Abstract
Although there are reports of narcolepsy type 1 caused by lesions of the central nervous system, there are far fewer reports of narcolepsy type 2 (NT2) caused by discrete brain lesions. We report a case of a patient in whom NT2 was diagnosed after a viral illness, and inflammatory lesions in the right thalamus and amygdala were found. In addition, symptoms of autonomic impairment developed and postural tachycardia syndrome was subsequently diagnosed in this patient. To our knowledge this is the first reported case of NT2 resulting from central nervous system lesions in these discrete locations, as well as the first reported case of postural tachycardia syndrome associated with narcolepsy.
Citation:
Kim P, During E, Miglis M. A case of narcolepsy type 2 and postural tachycardia syndrome secondary to lesions of the thalamus and amygdala. J Clin Sleep Med. 2018;14(3):479–481.
Keywords: autonomic, immune-mediated, inflammatory, narcolepsy, postural tachycardia syndrome
INTRODUCTION
Narcolepsy type 1 (NT1) is caused by selective loss of hypo-cretin-producing neurons in the lateral hypothalamus.1 The anatomy and pathophysiology of narcolepsy type 2 (NT2) is less understood, with heterogeneous clinical phenotypes. The available literature on NT2 caused by other neurological conditions, otherwise known as symptomatic or secondary narcolepsy, is limited, and there are no reports to our knowledge of postural tachycardia syndrome (POTS) associated with NT2. We present an illustrative case of discrete central nervous system lesions leading to secondary NT2 and POTS.
REPORT OF CASE
An 18-year-old woman with a prior diagnosis of attention-deficit hyperactivity disorder and anxiety was referred to the sleep clinic for evaluation of excessive daytime sleepiness that began approximately 5 days after an upper respiratory infection (URI). Her sleepiness progressed over several weeks to the point that she reported sleep attacks in both passive and active situations, despite sleeping up to 18 hours a day. She experienced vivid dreams and sleep paralysis, but denied hypnic hallucinations or cataplexy. Several weeks later she experienced daily headaches, difficulty concentrating, night sweats, palpitations, and orthostatic lightheadedness. Her prior medications included 20 mg of sustained-release amphetamine-dextro-amphetamine, 50 mg of sertraline, and an oral contraceptive, which she continued during her illness and without dose adjustment. Physical examination demonstrated a thin woman with a body mass index of 19 kg/m2. Her Epworth Sleepiness Scale score was 18. Oral examination revealed Mallampati class 4. Neurological examination was notable for hypoesthesia along the left hemiface and hemibody.
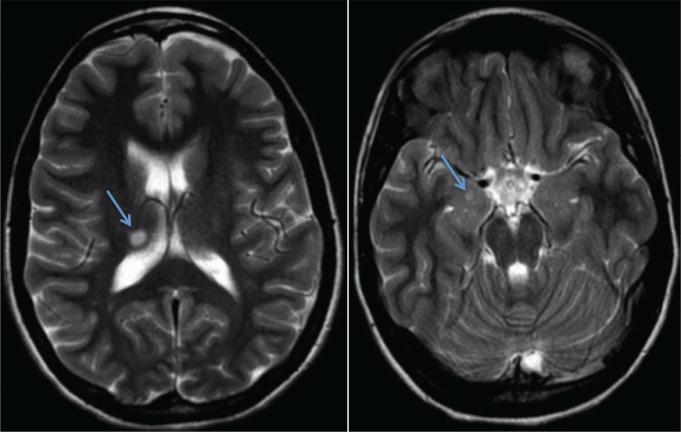
Brain magnetic resonance imaging with contrast, performed 4 months after symptom onset, demonstrated an 8 × 6 mm hyperintense lesion in the right thalamus and a 4 × 4 mm hyper-intense lesion in the right amygdala (Figure 1). Neither of these lesions enhanced with contrast. No lesions were visualized in the hypothalamus. Overnight polysomnogram performed at this time, after discontinuing all medications for 1 week, demonstrated a total sleep time of 8.8 hours, sleep onset latency of 10 minutes, sleep efficiency of 98%, and REM sleep latency of 278 minutes. There was no sleep-disordered breathing or periodic limb movements. A Multiple Sleep Latency Test (MSLT) performed the following day demonstrated a mean sleep latency of 4.6 minutes and 4 out of 4 sleep onset rapid eye movement periods. Her serum HLA-DQB1*06:02 was negative. Lumbar puncture performed 1 month later was normal, with no oligoclonal bands. Cerebrospinal fluid hypocretin was measured at this time and was also normal (335 pg/mL).
Figure 1. Brain magnetic resonance images.
Axial T2-weighted brain magnetic resonance images showing an 8 × 6 mm ovoid lesion in the right posterior superior thalamus (left image), and a 4 × 4 mm lesion in the right amygdala (right image). Lesions indicated by arrows.
Autonomic testing demonstrated an exaggerated postural tachycardia on 70-degree head-up tilt. The maximum recorded heart rate was 128 bpm, from a baseline of 72 bpm (56 beat increase), with a normal blood pressure response, consistent with POTS. Valsalva maneuver demonstrated an exaggerated blood pressure decrement in phase II. Heart rate variability with deep breathing and measures of sweat function with quantitative sudomotor axon reflex testing were both normal.
Prior to our evaluation she was treated with a 4-week course of prednisone, which had no effect on her symptoms. She was continued on her usual dose of amphetamine-dextroamphetamine; higher doses resulted in paradoxical sleepiness. Modafinil was added but later discontinued due to lack of efficacy. After 9 months of symptoms, sodium oxybate was added and titrated to 3 g twice nightly, resulting in significant improvement in her daytime sleepiness and sleep fragmentation. Propranolol was prescribed for her postural tachycardia, leading to improvement in her orthostatic symptoms.
DISCUSSION
We present the case of a patient in whom NT2 and POTS developed after a viral illness, likely because of immune-mediated lesions in the thalamus and amygdala. Although there are several reports of NT1 secondary to discrete central nervous system lesions, to our knowledge this represents one of few reported cases of NT2 due to this etiology. Most reports of secondary NT1 and excessive daytime sleepiness involve lesions in the hypothalamus, although bilateral thalamic lesions have also been described. Scammel and colleagues published the case of a patient in whom NT1 developed after a large postoperative stroke that involved the bilateral thalamus and amygdala; however, the hypothalamus and areas of the basal forebrain and rostral midbrain were also involved.2 Watson and colleagues described a case of a neurocysticercosis leading to NT2, but this lesion was located in the hypothalamus.3 Guilleminault and colleagues have reported on the “pseudo-hypersomnia” of patients with bilateral paramedian thalamic lesions,4 in which patients demonstrated reduced sleep onset latency during MSLT, but did not develop REM intrusion or sleep onset rapid eye movement periods, as our patient did. Bassetti and colleagues similarly described a series of patients with paramedian thalamic strokes and hypersomnia; however, those patients with unilateral lesions had mild hypersomnia that resolved by 8 months.5 There are no reports of unilateral thalamic lesions associated with secondary narcolepsy that we are aware of. Our patient's lesion was located in the lateral and posterior-dorsal thalamus, an area that contains the intralaminar nucleus, which projects to arousal centers including the reticular activating system, hypothalamus, and basal forebrain.6
Our patient's other lesion was located in the amygdala, an area that is particularly dense in hypocretin fibers and receptors. There are reciprocal connections between the amygdala and brainstem areas involved in the regulation of REM sleep, such as the pedunculopontine tegmentum and laterodorsal teg-mental nuclei; lesions in this region have been noted to impair arousal.7 Bilateral amygdala lesions in primates result in more consolidated sleep, greater sleep efficiency, increased total sleep, and increased REM density.8 In addition, and of particular interest in our case, the amygdala contains nuclei that project to autonomic centers in the hypothalamus and brainstem that regulate physiological responses to fear, stress, and emotion. Autonomic impairment has been described in narcolepsy, and hypocretin pathways project to areas in the brainstem and spinal cord that regulate sympathetic tone, including the rostral medulla, solitary nucleus, and sympathetic preganglionic neurons.9 Researchers have demonstrated that microinjection of hypocretin into these areas in mice can depolarize some of these synapses and result in tachycardia or hypertension.10
The pathogenesis of narcolepsy in our patient did not involve measurable hypocretin deficiency. In addition, although the imaging studies may lack sensitivity, no hypothalamic lesions were found. Although it is a possibility that the patient's MSLT findings were due to a rebound effect after discontinuing her stimulant and antidepressant medications, we think this is less likely given the fact that these medications were stopped 1 week prior to testing. In addition, the doses of these medications remained stable before and after her URI symptoms, whereas her hypersomnia began distinctly 5 days after URI symptoms and persisted without improvement. Given prior publications supporting the role of the amygdala and thalamus in the regulation of sleep and autonomic tone, we propose that our patient's lesions resulted in her unique presentation of hypersomnia and autonomic impairment.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. The authors report no conflicts of interest.
REFERENCES
- 1.Nishino S, Kanbayashi T. Symptomatic narcolepsy, cataplexy and hypersomnia, and their implications in the hypothalamic hypocretin/orexin system. Sleep Med Rev. 2005;9(4):269–310. doi: 10.1016/j.smrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Scammell TE, Nishino S, Mignot E, Saper CB. Narcolepsy and low CSF orexin (hypocretin) concentration after a diencephalic stroke. Neurology. 2001;56(12):1751–1753. doi: 10.1212/wnl.56.12.1751. [DOI] [PubMed] [Google Scholar]
- 3.Watson NF, Doherty MJ, Zunt JR. Secondary narcolepsy following neurocysticercosis infection. J Clin Sleep Med. 2005;1(1):41–42. [PubMed] [Google Scholar]
- 4.Guilleminault C, Quera-Salva MA, Goldberg MP. Pseudo-hypersomnia and pre-sleep behaviour with bilateral paramedian thalamic lesions. Brain. 1993;116(Pt 6):1549–1563. doi: 10.1093/brain/116.6.1549. [DOI] [PubMed] [Google Scholar]
- 5.Bassetti C, Mathis J, Gugger M, Lovblad KO, Hess CW. Hypersomnia following paramedian thalamic stroke: a report of 12 patients. Ann Neuro. 1996;39(4):471–480. doi: 10.1002/ana.410390409. [DOI] [PubMed] [Google Scholar]
- 6.Jang SH, Lim HW, Yeo SS. The neural connectivity of the intralaminar thalamic nuclei in the human brain: a diffusion tensor tractography study. Neurosci Lett. 2014;579:140–144. doi: 10.1016/j.neulet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Bisetti A, Cvetkovic V, Serafin M, et al. Excitatory action of hypocretin/ orexin on neurons of the central medial amygdala. Neuroscience. 2006;142(4):999–1004. doi: 10.1016/j.neuroscience.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 8.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879(1-2):130–138. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 9.Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala lesions reduce cataplexy in orexin knock-out mice. J Neurosci. 2013;33(23):9734–9742. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrive P. Orexin, orexin receptor antagonists and central cardiovascular control. Front Neurosci. 2013;7:257. doi: 10.3389/fnins.2013.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]