Abstract
Study Objectives:
Sleep disorders and sleep deficiency can increase the risk for cardiovascular disease. Less is known about whether multiple positive attributes of sleep health known as the SATED (satisfaction, alertness, timing, efficiency, and duration) model, can decrease future cardiovascular disease risks. We examined whether and how a variety of indicators of sleep health predicted 10-year estimated cardiometabolic risk scores (CRS) among employed adults.
Methods:
Workers in two industries—extended care (n = 1,275) and information technology (IT; n = 577)—reported on habitual sleep apnea symptoms and sleep sufficiency, and provided 1 week of actigraphy data including nighttime sleep duration, wake after sleep onset (WASO), sleep timing, and daytime napping. Workers also provided biomarkers to calculate future cardiometabolic risk.
Results:
More sleep apnea symptoms predicted higher CRS in both industries. More sleep sufficiency, less WASO, and less daytime napping (having no naps, fewer naps, and shorter nap duration) were also linked to lower CRS, but only in the extended care workers. There was no effect of sleep duration in both industries. In the IT employee sample, shorter sleep duration (≤ 6 hours versus 6–8 hours) and more naps strengthened the link between sleep apnea and CRS.
Conclusions:
Sleep health, measured by both subjective and objective methods, was associated with lower cardiometabolic disease risks among extended care workers (lower to middle wage workers). Sleep apnea was an important predictor of CRS; for the IT workers, the link between sleep apnea and CRS was exacerbated when they had poorer sleep health behaviors.
Citation:
Buxton OM, Lee S, Marino M, Beverly C, Almeida DM, Berkman L. Sleep health and predicted cardiometabolic risk scores in employed adults from two industries. J Clin Sleep Med. 2018;14(3):371–383.
Keywords: actigraphy, cardiometabolic risks, employees, sleep apnea, sleep health
BRIEF SUMMARY
Current Knowledge/Study Rationale: Prior research has shown that sleep deficiency (often focusing only on short sleep duration) and sleep apnea lead to the development of cardiometabolic disease. However, less is known about how positive attributes of the multidimensional aspects of sleep health predict future cardiometabolic risks in employed adult samples.
Study Impact: This study advances the literature on sleep health by examining the associations of positive attributes of sleep health with cardiometabolic risk scores, independent of and interacting with sleep apnea symptoms. Strengths of this study include the use of a variety of sleep health indicators measured by both self-reports and actigraphy and also focusing on midlife workers in the two different industry contexts (ie, information technology and extended care), which provides specific policy implications.
INTRODUCTION
Sleep is a multidimensional construct; sleep research has moved from testing the effect of a sleep disorder on individual health to examining multiple positive attributes that quantify “sleep health.”1 In prior literature, the opposite construct of sleep health, sleep deficiency, leads to the development of cardiometabolic disease by triggering metabolic stress and disturbing homeostatic regulation.2 Negative effects of sleep deficiency have been well documented across different types of studies. Controlled laboratory studies have shown that acute sleep loss for 1 week3,4 or even 1 night5 impairs metabolic function in healthy adults. National health surveil-lance data show us that short sleep is common in the United States.6 Observational and retrospective studies have shown that chronic sleep deficiency is associated with obesity, diabetes, hypertension, cardiovascular disease, and early mortality.7–12 However, sleep deficiency is framed negatively,1 and lacks a positively focused health promotion message considered more effective for public health messaging. This study focuses on positive attributes of sleep health and examines how they predict future cardiometabolic risk in two employed adult samples from different industries with different socioeconomic characteristics.
Sleep health is rarely defined in prior research, but a recent definition by Buysse1 provides a useful guideline to measure sleep health in five key dimensions—satisfaction with sleep, alertness during waking hours, timing of sleep, sleep efficiency, and sleep duration—known as the SATED scale. Based on this conceptualization, we examine self-reported sleep sufficiency (satisfaction with sleep) and actigraphy-assessed daytime napping (alertness during waking hours), sleep clock timing (timing of sleep), wake after sleep onset (WASO; sleep efficiency) and nighttime sleep duration (sleep duration). Using both self-reports and objective markers of sleep will provide a more comprehensive picture of perceived and measured sleep health. Daytime napping has been often forgotten in previous research, but it may relate to lack of alertness during waking hours. Specifically, napping patterns (eg, having naps, more frequent napping, and longer nap duration) may indicate lack of sleep restorativeness during nighttime and potentially signals increased sleep pressure during daytime.13 Less attention has been paid to sleep clock timing: whether and how regularity or variability of sleep timing indicates sleep health.14 The current study incorporates these multiple indicators of sleep health to advance the understanding of workers' multidimensional sleep health and their relative magnitudes for future cardiometabolic risks. To estimate future cardiometabolic risks, we use a validated risk score validated using Framingham Study (offspring) data to predict subsequent 10-year “hard” cardiovascular event risk.15
Sleep health is not merely the absence of a sleep disorder.1 To verify this, it is important to examine the effects of sleep health variables on cardiometabolic risks independent of the effect of a sleep disorder known to be a significant risk for cardiometabolic risks. Obstructive sleep apnea is a chronic sleep disorder associated with poor sleep quality and often reduced sleep duration2 and has been consistently linked to cardiometabolic disease in prior research.10,16,17 Approximately 30 million men and women in the United States have sleep apnea, and sleep apnea puts a strain on the heart by repeatedly causing oxygen levels to drop and blood pressure to surge while sleeping.18 Although sleep apnea is a risk factor for cardiometabolic disease, other positive sleep behaviors may independently contribute to decreasing cardiometabolic risks.11,19 Thus, this study tests the effects of multifaceted sleep health on 10-year estimated cardiometabolic risk scores (CRS), after controlling for the effect of sleep apnea symptoms.
Moreover, moving beyond testing the independent effects of sleep health and self-reported sleep apnea symptoms, in this study we further test the interactions between sleep health and sleep apnea symptoms on estimated future cardiometabolic risk. Individuals who suffer from severe sleep apnea may have a higher cardiometabolic risk. However, the link may become weaker (or stronger) if they exhibit good (or poor) sleep health. Each of the sleep apnea and sleep health variables may predict future cardiometabolic risks; but positive sleep health behaviors, such as having longer sleep duration and maintaining consistent sleep timing, may buffer the adverse effect of sleep apnea symptoms on the risk of developing cardiometabolic disease within 10 years. Therefore, we examine whether any of the sleep health indicators moderate the effect of sleep apnea symptoms on future cardiometabolic risks.
Prior research suggests disparities in sleep and cardio-metabolic health risks exist by socioeconomic status.11,20,21 In this study, we draw upon two industry samples—patient care workers in extended care settings and professional employees in a large information technology (IT) firm. Lower-wage workers in the extended care (nursing home) industry often work nonstandard, unpredictable schedules.22 Employees in the IT industry are generally considered white collar, involved mostly in software development, IT architecture, or engineering,23 and in relatively privileged jobs (by education and income) compared to other workers in the United States. Examining these two industry samples provides unique opportunities to compare the association between sleep health and CRS between different socioeconomic and work contexts.
This study addresses two specific hypotheses about the relationship between sleep health and CRS. We first examined whether and how a variety of sleep health indicators were uniquely associated with CRS, independent of sleep apnea symptoms. We hypothesized (H1) that greater sleep health (ie, more sleep sufficiency, consistent sleep midpoint times, less WASO, longer nighttime sleep, and less daytime napping) would be associated with lower CRS, after controlling for the effect of sleep apnea symptoms. Second, we examined whether there were interactive effects between sleep apnea symptoms and sleep health on CRS. We hypothesized (H2) that poorer sleep health would strengthen the link between sleep apnea symptoms and CRS.
METHODS
Participants
Data came from the Work, Family, and Health Study.24,25 The current study used baseline data from two industry samples recruited from an extended care (nursing home) industry partner and an IT industry partner. The extended care study cohort consisted of a primarily hourly workforce from 30 distinct worksites located in the northeastern United States. Eligible employees were involved in direct patient care, typically worked at least 22 h/wk, and did not do regular night work. Overall, of the 1,783 eligible employees with direct patient care responsibilities, 1,524 were recruited (85% participation rate). Among 211 eligible managers, 184 were recruited (87% participation rate). Of the 1,708 total workers, 1,485 workers provided biomarker data necessary to calculate CRS. Of these, 1,275 workers (1,210 employees + 65 managers) provided actigraphy-assessed data, and thus the final analytic sample for the extended care industry. The extended care sample workers included registered nurses and licensed practical nurses (29%), certified nursing assistants (65%), and other positions including administrators, educators, and food service aides (6%).
The IT study cohort consisted of employees located in teams within the IT division of a large Fortune 500 firm. Workers were eligible to participate if they were employees (not contractors) located in the metropolitan areas with the two largest worksites where data collection occurred. Of 1,171 eligible employees, 823 employees were recruited (70% participation rate). The employees were from 123 workgroups who reported to the same senior leadership or worked closely together on the same application.
To have a consistent sample with other studies that examined sleep and work-family conflict in this IT employee cohort,23,26 we excluded 24 employees across 5 workgroups as previously described.23,26 Therefore, 799 employees were the baseline cohort of IT employees (IT managers did not participate in biomarker and actigraphy-assessed substudies, and thus were not included in the current study). Of the 799 total employees, 703 employees provided biomarker data necessary to calculate CRS. Of these, 577 employees provided actigraphy-assessed data, and thus the final analytic sample for the IT industry.
Procedure
Trained field interviewers administered face-to-face computer-assisted personal interviews (CAPI) to employees from September 2009 through September 2010. Employees completed a 60-minute interview at the worksite and received a $20 incentive. All procedures were conducted in accordance with the principles in the Declaration of Helsinki, and approved by appropriate Institutional Review Boards.
Immediately following the CAPI, interviewers introduced the actigraphy data collection process and requested participation for an additional $20 incentive. If the participant agreed, the interviewer instructed them to wear the accelerometer (Spectrum, Philips-Respironics, Murrysville, Pennsylvania, United States) on their nondominant wrist for the next week except in situations in which the watch could be damaged (eg, excessive impact, extreme temperatures). Actigraphy data was downloaded using the Actiware Sleep Scoring Program (version 5.71, Philips-Respironics, Murrysville, Pennsylvania, United States). We used a standard validated algorithm27 and at least two members of the scoring team determined the validity of each recording based on study-specific standard sleep criteria applied similarly to all recordings.13,26,28 On average, participants had 6.61 and 6.72 days of valid actigraphy, in the extended care and IT worker samples, respectively.
Measures
Sleep
Self-reported sleep apnea symptoms: We calculated a sleep apnea score by summing (yes = 1, no = 0) responses to the following four items: “During the past month, (1) have you snored, or ever been told that you were snoring; (2) have you snored loudly, or ever been told that you were snoring loudly; (3) have you had, or ever been told that you were snorting or gasping; and (4) have you had, or ever been told that your breathing stops or you struggle for breath?” Higher scores indicated more sleep apnea symptoms (range = 0–4).
Self-reported sleep sufficiency: We measured self-reported sleep sufficiency by asking one item, “How often during the past 4 weeks did you get enough sleep to feel rested upon waking up?” This item28–31 is similar to current Centers for Disease Control and Prevention surveillance for state-level sleep sufficiency.32 Responses were rated on a scale ranging from 1 (never) to 5 (very often), such that higher scores indicated greater sleep sufficiency.
Actigraphy-assessed nighttime sleep duration: Sleep episodes were defined as beginning at the last epoch of high activity (> 10 activity counts) preceding at least five 30-second epochs of < 10 activity counts, indicating little to no movement. Any intervals identified that exceeded a 15-minute difference in duration or timing between the scorers were reviewed by the senior author and scorers until agreement. Among sleep periods each day, the longest sleep period of the day was defined as the main nighttime sleep. Habitual nighttime sleep duration was calculated in minutes per night by averaging nightly sleep duration across the actigraphy week. Considering that the association between nighttime sleep duration and health risks may be curvilinear (eg, U-shaped curve indicating both short and long sleep may have negative implications), we created sleep duration categories to compare the effects of shorter sleep (< 6 h/night) and longer sleep (> 8 h/night), compared to 6–8 h/night of sleep. The American Academy of Sleep Medicine and the Sleep Research Society recommend that 7–9 h/night of sleep is desirable for healthy adults for “optimal” health and well-being.33 In our samples of workers, few individuals exhibited more than 9 h/night of sleep (4% and 2% in the extended care and IT samples, respectively) and most had 6–8 h/night of sleep. To have comparable cell sizes, we thus coded longer sleep as > 8 h/night of sleep.
Actigraphy-assessed wake after sleep onset (WASO): WASO was computed as the average amount of time spent awake after sleep onset and before sleep offset during nighttime sleep, in minutes, as previously validated versus polysomnography.27 To compare the effect of moderate or long WASO versus less WASO, we recoded WASO in 3 categories: < 30 minutes (score = 0), 30 to < 45 minutes (score = 1), ≥ 45 minutes (score = 2), such that higher scores indicated longer WASO.
Actigraphy-assessed sleep timing: We assessed variability of sleep midpoint times. Sleep midpoint times were calculated based on the bedtime and wake time variables of each nighttime sleep. Bedtimes were determined by points of decreased activity levels and sudden, decreased light levels (lux). Wake times were determined the same way by finding the first epoch of sustained high activity (> 10 activity counts) after at least five 30-second epochs of < 10 activity counts. Midpoint times were centered at midnight (00:00 = 0), such that midpoint time of 2 indicated that a person's midpoint of sleep is 2:00 am. Then we created individual standard deviation (iSD) of sleep midpoint times around person means across the actigraphy week. Higher scores indicated greater variability of sleep timing, thus less sleep health. To account for potential interdependency between iSD and the level of sleep midpoint times, we controlled for person means of sleep midpoint times in our analysis.
Actigraphy-assessed having any naps, frequency of napping, and nap duration: After defining the main, nighttime sleep, all other sleep periods of the day were defined as nap episodes and were scored as such (1 = nap episode, 0 = main nighttime sleep episode, not nap). The scoring team members visually scored nap episodes by determining points of decreased activity levels as previously described.26 Briefly, a period of ≥ 30 minutes of low activity paired with a decrease in light levels during the time of low activity was confirmatory of a nap. If there was a period of ≥ 30 minutes of low activity without a decrease in light levels, scorers may still conclude a nap period, as the lack of light was not exclusionary criteria. Nap periods were required to be ≥ 30 minutes, intending to capture the intentional naps. Low activity periods of < 30 minutes may have been other quiet activities. Rapidly decreased light levels (lux) were considered confirmatory but not necessary or sufficient to define a nap. The accelerometer also had an on-wrist detection feature that allowed scorers to view when participants were not wearing the accelerometer. Scorers took into consideration the decrease in activity intensity within the context of the entire subject profile. First, a binary nap variable was coded as 1 if participants did not have any naps across the actigraphy week and 0 if participants had naps, to examine the effect of no napping (that may relate to more alertness during daytime). Second, for those who had any naps, the number of naps was summed across the week, such that higher scores indicate more naps. Third, the average nap duration during the week was calculated in minutes per day; this variable was used in the analysis only for those who had any naps.
Cardiometabolic Risks
A CRS was calculated based on modifiable risk factors in the widely used Framingham risk score (eg, age- and sex-specific strata use different score calculations). The score has been independently validated using the Framingham (offspring) data to predict subsequent 10-year cardiovascular event risk.15 Bio-markers measured as previously described24 included height and weight to calculate body mass index (BMI), blood pressure, and hemoglobin A1c. Seated blood pressure readings were collected three times at least 5 minutes apart during the interview, and before blood sampling, using wrist blood pressure monitors (HEM-637, Omron Healthcare, Bannockburn, Illinois, United States). BMI (kg/m2) was calculated based on height (Seca213/214 stadiometers, Seca North America, Hanover, Maryland, United States) and weight (Health-O-Meter 800KL, Jarden Corporation, Rye, New York, United States). Up to five blood spots were collected on barcoded filter paper (903 Protein Saver Paper, GE Healthcare Bio-Sciences Corp., Piscataway, New Jersey, United States) as previously described,34 air-dried, and sealed in a plastic bag for room temperature shipment with desiccant for storage at −86°C until assay for cholesterol as specifically validated for this study from serum to dried blood spot equivalents.35 Interviewers also collected a 1-μL blood droplet for immediate measurement of hemoglobin A1c levels (DCA Vantage Analyzer, Siemens Healthcare Diagnostics, Frimley, Camberley, United Kingdom). Tobacco consumption was self-reported and categorized as smokers or nonsmokers.
Covariates
To take into account possible differences in sleep and health risks by sociodemographic conditions, employee age (in 1-year intervals), sex (0 = female, 1 = male), marital/partner status (0 = unmarried, 1 = married or living with a partner), race (different categories by industry; extended care: 0 = White, 1 = Black, 2 = Hispanic, 3 = Other; IT: 0 = White, 1 = Asian/ Pacific Islander/Other, 3 = Other), providing care to adult relatives (0 = no, 1 = yes), range of current annual house -hold income (different categories by industry; extended care: 1 = less than $4,999 to 13 = more than $60,000; IT: 1 = less than $49,999 to 12 = more than $150,000), presence of children younger than 18 years in household (0 = no, 1 = yes) were included in all models. Moreover, total work hours were assessed from an item asking “About how many hours do you work in a typical week in this job?” and “On average, how many hours per week do you work at this other job(s)?” and summed if the respondent indicated he/she has an additional job to obtain total hours worked/week across all jobs. All continuous variables were centered at sample means.
Analytic Strategy
We used general linear regression models with identity link using Proc GLM in SAS version 9.4 (SAS Institute Inc., Cary, North Carolina, United States) to examine whether each of the sleep measures predict estimated CRS. Model 1 included sleep apnea symptoms in addition to a set of covariates (ie, age linear, age quadratic, sex, marital status, race, adult caregiving, household income range, living with children age 18 years or younger, total work hours, and managerial status). Model 2 contained variables in Model 1 and added six sleep variables that assess the five SATED dimensions of sleep health, including sleep sufficiency, having no naps (versus any naps), sleep midpoint times, variability in sleep midpoint times, WASO, and sleep duration. Model 3 contained variables in Model 1 and added number of naps or nap duration using a subsample of workers who had any naps. Because of high correlations between napping variables, we tested the associations of number of naps and nap duration separately in each model. In Model 4, interactions between sleep apnea and each of sleep health measures were tested. Where significant, the interaction was probed to describe differences at less (−1 standard deviation [SD]) and more (+1 SD) sleep apnea symptoms. Models were tested separately for each industry, given sociodemographic and structural differences between the IT and extended care industries36 that may influence workers' sleep patterns. We also explored interrelationships among age, sleep, and CRS using three-dimensional plots to depict the relative magnitudes of sleep health variables for CRS compared to known effects of age on CRS.
RESULTS
Descriptive Statistics
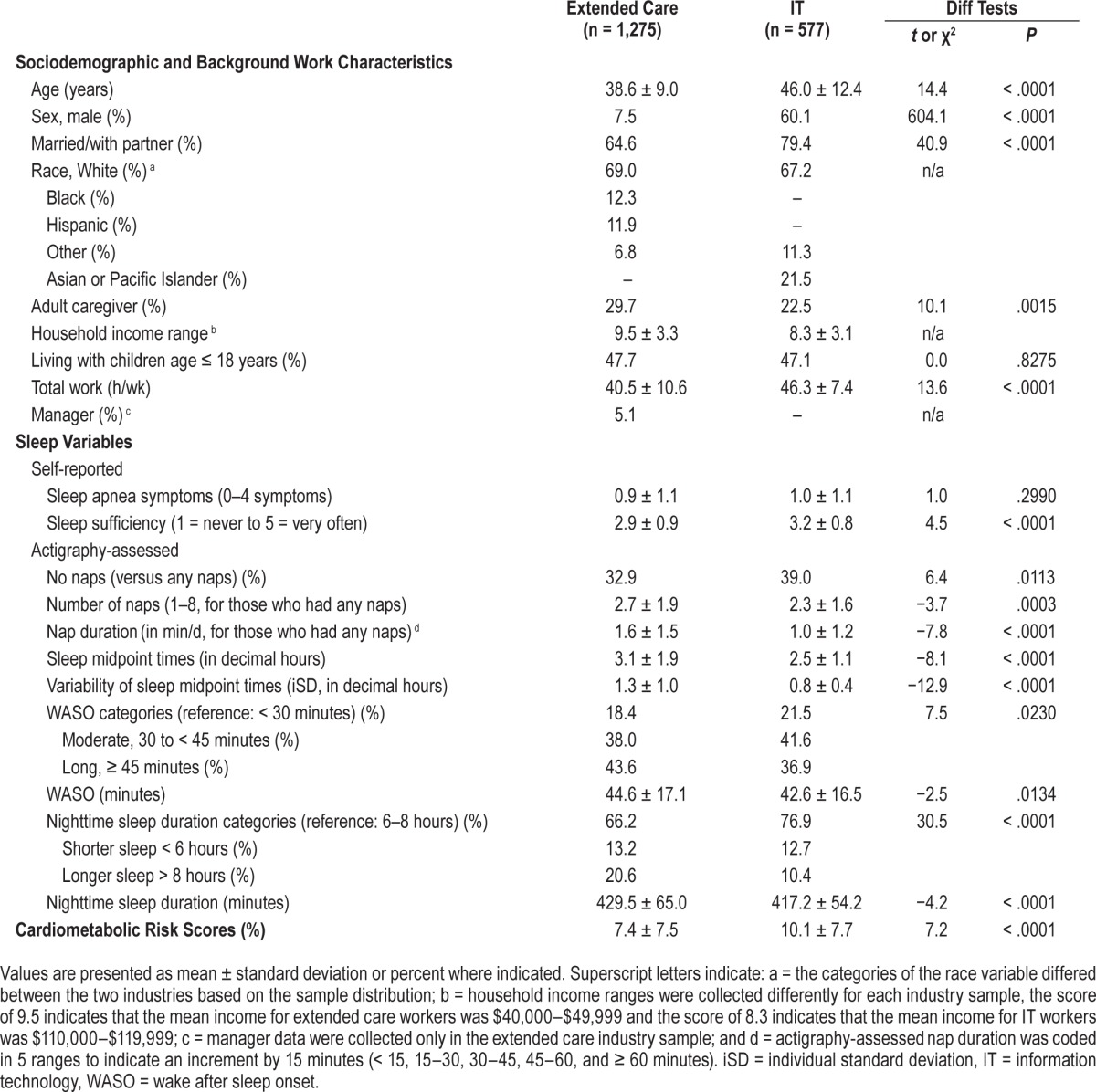
Sample characteristics of the extended care industry showed that the mean age was 38.62 years (standard deviation [SD] 12.41), and most (92%) were female, 65% were married or cohabiting, and 69% were White, 12.3% were African American, and 11.9% were Hispanic. The sample was primarily working class as indexed by household income: mean annual household income fell in the range of $40,000–$49,999 (mean = 9.54 [where 1 = less than $4,999 to 13 = more than $60,000] SD = 3.31). Nearly half (47.7%) were living with children younger than 18 years in the household. Mean total work hours per week in any jobs was 40.53 (SD = 10.59). Mean BMI was 29.43 kg/m2 (SD = 6.94); most (61%) fell below the obese range (BMI < 30 kg/m2).
For the IT workers, mean age was 46.01 years (SD = 9.02), and 60% were male, most (79%) were married or cohabiting, and 67% were White and 21.5% were Asian Pacific Islander. Mean annual household income fell in the range of $110,000– $119,999 (mean = 8.31 [where 1 = less than $49,999 to 12 = more than $150,000] SD = 3.10). Nearly half (47.1%) were living with children younger than 18 years in the household. Mean total work hours per week in any jobs was 46.01 (SD = 9.02). Mean BMI was 28.43 kg/m2 (SD = 5.68); most (67%) fell below the obese range.
Table 1 presents sociodemographic, work, and sleep descriptive statistics by industry. Extended care workers were more likely to have less sleep sufficiency, more WASO, longer sleep duration, later and more variable sleep midpoint times, and take any naps, more naps and longer naps compared to IT workers. However, the two industry worker groups did not significantly differ in their reports of sleep apnea symptoms. Moreover, extended care workers were more likely to have lower CRS than IT workers, mostly because there were more males and older workers in the IT sample. Because of different characteristics between the two industries, we examined correlations between sleep parameters and CRS by each industry. For extended care workers, more sleep apnea symptoms (r = .14, P < .001), more perceived sleep sufficiency (r = .08, P = .005), earlier midpoint times (r = −.08, P = .003), and more numbers of naps (r = .14, P < .001) were associated with higher CRS. For IT workers, more sleep apnea symptoms (r = .17, P < .001), earlier midpoint times (r = −.17, P < .001), and more numbers of naps (r = .13, P = .013) were associated with higher CRS. Across the industries, sleep parameters were not highly correlated each other, meaning that each of the sleep parameters is unique and independent. However, the weekly frequency of naps and average nap duration were highly correlated (r = .71, P < .001 for extended care industry and r = .76, P < .001 for the IT industry), and thus, for those who had any naps, we examined the effects of these two napping variables on CRS in separate models.
Table 1.
Sociodemographic, work, and sleep descriptive statistics by industry.
Main Effects of Sleep Apnea Symptoms on CRS
Table 2 shows results of general linear models examining the effect of covariates and sleep apnea symptoms on CRS (ie, Model 1). For both industry samples, there were significant linear and quadratic effects of age on CRS, such that an increase in age was associated with higher CRS and the rate of increases in CRS was accelerated in older ages. Biological sex was a strong predictor of CRS, with males having 7% to 8% higher CRS than females. In the extended care sample, white employees tended to have higher CRS than non-white employees, and those with less household income and having children younger than 18 years were more likely to have higher CRS. In the IT sample, other ethnic minorities (non-white, non-Asian Pacific Islander) had higher CRS.
Table 2.
Results of general linear models examining the associations between self-reported sleep apnea symptoms and estimated 10-year cardiometabolic risk scores.
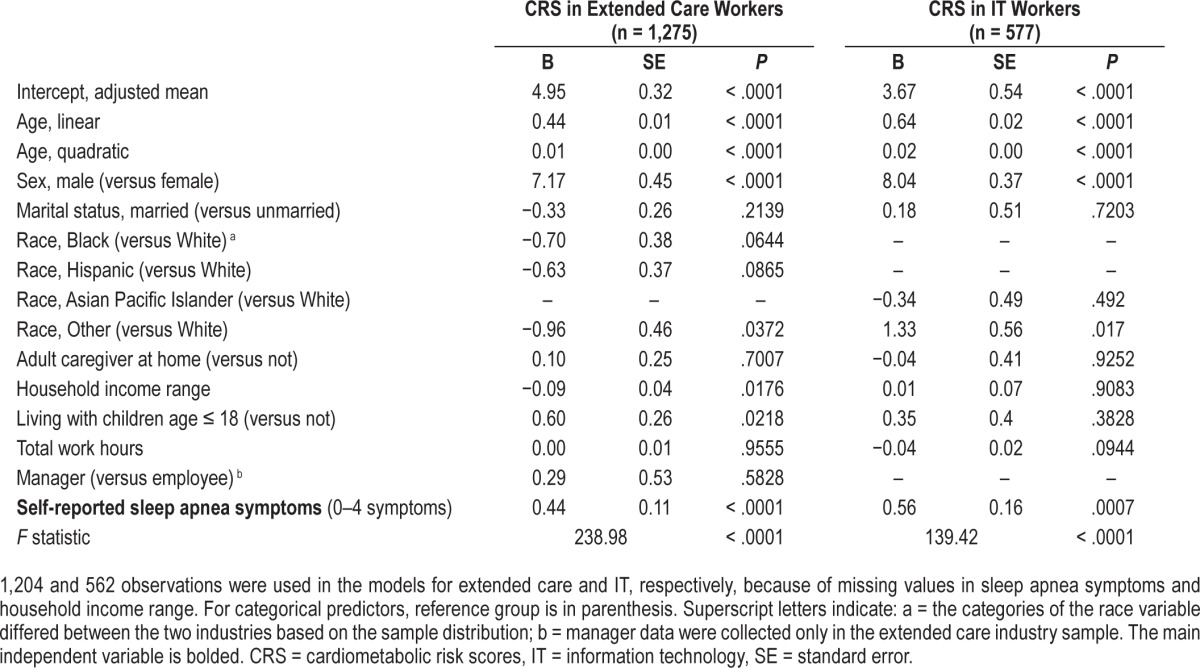
After controlling for the effects of these covariates, there were significant effects of sleep apnea symptoms on CRS in both industries. For extended care workers, one unit increase in sleep apnea symptoms was associated with 0.44% higher CRS. For IT workers, one unit increase in sleep apnea symptoms was associated with 0.56% higher CRS.
Main, Independent Effects of Sleep Health Indicators on CRS
Table 3 shows results of general linear models examining the unique effects of sleep sufficiency, having no naps (versus any naps), the level and variability of sleep midpoint times, WASO, and shorter or longer sleep duration (versus 6 to 8 hours) on CRS, beyond the effect of sleep apnea symptoms (ie, Model 2). After taking into account the effects of sociodemographic characteristics, work hours and sleep apnea symptoms, less perceived sleep sufficiency, having no naps, and more WASO were associated with higher CRS for the extended care workers, but not for the IT workers. Specifically, one unit increase in sleep sufficiency results in 0.27% lower CRS. Having no naps was associated with 0.74% lower CRS. Further, compared to having WASO less than 30 minutes, more WASO (more than 45 minutes) leads to 0.78% higher CRS. There was no effect of sleep duration, either shorter or longer sleep, on CRS, in both industries.
Table 3.
Results of general linear models examining the independent associations between self-reported and actigraphy-assessed sleep apnea and sleep health variables and estimated 10-year cardiometabolic risk scores.
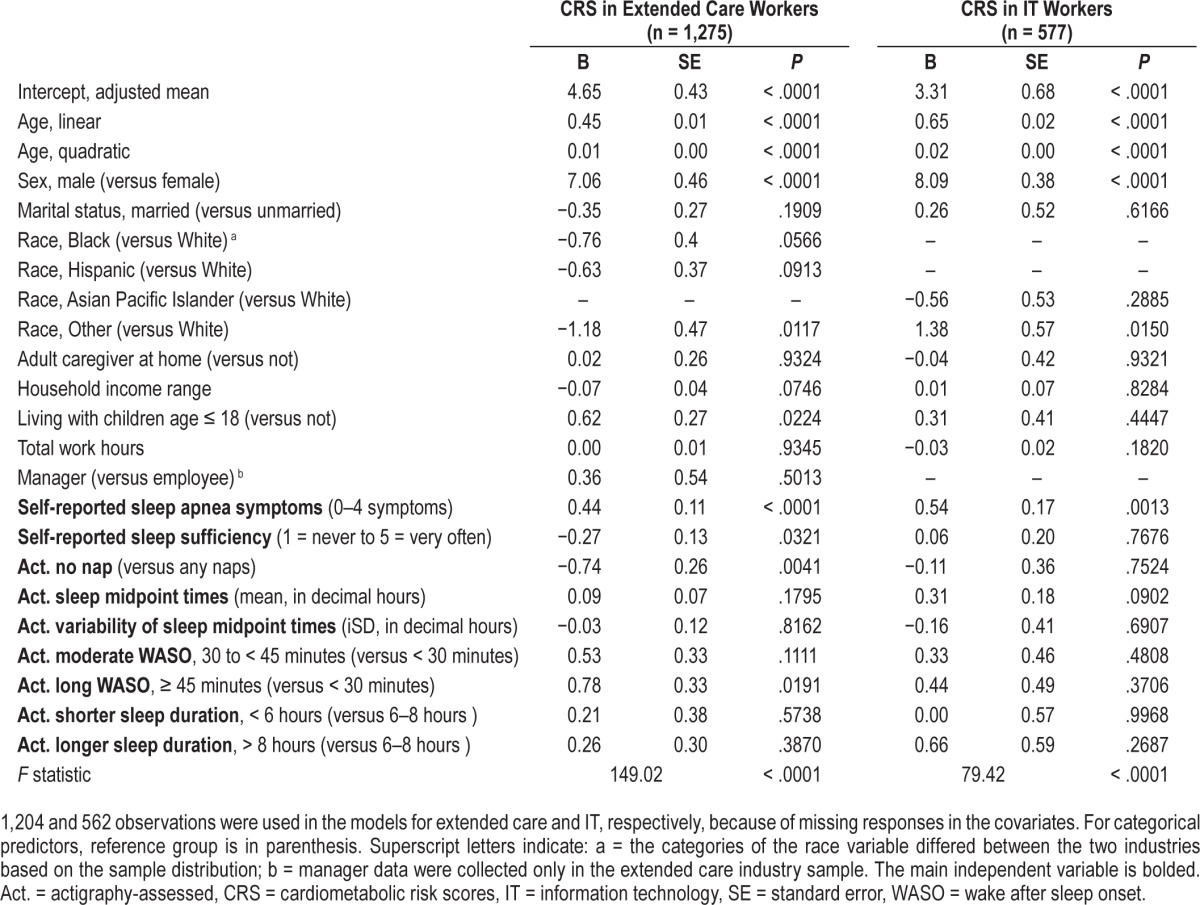
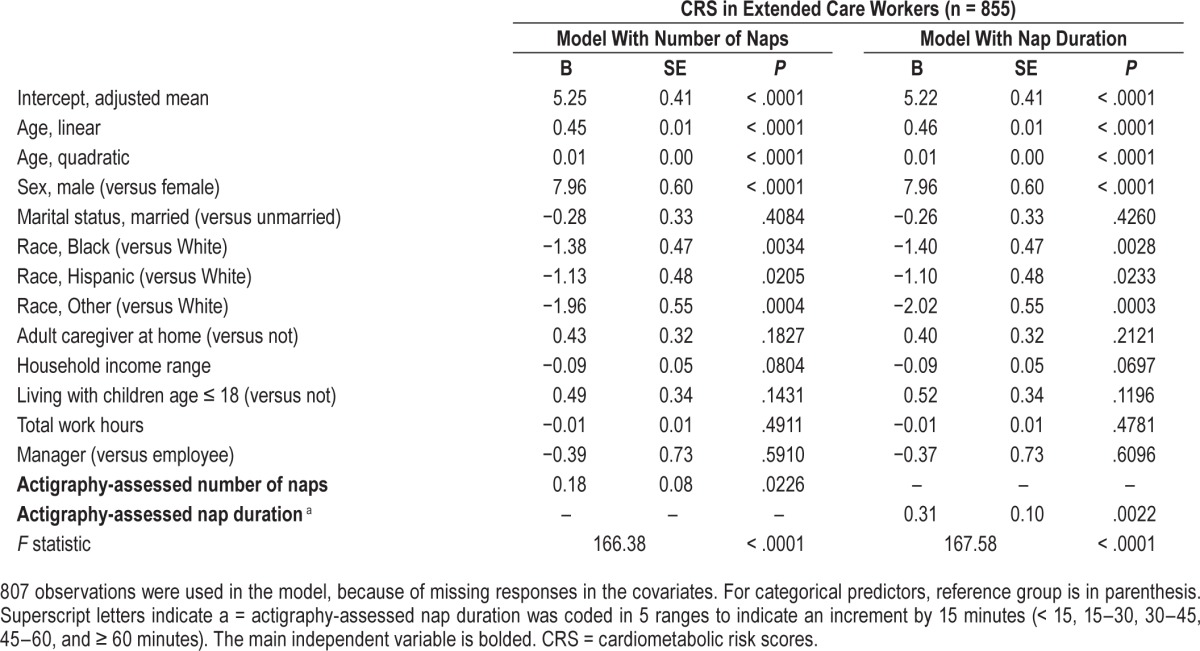
Next, the associations of number of daytime naps and nap duration with CRS were tested separately for each industry. Table 4 shows results for a subsample of extended care workers who had any naps during the actigraphy study week (ie, Model 3). After adjusting for all covariates from the prior model, one number increase in naps was linked to 0.18% higher CRS and 15 minutes increase in nap duration was associated with 0.31% increase in CRS among nappers in the extended care industry. There were no significant associations of these napping variables with CRS in the IT industry (data not shown). Thus, our H1 that greater sleep health would be linked to lower CRS was only supported in extended care workers, in the specific domains of more sleep sufficiency, less WASO, and less daytime napping (but not shorter nighttime sleep duration).
Table 4.
Results of general linear models examining the associations of actigraphy-assessed daytime napping with estimated 10-year cardiometabolic risk scores for extended care workers who had any naps.
In addition, we conducted supplementary analyses restricting our sample to those who had zero sleep apnea symptoms. For extended care workers who reported zero sleep apnea symptoms (n = 608, 48%), only having no naps significantly predicted lower CRS (B = −0.71, standard error [SE] = 0.36, P = .0465). Having shorter nap duration also tended to be associated with lower CRS (B = 0.28, SE = 0.14, P = .0551). For IT workers who reported zero apnea symptoms (n = 255, 44%), none of the sleep health variables turned out to be a significant predictor of CRS as a main effect. However, the interaction of daytime napping and nighttime sleep duration significantly influenced CRS. Specifically, having no naps and longer nighttime sleep (> 8 hours versus 6–8 hours) predicted lower CRS (B = −3.47, SE = 1.61, P = .0320), net of other sleep health variables; only 4% of the IT sample (n = 11) had no sleep apnea symptoms, no napping, and more than 8 hours of sleep per night. The interaction between napping and nighttime sleep duration was not significant in the extended care sample. There was no significant interaction effect of nap duration and nighttime sleep duration on CRS among those without sleep apnea symptoms in either industry.
Interaction Effects of Sleep Apnea Symptoms and Sleep Health on CRS
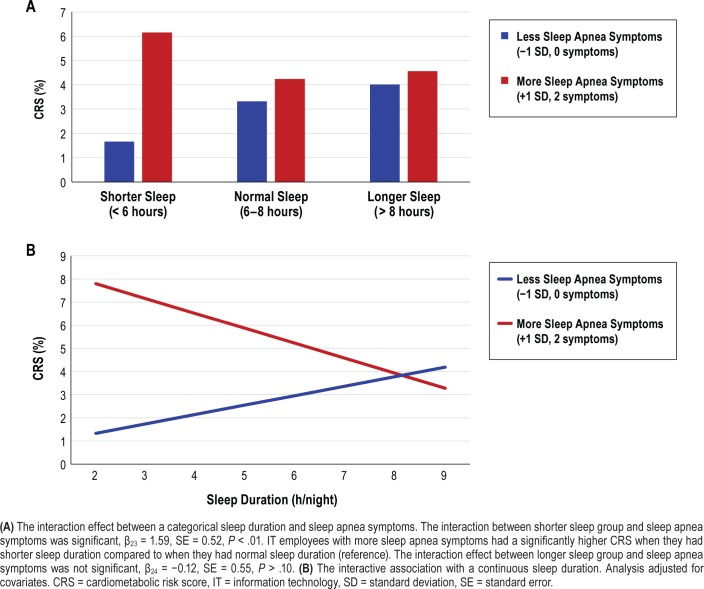
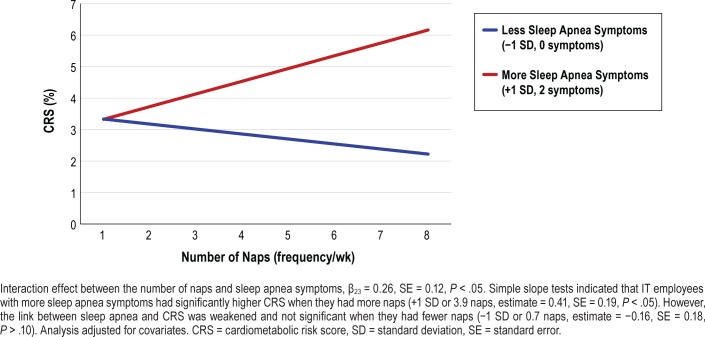
Last, we tested whether any of the sleep health indicators moderated the effect of sleep apnea symptoms on CRS (ie, Model 4). There were three significant interactions between sleep health indicators and sleep apnea symptoms predicting CRS, only in IT industry employees. First, the interaction effect between actigraphy-assessed WASO categories and sleep apnea symptoms was significant (moderate WASO × sleep apnea: β19 = −1.68, SE = 0.41, P < .001, long WASO × sleep apnea: β20 = −1.26, SE = 0.43, P < .01). However, simple slope tests showed that the link between sleep apnea symptoms and CRS was weaker in these relatively longer WASO groups compared to the shorter WASO group (ref: WASO < 30 minutes). Second, there was a significant interaction between actigraphy-assessed sleep duration and sleep apnea symptoms predicting CRS (β21 = 1.59, SE = 0.52, P < .01). Figure 1 shows that IT employees with more sleep apnea symptoms had significantly higher CRS when they had shorter sleep (< 6 hours) compared to when they had 6 to 8 hours of sleep. CRS did not significantly differ by longer sleep (> 8 hours) compared to 6 to 8 hours of sleep. Last, among nappers only, the interaction between nap frequency and sleep apnea symptoms also significantly predicted CRS (β23 = 0.26, SE = 0.12, P < .05). Figure 2 shows that IT employees with more sleep apnea symptoms had significantly higher CRS when they had more naps across the actigraphy week (+1 SD or 3.9 naps, estimate = 0.41, SE = 0.19, P < .05). However, the link between sleep apnea and CRS was weakened and not significant when they had fewer naps (−1 SD or 0.7 naps, estimate = −0.16, SE = 0.18, P > .10). These effects were not found for the extended care workers. There were no significant moderating effects of sleep sufficiency, having no nap (versus having any naps), and timing and variability of sleep midpoint. Thus, our H2 that sleep health would moderate the link between sleep apnea and CRS was supported in specific domains of sleep duration and number of naps (among the five domains of sleep health), for the IT sample.
Figure 1. Actigraphy-assessed sleep duration, sleep apnea symptoms, and the estimated 10-year CRS in information technology employees (n = 577).
Figure 2. Interactive effect between number of naps and sleep apnea symptoms on the estimated 10-year CRS in information technology employees who had any naps (n = 352).
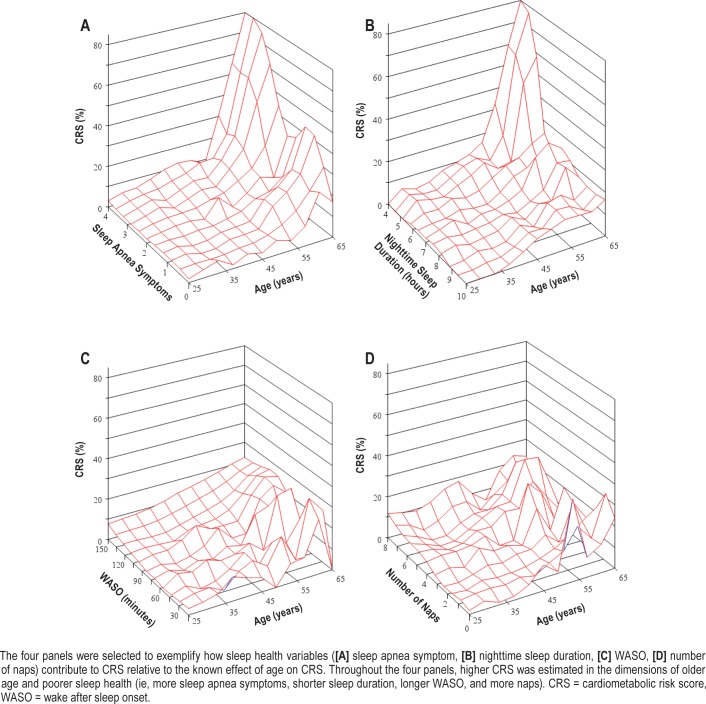
We further explored the associations among age, sleep health, and CRS to better understand how sleep health indicators contribute to CRS relative to the known effect of age on CRS. Figure 3 shows four examples with selected sleep health variables in the IT sample. Throughout the four panels, higher CRS was estimated in the dimensions of older age and poorer sleep health (ie, more sleep apnea symptoms, shorter sleep duration, longer WASO, and more napping).
Figure 3. Three-dimensional plots examining the associations between age, sleep health, and the estimated 10-year CRS in information technology employees (n = 577).
DISCUSSION
This study examined the effects of multiple positive attributes of sleep health on future CRS, independent of the effect of sleep apnea symptoms. Building upon recent conceptualizations of sleep health,1 we considered five key dimensions of sleep health: satisfaction (self-reported sleep sufficiency), alertness (actigraphy-assessed daytime naps, number of naps, and nap duration); timing of sleep (actigraphy-assessed sleep midpoint timing and variability in sleep midpoint), efficiency (actigraphy-assessed WASO), and duration (actigraphy-assessed nighttime sleep duration). We found that higher perceived sleep sufficiency, having no naps, and shorter WASO (versus longer WASO) uniquely predicted lower CRS, beyond the strong effect of sleep apnea symptoms on CRS. These effects were only apparent in the extended care industry sample. In the IT sample, however, some of the sleep health indicators (ie, longer sleep duration, fewer naps) moderated the association of sleep apnea symptoms with CRS. Strengths of this study include the use of a variety of sleep health indicators measured by both self-reports and actigraphy and also focusing on midlife workers in two different industry contexts.
Sleep Health Indicators Independently Predict Cardiometabolic Risks, But Only Among Workers in the Extended Care Industry
Independent of the strong effect of sleep apnea symptoms on CRS, more sleep sufficiency, less WASO, and less daytime napping (having no naps, fewer naps, and shorter nap duration) were also linked to lower CRS in the extended care workers. These associations were not found in the IT sample. The unadjusted, descriptive mean of CRS was lower in the extended care sample than in the IT sample (Table 1). However, the model-based estimated mean of CRS after adjusting for the effects of sociodemographic characteristics was higher in the extended care sample than in the IT sample (Table 2, see the intercepts). Workers in the extended care industry thus have a higher risk of the development of cardiovascular disease than those in the IT industry, net of background characteristics (eg, sex, age, race, household income, etc.) being equal. Given that workers in this industry were lower-wage and hourly workers providing direct care to patients,24,36 their stressful work conditions might have contributed to high adjusted CRS in this sample, on average. However, in the extended care sample, positive attributes of sleep health were additionally associated with lower CRS, suggesting the importance of promoting healthy sleep in this sample. For extended care workers, shift work and varying and unpredictable work schedules22 may frequently interfere with their sleep. Future workplace interventions should consider how to protect and promote workers' sleep in this industry. In contrast, in the IT sample, there were no significant main effects of sleep health variables on CRS. This may be because IT workers have more positive overall sleep health and this might have contributed to the null effect. Indeed, our sample of IT workers had greater sleep sufficiency, less napping, and earlier and less variable sleep midpoint, and a greater proportion had shorter WASO (< 30 min/night), compared to the extended care workers (Table 1).
Sleep Health Moderates the Adverse Effect of Sleep Apnea Among Workers in the IT Industry
We also found that, in the IT sample, some of the sleep health indicators (ie, shorter sleep and more naps) moderated the link between sleep apnea symptoms and CRS. IT employees with more sleep apnea symptoms had significantly higher CRS when they had shorter sleep (< 6 hours) compared to when they had 6 to 8 hours of sleep. Moreover, IT employees with more sleep apnea symptoms had significantly higher CRS when they had more naps (3.9 naps or more across the week). Importantly, the strong effect of sleep apnea on CRS was weakened and not significant when IT employees exhibited longer nighttime sleep duration and fewer daytime naps. To the best of our knowledge, this study is one of the first demonstrating that sleep health can buffer the adverse effect of sleep apnea symptoms on cardiovascular disease risk.
The National Healthy Sleep Awareness Project has recently warned that “sleep apnea hurts hearts.”18 Based on our findings, we suggest to add in this public awareness campaign that maintaining healthy sleep behaviors (eg, 6 to 8 hours of nighttime sleep for adults) can protect hearts, consistent with a recent American Heart Association Scientific Advisory.11,19 Although the protective effects of longer nighttime sleep and fewer naps were only found among workers in the IT industry where the work conditions are relatively privileged,23 IT workers might have been able to practice healthy sleep behaviors with more control over when and where they work than workers in other industries.13 These workers benefited from their healthy sleep, including no significant difference in the estimated risks of cardiovascular disease between more sleep apnea and less sleep apnea symptoms. Note that we did not find the moderating effects of sleep health on the link between sleep apnea symptoms and CRS in the extended care sample. This may relate to the demographic differences between the IT and extended care samples. Over 90% of the extended care workers were females and the mean age was younger than 40 years, which may relate to lower actual (though unobserved) sleep apnea prevalence in this sample. Compared to the extended care workers, IT workers consisted of more males and were older (Table 1), factors significantly associated with higher cardiovascular disease risk.15 Thus, the benefit of sleep health in decreasing cardiovascular disease risks may become more apparent among older workers (IT sample), as shown in the exploratory analyses revealing interactions between older age and poorer sleep health, and higher CRS (Figure 3). Future research may need to examine the positive and protective role of sleep health in future cardiovascular disease risk in diverse industry samples.
Limitations and Future Directions
The current study has limitations. Cross-sectional data constrain our ability to identify causality. Although our statistical models imply that sleep apnea symptoms and sleep health indicators predict risks of developing cardiovascular disease in the next 10 years, our design does not rule out reverse causality. Future research should include multiple time points and adopt experimental designs to attempt to identify causality. Sleep apnea symptoms were measured by self-reports. Future studies may consider incorporating diagnosed sleep apnea (by physician, or using an ambulatory diagnostic device) to examine the unique effects of sleep health indicators on CRS, independent of diagnosed sleep apnea. For example, some of the interactions between sleep apnea symptoms and sleep health found in this study may be confounded with the degree of apnea, such that participants with untreated severe apnea are prone to have many apneic arousals during nighttime sleep and longer naps during daytime. Our supplementary analyses showed that, among IT workers who had no sleep apnea symptoms, having no naps and longer nighttime sleep (> 8 hours versus 6 to 8 hours) predicted lower CRS. However, very few of the workers had no sleep apnea, no daytime naps, and more than 8 hours of sleep per night, suggesting the need to promote sleep health in working populations. Future analyses could extend this finding with a larger sample of workers and further test whether napping, particularly long napping and short nighttime sleep, is detrimental for someone with sleep apnea. Moreover, our sleep health indicators were selected based on the SATED scale1; however, the SATED scale has not been fully validated. It is necessary to have a population-based, validated scale of sleep health, using both self-reports and objective markers of sleep, as was done in the current study, but with the addition of objective apnea data. Last, our sample of workers was purposely selected from the IT and extended care industries, and thus the findings may not generalize to workers in other contexts, an area for future research.
CONCLUSIONS
This study advances the literature on sleep health examining the associations of positive attributes of sleep health with CRS, independent of and interacting with sleep apnea symptoms. Workers' poor sleep health may also lead to decreased productivity at work and increased health care costs in later life. Our findings support future research and interventions toward understanding the role of workers' sleep health for future health and well-being.
DISCLOSURE STATEMENT
All authors have reviewed and approved this manuscript. This research was conducted as part of the Work, Family, and Health Network (www.WorkFamilyHealthNetwork.org), which is funded by a cooperative agreement through the National Institutes of Health and the Centers for Disease Control and Prevention: Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01HD051217, U01HD051218, U01HD051256, U01HD051276), National Institute on Aging (U01AG027669), Office of Behavioral and Social Sciences Research, and National Institute for Occupational Safety and Health (U01OH008788, U01HD059773). Grants from the National Heart, Lung and Blood Institute (R01HL107240), the William T. Grant Foundation, Alfred P Sloan Foundation, and the Administration for Children and Families provided additional funding. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of these institutes and offices. The authors report no financial conflicts of interest. Outside of the current study, Dr. Buxton received subcontracts to Penn State from Mobile Sleep Technologies (National Science Foundation #1622766, National Institutes of Health R43AG056250).
ABBREVIATIONS
- BMI
body mass index
- CAPI
computer-assisted personal interviews
- CRS
cardiometabolic risk scores
- iSD
individual standard deviation
- IT
information technology
- SATED
satisfaction, alertness, timing, efficiency, and duration
- WASO
wake after sleep onset
REFERENCES
- 1.Buysse DJ. Sleep health: can we define it? Does it matter? Sleep. 2014;37(1):9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rangaraj VR, Knutson KL. Association between sleep deficiency and cardiometabolic disease: Implications for health disparities. Sleep Med. 2016;18:19–35. doi: 10.1016/j.sleep.2015.02.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for one week reduces insulin sensitivity in healthy men. Diabetes. 2010;59:2126–2133. doi: 10.2337/db09-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 5.Donga E, Van Dijk M, Van Dijk JG, et al. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab. 2010;95(6):2963–2968. doi: 10.1210/jc.2009-2430. [DOI] [PubMed] [Google Scholar]
- 6.Grandner MA, Malhotra A. Sleep as a vital sign: why medical practitioners need to routinely ask their patients about sleep. Sleep Health. 2015;1(1):11–12. doi: 10.1016/j.sleh.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangwisch JE, Heymsfield SB, Boden-Albala B, et al. Sleep duration as a risk factor for diabetes incidence in a large U.S. sample. Sleep. 2007;30(12):1667–1673. doi: 10.1093/sleep/30.12.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gottlieb DJ, Redline S, Nieto FJ, et al. Association of usual sleep duration with hypertension: the Sleep Heart Health Study. Sleep. 2006;29(8):1009–1014. doi: 10.1093/sleep/29.8.1009. [DOI] [PubMed] [Google Scholar]
- 9.Nishiura C, Noguchi J, Hashimoto H. Dietary patterns only partially explain the effect of short sleep duration on the incidence of obesity. Sleep. 2010;33(6):753–757. doi: 10.1093/sleep/33.6.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Itani O, Jike M, Watanabe N, Kaneita Y. Short sleep duration and health outcomes: a systematic review, meta-analysis, and meta-regression. Sleep Med. 2017;32:246–256. doi: 10.1016/j.sleep.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 11.St-Onge M-P, Grandner MA, Brown D, et al. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation. 2016;134(18):e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grandner MA, Hale L, Moore M, Patel NP. Mortality associated with short sleep duration: The evidence, the possible mechanisms, and the future. Sleep Med Rev. 2010;14(3):191–203. doi: 10.1016/j.smrv.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee S, Almeida DM, Berkman L, Olson R, Moen P, Buxton OM. Age differences in workplace intervention effects on employees' nighttime and daytime sleep. Sleep Health. 2016;2(4):289–296. doi: 10.1016/j.sleh.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bei B, Seeman TE, Carroll JE, Wiley JF. Sleep and physiological dysregulation: a closer look at sleep intraindividual variability. Sleep. 2017;40(9) doi: 10.1093/sleep/zsx109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marino M, Li Y, Pencina MJ, D'Agostino RB, Berkman LF, Buxton OM. Quantifying cardiometabolic risk using modifiable non-self-reported risk factors. Am J Prev Med. 2014;47(2):131–140. doi: 10.1016/j.amepre.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anothaisintawee T, Reutrakul S, Van Cauter E, Thakkinstian A. Sleep disturbances compared to traditional risk factors for diabetes development: Systematic review and meta-analysis. Sleep Med Rev. 2016;30:11–24. doi: 10.1016/j.smrv.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Pack AI, Gislason T. Obstructive sleep apnea and cardiovascular disease: a perspective and future directions. Prog Cardiovasc Dis. 2009;51(5):434–451. doi: 10.1016/j.pcad.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Sleep Education website. Sleep Apnea Hurts HEARTS Infographic. [Accessed January 26, 2018]. http://www.sleepeducation.org/healthysleep/infographics/sleep-apnea-hurts-hearts-infographic.
- 19.Buxton OM, Ness KM. Sleep as a pillar of cardiometabolic health. [Accessed January 29, 2018]. http://professional.heart.org/professional/ScienceNews/UCM_488194_Sleep-as-a-Pillar-of-Cardiometabolic-Health.jsp. Published September 19, 2016.
- 20.Whinnery J, Jackson N, Rattanaumpawan P, Grandner MA. Short and long sleep duration associated with race/ethnicity, sociodemographics, and socioeconomic position. Sleep. 2014;37(3):601–611. doi: 10.5665/sleep.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canivet C, Nilsson PM, Lindeberg SI, Karasek R, Östergren PO. Insomnia increases risk for cardiovascular events in women and in men with low socioeconomic status: A longitudinal, register-based study. J Psychosom Res. 2014;76(4):292–299. doi: 10.1016/j.jpsychores.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Keller SM. Effects of extended work shifts and shift work on patient safety, productivity, and employee health. Workplace Health Saf. 2009;57(12):497–502. doi: 10.3928/08910162-20091124-05. [DOI] [PubMed] [Google Scholar]
- 23.Kelly EL, Moen P, Oakes JM, et al. Changing work and work-family conflict: evidence from the Work, Family, and Health Network. Am Sociol Rev. 2014;79(3):485–516. doi: 10.1177/0003122414531435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bray J, Kelly E, Hammer L, et al. An integrative, multilevel, and transdisciplinary research approach to challenges of work, family, and health. Methods Rep RTI Press. 2013:1–38. doi: 10.3768/rtipress.2013.mr.0024.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King RB, Karuntzos G, Casper LM, et al. Work-family balance issues and work-leave policies. In: Gatchel RJ, Schultz IZ, editors. Handbook of Occupational Health and Wellness. New York, NY: Springer; 2013. pp. 323–340. [Google Scholar]
- 26.Buxton OM, Lee S, Beverly C, et al. Work-family conflict and employee sleep: evidence from IT workers in the Work, Family and Health Study. Sleep. 2016;39(10):1871–1882. doi: 10.5665/sleep.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marino M, Li Y, Rueschman MN, et al. Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep. 2013;36(11):1747–1755. doi: 10.5665/sleep.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson R, Crain TL, Bodner TE, et al. A workplace intervention improves sleep: results from the randomized controlled Work, Family, and Health Study. Sleep Health. 2015;1(1):55–65. doi: 10.1016/j.sleh.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buxton OM, Hopcia K, Sembajwe G, et al. Relationship of sleep deficiency to perceived pain and functional limitations in hospital patient care workers. J Occup Environ Med. 2012;54(7):851–858. doi: 10.1097/JOM.0b013e31824e6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buxton OM, Quintiliani LM, Yang MH, et al. Association of sleep adequacy with more healthful food choices and positive workplace experiences among motor freight workers. Am J Public Health. 2009;99(Suppl 3):636–643. doi: 10.2105/AJPH.2008.158501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crain TL, Hammer LB, Bodner T, et al. Work-family conflict, family-supportive supervisor behaviors (FSSB), and sleep outcomes. J Occup Health Psychol. 2014;19(2):155–167. doi: 10.1037/a0036010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention website. BRFSS Questionnaires. [Accessed January 29, 2018]. https://www.cdc.gov/brfss/questionnaires/index.htm. Updated January 18, 2018.
- 33.Watson NF, Badr MS, Belenky G, et al. Joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society on the recommended amount of sleep for a healthy adult: methodology and discussion. Sleep. 2015;38(8):1161–1183. doi: 10.5665/sleep.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ostler MW, Porter JH, Buxton OM. Dried blood spot collection of health biomarkers to maximize participation in population studies. J Vis Exp. 2014;(83):e50973. doi: 10.3791/50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Samuelsson LB, Hall MH, Mclean S, et al. Validation of biomarkers of CVD risk from dried blood spots in community-based research: methodologies and study-specific serum equivalencies. Biodemography Soc Biol. 2015;61(3):285–297. doi: 10.1080/19485565.2015.1068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kossek EE, Hammer LB, Kelly EL, Moen P. Designing work, family & health organizational change initiatives. Organ Dyn. 2014;43(1):53–63. doi: 10.1016/j.orgdyn.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]