Abstract
Study Objectives:
Recent evidence indicates that daytime sleep disturbance associated with night shift work may arise from both circadian misalignment and sleep reactivity to stress. This presents an important clinical challenge because there are limited means of predicting and distinguishing between the two mechanisms, and the respective treatments differ categorically; however, there is support that a polymorphism in the PERIOD3 gene (PER3) may indicate differences in vulnerability to daytime sleep disturbance in shift workers.
Methods:
We recruited 30 fixed night shift workers for laboratory assessments of circadian misalignment (dim light melatonin onset), sleep reactivity to stress (Ford Insomnia Response to Stress Test), daytime sleep disturbance (daytime Insomnia Severity Index), and PER3 genotype (PER34/4, PER35/−). The two mechanisms for daytime sleep disturbance (circadian misalignment and sleep reactivity to stress) were compared between PER3 genotypes.
Results:
Disturbed daytime sleep in the PER34/4 group was more likely related to sleep reactivity to stress, whereas disturbed sleep in the PER35/− group was more likely related to circadian misalignment. Exploratory analyses also revealed a blunted melatonin amplitude in the PER34/4 genotype group.
Conclusions:
This study provides further evidence for multiple mechanisms (ie, circadian misalignment versus sleep reactivity to stress) associated with daytime sleep disturbances in shift workers. Additionally, it provides the new finding that PER3 genotype may play an important role in individual vulnerability to the different mechanisms of daytime sleep disturbance in night shift workers.
Citation:
Cheng P, Tallent G, Burgess HJ, Tran KM, Roth T, Drake CL. Daytime sleep disturbance in night shift work and the role of PERIOD3. J Clin Sleep Med. 2018;14(3):393–400.
Keywords: circadian misalignment, insomnia, PERIOD3, shift work, stress
BRIEF SUMMARY
Current Knowledge/Study Rationale: Inadequate or disturbed sleep is a critical problem for health and safety in night shift workers. To inform the appropriate intervention strategies, this study explores the role of PER3 in predicting mechanisms of vulnerability to sleep disturbance.
Study Impact: Results suggest that PER3 genotypes may indeed differ in mechanism of vulnerability for daytime sleep disturbance. This suggests that PER3 genotype may inform intervention or prevention strategies that triage individuals for different and more targeted treatments.
INTRODUCTION
Sleep disturbance is a significant problem for shift workers due to the prevalence rate and the occupational and health consequences with which it is associated. Sleep disturbances among shift workers often include difficulties falling and staying asleep, and curtailment of total sleep duration by an average of 1 to 2 hours, particularly during the daytime sleep bout that occurs following a night shift.1–3 In fact, insomnia disorder is nearly twice as prevalent in night shift workers compared to the general population: whereas approximately 10% of the general population experience chronic insomnia,4,5 the prevalence of chronic insomnia was as high as 18.5% in fixed night shift workers.6 Consequences of disrupted sleep include reduced work productivity, higher absenteeism, and increased workplace injuries,7,8 as well as a higher economic burden associated with higher utilization of health care services.9,10
The elevated prevalence of daytime sleep disturbance in night shift workers is commonly attributed to the circadian disruption due to the nocturnal work schedule. The nocturnal work schedule operates in opposition to zeitgeber (eg, daylight) that synchronize the biological clock to a diurnal schedule. As such, many night shift workers are unable to significantly delay their circadian rhythms to match the nocturnal work schedule, resulting in circadian misalignment. Consequently, shift workers attempting to sleep following their night shift (commonly during the morning) experience interrupted sleep and reduced total sleep time because their circadian clocks are promoting alerting signals throughout the morning.
More recent evidence indicates that circadian misalignment may not be the only cause of daytime sleep disturbances in night shift workers. Disturbed sleep may also occur as a consequence of high trait sleep reactivity to stress, which is a heritable vulnerability to sleep disturbance in response to environmental stressors.11 Individuals with high sleep reactivity are more vulnerable to sleep difficulties following a wide range of disruptors including personal stress, anticipatory anxiety, caffeine, and alterations to one's habitual sleep-wake schedule.11–13 Both anecdotal and empirical evidence indicates that the nocturnal shift work schedule can be a significant personal and social stressor. Shift workers report less social engagement with family, friends, and hobbies14 despite the fact that some chose shift work to increase availability of parental care to children in two parent households (ie, “shift-parenting”). In addition to increased social isolation, working a nocturnal schedule requires significant lifestyle alterations that can contribute to increased stress. Indeed, prospective evidence indicates that in individuals reporting high sleep reactivity to stress prior to transitioning to shift work, shift work disorder (ie, insomnia and excessive sleepiness) is more than five times more likely to develop after starting shift work than in those reporting low sleep reactivity to stress.15
The disparate mechanisms by which sleep disturbances may arise in shift workers presents an important clinical challenge because treatments that target circadian misalignment (eg, bright light therapy, melatonin) differ categorically from treatments that target sleep reactivity (eg, cognitive behavioral therapy, mindfulness-based therapies). Currently, there are limited means of predicting and distinguishing between shift workers whose sleep disturbance is associated with circadian mis-alignment versus sleep reactivity to stress. However, emerging evidence suggests that a polymorphism in the PERIOD3 gene (PER3) may indicate differences in vulnerability to insomnia in shift workers. PER3 belongs to the family of period genes that regulate circadian rhythms through a core feedback loop.16 This variable-number tandem repeat (VNTR) polymorphism consists of a sequence of 18 amino acids in the coding area that is repeated either four (4-repeat) or five (5-repeat) times. Individuals may be homozygous, carrying copies of the same allele on both chromosomes (PER34/4 or PER35/5), or heterozygous, carrying one copy of each allele (PER35/4). This VNTR polymorphism affects the availability of phosphorylation sites, and proteins transcribed from the 4-repeat allele have less phosphorylation sites. Thus, these proteins are phosphorylated at a lower rate.17 This results in a prolonged feedback loop, which can lengthen the period of circadian oscillation.18
A number of research findings suggest that shift workers with different PER3 genotypes may also differ in their vulnerability to sleep disturbance (ie, circadian misalignment versus sleep reactivity to stress). First, observational studies have found an overrepresentation of the PER34/4 genotype in late chronotypes17,19,20 and those with delayed sleep phase syndrome.17 Late chronotypes have a nocturnal preference (more daytime sleepiness, more nocturnal alertness), suggesting reduced vulnerability to circadian misalignment during night work. As such, those with the PER34/4 genotype might also be less vulnerable to circadian misalignment during night work due to a tendency toward a later chronotype, perhaps as a consequence of a longer circadian period. However, studies comparing physiological phase markers (eg, melatonin onset, mRNA of BMAL1, PER2, and PER3 in leukocytes) have not found significant differences between PER3 genotypes.21,22 Instead, evidence suggests that the PER3 genotypes differ in the extent to which behaviors such as sleep timing are dictated by circadian phase. Specifically, those with the PER34/4 genotype exhibit sleep timing that is less rigidly controlled by their circadian phase as compared to the other PER3 phenotypes,21 such that sleep timing in individuals with the PER34/4 genotype show much more variation given the same circadian phase.
Given that PER34/4 genotypes are under less rigid circadian control, PER34/4 may also be less likely than the other PER3 genotypes to experience sleep disturbance associated with circadian misalignment. Instead, sleep disturbances reported by PER34/4 shift workers may be associated with sleep reactivity to the stressors associated with shift work. Indeed, differences in vulnerability to sleep disturbances by PER3 genotype have been observed in non-shift work samples faced with environmental or physiological stressors. For example, women with the PER34/4 genotype reported curtailed sleep duration associated with transient mood disturbance.23 Another study found that individuals living in high-crime neighborhoods were more likely to report sleep related anxiety and more severe insomnia symptoms if they possessed the 4-repeat allele.24 A different study demonstrated that insomnia associated with alcohol dependence was more severe in PER34/4 relative to carriers of the 5-repeat allele (PER35/−).25 Together, the evidence suggests that PER3 genotype in shift workers may also show differences in vulnerability to insomnia.
To investigate the relationship between PER3 genotype and insomnia in shift workers, this study aimed to characterize differences between PER3 genotypes in the relationship of insomnia, circadian misalignment, and sleep reactivity to stress. Given evidence in other populations of differential vulnerability to sleep reactivity to stress in PER3 genotypes, we hypothesized that sleep disturbance in night shift workers homozygous for the 4-repeat allele (PER34/4) would show a stronger relationship with sleep reactivity to stress than carriers of the 5-repeat allele (PER35/−). Additionally, exploratory analyses were conducted to examine potential differences in melatonin phase, amplitude, and duration between PER3 genotypes.
METHODS
Participants
Participants were recruited for a larger observational study examining cardiac and metabolic risks in shift workers, and thus targeted shift workers with no medical history of cardiovascular illness or diabetes. Recruitment was conducted through use of newsletters and flyers distributed throughout the community and health system in the Detroit metropolitan area. A total of 125 prospective participants were invited to complete an online prescreening survey, 40 of whom were eligible for an in-person interview. During this interview, information about the subject's sleep schedule and shift work was obtained, questionnaires were administered, and the Structured Clinical Interview for DSM Disorders was administered by a doctoral-level clinical psychologist. Informed consent was also given by all participants.
Participants were included in the study if they worked the night shift at least 3 nights a week for a duration of 6 to 12 hours, and their shift occurred between the hours of 6:00 pm and 9:00 am. Participants were excluded for any past diagnoses of insomnia, sleep disorders not related to their shift work, major medical illnesses (diabetes, hypertension, liver conditions, disorders of the central nervous system), or body mass index ≥ 35 kg/m2. Participants also were excluded for any recreational drug use, use of medications acting on the central nervous system, alcohol dependence (≥ 4 drinks per day), heavy tobacco use (≥ 10 cigarettes per day), or caffeine use greater than five to six 100-mg servings per day because such substances may interfere with collection of data (eg, glucose tolerance, sleepiness, circadian phase, blood pressure, cortisol) in the larger study. Participants were also asked to refrain from use of alcohol, caffeine, and tobacco for 24 hours prior to their laboratory visit. The final sample included 30 participants (22 female) with a mean age of 38.8 years (standard deviation [SD] = 10.3; range = 24–57). Health care worker was the predominant occupation (n = 24), but occupations also included manufacturing (n = 2), food service (n = 2), information technology (n = 1), and security (n = 1). All procedures were ap -proved by the Henry Ford Health System Institutional Review Board. Informed consent was also given by all participants before any procedures were executed.
Procedures
Participants arrived at the laboratory at 7:30 am, following a typical night shift, and completed symptom questionnaires (listed in the following paragraphs) before being given an 8-hour sleep opportunity with time in bed enforced between 8:30 am and 4:30 pm. Lights-on was 4:30 pm, and salivary samples for genotyping were collected. Participants then remained in the laboratory for 24 hours after awakening to allow for hourly collection of melatonin (see the section titled Circadian Phase Assessment).
Questionnaires
Daytime Sleep Disturbance
The Insomnia Severity Index (ISI) was used to determine the severity of symptoms of insomnia26 occurring during participants' daytime sleep schedules. The measure uses five-point Likert scales (from 0 to 4) to assess the severity of insomnia (ie, difficulty falling asleep, staying asleep, and having refreshing sleep) over the prior 2 weeks. It uses similar 5-point scales to gauge how satisfied/dissatisfied an individual is with his or her sleep, how noticeable the sleep problems are to the individual, how worried/distressed the individual is regarding sleep, and the extent to which the sleep problem interferes with daily functioning. The self-report measure was modified according to previous studies of shift workers to only assess sleep disturbance during daytime sleep.27 The score from each item was summed, creating a total score for daytime sleep that indicated severity of insomnia symptoms, which was mean centered for analysis.
Sleep Reactivity to Stress
The Ford Insomnia Response to Stress Test (FIRST) was administered in order to assess vulnerability to sleep disturbances due to trait sleep reactivity.11 The FIRST is a self-report questionnaire that assesses the degree to which an individual's sleep is vulnerable to nine common stressors (eg, an important meeting the next day) by asking how likely it is that each stressor would cause difficulty sleeping for that individual. The degree of sleep reactivity to each item was indicated on a four-point Likert scale (Not likely, Somewhat likely, Moderately likely, Very likely), and scores were summed for each individual. Individual scores, reflecting degree of sleep reactivity to stress, were mean centered for analysis.
Physiological Measures
PER3 Genotype
Each participant was instructed to deposit 2 mL of saliva into an Oragene OG-500 collection kit for the purpose of sequencing of the PER3 gene. Saliva samples were then sent to the Genomics Lab, part of the Research Technology Support Facility at Michigan State University, for analysis. Genomic DNA was extracted, and a VNTR in the PER3 gene was characterized as being either the 4-repeat or 5-repeat allele. In order to analyze the effect of carrying the 4-repeat allele in moderating relationships among studied measures, participants were categorized as either homozygous for the 4-repeat allele (PER34/4; n = 10), or not (PER35/−; n = 20). The PER35/− group comprised 19 heterozygous (PER35/4) and 1 homozygous (PER35/5) individuals.
Circadian Phase Assessment
Hourly salivary samples were collected under dim light conditions (< 10 lux) for 24 hours,28 and dim light melatonin onset (DLMO) was determined from these assays. Saliva samples were collected using Salivette tubes (SARSTEDT AG & Co., Nümbrecht, Germany) with cotton inserts. Cotton inserts were placed by participants near the salivary glands under the tongue, with the instruction to saturate the cotton insert. Duration of saturation was no less than 2 minutes, with longer saturation used in some cases to ensure a minimum of 0.5 mL of saliva could be centrifuged and frozen. Samples were submitted to SolidPhase, Inc. (Portland, Oregon, United States) to undergo radioimmunoassay for melatonin.
During sample collection, participants were instructed to remain in a semireclined and comfortable position prior to and during sample collection. Food or drinks were not allowed within 10 minutes prior to measurements, and foods that have been suggested to interfere with melatonin measurement, such as bananas and mustard, were not allowed during the study. Between sample collections, participants were allowed to perform activities such as reading, listening to music, and using their personal electronic devices as long as backlit screens were kept below 10 lux (as measured from the angle of gaze). The threshold used for DLMO was calculated based on two SD above the mean melatonin values during the “biological day.” Mean and SD were calculated using the lowest set of 5 consecutive values over the 24-hour collection period. The time at which melatonin concentration surpassed this threshold was extrapolated using a linear estimate.
Circadian misalignment was operationalized as the difference in hours between self-reported habitual sleep onset for daytime sleep following a night shift (presented in Table 1) and DLMO. Habitual daytime sleep was selected as a point of reference for DLMO to match the outcome variable of daytime sleep disruption in analyses. To maintain a continuous relationship of time before and after midnight, an event occurring after midnight was recorded as a number above 24 (eg, 1:00 am recorded as 25), such that the difference between 1:00 am and 11:00 pm (ie, 25 minus 23) would be 2 hours. Because daytime sleep was conceptualized as occurring after the night shift (ie, after midnight), timing of daytime sleep was always greater than 24 (eg, 10:00 am recorded as 34). This operationalization is theoretically comparable to phase angle, such that larger numbers indicate a longer period of wakefulness between DLMO and habitual daytime sleep onset.
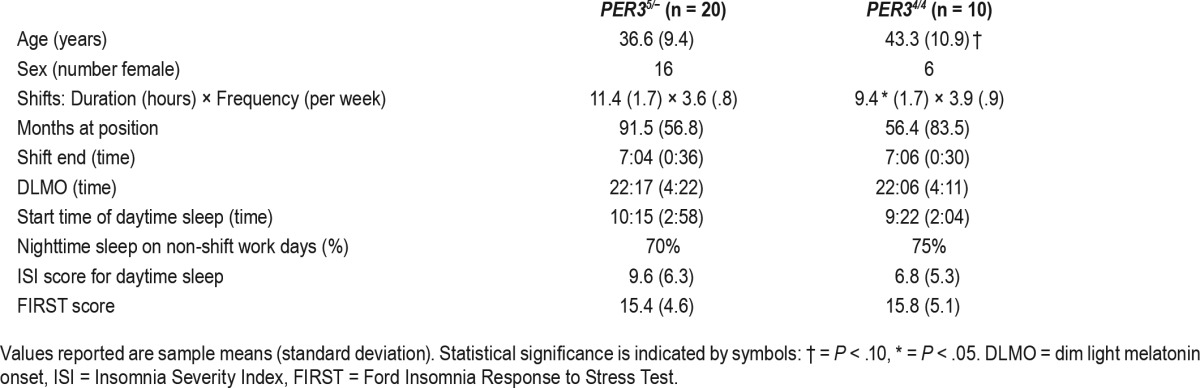
Table 1.
Demographic variables by PER3 genotype.
Analytical Approach
Sleep Disturbance and PER3
The influence of PER3 genotype on insomnia severity (ISI score) was tested using a linear regression with sleep reactivity (FIRST), circadian misalignment (time of habitual daytime sleep minus time of DLMO), and PER3 genotype (PER34/4, PER35/−) as fixed effects, including the interaction between FIRST × PER3 genotype, and circadian misalignment × PER3 genotype. Given the cyclical nature of circadian rhythms, polynomial models were also tested for fit between the dependent variable and circadian misalignment as a predictor. Results indicated that the first-order linear model produced the best fit as indicated by both model significance and highest adjusted-R2 value, F1,28 = 5.46, P < .05, adjusted-R2 = .133, compared to a second-order (ie, quadratic) model, F2,27 = 2.82, P = .08, adjusted-R2 = .112, and compared to a third-order (ie, cubic) model, F3,26 = 1.84, P = .16, adjusted-R2 = .080. As such, a linear model was selected for final analyses. Covariates were also tested during model specification (see Sample Characteristics in the Results section), and were removed from the final model if not significant. Both circadian misalignment and FIRST were centered, and standardized betas were reported (unless otherwise specified) to enable comparison of effect size across the independent variables.
Circadian Rhythm of Melatonin and PER3
Melatonin rhythms were modeled using a polynomial marginal regression model to capture the nonlinearity of melatonin values across a 24-hour period. A marginal model was selected because it offers similar advantages to a mixed-effects model over a repeated-measures analysis of variance (eg, no assumption of sphericity, more robust handling of missing data) without needing to specify random effects.29 A first-order autoregressive covariance structure was selected to account for residuals that are correlated in a time-decreasing manner. A third-order polynomial model (ie, has two inflection points) was selected based on the cyclical rhythm of melatonin (ie, one inflection point reflecting the increase of melatonin near DLMO, and a second inflection point reflecting peak melatonin during the biological night). Goodness of fit for the third-order polynomial model was also confirmed via comparison with both first- and second-order polynomial models using likelihood ratio tests (third- versus first-order: χ25 = 157.07, P < .001; third- versus second-order: χ26 = 161.50, P < .001). Time was centered at each individual's DLMO. PER3 genotype was entered into the final model as a moderator to test for differences in melatonin rhythms between groups. Covariates were also tested during model specification as mentioned, and were removed from the final model if not significant. Additionally, melatonin onset (DLMO), offset (DLMOff), and duration (difference between DLMO and DLMOff) was compared between PER3 genotype groups.
RESULTS
Sample Characteristics
The study included 30 permanent night-shift workers (22 female) with a mean age of 38.8 years (SD = 10.3). Of the participants, 20 were carriers of the 5-repeat allele of PER3 (PER35/−), and 10 were homozygous for the 4-repeat allele (PER34/4). PER3 genotypes did not differ by demographic variables or daytime insomnia severity (see Table 1). PER3 genotypes also did not differ in duration of working the night shift; however, shift schedules did differ between genotype groups, with the PER34/4 group reporting a shorter average length of shift (mean = 9.4 hours, standard error of the mean [SEM] = 0.5) relative to the PER35/− group (mean = 11.3 hours, SEM = 0.4). As such, the length of shift was tested as a covariate in all subsequent analyses, along with age and sex, particularly given the overrepresentation of females in this sample. Season at the time of testing was also included as a covariate to control for differences in melatonin rhythms associated with changes in scotophase, and was operationalized as an ordinal variable from summer to winter in order of increasing scotophase. No differences in season at the time of testing were found between PER3 genotypes.
PER3 and Insomnia
Model testing did not show a significant effect of age (P = .71) or season (P = .95), and thus both were removed as covariates; however, sex was marginally significant (P = .10), and was retained in the final model to account for the overrepresentation of females in the sample. Shift schedule was also a marginally significant covariate (Duration × Frequency, P = .056) and was thus also retained in the final model. The effect indicated that although increased shift duration predicted more sleep disturbance at mean shift frequency (mean = 3.67 shifts per week), this effect was mitigated with higher shift frequency. The final model included the interactions of circadian misalignment × PER3 genotype and FIRST × PER3 genotype, all lower-order terms, along with shift schedule and sex as covariates.
Results from the multiple regression indicated that the final model was significant, F9,20 = 2.98, R2 = 0.57, P < .05, suggesting goodness of fit. Examination of fixed effects revealed that the two interaction terms of interest were significant: PER3 geno-type × circadian misalignment, t22 = 2.16, P < .05, and PER3 genotype × FIRST, t22 = −2.81, P < .05. The PER3 genotype × circadian misalignment interaction indicated that the sleep disturbance in the PER34/4 genotype was not as strongly associated with circadian misalignment as it was for the PER35/− genotype. Specifically, the beta coefficient for the effect of PER35/− group indicated a large increase in sleep disturbance with more circadian misalignment, β = 0.67, P < .01; however, the same relationship in the PER34/4 group was close to zero and nonsignificant, β = −0.05, (see Figure 1A). In contrast, the PER3 genotype × FIRST interaction indicated that sleep disturbance in the PER34/4 genotype was more strongly associated with sleep reactivity to stress compared to the PER35/− genotype. Specifically, the beta coefficient for the marginal effect of the PER34/4 group indicated a large increase in sleep disturbance with higher FIRST scores, β = 1.01, P < .01; however, the same relationship in the PER35/− group was nonsignificant, β = −0.17 (see Figure 1B).
Figure 1. Relationships between severity of daytime sleep disturbance, circadian misalignment, and trait sleep reactivity to stress.
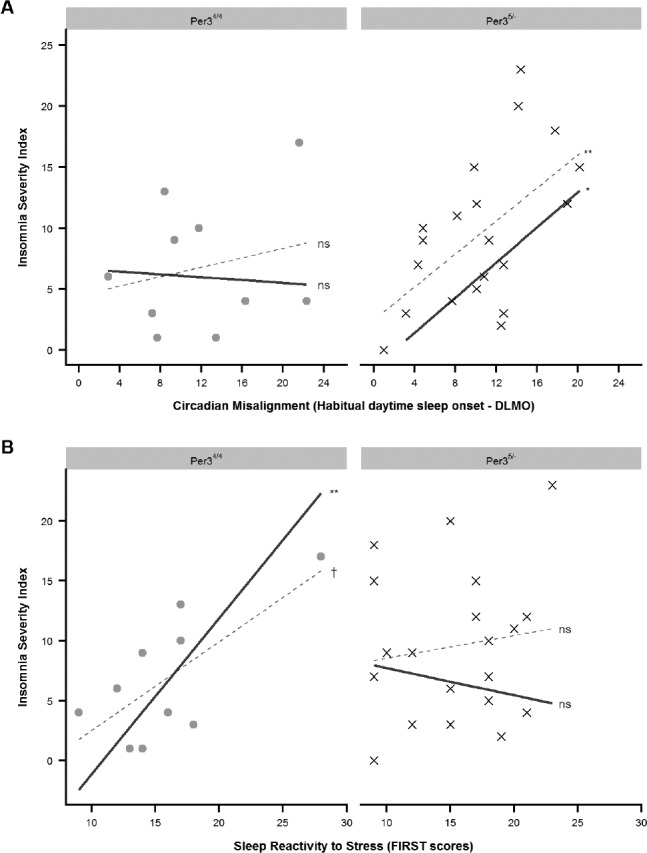
(A) Graphical representation of the relationship between severity of daytime sleep disturbance and circadian misalignment between PER3 genotypes. Daytime sleep disturbance was measured by the ISI. Circadian misalignment was operationalized as the difference in hours between onset of habitual bedtime sleep and dim light melatonin onset. Note that the timing of events (DLMO and sleep) occurring after midnight increased above 24 (eg, 1:00 AM recorded as 25) to maintain a linear relationship of time across days. Dotted trend lines represent the unadjusted correlation, and solid lines represent the predicted values after adjusting for all variables in the statistical model. Statistical significance of the trend lines is indicated by symbols: † = P < .10, * = P < .05, ** = P < .01. (B) Graphical representation of the relationship between severity of sleep disturbance and trait sleep reactivity to stress as measured by the FIRST. Dotted trend lines represent the unadjusted correlation, and solid lines represent the predicted values after adjusting for all variables in the statistical model. Statistical significance of the trend lines is indicated by symbols: † = P < .10, * = P < .05, ** = P < .01. DLMO = dim light melatonin onset, FIRST = Ford Insomnia Response to Stress Test, ISI = Insomnia Severity Index.
PER3 and Melatonin Rhythms
Exploratory analyses were also conducted to examine potential differences in melatonin rhythms between PER3 genotypes under shift work conditions. Melatonin values over 24 hours were modeled using a third-order marginal linear regression model. Sex, age, and shift characteristics were also tested as covariates during model specification. Sex (P = .4), shift characteristic (shift duration × frequency: P = .13), and age (P = .12) were removed due to nonsignificance. Model testing did reveal that season was a significant covariate and was thus retained in the final model.
The final model included PER3 genotype (PER34/4, PER35/−) as a moderator of Hour3 along with lower-order terms as independent variables, and melatonin values as the dependent variable, and season as a covariate. A significant Hour3 × PER3 interaction, t750 = 2.37, P < .05, indicated that the melatonin rhythms differed between PER3 genotype groups. Graphic representation of the predicted values suggested a blunted melatonin profile in the PER34/4 (see Figure 2), which was confirmed with a post hoc analysis using peak melatonin values, t26.43 = −2.40, P < .05. Comparison of average peak melatonin values indicated that the PER34/4 group (7.90 pg/mL ± 1.44 SEM) show a 40% decrease in peak melatonin compared to the PER35/− group (13.14 pg/mL ± 1.64 SEM). Additionally, we also identified the number of “low secretors” of melatonin using a criteria of peak value ≤ 5 pg/mL. Comparison by group indicated that 30% (3 of 10) of the PER34/4 were low secretors whereas only 10% (2 of 20) was detected in the PER35/− group; in other words, there were three times as many low secretors in PER34/4 compared to the PER35/− group.
Figure 2. Melatonin values across the 24-hour experimental period compared by PER3 genotype groups.
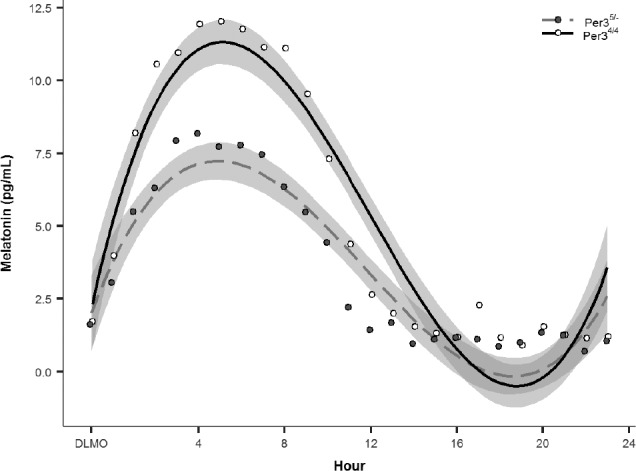
Values were aligned relative to DLMO for comparison of rhythmic activity. Shaded grey areas represent standard error of the predicted values from the statistical model. DLMO = dim light melatonin onset.
Exploratory analyses also examined melatonin onset (DLMO), offset (DLMOff), and duration. Group comparison between PER3 genotypes using t tests did not reveal any significance differences.
DISCUSSION
This study aimed to examine if the VNTR polymorphism in the PERIOD3 gene might be associated with different patho-physiological vulnerabilities to insomnia associated with shift work (ie, circadian misalignment and sleep reactivity). Results suggested that disturbed sleep in the PER35/− group was more likely related to circadian misalignment, whereas disturbed sleep in the PER34/4 group was more likely related to sleep reactivity to stress. This study also provided further evidence that insomnia in shift work may arise from both circadian misalignment and sleep reactivity to stress. Although limited by the observational design, the evidence suggests that PER3 genotypes may differ in the mechanism of risk for insomnia in shift work; whereas insomnia in PER35/− shift workers is more closely associated with circadian phase as typically expected, insomnia in PER34/4 individuals may more likely arise from trait sleep reactivity (eg, difficulty winding down for sleep due to stress related to the shift work schedule).
The finding of differential risk for sleep disturbance in shift work between PER3 genotypes is consistent with prior research indicating that the relationship between sleep timing and circadian phase is less rigid in the PER34/4 compared to the PER35/5 genotype.21 As such, circadian misalignment would have a weaker effect on daytime sleep disturbance in individuals with the PER34/4 genotype. Although this could be protective for some, the relatively reduced influence of circa-dian misalignment may also unmask other risk factors for insomnia, such as sleep reactivity to stress. Indeed, results from this study showed that complaints of sleep disturbance in the PER34/4 genotype were more strongly associated with sleep reactivity to stress compared to circadian misalignment.
Additionally, this study found a blunted melatonin amplitude in the PER34/4 genotype. There was also a larger proportion of individuals with low melatonin secretion in the PER34/4 group. Though previous research has not found baseline differences in melatonin amplitude between PER3 genotypes in the general population,21,22 other studies have shown that melatonin amplitude may become blunted in certain individuals who experience circadian disruption from re-entrainment to a different light-dark cycle.30,31 As such, these results may reflect the role of PER3 genotype as a contributing factor to individual differences in response to circadian re-entrainment.
The blunted melatonin amplitude found in the PER34/4 genotype may also be related to the differential relationship between sleep disturbance and circadian misalignment versus sleep reactivity to stress. Dijk et al.30 demonstrated that those who exhibit a blunted melatonin amplitude following changes in the light-dark cycle also showed blunted amplitude of body temperature and cortisol, suggesting an overall reduction in circadian amplitude. Moreover, those who show a blunted melatonin amplitude also exhibited an attenuation of circadian rhythmicity in behavioral outputs (eg, alertness, performance, etc.) Together, these results may suggest that in addition to being under less rigid circadian control at baseline, those with the PER34/4 genotype may experience further decoupling of circadian control (via reduced melatonin amplitude) following a disruption in the light-dark cycle. Consequentially, insomnia in shift workers with the PER34/4 genotype may be less likely affected by their circadian phase (ie, circadian misalignment) as compared to other risk factors of insomnia such as sleep reactivity to stress.
Though results are consistent with the extant literature, the evidence should be considered preliminary due to the limitations of the observational design in a single 24-hour period. In particular, melatonin rhythms are responsive to dynamic photoperiods, with the added complexities of significant individual differences in the degree of response to changes in the environment.32 Furthermore, there is a range of factors that can modulate individual melatonin profiles, including behavioral factors such as sleep deprivation,33 social factors such as employment,34 and biological factors such as structural and functional differences in the pineal gland.35–37 Results from this study suggest that the VNTR polymorphism in the PER3 gene may be an additional factor that contributes to individual differences in melatonin rhythms in response to shift work.
Future research should employ experimental and longitudinal designs to confirm differences observed in this study, and determine how PER3 genotype may inform strategies for preventing insomnia in shift workers. For example, PER3 genotype may be helpful in identifying individuals who may benefit from bright light interventions during night work to reduce insomnia during daytime sleep due to circadian misalignment, versus those who may benefit from cognitive behavioral therapy to reduce sleep disturbance in response to shift work related stressors. Future studies may also employ objective measures of sleep such as wrist actigraphy or polysomnography for increased resolution of sleep difficulties (eg, difficulty initiating versus maintaining sleep, differences between daytime and nighttime sleep) across a longer period of time (eg, weeks to months).
Limitations
A few considerations should be made in interpreting the results of this study. First, the sample was disproportionately female largely because of recruitment in a hospital setting that includes a large sample of nurses. Although this may limit generalizability, the preponderance of women in this sample is representative of the health care and social assistance occupations where almost 80% of the workforce are women.38 The severity of sleep disturbance in this sample was also lower, with mean values by PER3 groups falling below the clinical threshold (ISI < 15); however, the rate of clinically significant insomnia (ISI ≥ 15) in this sample was 20% (6 of 30 participants), which is consistent with prior research indicating that the prevalence rate of insomnia was approximately 18% in night shift workers.6
Another limitation is that the sample size in this study was relatively small; however, it is among a minority of studies that have examined circadian phase in a fixed night-shift sample versus a simulated shift work paradigm. Although a simulated shift work paradigm is an effective model of acute circadian misalignment, research utilizing a sample of workers who have been stable on the night shift for months or years have greater generalizability. A consequence of the smaller sample size —in conjunction with the distribution of circadian misalignment in shift workers—is that some of our analyses were better described using a linear model despite the cyclical nature of circadian rhythms. As such, interpretations of these findings should be made in the context of circadian rhythms despite the linear model; principally, with the understanding that the adverse effect of circadian misalignment does not increase linearly in an unbounded fashion, but instead likely begins to decrease as the degree of “misalignment” approaches hour 24 (ie, same circadian position as hour 0). Future research with larger samples may better reflect this cyclical relationship with time.
Finally, this study combined both heterozygous (PER34/5) and homozygous (PER35/5) carriers of the 5-repeat allele together (PER35/−). This approach may have diminished the magnitude of the effects associated with the 5-repeat allele; however, the 5-repeat allele occurs at a much lower frequency than that of the 4-repeat allele in most populations.39 Although the population distribution of PER3 genotypes in night shift workers has not been adequately established, the distribution of PER3 genotypes in our sample matches that of a prior study with approximately twice the sample size of police officers engaged in shift work (approximately one-third PER34/4 and two-thirds PER35/−).40 Furthermore, a large study of 432 Norwegian college students also found that the PER35/5 genotype only occurred in approximately 10% of the sample.41 This suggests that a significantly larger sample of shift workers would be required to achieve adequate power for comparisons between all three genotypes and would likely require genetic prescreening of participants.
CONCLUSIONS
This study provides further evidence for multiple mechanisms (ie, circadian misalignment versus sleep reactivity to stress) associated with daytime sleep disturbances in shift workers. Additionally, it provides the new finding that PER3 genotype may play an important role in individual vulnerability to the different causes of daytime sleep disturbance in shift workers. Specifically, PER34/4 individuals may be more vulnerable to insomnia associated with trait sleep reactivity, whereas PER35/− individuals may be more vulnerable to insomnia associated with circadian misalignment. Future studies may delineate the therapeutic and economic implications of such findings.
DISCLOSURE STATEMENT
This study was funded by Teva Pharmaceuticals Industries. Funding for the first author (PC) is provided by the National Institutes of Health (K23HL138166). This manuscript has been read and approved by all authors listed. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the technical staff of Henry Ford Hospital Sleep Center for their invaluable assistance in the completion of the present study. The authors also thank Olivia Walch for providing consultation on this project.
ABBREVIATIONS
- DLMO
dim light melatonin onset
- DLMOff
dim light melatonin offset
- FIRST
Ford Insomnia Response to Stress Test
- ISI
Insomnia Severity Index
- PER3
PERIOD3 gene
- VNTR
variable-number tandem repeat
REFERENCES
- 1.Allen AH, Park JE, Adhami N, et al. Impact of work schedules on sleep duration of critical care nurses. Am J Crit Care. 2014;23(4):290–295. doi: 10.4037/ajcc2014876. [DOI] [PubMed] [Google Scholar]
- 2.Torsvall L, Akerstedt T, Gillander K, Knutsson A. Sleep on the night shift: 24-hour EEG monitoring of spontaneous sleep/wake behavior. Psychophysiology. 1989;26(3):352–358. doi: 10.1111/j.1469-8986.1989.tb01934.x. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson RT. How fast should the night shift rotate? Ergonomics. 1992;35(12):1425–1446. doi: 10.1080/00140139208967412. [DOI] [PubMed] [Google Scholar]
- 4.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 5.Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–S10. [PMC free article] [PubMed] [Google Scholar]
- 6.Drake C, Roehrs T, Richardson G, Walsh JK, Roth T. Shift work sleep disorder: prevalence and consequences beyond that of symptomatic day workers. Sleep. 2004;27(8):1453–1462. doi: 10.1093/sleep/27.8.1453. [DOI] [PubMed] [Google Scholar]
- 7.Daley M, Morin CM, LeBlanc M, Grégoire JP, Savard J, Baillargeon L. Insomnia and its relationship to health-care utilization, work absenteeism, productivity and accidents. Sleep Med. 2009;10(4):427–438. doi: 10.1016/j.sleep.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 8.Kleinman NL, Brook RA, Doan JF, Melkonian AK, Baran RW. Health benefit costs and absenteeism due to insomnia from the employer's perspective: a retrospective, case-control, database study. J Clin Psychiatry. 2009;70(8):1098–1104. doi: 10.4088/JCP.08m04264. [DOI] [PubMed] [Google Scholar]
- 9.Anderson LH, Whitebird RR, Schultz J, McEvoy CE, Kreitzer MJ, Gross CR. Healthcare utilization and costs in persons with insomnia in a managed care population. Am J Manag Care. 2014;20(5):e157–e165. [PubMed] [Google Scholar]
- 10.Wickwire EM, Shaya FT, Scharf SM. Health economics of insomnia treatments: the return on investment for a good night's sleep. Sleep Med Rev. 2016;30:72–82. doi: 10.1016/j.smrv.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27(2):285–292. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- 12.Drake CL, Friedman NP, Wright KP, Jr, Roth T. Sleep reactivity and insomnia: genetic and environmental influences. Sleep. 2011;34(9):1179–1188. doi: 10.5665/SLEEP.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drake C, Pillai V, Roth T. Stress and sleep reactivity: a prospective investigation of the stress-diathesis model of insomnia. Sleep. 2014;37(8):1295–1304. doi: 10.5665/sleep.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes M. Making Time Use Explicit in an Investigation of Social Exclusion in the UK. Swindon: ESRC; 2011. RES-061-23-0122. [Google Scholar]
- 15.Kalmbach DA, Pillai V, Cheng P, Arnedt JT, Drake CL. Shift work disorder, depression, and anxiety in the transition to rotating shifts: the role of sleep reactivity. Sleep Med. 2015;16(12):1532–1538. doi: 10.1016/j.sleep.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dijk DJ, Archer SN. PERIOD3, circadian phenotypes, and sleep homeostasis. Sleep Med Rev. 2010;14(3):151–160. doi: 10.1016/j.smrv.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Archer SN, Robilliard DL, Skene DJ, et al. A length polymorphism in the circadian clock gene Per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–415. doi: 10.1093/sleep/26.4.413. [DOI] [PubMed] [Google Scholar]
- 18.Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8(2):139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- 19.Jones KHS, Ellis J, Von Schantz M, Skene DJ, Dijk D-J, Archer SN. Age-related change in the association between a polymorphism in the PER3 gene and preferred timing of sleep and waking activities. J Sleep Res. 2007;16(1):12–16. doi: 10.1111/j.1365-2869.2007.00561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pereira DS, Tufik S, Louzada FM, et al. Association of the length polymorphism in the human Per3 gene with the delayed sleep-phase syndrome: does latitude have an influence upon it? Sleep. 2005;28(1):29–32. [PubMed] [Google Scholar]
- 21.Archer SN, Viola AU, Kyriakopoulou V, von Schantz M, Dijk D-J. Inter-individual differences in habitual sleep timing and entrained phase of endogenous circadian rhythms of BMAL1, PER2 and PER3 mRNA in human leukocytes. Sleep. 2008;31(5):608–617. doi: 10.1093/sleep/31.5.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Viola AU, Archer SN, James LM, et al. PER3 polymorphism predicts sleep structure and waking performance. Curr Biol. 2007;17(7):613–618. doi: 10.1016/j.cub.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 23.Viena T, Gobin C, Fins A, Craddock T, Tartar A, Tartar J. A PER3 polymorphism interacts with sleep duration to influence transient mood states in women. J Circadian Rhythms. 2016;14:3. doi: 10.5334/jcr.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson MR, Akeeb A, Lavela J, Chen Y, Mellman TA. Period 3 gene polymorphism and sleep adaptation to stressful urban environments. J Sleep Res. 2017;26(1):115–118. doi: 10.1111/jsr.12451. [DOI] [PubMed] [Google Scholar]
- 25.Brower KJ, Wojnar M, Sliwerska E, Armitage R, Burmeister M. PER3 polymorphism and insomnia severity in alcohol dependence. Sleep. 2012;35(4):571–577. doi: 10.5665/sleep.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 27.Gumenyuk V, Belcher R, Drake CL, Roth T. Differential sleep, sleepiness, and neurophysiology in the insomnia phenotypes of shift work disorder. Sleep. 2015;38(1):119–126. doi: 10.5665/sleep.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6(1):93–102. doi: 10.3109/07420528909059144. [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom MJ, Bates DM. Nonlinear mixed effects models for repeated measures data. Biometrics. 1990;46(3):673–687. [PubMed] [Google Scholar]
- 30.Dijk DJ, Duffy JF, Silva EJ, Shanahan TL, Boivin DB, Czeisler CA. Amplitude reduction and phase shifts of melatonin, cortisol and other circadian rhythms after a gradual advance of sleep and light exposure in humans. PLoS One. 2012;7(2):e30037. doi: 10.1371/journal.pone.0030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumont M, Paquet J. Progressive decrease of melatonin production over consecutive days of simulated night work. Chronobiol Int. 2014;31(10):1231–1238. doi: 10.3109/07420528.2014.957304. [DOI] [PubMed] [Google Scholar]
- 32.Wehr TA. Melatonin and seasonal rhythms. J Biol Rhythms. 1997;12(6):518–527. doi: 10.1177/074873049701200605. [DOI] [PubMed] [Google Scholar]
- 33.Zeitzer JM, Duffy JF, Lockley SW, Dijk D-J, Czeisler CA. Plasma melatonin rhythms in young and older humans during sleep, sleep deprivation, and wake. Sleep. 2007;30(11):1437–1443. doi: 10.1093/sleep/30.11.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burgess HJ, Fogg LF. Individual differences in the amount and timing of salivary melatonin secretion. PLoS One. 2008;3(8):e3055. doi: 10.1371/journal.pone.0003055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunet AG, Brunet AG, Malpaux B, Daveau A, Taragnat C, Chemineau P. Genetic variability in melatonin secretion originates in the number of pinealocytes in sheep. J Endocrinol. 2002;172(2):397–404. doi: 10.1677/joe.0.1720397. [DOI] [PubMed] [Google Scholar]
- 36.Kunz D, Schmitz S, Mahlberg R, et al. A new concept for melatonin deficit: on pineal calcification and melatonin excretion. Neuropsychopharmacology. 1999;21(6):765–772. doi: 10.1016/S0893-133X(99)00069-X. [DOI] [PubMed] [Google Scholar]
- 37.Tessonneaud A, Locatelli A, Caldani M, Viguier-Martinez MC. Bilateral lesions of the suprachiasmatic nuclei alter the nocturnal melatonin secretion in sheep. J Neuroendocrinol. 1995;7(2):145–152. doi: 10.1111/j.1365-2826.1995.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 38.United States Department of Labor website. Labor Force Statistics from the Current Population Survey. [Accessed January 31, 2018]. https://www.bls.gov/cps/
- 39.Nadkarni NA, Weale ME, Von Schantz M, Thomas MG. Evolution of a length polymorphism in the human PER3 gene, a component of the circadian system. J Biol Rhythms. 2005;20(6):490–499. doi: 10.1177/0748730405281332. [DOI] [PubMed] [Google Scholar]
- 40.Wirth M, Burch J, Violanti J, et al. Association of the Period3 clock gene length polymorphism with salivary cortisol secretion among police officers. Neuro Endocrinol Lett. 2013;34(1):27–37. [PMC free article] [PubMed] [Google Scholar]
- 41.Osland TM, Bjorvatn B, Steen VM, Pallesen S. Association study of a variable-number tandem repeat polymorphism in the clock gene PERIOD3 and chronotype in Norwegian university students. Chronobiol Int. 2011;28(9):764–770. doi: 10.3109/07420528.2011.607375. [DOI] [PubMed] [Google Scholar]


