Abstract
Background
Arterial stiffening may underlie the association between depression and cardiovascular disease (CVD), but reported data are inconsistent. We investigated the associations between aortic stiffness and major depressive disorder (MDD) and depressive symptoms, and whether these differed by sex and age.
Methods
We measured carotid to femoral pulse wave velocity (cfPWV) using applanation tonometry, and we assessed depression using the Mini-International Neuropsychiatric Interview (MINI) and the Patient Health Questionnaire-9 (PHQ-9) in a cohort of participants from The Maastricht Study. Logistic and negative binominal models were adjusted for age, type 2 diabetes mellitus (T2DM), mean arterial pressure (MAP) and CVD risk factors.
Results
We included 2757 participants in our analyses (48.8% men, mean age 59.8 ± 8.1 yr, 27% T2DM). We found that cfPWV was associated with MDD in men (fully adjusted odds ratio [OR] 2.36, 95% confidence interval [CI] 1.45–3.84), but not in women (OR 1.57, 95% CI 0.93–2.66), aged 60 years or younger. The ORs were not significant in individuals older than 60 years (men: OR 1.03, 95% CI 0.63–1.68; women: OR 0.64, 95% CI 0.32–1.31). Similarly, cfPWV was associated with a higher PHQ-9 score in men (rate ratio 1.28, 95% CI 1.09–1.52), but not in women (rate ratio 1.11, 95% CI 0.99–1.23), aged 60 years or younger. Associations were not significant in individuals older than 60 years (men: rate ratio 0.96, 95% CI 0.84–1.08; women: rate ratio 1.00, 95% CI 0.90–1.12).
Limitations
We cannot rule out reversed causation in this cross-sectional study.
Conclusion
Greater aortic stiffness is associated with MDD and depressive symptoms among middle-aged men and to a lesser extent in women, whereas this association was not observed in old age.
Introduction
Arterial stiffening is considered a potential mechanism that could underlie the consistently observed association between depression and cardiovascular disease (CVD).1–3 Arterial stiffening may cause damage to the cerebral vasculature, which is characterized by low impedance, as it increases pressure and flow pulsatility, which are harmful to the cerebral microcirculation.4 The vascular depression hypothesis states that the accumulation of (micro)vascular damage in the frontal–subcortical mood regulatory circuit of the brain may ultimately cause depression.5,6 High pulse wave velocity has indeed been associated with cerebral small vessel disease7 and could consequently be involved in the pathophysiology of depression.
Conflicting results have been reported on the association between aortic stiffness and both the presence of major depressive disorder (MDD)8–10 and depressive symptoms,11,12 which may be explained by suboptimal study designs and/or assessment of arterial stiffness and depression. A significant association between aortic stiffness and depression was observed in the population-based Rotterdam Study10 and the AGES-Reykjavik Study,11 whereas no association was found with depressive symptoms in the Health ABC Study.12 This contradiction could be explained by differences in the age range between these study populations (≥ 55 yr in the Rotterdam Study v. 70–79 yr in the Health ABC Study), and by the use in the latter study of questionnaire data, which are less accurate than a diagnostic interview, as an estimate of depressive symptoms. In addition, (borderline) significant associations between aortic stiffness and MDD were reported in 2 relatively small cross-sectional studies.8,9 Both the statistical power and the generalizability of the results might have been hampered in these studies because they had a case–control design. Additionally, one study9 used less accurate measures to assess aortic stiffness, and the population of the other study8 was rather small. Moreover, a recent systematic review has shown that the association between depression and stroke is heterogeneous and differs among subgroups of age, body mass index (BMI), smoking status, and type of depression measurement.13 Furthermore, the Framingham Study has shown that the association between CVD and depression is stronger in middle age (< 65 yr), than in old age (≥ 65 yr).14 The association between intermediate measures of cardiovascular risk, such as aortic stiffness, and depression could also differ in middle age versus old age. However, none of the previous studies evaluated whether this association differs according to age. In addition, men and women appear to differ in both their cardiovascular risk profiles and depressive symptoms. Aortic stiffness is known to be substantially greater in men than in women,15,16 whereas depression appears to be more prevalent among women than men.17 These sex differences may be even more prominent in middle age, when hormonal differences between the sexes are greatest and when women may have the highest cardiovascular protection from reproductive hormones. To date, 3 out of 4 studies have investigated the effect of sex on the association between cfPWV and depression or depressive symptoms, but their populations were older adults (mean age > 65 yr); none of them reported any significant differences. Of these 4 studies, only the Rotterdam Study10 used a structured interview to diagnose MDD.
In view of these findings we investigated the association between cfPWV and both MDD and depressive symptoms in a population-based cohort study of adults aged 40–75 years. We specifically examined whether any associations differed according to age and sex.
Methods
Study population
We used data from The Maastricht Study, an observational prospective population-based cohort study. Its rationale and methodology have been described previously.18 In brief, the study focuses on the etiology, pathophysiology, complications and comorbidities of type 2 diabetes mellitus (T2DM) and is characterized by an extensive phenotyping approach. Eligible for participation were all individuals aged 40–75 years and living in the southern part of the Netherlands. Participants were recruited through mass media campaigns and from the municipal registries and the regional Diabetes Patient Registry via mailings. Recruitment was stratified according to known T2DM status, with an oversampling of individuals with T2DM for reasons of efficiency. The present report includes cross-sectional data from participants who completed the baseline survey between November 2010 and September 2013. The examinations of each participant were performed within a time window of 3 months. The study has been approved by the institutional medical ethical committee (NL31329.068.10) and the Minister of Health, Welfare and Sports of the Netherlands (Permit 131088–105234-PG). All participants gave written informed consent.
Carotid to femoral pulse wave velocity
All measurements were carried out by trained vascular technicians who were unaware of the participants’ clinical or diabetes status; measurements took place in a dark, quiet, temperature-controlled room (21–23°C). Participants were asked to refrain from smoking and drinking coffee, tea or alcoholic beverages in the 3 hours before the study. They were allowed to have a light meal (breakfast or lunch). All measurements were performed with the participant in a supine position after 10 minutes of rest. Talking or sleeping was not allowed during the examination.
We determined carotid to femoral pulse wave velocity ( cfPWV) according to recent guidelines19 using applanation tonometry (SphygmoCor, Atcor Medical). Pressure waveforms were determined at the right common carotid and right common femoral arteries. The difference in the time of pulse arrival from the R-wave of the electrocardiogram between the 2 sites (transit time) was determined using the intersecting tangents algorithm. The pulse wave travel distance was calculated as 80% of the direct straight distance (measured with an infantometer) between the 2 arterial sites. We used the median of 3 consecutive cfPWV (defined as travelled distance or transit time) recordings in the analyses. Brachial systolic, diastolic, mean arterial pressure (MAP) and heart rate were determined repeatedly during cfPWV assessment with a 5-minute interval using an oscillometric device (Accutorr Plus, Datascope Inc.), and we calculated the average of these measurements.
We assessed the reproducibility of cfPWV measurements in 12 individuals (6 men, mean age 60.8 ± 6.8 yr, 6 individuals with T2DM) who were examined by 2 observers on 2 occasions spaced 1 week apart. The intra- and interobserver intraclass correlation coefficients (ICC) were 0.87 and 0.69.
Assessment of depression
We assessed MDD using the Mini-International Neuropsychiatric Interview (MINI).20 The MINI is a short diagnostic interview used to assess the presence of minor depression or MDD in the preceding 2 weeks according to the DSM-IV.21 We diagnosed MDD if participants had at least 1 core symptom (depressed mood or anhedonia) and at least 4 other symptoms of depression (weight change, change in appetite, insomnia/hypersomnia, psychomotor agitation/retardation, fatigue/loss of energy, guilt/worthlessness, diminished ability to think/concentrate or indecisiveness and suicidal thoughts/plans). For sensitivity analyses we defined a minor depressive episode as the presence of at least 1 core symptom and 1–3 other symptoms of depression, and we defined early-onset depression as onset of MDD before the age of 40 years.22
Depressive symptoms were assessed using a validated Dutch version of the 9-item Patient Health Questionnaire (PHQ-9).23 The PHQ-9 is a self-administered questionnaire based on the DSM-IV21 criteria for MDD. It comprises 9 items rated on a 4-point scale ranging from 0 to 3 (0 = not at all; 3 = nearly every day). Response options can generate a continuous score ranging from 0 (no symptoms) to 27 (all symptoms present nearly every day). Both cognitive symptoms of depression, comprising thoughts about oneself and problems of the mind, and somatic symptoms of depression, comprising various bodily sensations perceived as unpleasant or worrisome, are measured with the PHQ-9.24
General characteristics and covariates
We used web-based questionnaires to obtain information regarding smoking status (never/former/current), alcohol consumption, educational level, physical activity and prior CVD.18 Alcohol consumption was classified as none, low (1–7 glasses/wk for women and 1–14 glasses/wk for men) and high (> 7 glasses/wk for women and > 14 glasses/wk for men). Educational level was was classified as low (no education, primary education, lower vocational education), intermediate (intermediate general secondary education, intermediate vocational education, higher general secondary education), or high (higher vocational education or university). Physical activity was assessed by means of a modified version of the Champs questionnaire.18 Prior CVD was defined as a history of myocardial infarction; stroke; or vascular surgery (including angioplasty) on coronary, carotid, abdominal aortic, or peripheral arteries based on the Rose questionnaire.25 Glucose metabolism status was assessed according to World Health Organization criteria26 using a 75 g oral glucose tolerance test. Both fasting glucose and 2-hour postload glucose concentrations were analyzed using a standard enzymatic hexokinase reference method. Serum concentrations of total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides were measured using an automatic analyzer (Beckman Synchron LX20, Beckman Coulter Inc.). We estimated the glomerular filtration rate (GFR) using the CKD-EPI equation based on the combination of serum creatinine and serum cystatin C.18 Body mass index, waist:hip ratio and medication use were determined as described previously.18 Use of either hormone replacement therapy or the contraceptive pill was considered to be exogenous estrogen medication. Blood pressure was calculated as the average of at least 3 blood pressure readings (Omron 705IT) performed after a minimum of 10 minutes of seated rest. Hypertension was defined as systolic blood pressure greater than 140 mm Hg or as diastolic blood pressure greater than 90 mm Hg and/or the use of blood pressure–lowering medication.
Statistical analysis
All analyses were performed using IBM SPSS Statistics software version 22.0 (IBM Corp.). To account for the differences in the distribution of the cfPWV between the sexes, the cfPWV was introduced as a sex-specific Z-score in all models.
We compared baseline characteristics of the study population between individuals with and without MDD; we used a t test for normally distributed continuous variables, a Mann–Whitney test for non-normally distributed variables, and a χ2 test for dichotomous or ordinal variables. We considered results to be significant at p < 0.05, 2-tailed. We used logistic regression analyses to investigate the association between cfPWV and MDD. We used negative binomial regression analyses to examine the association between cfPWV and depressive symptoms in order to account for the marked floor effect of the PHQ-9 distribution.27 Both regression analyses were adjusted for multiple confounders. Model 1 was the crude model; model 2 was adjusted for age, sex and T2DM; model 3 was additionally adjusted for MAP; and model 4 was additionally adjusted for smoking, alcohol use, BMI, mean heart rate, hypertension, prior CVD and the use of lipid-modifying medication. Models were adjusted for T2DM to account for the oversampling of these individuals in the Maastricht Study. Additionally, T2DM has been associated with both greater aortic stiffness28 and a greater chance of MDD developing,29 therefore we considered T2DM to be a confounder. As MAP and mean heart rate directly influence stiffness measurements, they were accounted for in the models. The following cardiovascular risk factors, which have been associated with both arterial stiffening and depression, were also added as confounders: smoking, high alcohol intake, BMI, hypertension, prior CVD and the use of lipid-modifying medication. Interactions for age, sex and T2DM were assessed in model 2 and were considered to be significant at p < 0.10.
Results
Study population
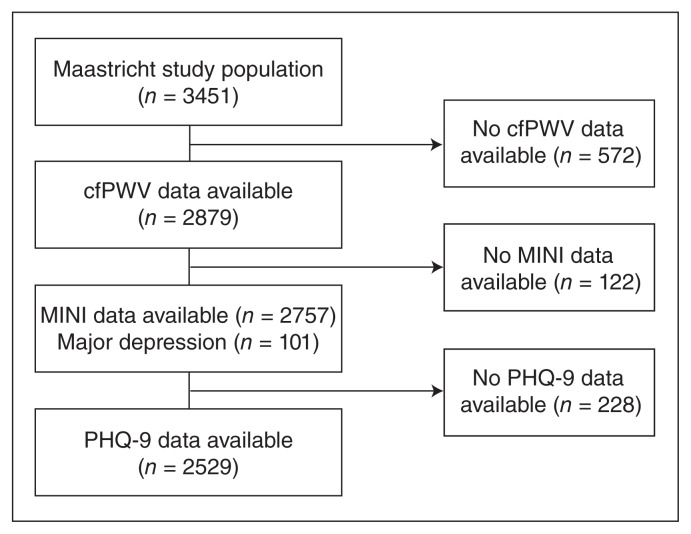
The present report includes cross-sectional data from the first 3451 participants of The Maastricht Study. Participants were mainly white (98.6%). Figure 1 shows the flow of participants through the study. After exclusion of individuals without data on cfPWV (n = 572), the MINI diagnostic interview and PHQ-9 were available in 2757 and 2529 participants, respectively. Individuals with missing data on covariates, such as prior CVD, smoking status and alcohol use (n = 111), were older, more frequently had T2DM and had a higher cfPWV than the individuals included in the final models (Appendix 1, Table S1, available at jpn.ca/160246-a1). Table 1 shows the demographic and clinical characteristics of the study population according to the presence of MDD. The mean age of participants was 59.8 ± 8.1 years, and 48.8% of participants were women. In total 101 (3.6%) participants had MDD.
Fig. 1.
Selection of participants for inclusion in our analyses. cfPWV = carotid to femoral pulse wave velocity; MINI = Mini-International Neuropsychiatric Interview; PHQ-9 = Patient Health Questionnaire-9.
Table 1.
Demographic and clinical characteristics of the study population
| Group; mean ± SD or median [IQR]* | ||
|---|---|---|
|
|
||
| Characteristic | No depressive disorder (n = 2656) | MDD (n = 101) |
| Age, yr | 59.9 ± 8.1 | 58.6 ± 8.1 |
| Female sex, % | 48.6 | 47.5 |
| Education level, low/medium/high, % | 32/29/39 | 49/30/22 |
| Cardio-metabolic risk factors | ||
| BMI, kg/m2 | 26.9 ± 4.3 | 28.4 ± 5.1 |
| Waist:hip ratio | 0.94 ± 0.09 | 0.96 ± 0.10 |
| Glucose metabolism status, NGM/IGM/TDM2, % | 57/15/27 | 41/10/49 |
| Total cholesterol, mmol/L | 5.24 ± 1.16 | 5.06 ± 1.13 |
| HDL cholesterol, mmol/L | 1.52 ± 0.47 | 1.36 ± 0.45 |
| LDL cholesterol, mmol/L | 3.10 ± 1.03 | 2.99 ± 1.14 |
| Total:HDL cholesterol ratio | 3.70 ±1.18 | 4.03 ± 1.35 |
| Systolic blood pressure, mm Hg | 135 ± 18 | 136 ± 18 |
| Diastolic blood pressure, mm Hg | 76 ± 10 | 77 ± 11 |
| Mean arterial pressure, mm Hg | 97 ± 10 | 97 ± 11 |
| Heart rate, bpm | 63 ± 9 | 65 ± 10 |
| Hypertension, % | 55.2 | 58.4 |
| Prior CVD, % | 16.1 | 21.6 |
| eGFR, mL/min/1.73 m2 | 88.3 ± 14.7 | 87.8 ± 16.0 |
| Lifestyle factors | ||
| Smoking, never/former/current, % | 34/53/13 | 22/52/27 |
| Alcohol use, none/low/high, % | 17/56/27 | 35/43/22 |
| Physical activity, hours/wk† | 13.3 [8.3–18.8] | 10.5 [6.0–16.5] |
| High-intensity physical activity, hours/wk† | 4.5 [2.3–8.0] | 3.0 [0.8–5.5] |
| Medication use | ||
| Glucose lowering drugs, % | 21.7 | 42.6 |
| Anti-hypertensive drugs, % | 38.5 | 46.5 |
| Lipid modifying drugs, % | 36.2 | 43.6 |
| Antidepressive drugs, %‡ | 6.4 (170) | 25.7 (26) |
| Stiffness related indices | ||
| cfPWV, m/s | 8.99 ± 2.14 | 9.52 ± 2.84 |
| Pulse pressure, mm Hg | 51 ± 10 | 51 ± 10 |
| Depressive symptoms | ||
| PHQ-9 score | 2.0 [0.0–4.0] | 10.0 [7.0–16.0] |
BMI = body mass index; cfPWV = carotid to femoral pulse wave velocity; CVD = cardiovascular disease; eGFR = estimated glomerular filtration rate; HDL = high-density lipoprotein; IGM = impaired glucose metabolism; IQR = interquartile range; LDL = low-density lipoprotein; MDD = major depressive disorder; NGM = normal glucose metabolism; PHQ-9 = Patient Health Questionnaire-9; SD = standard deviation; TDM2 = type 2 diabetes mellitus.
Unless indicated otherwise.
Data on physical activity were available for 2409 participants.
Antidepressive drugs according to sex: 8.9% in nondepressed women v. 4.0% in nondepressed men and 33.3% in depressed women v. 18.9% in depressed men.
Participants with MDD smoked more often, used less alcohol and were less physically active than participants without MDD. They had a worse metabolic risk factor profile, including a greater likelihood of having T2DM, higher BMI, lower HDL cholesterol and higher total cholesterol:HDL ratio. Other cardiovascular risk factors, such as the GFR, prior CVD, systolic and diastolic blood pressure, hypertension, the use of blood pressure–lowering and lipid-modifying medication, did not differ between individuals with and without MDD. The cfPWV was slightly higher in patients with MDD than in those without MDD (p = 0.07).
Association between cfPWV and MDD
Our results showed that cfPWV was significantly associated with MDD after adjustment for age, sex and T2DM (odds ratio [OR] 1.24, 95% confidence interval [CI] 1.01–1.51). The association between cfPWV and MDD differed with age (p = 0.043) and sex (p = 0.015); no significant interaction was observed for T2DM (p = 0.22). Analyses were, therefore, stratified according to age (in 5-yr categories) and sex. Based on the results we stratified the analyses according to the median age of the population (≤ 60 yr v. > 60 yr; analyses for the full population and according to the cut-offs of 55, 60 and 65 yr are shown in Appendix 1, Table S7).
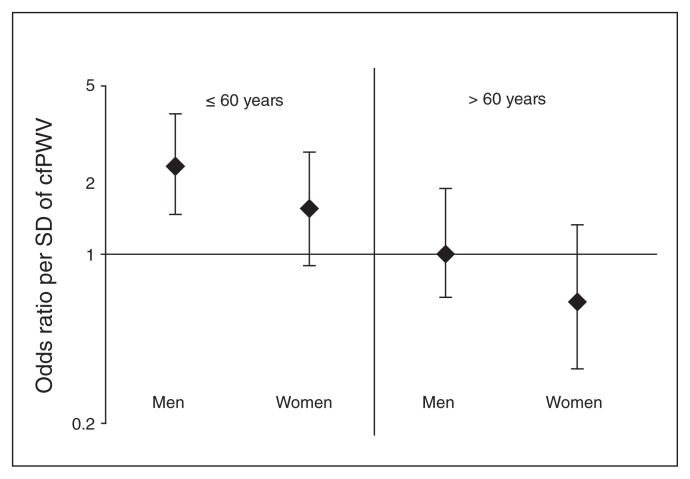
As shown in Table 2, in men aged 60 years or younger, cfPWV was significantly associated with MDD after adjusting for age, T2DM and MAP (OR 2.23, 95% CI 1.42–3.52). This OR was slightly higher in the fully adjusted model (OR 2.36, 95% CI 1.45–3.84). In women aged 60 years or younger, cfPWV was not significantly associated with MDD after adjusting for age, T2DM and MAP (OR 1.34, 95% CI 0.84–2.15). The fully adjusted OR remained nonsignificant (OR 1.57, 95% CI 0.93–2.66). In both men and women older than 60 years, cfPWV was not significantly associated with MDD, and the fully adjusted ORs were considerably lower in women than in men (Table 2 and Fig. 2).
Table 2.
Association between carotid to femoral pulse wave velocity and major depressive disorder stratified by age and sex
| Age ≤ 60 yr; OR (95% CI) | Age > 60 yr; OR (95% CI) | |||
|---|---|---|---|---|
|
|
|
|||
| Model* | Men (n = 612, 27 cases) | Women (n = 746, 35 cases) | Men (n = 800, 21 cases) | Women (n = 599, 18 cases) |
| Model 1 | 2.49 (1.64–3.78) | 1.42 (0.95–2.10) | 1.41 (1.04–1.91) | 0.72 (0.44–1.19) |
| Model 2 | 2.37 (1.54–3.64) | 1.27 (0.84–1.93) | 1.37 (0.99–1.90) | 0.54 (0.31–0.94) |
| Model 3 | 2.23 (1.42–3.52) | 1.34 (0.84–2.15) | 1.33 (0.93–1.90) | 0.60 (0.33–1.08) |
| Model 4† | 2.36 (1.45–3.84) | 1.57 (0.93–2.66) | 1.03 (0.63–1.68) | 0.64 (0.32–1.31) |
CI = confidence interval; OR = odds ratio.
Model 1 = crude; Model 2 = adjusted for age, type 2 diabetes; Model 3 = additionally adjusted for mean arterial pressure; Model 4 = additionally adjusted for heart rate, body mass index, lipid medication, smoking, alcohol use, prior cardiovascular disease and hypertension.
A total of 111 participants (7 cases) equally distributed among the groups had missing values on covariates and dropped out of the analyses.
Fig. 2.
The association between carotid to femoral pulse wave velocity (cfPWV) and major depressive disorder stratified by age and sex. Men and women aged 60 years or younger have a higher risk of major depressive disorder, although this association was not significant for women. The presented odds ratios (ORs) were adjusted for age, type 2 diabetes, mean arterial pressure, heart rate, body mass index, lipid medication, smoking, prior cardiovascular disease, alcohol use and hypertension. SD = standard deviation.
Associations between cfPWV and depressive symptoms
We found that cfPWV was significantly associated with more depressive symptoms after adjustment for age, sex and T2DM (rate ratio [RR] 1.07 95% CI 1.01–1.13). The association between cfPWV and depressive symptoms differed with age (p = 0.036); however, no significant interactions were observed for sex (p = 0.59) or T2DM (p = 0.94). For consistency reasons, we stratified the results according to both age and sex.
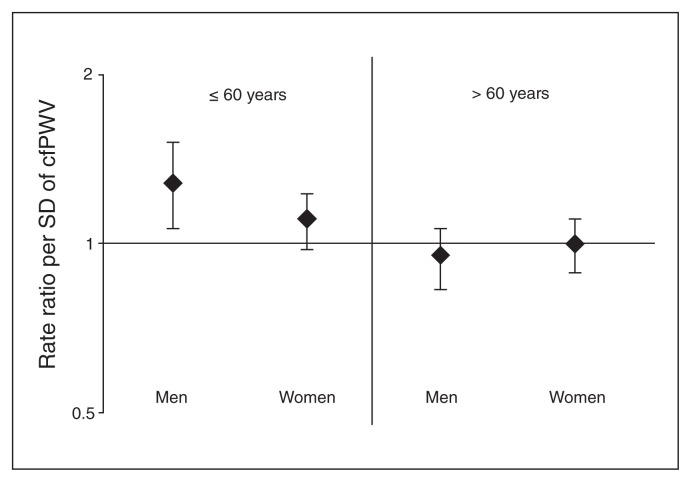
As shown in Table 3, in men aged 60 years or younger, cfPWV was significantly associated with more depressive symptoms after adjustment for age, T2DM and MAP (RR 1.28, 95% CI 1.09–1.52). This association remained unchanged in the fully adjusted model (RR 1.28, 95% CI 1.09–1.52). In women aged 60 years or younger, cfPWV was significantly associated with more depressive symptoms in the crude model (RR 1.13, 95% CI 1.03–1.23). This association was attenuated by additional adjustments for age, sex and especially T2DM (RR 1.09, 95% CI 0.996–1.21). In both men and women older than 60 years cfPWV was not significantly associated with depressive symptoms (Table 3 and Fig. 3).
Table 3.
The association between cfPWV and depressive symptoms stratified by age and sex
| Age ≤ 60 years; rate ratio (95% CI) | Age > 60 years; rate ratio (95% CI) | |||
|---|---|---|---|---|
|
|
|
|||
| Model* | Men (n = 582) | Women (n = 722) | Men (n = 761) | Women (n = 579) |
| Model 1 | 1.28 (1.11–1.48) | 1.13 (1.03–1.23) | 1.12 (1.01–1.23) | 1.02 (0.93–1.12) |
| Model 2 | 1.22 (1.04–1.42) | 1.09 (0.996–1.21) | 1.07 (0.96–1.20) | 0.98 (0.90–1.07) |
| Model 3 | 1.28 (1.09–1.52) | 1.12 (0.996–1.25) | 1.04 (0.92–1.17) | 1.00 (0.90–1.11) |
| Model 4† | 1.28 (1.09–1.52) | 1.11 (0.99–1.23) | 0.96 (0.84–1.08) | 1.00 (0.90–1.12) |
CI = confidence interval.
Model 1 = crude; Model 2 = adjusted for age, type 2 diabetes; Model 3 = additionally adjusted for mean arterial pressure; Model 4 = additionally adjusted for heart rate, body mass index, lipid medication, smoking, alcohol use, prior cardiovascular disease and hypertension.
A total of 90 participants equally distributed among the groups had missing values on covariates and dropped out of the analyses.
Fig. 3.
The association between carotid to femoral pulse wave velocity (cfPWV) and depressive symptoms stratified by age and sex. Men and women aged 60 years or younger had a higher risk of depressive symptoms, although this association was not statistically significant for women. Rate ratios were adjusted for age, type 2 diabetes, mean arterial pressure, heart rate, body mass index, lipid medication, smoking, prior cardiovascular disease, alcohol use and hypertension. SD = standard deviation.
Additional analyses
When we excluded individuals with extreme cfPWV values (± 3 standard deviations; outlier analyses) the association between cfPWV and MDD and depressive symptoms did not materially change. To acknowledge differences in etiology between early- and late-onset depression, we excluded individuals with a first depression episode before 40 years of age (n = 407); this did not change the results (Appendix 1, Tables S2 and S3). Also, when we excluded all participants who used antidepressants (n = 196) the results did not materially change (Appendix 1, Tables S2 and S3). Antidepressants could be prescribed for both depressive and anxiety disorders, therefore we additionally adjusted the models for antidepressant use; again this did not materially change the results (Appendix 1, Tables S4 and S5). To adjust for various comorbidities of depression, models were additionally adjusted for antipsychotic, anxiolytic and sleep medication, but this did not change the observed associations. When major and minor depression were combined as an outcome measure, the results did not change (Appendix 1, Table S6). To exclude the possibility that T2DM oversampling biased the associations, the analyses were repeated in individuals without T2DM (n = 2008); the results remained unchanged ( Appendix 1, Tables S2 and S3).
When we adjusted the analyses for education level and moderate to vigorous self-reported physical activity (data available in 2409 participants), the results did not materially change (Appendix 1, Tables S4 and S5). Finally, adjustment for the use of exogenous hormone replacement medication did not materially alter the observed ORs and RRs in women.
Discussion
The present study showed that greater aortic stiffness was associated with MDD and the presence of depressive symptoms in men aged 60 years or younger, and to a lesser extent in women of the same age, although in women the association was not statistically significant. We found no association between aortic stiffness and depression in men and women older than 60 years.
The association in men aged 60 years or younger was independent of other cardiovascular risk factors. Most studies to date have analyzed the association in populations older than 60 years. Only the study by Seldenrijk and colleagues9 included participants between the ages of 20 and 66 years and observed an association between the augmentation index, which is a composite measure of early arterial wave reflection due to greater arterial stiffness, and MDD. However, the authors did not evaluate whether the association differed according to age because of the relatively young study sample. Of the studies that included participants older than 60 years, 2 studies8,12 did not find significant associations between aortic stiffness and depression in older participants. Of these, one study8 had a small sample size (n = 46) including few cases (n = 25), and the other study12 included participants from only a limited age range (70–79 yr). Aortic stiffness was associated with both a depressive disorder and depressive symptoms in The Rotterdam Study10 and with depressive symptoms in the AGES-Reykjavik Study, as diagnostic interviews were not available.11 The observed associations were, however, relatively small compared with our results in individuals aged 60 years or younger. It may be plausible to assume that the association between aortic stiffness and depression may be overshadowed in later life by the development of CVD, especially in individuals at risk owing to greater aortic stiffness. In later life other factors, such as CVD and atherosclerosis, may play a more prominent role in the development of depression than aortic stiffness. In a longitudinal study, Kim and colleagues30 found, for example, that CVD, including pre-existing heart disease and stroke but not hypertension, was associated with incident depression in community participants aged 65 years and older. Stroke could lead to depression through direct damage to the cortical and subcortical areas of the brain that are involved in mood regulation.31 In patients with myocardial infarction comorbid carotid atherosclerosis, 32 rather than aortic stiffness, could accelerate microvascular damage in the mood regulatory regions of the brain and thus lead to vascular depression. Myocardial infarction is, for example, associated with an increased risk of cerebrovascular disease, independent of age or hypertension.33
The association between aortic stiffness and depression was stronger in men than in women aged 60 years or younger. Although the association was significant only in middle-aged men, we cannot rule out the presence of an association in middle-aged women. Only a few previous large cross-sectional studies carried out interaction analyses for sex,10–12 and none reported any significant differences. However, the mean age of these study populations was 70 years or older, whereas our observed sex interaction occurred in patients aged 60 years or younger. A possible explanation for the interaction might be that in women aged 60 years or younger, hormonal fluctuations, as seen during the menstrual cycle and perimenopause, rather than aortic stiffness could predispose them to depression.34 Reproductive hormones are also thought to have a protective effect on the vasculature, especially before menopause,15,16 thus further diminishing the association between cfPWV and depression in women. Exogenous hormone supplementation, such as hormone replacement therapy or contraception, could also be a confounder; however, in our study use of such medication was infrequent and did not affect the observed associations. An alternative explanation may be the substantial heterogeneity among those with depressive disorder, which might result in important differences of this diagnosis between men and women. Also, the possibility that the described sex differences were due to chance cannot be ruled out.
We found that aortic stiffness was associated more strongly with MDD than with depressive symptoms. This could be attributed to the misclassification of depressive symptoms as MDD by the PHQ-9.10,24 Furthermore, the association between aortic stiffness and depressive symptoms may not be linear; it could be stronger in individuals who fulfil the diagnostic criteria for MDD. This threshold of severity could be captured better by a diagnostic instrument, such as the MINI. Finally, the weaker association between aortic stiffness and depressive symptoms could also explain the lack of interaction with sex in these analyses.
Multiple mechanisms could explain the association between aortic stiffness and depression. First, according to the vascular depression hypothesis, cardiovascular risk factors, such as arterial stiffness, may lead to the accumulation of cerebral small vessel lesions in the mood regulatory centres of the brain,35 which may result in the development of depression.5,6 A recent study by van Sloten and colleagues7 indeed showed that the presence of supratentorial cerebral white matter hyperintensities and subcortical infarcts together explained part of the association between cfPWV and depression. Second, arterial stiffness may lead to greater oscillations in wall shear stress, which may induce endothelial dysfunction as well as the production of low-grade inflammatory markers by the endothelium.36 Third, endothelial dysfunction could lead to depression through a disturbance of the process of neurogenesis,37,38 through small vessel disease,39 or speculatively through decreased cerebrovascular reactivity.40 Systemic low-grade inflammation on the other hand, could via multiple pathways stimulate the production of proinflammatory markers by microglial cells in the brain, which could induce symptoms of depression.41–43 Fourth, other factors, such as autonomic and hypothalamic–pituitary–adrenal axis dysfunction may underlie both arterial stiffness44,45 and depression.46,47 Finally, behavioural risk factors, such as high alcohol intake, smoking and physical inactivity, are bidirectionally associated with depression3,48–50 and may also lead to arterial stiffening,51–53 thus confounding the association. However, in our study additional adjustments for alcohol use, smoking and physical activity did not materially alter the results.
Limitations
The strengths of this study include its large sample size; the large number of participants with depression; and the advanced phenotyping, including the use of the gold standard method to assess aortic stiffness, the assessment of depression with the help of a questionnaire as well as a diagnostic interview, and the measurement of many potential confounding factors that could be accounted for in the fully adjusted models. However, owing to the cross-sectional design, reversed causation cannot be ruled out. For example, treatment for MDD has been shown to improve arterial stiffness.54 Also, aortic stiffness has been associated with current, but not remitted, depression.9 Additionally, misclassification of MDD could have occurred. Most participants who used antidepressants (87%) did not have MDD. We could not discriminate between antidepressants prescribed for anxiety, depression, or neuropathic pain, as indications were not recorded. Yet, the exclusion of participants who used antidepressants (n = 196) or additional adjustments for antidepressant use did not alter our results. Selection bias may have further influenced our results, as participants with severe MDD were unlikely to be included in the study. Therefore, our results may be an underestimation of the actual association. Aditionally, the individuals with missing data on covariates were excluded in the final models. However, the percentage of missing data was fairly small (4%), therefore imputation of missing data was unlikely to influence the results.55 Furthermore, in participants older than 60 years, competing risk could have resulted in an underrepresentation of depression cases, as participants with greater aortic stiffness are at increased risk for fatal CVD. Although we adjusted for multiple confounders, residual confounding due to other chronic medical conditions, such as asthma, chronic obstructive pulmonary disease, cancer, or arthritis, which may be comorbid to depression56 and which could lead to arterial stiffening, cannot be ruled out. Finally, the association between CVD markers and depression may differ according to race;57–60 however, our study population consisted mainly of white people, therefore we could not investigate such effects.
Conclusion
Our study shows that greater aortic stiffness is associated with MDD and depressive symptoms in middle-aged men and to a lesser extent in middle-aged women, whereas this association was not observed in old age.
References
- 1.Van Dooren FEP, Nefs G, Schram MT, et al. Depression and risk of mortality in peope with diabetes mellitus: a systematic review and meta-analysis. PLoS ONE. 2013;8:1210–25. doi: 10.1371/journal.pone.0057058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan A, Sun Q, Okereke OI, et al. Depression and risk of stroke morbidity and mortliaty: a meta-analysis and systematic review. JAMA. 2011;306:1241–9. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J. 2006;27:2763–74. doi: 10.1093/eurheartj/ehl338. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF. Arterial stiffness and hypertension. Hypertension. 2014;64:13–8. doi: 10.1161/HYPERTENSIONAHA.114.00921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Meyers BS, Young RC, et al. Vascular depression hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan KR, Hays JC, Blazer DG. MRI-defined vascular depression. Am J Psychiatry. 1997;154:497–501. doi: 10.1176/ajp.154.4.497. [DOI] [PubMed] [Google Scholar]
- 7.Van Sloten TT, Protogeru AD, Henry RM, et al. Association between arterial stiffness, cerebral small vessel disease and cognitive impairment: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;53:121–30. doi: 10.1016/j.neubiorev.2015.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paranthaman R, Greenstein AS, Burns AS, et al. Vascular function in older adults with depressive disorder. Biol Psychiatry. 2010;68:133–9. doi: 10.1016/j.biopsych.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 9.Seldenrijk A, van Hout HP, van Marwijk HW, et al. Depression, anxiety, and arterial stiffness. Biol Psychiatry. 2011;69:795–803. doi: 10.1016/j.biopsych.2010.12.034. [DOI] [PubMed] [Google Scholar]
- 10.Tiemeier H, Breteler MM, van Popele NM, et al. Late-life depression is associated with arterial stiffness: a population-based study. J Am Geriatr Soc. 2003;51:1105–10. doi: 10.1046/j.1532-5415.2003.51359.x. [DOI] [PubMed] [Google Scholar]
- 11.Van Sloten TT, Mitchell GF, Sigurdsson S, et al. Associations between arterial stiffness, depressive symptoms and cerebral small vessel disease: cross-sectional findings from the AGES-Reykjavik Study. J Psychiatry Neurosci. 2016;14:162–8. doi: 10.1503/jpn.140334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis TT, Sutto-Tyrell K, Penninx BW, et al. Race, psychosocial factors, and arortic pulse wave velocity: The Health, Aging, and Body Coposition Study. J Gerontol A Biol Sci Med Sci. 2010;65:1079–85. doi: 10.1093/gerona/glq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan A, Sun Q, Okereke OI, et al. Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA. 2011;306:1241–9. doi: 10.1001/jama.2011.1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salaycik KJ, Kelly-Hayes M, Beiser A, et al. Depressive symptoms and risk of stroke: the Framingham Study. Stroke. 2007;38:16–21. doi: 10.1161/01.STR.0000251695.39877.ca. [DOI] [PubMed] [Google Scholar]
- 15.Franklin SS, Gustin W, Wong ND, et al. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–15. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- 16.Segers P, Rietzschel ER, De Buyzere ML, et al. Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle-aged men and women. Hypertension. 2007;49:1248–55. doi: 10.1161/HYPERTENSIONAHA.106.085480. [DOI] [PubMed] [Google Scholar]
- 17.Weissman MM, Bland R, Joyce PR, et al. Sex differences in rates of depression: cross-national perspectives. J Affect Disord. 1993;29:77–84. doi: 10.1016/0165-0327(93)90025-f. [DOI] [PubMed] [Google Scholar]
- 18.Schram MT, Sep SJ, van der Kallen CJ, et al. The Maastricht Study: an extensive phenotyping study on determinants of type 2 diabetes, its complications and its comorbidities. Eur J Epidemiol. 2014;29:439–51. doi: 10.1007/s10654-014-9889-0. [DOI] [PubMed] [Google Scholar]
- 19.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30:445–8. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 20.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 21.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th edition. Washington (DC): APA; 2000. [Google Scholar]
- 22.Korten NC, Comijs HC, Lamers F, et al. Early and late onset depression in young and middle aged adults: Differential symptomatology, characteristics and risk factors? J Affect Disord. 2012;138:259–67. doi: 10.1016/j.jad.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen EP, Köhler S, Stehouwer CD, et al. The Patient Health Questionnaire-9 as a screening tool for depression in individuals with type 2 diabetes mellitus: The Maastricht Study. J Am Geriatr Soc. 2016;64:e201–6. doi: 10.1111/jgs.14388. [DOI] [PubMed] [Google Scholar]
- 25.Leng GC, Fowkes FG. The Edinburgh Claudication Questionnaire: an improved version of the WHO/Rose Questionnaire for use in epidemiological surverys. J Clin Epidemiol. 1992;45:1101–9. doi: 10.1016/0895-4356(92)90150-l. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva (Switzerland): WHO; 2006. [accessed 2017 Oct 3]. Available: www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf. [Google Scholar]
- 27.Sachdev PS, Parslow RA, Lux O, et al. Relationship of homocysteine, folic acid and vitamin B12 with depression in a middle-aged community sample. Psychol Med. 2005;35:529–38. doi: 10.1017/s0033291704003721. [DOI] [PubMed] [Google Scholar]
- 28.Schram MT, Henry RM, van Dijk RA, et al. Increased central artery stiffness in impaired glucose metabolism and type 2 diabetes: the Hoorn Study. Hypertension. 2004;43:176–81. doi: 10.1161/01.HYP.0000111829.46090.92. [DOI] [PubMed] [Google Scholar]
- 29.Mezuk B, Eaton WW, Albrecht S, et al. Depression and type 2 diabetes over the lifespan: a meta-analysis. Diabetes Care. 2008;31:2383–90. doi: 10.2337/dc08-0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JM, Stewart R, Sung-Wan K, et al. Vascular risk factors and incident late-life depression in a Korean population. Br J Psychiatry. 2006;189:26–30. doi: 10.1192/bjp.bp.105.015032. [DOI] [PubMed] [Google Scholar]
- 31.Robinson RG, Jorge RE. Post-stroke depression: a review. Am J Psychiatry. 2015;173:221–31. doi: 10.1176/appi.ajp.2015.15030363. [DOI] [PubMed] [Google Scholar]
- 32.Hess DC, D’Cruz IA, Adams RJ, et al. Coronary artery disease, myocardial infarction, and brain embolism. Neurol Clin. 1993;11:399–417. [PubMed] [Google Scholar]
- 33.Witt BJ, Brown RD, Jr, Jacobsen SJ, et al. A community-based study of stroke incidence after myocardial infarction. Ann Intern Med. 2005;143:785–92. doi: 10.7326/0003-4819-143-11-200512060-00006. [DOI] [PubMed] [Google Scholar]
- 34.Gordon JL, Girdler SS. Hormone replacement therapy in the treatment of perimenopausal depression. Curr Psychiatry Rep. 2014;16:517–25. doi: 10.1007/s11920-014-0517-1. [DOI] [PubMed] [Google Scholar]
- 35.Mitchell GF, van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility — Reykjavik study. Brain. 2011;134:3398–407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chappell DC, Varner SE, Nerem RM, et al. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–9. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 37.Shen Q, Goderie SK, Jin L, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–40. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 38.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–60. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 39.Hoth KF, Tate DF, Poppas A, et al. Endothelial function and white matter hyperintensities in older adults with cardiovascular disease. Stroke. 2007;38:308–12. doi: 10.1161/01.STR.0000254517.04275.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lemke H, de Castro AG, Schlattmann P, et al. Cerebrovascular reactivity over time-course — from major depressive disorder to remission. J Psychiatr Res. 2010;44:132–6. doi: 10.1016/j.jpsychires.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Dantzer R, O’Conner JC, Freund GG, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:45–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alexopoulos GS, Morimoto SS. The inflammation hypothesis in geriatric depression. Int J Geriatr Psychiatry. 2011;26:1109–18. doi: 10.1002/gps.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Ittersum FJ, Schram MT, van der Heijden-Spek JJ, et al. Autonomic nervous function, arterial stiffness and blood pressure in patients with Type I diabetes mellitus and normal urinary albumin excretion. J Hum Hypertens. 2004;18:761–8. doi: 10.1038/sj.jhh.1001751. [DOI] [PubMed] [Google Scholar]
- 45.Himeno A, Satoh-Asahara N, Usui T, et al. Salivary cortisol levels are associated with outcomes of weight reduction therapy in obese Japanese patients. Metabolism. 2012;61:255–61. doi: 10.1016/j.metabol.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 46.Rottenberg J. Cardiac vagal control in depression: a critical analysis. Biol Psychol. 2007;74:200–11. doi: 10.1016/j.biopsycho.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Bremmer MA, Deeg DJ, Beekman AT, et al. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry. 2007;62:479–86. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 48.Luijendijk HJ, Stricker BH, Hofman A, et al. Cerebrovascular risk factors and incident depression in community-dwelling elderly. Acta Psychiatr Scand. 2008;118:139–48. doi: 10.1111/j.1600-0447.2008.01189.x. [DOI] [PubMed] [Google Scholar]
- 49.Chang SC, Pan A, Kawachi I, et al. Risk factors for late-life depression: a prospective cohort study among older women. Prev Med. 2016;91:144–51. doi: 10.1016/j.ypmed.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adamson BC, Yang Y, Motl RW. Association between compliance with physical activity guidelines, sedentary behavior and depressive symptoms. Prev Med. 2016;91:152–7. doi: 10.1016/j.ypmed.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 51.Saini S, Saxena Y, Gupta R. Arterial compliance and autonomic functions in adult male smokers. J Clin Diagn Res. 2016;10:12–6. doi: 10.7860/JCDR/2016/19547.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kesse-Guyot E, Vergnaud AC, Fezeu L, et al. Associations between dietary patterns and arterial stiffness, carotid artery intima-media thickness and atherosclerosis. Eur J Cardiovasc Prev Rehabil. 2010;17:718–24. doi: 10.1097/HJR.0b013e32833a197f. [DOI] [PubMed] [Google Scholar]
- 53.Endes S, Schaffner E, Caviezel S, et al. Long-term physical activity is associated with reduced arterial stiffness in older adults: longitudinal results of the SAPALDIA cohort study. Age Ageing. 2016;45:110–5. doi: 10.1093/ageing/afv172. [DOI] [PubMed] [Google Scholar]
- 54.Oulis P, Kouzoupis A, Kyrkou K, et al. Reversal of increased arterial stiffness in severely depressed women after 6-week antidepressant treatment. J Affect Disord. 2010;122:164–6. doi: 10.1016/j.jad.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- 56.Watkins DC, Assari S, Johnson-Lawrence V. Race and ethnic group differences in comorbid major depressive disorder, generalized anxiety disorder, and chronic medical conditions. J Racial Ethn Health Disparities. 2015;2:385–94. doi: 10.1007/s40615-015-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assari S. Race and ethnic differences in additive and multiplicative effects of depression and anxiety on cardiovascular risk. Int J Prev Med. 2016;7:22. doi: 10.4103/2008-7802.173931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Case SM, Stewart JC. Race/ethnicity moderates the relationship between depressive symptom severity and C-reactive protein: 2005–2010 NHANES data. Brain Behav Immun. 2014;41:101–8. doi: 10.1016/j.bbi.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Lewis TT, Guo H, Lunos S, et al. Depressive symptoms and cardiovascular mortality in older black and white adults: evidence for a differential association by race. Circ Cardiovasc Qual Outcomes. 2011;4:293–9. doi: 10.1161/CIRCOUTCOMES.110.957548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Assari S, Sonnega A. Racial differences in the predictive role of high depressive symptoms on incident heart disease over 18 years: results from the Health and Retirement Study. Rev Cardiovasc Med. 2017;6:e34767. [Google Scholar]