood vessels. Am J Physiol. 1967;212(5):1070–1080. doi: 10.1152/ajplegacy.1967.212.5.1070. [DOI] [PubMed] [Google Scholar]
- 38.Bickler PE, Litt L, Banville DL, Severinghaus JW. Effects of acetazolamide on cerebral acid-base balance. J Appl Physiol. 1988;65(1):422–427. doi: 10.1152/jappl.1988.65.1.422. [DOI] [PubMed] [Google Scholar]
- 39.Brest AN, Onesti G, Sekine G, Kodama R, Moyer JH. Acetazolamide alone and in combination with reserpine in the treatment of hypertension. Angiology. 1961;12:589–592. doi: 10.1177/000331976101201107. [DOI] [PubMed] [Google Scholar]
- 40.Pickkers P, Garcha RS, Schachter M, Smits P, Hughes AD. Inhibition of carbonic anhydrase accounts for the direct vascular effects of hydrochlorothiazide. Hypertension. 1999;33(4):1043–1048. doi: 10.1161/01.hyp.33.4.1043. [DOI] [PubMed] [Google Scholar]
- 41.Chobanyan-Jurgens K, Schwarz A, Bohmer A, et al. Renal carbonic anhydrases are involved in the reabsorption of endogenous nitrite. Nitric Oxide. 2012;26(2):126–131. doi: 10.1016/j.niox.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Javaheri S, Sands SA, Edwards BA. Acetazolamide attenuates Hunter-Cheyne-Stokes breathing but augments the hypercapnic ventilatory response in patients with heart failure. Ann Am Thorac Soc. 2013;11(1):80–86. doi: 10.1513/AnnalsATS.201306-201OC. [DOI] [PubMed] [Google Scholar]
- 43.Redolfi S, Yumino D, Ruttanaumpawan P, et al. Relationship between overnight rostral fluid shift and obstructive sleep apnea in nonobese men. Am J Respir Crit Care Med. 2009;179(3):241–246. doi: 10.1164/rccm.200807-1076OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.