SUMMARY
Interleukin-6 (IL6) plays a central role in multiple myeloma pathogenesis and confers resistance to corticosteroid-induced apoptosis. We therefore evaluated the efficacy and safety of siltuximab, an anti-IL6 monoclonal antibody, alone and in combination with dexamethasone, for patients with relapsed or refractory multiple myeloma who had ≥2 prior lines of therapy, one of which had to be bortezomib-based. Fourteen initial patients received siltuximab alone, 10 of whom had dexamethasone added for suboptimal response; 39 subsequent patients were treated with concurrent siltuximab and dexamethasone. Patients received a median of 4 prior lines of therapy, 83% were relapsed and refractory, and 70% refractory to their last dexamethasone-containing regimen. Suppression of serum C-reactive protein levels, a surrogate marker of IL6 inhibition, was demonstrated. There were no responses to siltuximab but combination therapy yielded a partial (17%) + minimal (6%) response rate of 23%, with responses seen in dexamethasone-refractory disease. The median time to progression, progression-free survival and overall survival for combination therapy was 4.4, 3.7 and 20.4 months, respectively. Haematological toxicity was common but manageable. Infections occurred in 57% of combination-treated patients, including ≥grade 3 infections in 18%. Further study of siltuximab in modern corticosteroid-containing myeloma regimens is warranted, with special attention to infection-related toxicity.
Keywords: Interleukin-6, siltuximab, multiple myeloma, dexamethasone, targeted therapy
Introduction
Although the advent of the immunomodulatory drugs, thalidomide and lenalidomide, the proteasome inhibitor bortezomib, and autologous stem cell transplantation has significantly improved outcomes for patients with multiple myeloma, the majority of patients will experience repeated relapses and ultimately succumb to refractory disease (Kumar, et al 2004). As such, novel therapies are needed for this patient population.
Interleukin-6 (IL6) is a pleiotropic cytokine that has been shown to play a critical role in the pathogenesis of multiple myeloma (Anderson, et al 1989, Kawano, et al 1988, Klein, et al 1989, Uchiyama, et al 1993). Pre-clinical studies have demonstrated that IL6 not only contributes to multiple myeloma cell proliferation and survival but also to resistance to chemotherapeutics, including bortezomib and melphalan (Hunsucker, et al 2011, Voorhees, et al 2007). In particular, IL6 conferred striking resistance to corticosteroid-induced apoptosis (Chauhan, et al 1997, Hardin, et al 1994, Juge-Morineau, et al 1995, Lichtenstein, et al 1995, Rowley, et al 2000). Although multiple growth factors have been implicated in corticosteroid resistance (Ferlin-Bezombes, et al 1998, Juge-Morineau, et al 1995, Liu, et al 1999, Moreaux, et al 2004, Xu, et al 1997), inhibition of IL6 was able to sensitize multiple myeloma cells to dexamethasone-induced cell death even when grown in the presence of bone marrow stromal cells (Cheung and Van Ness 2001, Grigorieva, et al 1998, Honemann, et al 2001). Additionally, studies in the severe combined immunodeficient mouse-human (SCID-hu) chimera mouse model of human multiple myeloma have also demonstrated synergistic activity between anti-IL6 therapy and dexamethasone (Fulciniti, et al 2009, Tassone, et al 2005). As such, inhibition of IL6 is an attractive approach to the treatment of multiple myeloma, particularly as a way of overcoming corticosteroid resistance.
Siltuximab is a chimeric monoclonal antibody with strong affinity and specificity for human IL6. We have previously demonstrated strong synergistic activity of siltuximab and dexamethasone in multiple myeloma cell lines grown in the presence of bone marrow stroma and in patient multiple myeloma cells, including those derived from patients with corticosteroid-resistant disease (Voorhees, et al 2009). An early phase 1 study evaluated escalating doses of siltuximab in patients with relapsed or relapsed and refractory multiple myeloma who had received at least 2 prior lines of chemotherapy (van Zaanen, et al 1998). Siltuximab was given as 14 daily 2-hour infusions on a 28-day cycle for a maximum of 2 cycles. The median half-life of siltuximab was 17.8 days, and no human anti-chimeric antibodies were noted. C-reactive protein (CRP), a surrogate marker of IL6 activity, decreased to undetectable levels in 11 of 12 patients. Treatment was well tolerated but no responses were seen. More recently, another phase 1 dose escalation study of single-agent siltuximab was conducted in patients with B-cell non-Hodgkin lymphoma, Castleman disease, or multiple myeloma utilizing an intermittent dose schedule (i.e., weekly, every 2 weeks or every 3 weeks). Treatment was well tolerated, even after prolonged dosing, and no dose-limiting toxicities were seen (Kurzrock, et al 2011). Clinical activity was encouraging in multicentric Castleman disease, with 8 of 11 patients treated at the highest dose level of 12 mg/kg every 2 or 3 weeks experiencing a radiological response and all patients deriving clinical benefit (van Rhee, et al 2010). Of the 13 patients with multiple myeloma on that study, 2 achieved complete responses and 1 had prolonged stabilization of disease (Kurzrock, et al 2011).
Given the pre-clinical and preliminary clinical data, we conducted an open-label multicentre, single-arm phase 2 study to evaluate the efficacy and safety of siltuximab, both as a single agent and in combination with dexamethasone, for patients with bortezomib-pretreated multiple myeloma relapsing after or refractory to at least 2 prior lines of therapy.
Methods
Patients
Study patients were at least 18 years of age and had received ≥2 prior lines of therapy, with documented relapse after or progression on the last line of therapy. Prior therapy with a bortezomib-containing regimen was required. Other eligibility criteria included measurable disease, an Eastern Cooperative Oncology Group performance status of ≤2, adequate bone marrow reserves (absolute neutrophil count ≥1.0 × 109/l, haemoglobin ≥75 g/l and platelet count ≥50 × 109/l), adequate liver function (aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase levels ≤3 times the upper limit of normal, total bilirubin ≤2 times the upper limit of normal), and a calculated creatinine clearance of ≥20 ml/min. Patients of childbearing potential were required to use adequate birth control measures, and female patients of childbearing potential had to have a negative serum pregnancy test at the time of screening.
Patients provided written informed consent before study entry. Each of the participating centres obtained approval through their Institutional Review Boards or Ethical Committees, and the study was conducted in accordance with the Declaration of Helsinki.
Study design and treatment
The study included 2 treatment plans, A and B. Patients on treatment plans A and B received siltuximab (Janssen Biotech, Inc., Horsham, PA, USA) at a dose of 6 mg/kg on day 1 and 15 of a 28-day cycle as a 2-h intravenous infusion. The rationale for the dose and schedule was based on pharmacokinetic and pharmacodynamic modelling from a 3-part phase 1/2 study of siltuximab in metastatic renal cell carcinoma, which revealed that a dose of 6 mg/kg once every 2 weeks would decrease CRP levels throughout the duration of the treatment period (Puchalski, et al 2010). For treatment plan A, pulse dexamethasone was added after cycle 1 for disease progression and after cycle 2 for less than a partial response. If no partial responses or better were seen with single-agent siltuximab in the first 14 patients on treatment plan A, subsequent patients would be treated per treatment plan B, in which siltuximab and pulse dexamethasone were administered concurrently beginning with cycle 1 of treatment. Pulse dexamethasone consisted of 40 mg on days 1 − 4, 9 − 12, and 17 − 20 for the first four 28-day cycles and days 1 − 4 thereafter. Prophylactic antibiotic therapy (e.g. trimethoprim-sulfamethoxazole) was required for the duration of dexamethasone therapy. The planned duration of treatment was 12 cycles; however, patients with stable disease or better were allowed to remain on therapy beyond 12 cycles. Siltuximab was not dose reduced during treatment. However prior to re-dosing, the absolute neutrophil count had to be ≥0.5 × 109/l, haemoglobin ≥75 g/l, and platelet count ≥50 × 109/l, and all other clinically-significant toxicity had to recover to ≤grade 2 or baseline. Siltuximab treatment could be delayed up to 14 days; no more than 2 toxicity-related dose delays were allowed. Dexamethasone could be stepwise dose reduced to a minimum of 20 mg on days 1-4 in the presence of steroid-induced toxicity.
Data were collected and analysed by medical and statistical representatives from Janssen Research & Development and the investigators. All investigators had access to the primary data and participated in the preparation of the manuscript. Participating institutions received clinical grant support for the conduct of the study.
Assessment of efficacy
The primary efficacy endpoint was the overall response rate (≥partial response rate), as per European Group for Blood and Marrow Transplant (EBMT) criteria (Blade, et al 1998), to either single-agent siltuximab or the combination of siltuximab and dexamethasone, depending on whether treatment plan A or B was utilized. Patients were evaluable for response if they received at least one dose of siltuximab and had at least one confirmed post-baseline disease assessment. Response was assessed on day 22 of each cycle by the investigator using M-protein results from a central laboratory, and confirmed by the sponsor. Secondary efficacy endpoints included time to response, duration of response, time to progression, progression-free survival and overall survival. Minimal responses were also evaluated, due to its proven clinical benefit in this patient population (Anderson, et al 2008, Niesvizky, et al 2008). An assessment of overall response rate utilizing the International Myeloma Working Group (IMWG) uniform response criteria was also performed (Durie, et al 2006).
Time to response was defined as the time from the first administration of protocol therapy to the time of first confirmed response. Duration of response was defined as the time from the first documented response to the time of first documented disease progression. Time to progression was defined as the time interval from the first administration of protocol therapy to first documented disease progression. Progression-free survival was defined as the time interval from the first administration of protocol therapy to first documented disease progression or death, whichever occurred first. Relapsed and refractory disease was defined as disease that is nonresponsive while on therapy or progresses within 60 days of the last dose of therapy in patients who have achieved ≥minimal response at some point in their disease course (Anderson, et al 2008, Rajkumar, et al 2011). Dexamethasone-refractory disease was defined as less than a minimal response to any prior dexamethasone-containing therapy or disease progression within 60 days of completing the last dexamethasone-based therapy.
Assessment of safety, pharmacokinetic, pharmacodynamic and biomarker end points
Patients who received ≥1 dose of siltuximab were evaluable for safety. Adverse events were assessed at each study visit and graded for intensity per National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf). Adverse events were recorded from the time of obtaining informed consent to 30 days after administration of the last study agent. Safety endpoints included the incidence of all adverse events, ≥grade 3 adverse events, serious adverse events, deaths, and clinically-significant changes in vital signs, safety-related laboratory parameters and electrocardiograms.
Serum samples for pharmacokinetic evaluation of siltuximab were obtained pre- and post-dose on day 1 and on day 8 of cycles 1 − 3. Siltuximab serum concentrations were determined using an electrochemiluminescent immunoassay format on the Meso Scale Discovery Platform with a lower limit of quantification of 0.045 μg/ml. Serum samples to evaluate anti-siltuximab antibodies were obtained at baseline, the time of siltuximab discontinuation, and every 3 months up to 3 times after the last dose. Antibodies to siltuximab were determined using a validated assay (Rossi, et al 2010). CRP levels were drawn with each siltuximab administration for cycles 1 − 3 and on day 1 of each subsequent cycle. Exploratory pharmacodynamic markers, including but not limited to hepcidin, were obtained on day 1 of cycles 1 − 3. CRP was analysed at a central laboratory. Hepcidin was tested using a polyclonal antibody-based competitive enzyme-linked immunosorbent assay (Intrinsic LifeSciences, LLC, La Jolla, CA, USA).
Statistical analysis
The original sample size was 40 patients. The study was later amended to allow enrollment of a total of 90 patients to better estimate the response rate with greater precision. Due to slow accrual, the study was closed after enrollment of 55 patients. No formal hypothesis testing was planned. Descriptive statistics were used to summarize the data. For continuous parameters, number of observations, mean, standard deviation, median and range were used. For discrete parameters, frequency was summarized. Time-to-event endpoints were evaluated as per the Kaplan-Meier method. Efficacy and safety data were analysed separately for those receiving treatment plan A and B. In addition, data from patients who received dexamethasone in treatment plan A were combined with treatment plan B to provide a more comprehensive assessment of efficacy and safety with the combination of siltuximab and dexamethasone. For those patients on treatment plan A who received dexamethasone, time-to-event analyses were performed from the time that combination therapy was initiated. Ad-hoc comparison of some specific baseline characteristics were conducted between treatment plans A and B based on Fisher’s exact test. The differences were tested at a two-sided alpha level of 0.05.
Results
Patient and treatment information
All patients had completed protocol therapy at the time of data cut-off. From September 2006 to May 2009, a total of 55 patients with relapsed or refractory multiple myeloma were enrolled at 10 treatment centres in the United States and The Netherlands. Two patients were not treated on study: one due to the development of an adverse event; the other due to ineligibility. Fourteen patients were treated with single agent siltuximab per treatment plan A, 4 of whom had dexamethasone added to their therapy after cycle 1 and 6 after cycle 2 for lack of response. Thirty-nine patients were treated with concurrent siltuximab and dexamethasone beginning with cycle 1. In all treated patients, the median number of treatment cycles administered was 4 (range 1 – 24). Thirty-two patients (60%) received 1 – 4 cycles, 11 patients (21%) received 5 – 8 cycles, 8 patients (15%) received 9 − 12 cycles, and 2 patients (4%) received greater than 12 cycles. Of the 53 patients treated with siltuximab, 37 (70%) discontinued therapy due to disease progression, 12 (23%) due to adverse events, and 4 (8%) for other reasons. The median age of all treated patients was 65 years (range 43 – 89), and the median time from diagnosis was 4.0 years (range 0.7 – 13.2) (Table I). The median beta2-microglobulin was 4.2 mg/l (range 1.3 – 15.6), and 37% and 29% of patients had International Staging System stage II or III disease at study entry, respectively. Patients had received a median of 4 prior lines of therapy (range 2 – 9) and 45% of the treated patients had received ≥5 prior lines of therapy. All patients had received prior bortezomib and corticosteroid therapy, 89% prior thalidomide and/or lenalidomide, 91% alkylating agents, 68% anthracyclines and 66% high-dose melphalan with stem cell rescue. Eighty-three percent of patients had relapsed and refractory disease at the time of study enrollment, as defined by less than a minimal response to their last therapy (43%) or disease progression within 60 days of their last treatment (40%). Of the 50 patients previously treated with dexamethasone, 70% were refractory to their last dexamethasone-containing therapy. Baseline characteristics were similar between patients on treatment plans A and B, although a higher proportion of patients on treatment plan B had relapsed and refractory disease (P=0.2218), as well as disease refractory to their last dexamethasone-containing regimen (P=0.0031).
Table I.
Baseline patient demographics, disease characteristics and treatment history
| Treatment Plan A (N=14) | Treatment Plan B (N=39) | Treatment Plans A and B (N=53) | |
|---|---|---|---|
| Median age, years (range) | 62 (48 – 89) | 65 (43 – 83) | 65 (43 – 89) |
| Sex, N (%) | |||
| Male | 7 (50) | 23 (59) | 30 (57) |
| Female | 7 (50) | 16 (41) | 23 (43) |
| Race (%) | |||
| Caucasian | 8 (57) | 28 (72) | 36 (68) |
| Black | 2 (14) | 5 (13) | 7 (13) |
| Asian | 0 | 3 (8) | 3 (6) |
| Other | 4 (29) | 3 (8) | 7 (13) |
| ECOG Performance Status score, N (%) | |||
| 0 | 5 (36) | 10 (26) | 15 (28) |
| 1 | 8 (57) | 26 (67) | 34 (64) |
| 2 | 1 (7) | 3 (8) | 4 (8) |
| Median time from diagnosis, years (range) | 4.1 (2.4 – 12.8) | 3.9 (0.7 – 13.2) | 4.0 (0.7 – 13.2) |
| Ig isotype, N (%) | |||
| IgG | 8 (57) | 26 (67) | 34 (64) |
| IgA | 5 (36) | 7 (18) | 12 (23) |
| Light chain | 0 | 5 (13) | 5 (9) |
| International Staging System stage at baseline, N (%) | |||
| I | 4 (31) | 14 (36) | 18 (35) |
| II | 4 (31) | 15 (39) | 19 (37) |
| III | 5 (39) | 10 (26) | 15 (29) |
| Median creatinine clearance, ml/min (range) | 62 (45 – 179) | 70 (27 – 168) | 67 (27 – 179) |
| Relapsed and refractory disease, N (%) | 10 (71) | 34 (87) | 44 (83) |
| PD on last prior therapy | 6 (43) | 14 (36) | 20 (38) |
| SD on last prior therapy | 0 | 3 (8) | 3 (6) |
| PD within 60 days of last prior therapy | 4 (29) | 17 (44) | 21 (40) |
| Refractory to last dexamethasone-based therapy, N/total (%) | 4/12 (33) | 31/38 (82) | 35/50 (70) |
| Median prior lines of therapy, (range) | 4 (2 – 8) | 4 (2 – 9) | 4 (2 – 9) |
| ≥5 prior lines of therapy, N (%) | 5 (36) | 19 (49) | 24 (45) |
| Prior treatment regimens, N (%) | |||
| Bortezomib | 14 (100) | 39 (100) | 53 (100) |
| Corticosteroids | 14 (100) | 39 (100) | 53 (100) |
| IMiDs | 12 (86) | 35 (90) | 47 (89) |
| Alkylating agents | 14 (100) | 34 (87) | 48 (91) |
| Anthracyclines | 10 (71) | 26 (67) | 36 (68) |
| ASCT | 8 (57) | 27 (69) | 35 (66) |
ASCT: Autologous stem cell transplantation. ECOG: Eastern Cooperative Oncology group. Ig: Immunoglobulin. IMiDs: Immunomodulatory drugs. PD: Progressive disease. SD: Stable disease. Percentages may not sum correctly due to rounding.
Pharmacokinetic, pharmacodynamic and immunogenicity analyses
The similar mean siltuximab concentrations (±standard deviation) of 44.81 μg/ml (±15.21) and 44.51 μg/ml (±19.80) for patients on day 8 of cycle 1 on treatment plans A and B, respectively, suggest that dexamethasone did not impact siltuximab pharmacokinetics. Antibodies to siltuximab were not detected in any of the 36 patients who had appropriate samples for evaluation. Mean CRP levels promptly decreased by cycle 1, day 15 and reached the lower limit of quantification (0.2 mg/l) by cycle 2, day 15 (Fig 1) for all treated patients. A linear mixed effect model, with the patient identifier as a random effect and the visit as a fixed effect, was fitted on the data (number of patients per visit >12). The CRP value was significantly different on all visits after cycle 1 day 1 compared with cycle 1 day 1 (P<0.04).The random effect showed 4 outliers; when these were removed, the P-values dropped even lower (P<10−6) for all visits. Hepcidin is a 25 kD peptide that plays a critical role in iron homeostasis and is also induced by IL6 (Nemeth, et al 2004). Mean hepcidin levels ±standard deviation dropped from 240.33 ng/ml ± 327.00 at cycle 1, day 1 to 195.20 ng/ml ± 271.86 at cycle 2, day 1 and 104.36 ng/ml ± 92.06 at cycle 3, day 1. The same statistical analysis was also applied to the hepcidin values with the following results: no significant changes were detected between cycle 2 day 1 and cycle 1 day 1 (P=0.5243). The difference between cycle 3 day 1 and cycle 1 day 1 was larger, but did not reach the significance threshold (P=0.0602).
Figure 1.
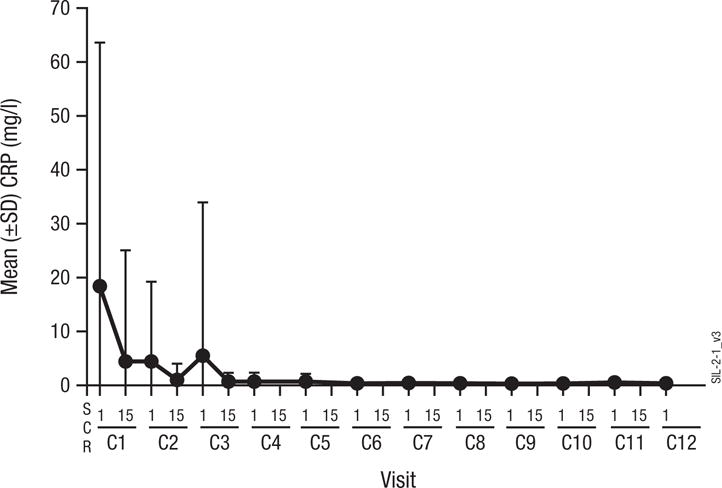
Mean serum C-reactive protein (CRP) concentrations by visit in treated patients. C: cycle. SCR: screening. SD: standard deviation.
Efficacy
The median duration of study follow-up was 13.9 months (range 0.5 – 37.7). Thirteen patients on treatment plan A and 38 on treatment plan B were evaluable for response. There were no responses to single-agent siltuximab, with 8 patients achieving stable disease and 5 disease progression. The overall response rate (≥partial response rate) per EBMT criteria for those on treatment plan B was 8% (95% confidence interval 2% − 21%), with 8% of patients achieving a minimal response as their best response and 68% stable disease (Table II). Of the 9 evaluable patients on treatment plan A who had dexamethasone added to siltuximab therapy for lack of response, 5 achieved a partial response. Thus, the overall response rate for patients receiving combination therapy on either plan A or B was 17% (8 patients, 95% confidence interval 8% − 31%). No very good partial or complete responses were seen. Of the 8 patients achieving a partial response on combination therapy, 7 had disease that was refractory to their most recent line of therapy. The median time to response was 1.6 months (range 0.7 – 8.1 months) and the median duration of response 5.9 months (95% confidence interval 4.8 – 12 months). The clinical benefit rate (≥minimal response rate) for patients receiving combination therapy was 23%. Utilizing updated IMWG uniform response criteria, the clinical benefit rate for patients receiving combination therapy was 28%.
Table II.
Response assessment of siltuximab with and without dexamethasone
| Treatment Plan A Siltuximab |
Treatment Plan B (Siltuximab + Dexamethasone) | Combination Siltuximab + Dexamethasonea | ||
|---|---|---|---|---|
| Before Dex | After Dex | |||
| Evaluable for response, N | 13 | 9 | 38 | 47 |
| Overall response rate (≥PR), N (%) | ||||
| PR by EBMT | 0 (0) | 5 (56) | 3 (8) | 8 (17) |
| PR by IMWG | 0 (0) | 5 (56) | 4 (11) | 9 (19) |
| Clinical Benefit Rate (≥MR), N (%) | ||||
| PR + MR by EBMT | 0 (0) | 5 (56) | 6 (16) | 11 (23) |
| PR +MR by IMWG | 0 (0) | 5 (56) | 8 (21) | 13 (28) |
| Stable disease by EBMT, N (%) | 8 (62) | 1 (11) | 26 (68) | 27 (57) |
| Disease progression, N (%) | 5 (39) | 2 (22) | 6 (16) | 8 (17) |
| Time to response (months), median (range) | N/A | 1.7 (0.7 – 8.1) | 1.5 (0.7 – 1.6) | 1.6 (0.7 – 8.1) |
| Duration of response (months), median (95% CI) | N/A | 12.0 (3.0 – 16.6) | 5.9 (4.9 – 9.9) | 5.9 (4.8 – 12.0) |
CI: Confidence interval. Dex: Dexamethasone. EBMT: European Group for Blood and Marrow Transplantation. IMWG: International Myeloma Working Group Criteria. MR: Minimal response. N/A: Not applicable. PR: Partial response.
Includes patients in Treatment Plan A who received siltuximab plus dexamethasone and all patients in Treatment Plan B.
Given the role of IL6 in corticosteroid resistance, we evaluated the efficacy of siltuximab and dexamethasone for those patients with previously documented resistance to dexamethasone-based therapy. Five of 32 evaluable patients (16%) with disease refractory to their most recent dexamethasone-containing therapy had ≥minimal response with siltuximab and dexamethasone, whereas 5 of 12 evaluable patients (42%) with disease sensitive to their last dexamethasone-based regimen responded (P=0.1054). Additionally, of 10 patients with a minimal or partial response, 7 had less than a minimal response to a prior dexamethasone-based therapy.
The median time to progression for patients receiving combination siltuximab and dexamethasone was 4.4 months (Fig 2A, 95% confidence interval 2.8 – 5.6 months). The median progression-free and overall survival was 3.7 months (95% confidence interval 2.8 – 4.9 months) and 20.4 months (Fig 2B, 95% confidence interval 11.4 – 32.3 months) for patients receiving combination therapy.
Figure 2.
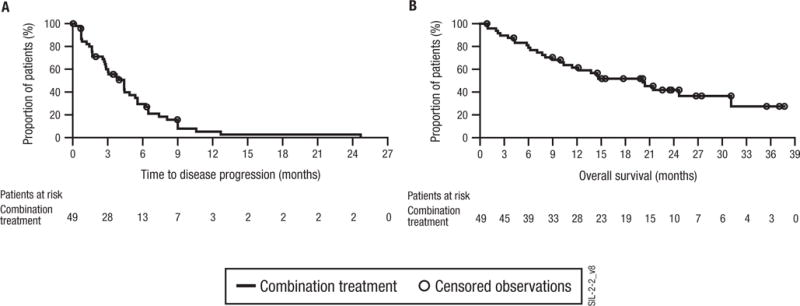
Kaplan-Meier curves for (A) time to progression and (B) overall survival in combination-treated patients. Time-to-event analyses for those on treatment plan A were measured from the start of dexamethasone therapy.
Safety
Fifty-three patients received at least 1 dose of siltuximab and were evaluable for safety. All 49 patients who received combination therapy experienced at least 1 adverse event, with 90% and 92% of patients experiencing adverse events at least reasonably related to siltuximab and dexamethasone, respectively. Haematological toxicity was common with neutropenia, anaemia and thrombocytopenia occurring in 29%, 35% and 49% of patients, respectively (Table III). However, grade 3 or 4 neutropenia was only seen in 15% and 3% and grade 3 or 4 thrombocytopenia in 14% and 12% of patients, respectively. No episodes of febrile neutropenia occurred. Common non-haematological toxicities included fatigue (43%), abnormal hepatic function (31%), diarrhoea and peripheral oedema (29% each), dyspnea (27%), dizziness (25%), nausea and insomnia (22% each), constipation and weight increase (20% each), and myalgia and enzyme abnormality (16% each). Hypertriglyceridaemia has been seen with the anti-IL6 receptor monoclonal antibody, tocilizumab (Maini, et al 2006). In the present study, 7 patients (14%) experienced hypertriglyceridaemia; all but 1 episode was less than grade 3 in severity. Only 2 patients treated with combination therapy had an infusion-related reaction, both of which were ≤grade 2 in severity. No venous thromboembolic events occurred. Fifty-seven percent of patients who received combination therapy had 1 or more infection-related adverse events that included upper respiratory infections (14%), cellulitis, oral candidiasis, and pneumonia (8% each), herpes zoster reactivation and urinary tract infection (6% each), and septic shock (4%). One patient had reactivation of hepatitis B. In total, 12% and 6% of patients experienced grade 3 or 4 infections, respectively.
Table III.
Adverse events occurring in ≥15% of patients on siltuximab alone and with dexamethasone
| Patients with adverse event (%) | Siltuximab Alone (Treatment Plan A before Dexamethasone) N=14 |
Combination Siltuximab and Dexamethasone N=49 |
||||
|---|---|---|---|---|---|---|
| All | Gr3 | Gr4 | All | Gr3 | Gr4 | |
| Thrombocytopenia | 36 | 14 | 14 | 49 | 14 | 12 |
| Fatigue | 36 | 0 | 0 | 43 | 4 | 4 |
| Anaemia | 57 | 36 | 7 | 35 | 14 | 2 |
| Hepatic function abnormal | 14 | 0 | 0 | 31 | 6 | 2 |
| Neutropenia | 21 | 7 | 0 | 29 | 14 | 4 |
| Diarrhoea | 50 | 0 | 0 | 29 | 0 | 0 |
| Peripheral oedema | 0 | 0 | 0 | 29 | 0 | 0 |
| Dyspnea | 14 | 0 | 0 | 27 | 4 | 0 |
| Dizziness | 7 | 0 | 0 | 25 | 0 | 0 |
| Nausea | 29 | 0 | 0 | 22 | 0 | 0 |
| Leucopenia | 14 | 7 | 0 | 22 | 4 | 0 |
| Insomnia | 0 | 0 | 0 | 22 | 2 | 0 |
| Constipation | 7 | 0 | 0 | 20 | 0 | 0 |
| Weight increase | 0 | 0 | 0 | 20 | 2 | 0 |
| Myalgia | 7 | 0 | 0 | 16 | 0 | 0 |
| Enzyme abnormality | 0 | 0 | 0 | 16 | 0 | 0 |
Seventy-four percent of patients receiving combination therapy had ≥grade 3 adverse events. In addition to the ≥grade 3 haematological and infection-related adverse events noted above, common ≥grade 3 adverse events included fatigue and abnormal hepatic function (8% each). Grade 4 events occurred in 29% of patients, whereas serious adverse events were noted in 41% of patients. Forty-one percent of patients on combination therapy required temporary withholding of siltuximab due to adverse events, while 47% of patients had dexamethasone held or dose reduced due to adverse events. Adverse events necessitating discontinuation of therapy occurred in 12 (25%) of the 49 patients treated with siltuximab and dexamethasone, but no single event accounted for discontinuation in more than 2 patients. Five patients died during the study period; 3 due to disease progression and 2 due to infection, one of which was felt to be reasonably attributable to protocol therapy.
Discussion
We evaluated the safety and efficacy of siltuximab with or without dexamethasone for patients with relapsed or refractory multiple myeloma. Unfortunately, no responses were seen in the first 14 evaluable patients treated with single-agent siltuximab, with the caveat that a response to monotherapy would have been difficult to discern given the introduction of dexamethasone within the first 2 cycles for lack of response. This is in contrast to the phase 1 study of single-agent siltuximab in patients with multiple myeloma, B-cell non-Hodgkin lymphoma or Castleman disease in which 2 of 13 patients with multiple myeloma achieved complete responses on siltuximab (Kurzrock, et al 2011). Although the overall response rate to combination siltuximab and dexamethasone in treatment plan B was only 8%, the overall response rate was 17% for all patients treated with siltuximab and dexamethasone combination therapy. Moreover, the clinical benefit rate (≥minimal response rate) was 23%. When using the updated international uniform response criteria, the clinical benefit rate was 28%. The median time to progression and overall survival for patients receiving combination therapy was 4.4 and 20.4 months, respectively, with better outcomes seen for those with responsive disease.
Several factors may have contributed to the modest response rate of combination siltuximab and dexamethasone in this study. First, multiple myeloma becomes increasingly independent of the bone marrow microenvironment for survival in the relapsed/refractory setting, which in part explains the increased incidence of extramedullary disease over time. Additionally, mutations inherent to the myeloma cells may confer corticosteroid resistance. A salient example is the presence of RAS mutations in advanced multiple myeloma that in pre-clinical models lead to dexamethasone resistance (Rowley, et al 2000). As such, anti-IL6 therapy may be better positioned earlier in the course of the disease. Second, although systemic IL6 inhibition in the present study was readily apparent, as demonstrated by a decrease in serum CRP levels, IL6 inhibition at the tumoural level may have been suboptimal. In the above-mentioned phase 1 study of siltuximab monotherapy, radiological responses were seen in 8 of 11 patients with Castleman disease treated at the highest dose of 12 mg/kg once every 2 or 3 weeks (van Rhee, et al 2010). The 2 patients with multiple myeloma who achieved complete responses to single-agent therapy in the phase 1 study were also treated at a higher siltuximab dose intensity, utilizing either 6 mg/kg weekly or 12 mg/kg once every 3 weeks (unpublished observations). Notably, there was a higher response rate seen in patients on treatment plan A of this study compared with those on treatment plan B. CRP levels were not maximally inhibited until cycle 2, day 15. As such, it is tempting to speculate that the improved response rate in treatment plan A was the result of more optimal IL6 inhibition at the time that dexamethasone was introduced. However, it should be noted that a smaller proportion of patients on treatment plan A had disease refractory to their last dexamethasone-containing therapy, which could readily explain the difference. Nonetheless, given the fact that patients had received a median of 4 prior lines of therapy and 83% had relapsed and refractory disease, the 17% overall response rate and 23% clinical benefit rate seen in this study are noteworthy.
Interestingly, responses were seen in patients with multiple myeloma refractory to their last dexamethasone-based therapy, suggesting the possibility that inhibition of IL6 signalling with siltuximab can overcome corticosteroid resistance in some instances. This would support data from a large body of pre-clinical work demonstrating the ability to restore corticosteroid sensitivity via anti-IL6 therapy (Cheung and Van Ness 2001, Fulciniti, et al 2009, Grigorieva, et al 1998, Honemann, et al 2001, Voorhees, et al 2009). However, we cannot rule out the possibility that differences in response to dexamethasone-based therapy were due to differences in dexamethasone dose intensity between regimens, as this level of information could not be readily established from the medical records. We are also limited by the lack of a true control arm that would enable us to distinguish the role of siltuximab in the observed response rates. Certainly, randomized studies are needed to determine if siltuximab can overcome corticosteroid resistance.
Although haematological toxicity was common, grade 3 and 4 haematological adverse events were infrequent. Our results are in line with those from the phase 1 study of siltuximab, in which grade 3 or higher possibly related neutropenia and thrombocytopenia were seen in only 16% and 4% of patients, respectively (unpublished observations). Non-haematological toxicities were largely in line with expected toxicities of dexamethasone (e.g. fatigue, peripheral oedema, dizziness, myalgias, insomnia, and weight increase). However, infections occurred at a high rate, with grade 3 and 4 infections seen in 12% and 6% of patients, respectively. Two patients died of sepsis, one of which was attributed to protocol therapy. Certainly, infection-related complications are common in patients with advanced multiple myeloma, and high-dose pulse dexamethasone is immunosuppressive in and of itself. However, IL6 does play a critical role in immunity and could potentially further increase risk. Results from a randomized phase 2 study of bortezomib versus bortezomib and siltuximab were recently presented (Orlowski, et al 2012) and did not demonstrate a difference in grade 3 or higher infections between the two treatment arms (14% and 16%, respectively). Nonetheless, careful attention to infection-related toxicity with siltuximab-based therapy will be critical moving forward.
In conclusion, the combination of siltuximab and dexamethasone had clinical activity in heavily-pretreated multiple myeloma, including patients with relapsed and refractory disease. Responses were seen in dexamethasone-refractory disease, suggesting that siltuximab may be able to overcome corticosteroid resistance in some cases. Randomized studies incorporating siltuximab into current multiple myeloma therapy will be needed to better establish its role in therapy, with careful attention to infection-related toxicity. In this regard, a randomized phase 2 clinical trial of melphalan, prednisone and bortezomib with or without siltuximab at optimal dose intensity is being conducted in newly-diagnosed multiple myeloma and should better determine the role of IL6 inhibition in multiple myeloma therapy.
Acknowledgments
Peter Voorhees would like to acknowledge support from the National Institutes of Health and the National Center for Research Resources (K12 RR17667 and KL2 RR025746). The authors thank Jennifer Han of Janssen Services, LLC for providing editorial assistance with the manuscript.
Footnotes
Author’s Contributions
PMV performed the research, designed the research study, analysed the data and wrote the paper. RFM, SJ, GS, AK, SL, RCF, SZ, PWW, and SKT performed the research and analysed the data. PS and RZO performed the research, designed the research study, and analysed the data. BK, BH, TC, XQ, HvdV, and HX designed the research study and analysed the data.
Conflicts of Interest
PMV has been a consultant/served on an advisory board for Abbott Laboratories, Med Immune, and Celgene; has received honoraria from Celgene and Millenium Pharmaceuticals; and has received research funding from Janssen, Merck, Celgene, and Pfizer. PS has received research support from Janssen, Celgene, and Onyx. AK has been on the speakers bureau for MLN, Celgene, and Onyx. BK, BH, TC, XQ, HvdV, and HX are employees of Janssen, a Johnson & Johnson company, and own Johnson & Johnson stock. All other authors have no conflicts of interest.
References
- Anderson KC, Jones RM, Morimoto C, Leavitt P, Barut BA. Response patterns of purified myeloma cells to hematopoietic growth factors. Blood. 1989;73:1915–1924. [PubMed] [Google Scholar]
- Anderson KC, Kyle RA, Rajkumar SV, Stewart AK, Weber D, Richardson P, on behalf of the ASHTM/FDA Panel on Clinical Endpoints in Multiple Myeloma Clinically relevant end points and new drug approvals for myeloma. Leukemia. 2008;22:231–239. doi: 10.1038/sj.leu.2405016. [DOI] [PubMed] [Google Scholar]
- Blade J, Samson D, Reece D, Apperley J, Bjorkstrand B, Gahrton G, Gertz M, Giralt S, Jagannath S, Vesole D. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. British Journal of Haematology. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Pandey P, Ogata A, Teoh G, Treon S, Urashima M, Kharbanda S, Anderson KC. Dexamethasone induces apoptosis of multiple myeloma cells in a JNK/SAP kinase independent mechanism. Oncogene. 1997;15:837–843. doi: 10.1038/sj.onc.1201253. [DOI] [PubMed] [Google Scholar]
- Cheung WC, Van Ness B. The bone marrow stromal microenvironment influences myeloma therapeutic response in vitro. Leukemia. 2001;15:264–271. doi: 10.1038/sj.leu.2402022. [DOI] [PubMed] [Google Scholar]
- Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV, International Myeloma Working, G International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- Ferlin-Bezombes M, Jourdan M, Liautard J, Brochier J, Rossi JF, Klein B. IFN-alpha is a survival factor for human myeloma cells and reduces dexamethasone-induced apoptosis. Journal of Immunology. 1998;161:2692–2699. [PubMed] [Google Scholar]
- Fulciniti M, Hideshima T, Vermot-Desroches C, Pozzi S, Nanjappa P, Shen Z, Patel N, Smith ES, Wang W, Prabhala R, Tai YT, Tassone P, Anderson KC, Munshi NC. A high-affinity fully human anti-IL-6 mAb, 1339, for the treatment of multiple myeloma. Clinical Cancer Research. 2009;15:7144–7152. doi: 10.1158/1078-0432.CCR-09-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigorieva I, Thomas X, Epstein J. The bone marrow stromal environment is a major factor in myeloma cell resistance to dexamethasone. Experimental Hematology. 1998;26:597–603. [PubMed] [Google Scholar]
- Hardin J, MacLeod S, Grigorieva I, Chang R, Barlogie B, Xiao H, Epstein J. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]
- Honemann D, Chatterjee M, Savino R, Bommert K, Burger R, Gramatzki M, Dorken B, Bargou RC. The IL-6 receptor antagonist SANT-7 overcomes bone marrow stromal cell-mediated drug resistance of multiple myeloma cells. International Journal of Cancer. 2001;93:674–680. doi: 10.1002/ijc.1388. [DOI] [PubMed] [Google Scholar]
- Hunsucker SA, Magarotto V, Kuhn DJ, Kornblau SM, Wang M, Weber DM, Thomas SK, Shah JJ, Voorhees PM, Xie H, Cornfeld M, Nemeth JA, Orlowski RZ. Blockade of interleukin-6 signalling with siltuximab enhances melphalan cytotoxicity in preclinical models of multiple myeloma. British Journal of Haematology. 2011;152:579–592. doi: 10.1111/j.1365-2141.2010.08533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge-Morineau N, Francois S, Puthier D, Godard A, Bataille R, Amiot M. The gp 130 family cytokines IL-6, LIF and OSM but not IL-11 can reverse the anti-proliferative effect of dexamethasone on human myeloma cells. British Journal of Haematology. 1995;90:707–710. doi: 10.1111/j.1365-2141.1995.tb05605.x. [DOI] [PubMed] [Google Scholar]
- Kawano M, Hirano T, Matsuda T, Taga T, Horii Y, Iwato K, Asaoku H, Tang B, Tanabe O, Tanaka H, Kuramoto A, Kishimoto T. Autocrine generation and requirement of BSF-2/IL-6 for human multiple myelomas. Nature. 1988;332:83–85. doi: 10.1038/332083a0. [DOI] [PubMed] [Google Scholar]
- Klein B, Zhang XG, Jourdan M, Content J, Houssiau F, Aarden L, Piechaczyk M, Bataille R. Paracrine rather than autocrine regulation of myeloma-cell growth and differentiation by interleukin-6. Blood. 1989;73:517–526. [PubMed] [Google Scholar]
- Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Witzig TE, Lust JA, Larson DR, Kyle RA, Greipp PR. Clinical course of patients with relapsed multiple myeloma. Mayo Clinic Proceedings. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- Kurzrock R, Voorhees PM, Casper C, Furman RR, Fayad L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani S, van de Velde H, Qin X, Qi M, Cornfeld MJ, van Rhee F. Long-Term Safety in a Phase 1 Study of Siltuximab (CNTO 328), an Anti-Interleukin-6 Monoclonal Antibody, in Patients with B-Cell Non-Hodgkin’s Lymphoma, Multiple Myeloma, or Castleman’s Disease. Blood (ASH Annual Meeting Abstracts) 2011;118 Abstract 3959. [Google Scholar]
- Lichtenstein A, Tu Y, Fady C, Vescio R, Berenson J. Interleukin-6 inhibits apoptosis of malignant plasma cells. Cell Immunology. 1995;162:248–255. doi: 10.1006/cimm.1995.1076. [DOI] [PubMed] [Google Scholar]
- Liu P, Oken M, Van Ness B. Interferon-alpha protects myeloma cell lines from dexamethasone-induced apoptosis. Leukemia. 1999;13:473–480. doi: 10.1038/sj.leu.2401334. [DOI] [PubMed] [Google Scholar]
- Maini RN, Taylor PC, Szechinski J, Pavelka K, Broll J, Balint G, Emery P, Raemen F, Petersen J, Smolen J, Thomson D, Kishimoto T, Group, C.S Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate. Arthritis & Rheumatism. 2006;54:2817–2829. doi: 10.1002/art.22033. [DOI] [PubMed] [Google Scholar]
- Moreaux J, Legouffe E, Jourdan E, Quittet P, Reme T, Lugagne C, Moine P, Rossi JF, Klein B, Tarte K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood. 2004;103:3148–3157. doi: 10.1182/blood-2003-06-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E, Rivera S, Gabayan V, Keller C, Taudorf S, Pedersen BK, Ganz T. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. Journal of Clinical Investigation. 2004;113:1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesvizky R, Richardson PG, Rajkumar SV, Coleman M, Rosinol L, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Boral AL, Esseltine DL, Anderson KC, Blade J. The relationship between quality of response and clinical benefit for patients treated on the bortezomib arm of the international, randomized, phase 3 APEX trial in relapsed multiple myeloma. British Journal of Haematology. 2008;143:46–53. doi: 10.1111/j.1365-2141.2008.07303.x. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Gercheva L, Williams C, Sutherland HJ, Robak T, Masszi T, Goranova-Marinova V, Dimopoulos MA, Cavenagh JD, Spicka I, Maiolino A, Suvorov A, Blade J, Samoilova OS, Van De Velde H, Bandekar R, Kranenburg B, Xie H, Rossi JF. Phase II, randomized, double blind, placebo-controlled study comparing siltuximab plus bortezomib versus bortezomib alone in pts with relapsed/refractory multiple myeloma. Journal of Clinical Oncology (ASCO Meeting Abstracts) 2012;30:8018. doi: 10.1002/ajh.23868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM. Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clinical Cancer Research. 2010;16:1652–1661. doi: 10.1158/1078-0432.CCR-09-2581. [DOI] [PubMed] [Google Scholar]
- Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D, Jagannath S, Facon T, Avet-Loiseau H, Lonial S, Palumbo A, Zonder J, Ludwig H, Vesole D, Sezer O, Munshi NC, San Miguel J, International Myeloma Workshop Consensus, P Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117:4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B. A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer. British Journal of Cancer. 2010;103:1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley M, Liu P, Van Ness B. Heterogeneity in therapeutic response of genetically altered myeloma cell lines to interleukin 6, dexamethasone, doxorubicin, and melphalan. Blood. 2000;96:3175–3180. [PubMed] [Google Scholar]
- Tassone P, Neri P, Burger R, Savino R, Shammas M, Catley L, Podar K, Chauhan D, Masciari S, Gozzini A, Tagliaferri P, Venuta S, Munshi NC, Anderson KC. Combination therapy with interleukin-6 receptor superantagonist Sant7 and dexamethasone induces antitumor effects in a novel SCID-hu In vivo model of human multiple myeloma. Clinical Cancer Research. 2005;11:4251–4258. doi: 10.1158/1078-0432.CCR-04-2611. [DOI] [PubMed] [Google Scholar]
- Uchiyama H, Barut BA, Mohrbacher AF, Chauhan D, Anderson KC. Adhesion of human myeloma-derived cell lines to bone marrow stromal cells stimulates interleukin-6 secretion. Blood. 1993;82:3712–3720. [PubMed] [Google Scholar]
- van Rhee F, Fayad L, Voorhees P, Furman R, Lonial S, Borghaei H, Sokol L, Crawford J, Cornfeld M, Qi M, Qin X, Herring J, Casper C, Kurzrock R. Siltuximab, a novel anti-interleukin-6 monoclonal antibody, for Castleman’s disease. Journal of Clinical Oncology. 2010;28:3701–3708. doi: 10.1200/JCO.2009.27.2377. [DOI] [PubMed] [Google Scholar]
- van Zaanen HC, Lokhorst HM, Aarden LA, Rensink HJ, Warnaar SO, van der Lelie J, van Oers MH. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. British Journal of Haematology. 1998;102:783–790. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Chen Q, Kuhn DJ, Small GW, Hunsucker SA, Strader JS, Corringham RE, Zaki MH, Nemeth JA, Orlowski RZ. Inhibition of interleukin-6 signaling with CNTO 328 enhances the activity of bortezomib in preclinical models of multiple myeloma. Clinical Cancer Research. 2007;13:6469–6478. doi: 10.1158/1078-0432.CCR-07-1293. [DOI] [PubMed] [Google Scholar]
- Voorhees PM, Chen Q, Small GW, Kuhn DJ, Hunsucker SA, Nemeth JA, Orlowski RZ. Targeted inhibition of interleukin-6 with CNTO 328 sensitizes pre-clinical models of multiple myeloma to dexamethasone-mediated cell death. British Journal of Haematology. 2009;145:481–490. doi: 10.1111/j.1365-2141.2009.07647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Gardner A, Tu Y, Michl P, Prager D, Lichtenstein A. Multiple myeloma cells are protected against dexamethasone-induced apoptosis by insulin-like growth factors. British Journal of Haematology. 1997;97:429–440. doi: 10.1046/j.1365-2141.1997.592708.x. [DOI] [PubMed] [Google Scholar]


