Abstract
Background
Higher body mass index (BMI) and obesity is common among youth with bipolar disorder (BD) and is associated with greater psychiatric illness severity, including suicidality. Obesity has been associated with frontal, temporal and subcortical volumetric reductions in adults with BD. We examined the neurostructural correlates of BMI in adolescents early in their course of BD.
Methods
We processed T1-weighted images of adolescents with BD and psychiatrically healthy controls using FreeSurfer to derive a priori region of interest (ROI) volumes/cortical thickness for the frontal lobe (FL), prefrontal cortex (PFC) and orbitofrontal cortex (OFC) as well as volumes for the amygdala and hippocampus. General linear models assessed the association between BMI and the ROIs, controlling for age, sex and intracranial volume. We also conducted exploratory within-BD group and whole brain vertex-wise analyses.
Results
We included 40 adolescents with BD and 48 controls in our analyses. In addition to a main effect of BMI on the ROIs, there were significant diagnosis × BMI interaction effects on FL volumes. In the BD group only, BMI was negatively associated with FL, OFC and PFC cortical thickness. Whole brain analysis of BMI–volume correlations revealed 2 significant interaction clusters: 1 in the medial OFC and 1 in the caudal anterior cingulate cortex, with BD showing a stronger negative correlation.
Limitations
Reliance on BMI rather than a more nuanced measure of obesity may have influenced the findings.
Conclusion
Our results suggest that elevated BMI among adolescents with BD is associated with frontal neurostructural differences that are not observed in controls. Prospective studies examining the direction of the observed associations and the effect of BMI optimization on brain structure in adolescents with BD are warranted.
Introduction
Overweight and obesity are common among youth and adults with bipolar disorder (BD) in clinical as well as in largely untreated epidemiologic samples.1–6 Elevated body mass index (BMI) is associated with hypertension, diabetes, certain types of cancer and cardiovascular disease (CVD).7–9 The latter is particularly concerning because CVD is excessively prevalent and occurs prematurely in individuals with BD,4,10 recently leading the American Heart Association to position BD as an illness that predisposes youth to premature CVD and atherosclerosis.11
In addition to its adverse physical health correlates, obesity has been associated with a more pernicious course of BD in adults, including increased frequency of mood episodes, increased treatment resistance, poor cognitive function and increased suicidality.2,12–14 Similar to findings in adults with BD, overweight/obesity in adolescents with BD has been associated with greater prevalence of substance use disorder, self-injurious behaviour, suicidality, neurocognitive dysfunction and greater number of psychiatric hospital admissions.2,6,15,16 Recent general population data from the United States indicated that although overweight/obesity was not more common among adolescents with BD than in other adolescents, in this largely untreated sample it was nonetheless associated with indicators of BD severity, such as suicide attempts and psychiatric hospital admission.5 Nevertheless, the brain phenotypes and the putative neurobiological mechanisms underlying the link between obesity and increased illness burden are relatively unknown.
The neurostructural correlates of BMI and other proxies of obesity (e.g., waist circumference and total body fat) have been studied and reviewed extensively in the general population.17 In youth, BMI is most consistently associated with reduced grey matter volume in the frontal lobe (FL), specifically in the prefrontal cortex (PFC) and orbitofrontal cortex (OFC).17–22 There is also evidence of reduced volume of medial temporal lobe (MTL) structures, such as the hippocampus and parahippocampal gyrus in overweight and obese adolescents.23 The FL regions are of particular relevance to BD because they are associated with higher-level cognitive processes, such as executive functioning, decisionmaking and inhibitory control, and are also known to modulate emotion processing.17,24
Bipolar disorder is also associated with differences in brain structure. A recent-meta analysis established that adolescents with BD have smaller amygdalas than healthy controls.25 Smaller hippocampal volumes as well as reduced grey matter and thinner cortex in the PFC have also been reported in youth with BD.26–28 In adults, a recent systematic review of studies examining cortical thickness showed that BD was associated with cortical thinning, predominantly in the left anterior cingulate cortex, left superior temporal gyrus and bilateral PFC.29 In contrast, a meta-analysis of studies examining regional volumes in adults concluded that, besides right lateral ventricular enlargement, no other regional volumes were significantly different in individuals with BD.30 Overlapping findings between youth and adult cortical thickness studies, but mixed findings between youth and adult volumetric studies, suggest volume and cortical thickness may follow different neurodevelopmental trajectories.
Despite the large number of brain structure studies examining obesity and BD independently, investigation of the association between obesity and BD from a neuroimaging perspective is limited. One group recently reported voxel-based morphometry (VBM) findings that higher BMI in patients with BD was associated with reduced grey matter and white matter volume in clusters within the frontal, temporal and subcortical limbic structures in contrast to healthy-weight controls who showed reduced occipital lobe grey matter only,31,32 but this topic has not yet been addressed in adolescents. In addition to the importance of examining this topic in adolescents from a developmental perspective, focusing on adolescents offers the advantage of reduced confounding variables, as adolescents have shorter duration of BD-related symptoms, stressors and treatments and less impact of age-related neurodegeneration and chronic diseases of aging.
The purpose of this study was to ascertain the association between BMI and brain structure (volume and cortical thickness) of regions of interest (ROIs) selected a priori among adolescents with and without BD. The selected ROIs have been implicated in both BD and obesity and include the cortical (FL, PFC, and OFC) and subcortical (amygdala and hippocampus) regions. Of note, for cortical ROIs, we examined both volume and cortical thickness as separate measures.
We hypothesized that there would be a negative main effect of BMI in predicting ROI measures. We also hypothesized that there would be a group × BMI interaction effect in predicting ROI measures such that the negative correlation between BMI and ROI measures would be greater in adolescents with BD than in psychiatrically healthy control participants.
Methods
Participant selection
This study included adolescents with BD (type I, II or not otherwise specified [NOS]) and psychiatrically healthy controls between the ages of 13 and 20 years. We recruited participants with BD primarily from within the Centre for Youth Bipolar Disorder (CYBD), a subspecialty clinical research program at Sunnybrook Health Sciences Centre in Toronto, Canada. To be included, they had to meet DSM-5 diagnostic criteria for BD (I, II, or NOS). Briefly, BD-I requires at least 1 episode of mania (severe elation or irritability accompanied by other symptoms), usually alternating with episodes of depression, whereas BD-II involves milder hypomanic episodes alternating with periods of depression.
We used operationalized criteria for BD-NOS based on the Course and Outcome of Bipolar Youth (COBY) study:33 individuals with BD-NOS had at least 4 lifetime days with at least 4 hours of either elated mood with the addition of 2 associated DSM-5 manic symptoms or irritable mood with the addition of 3 associated DSM-5 manic symptoms, with a clear change in functioning. We recruited control participants from the community via advertisements (newspaper ads, local print media) on public transit and in institutions in the Greater Toronto Area. To be included, they had to have no history of major psychiatric disorders (i.e., no lifetime mood or psychotic disorders, no alcohol or drug dependence in the past 3 months and no anxiety disorders in the past 3 months) and no family history of BD or psychosis (first or second-degree relatives). Psychiatric diagnoses were assessed using the Schedule for Affective Disorders and Schizophrenia for School Aged Children (6–18 Years) — Present and Lifetime version (K-SADS-PL), a semistructured interview tool intended to obtain current episode and lifetime history of psychiatric disorders.34
The KSADS Depression Rating Scale (DRS)35 and KSADS Mania Rating Scale (MRS)36 were used as measures of depressive and hypomanic symptom severity, respectively. Individuals were classified as hypomanic if they had an MRS score of 12 or higher, depressed if they had a DRS score of 13 or higher, mixed if both scores were above threshold, or euthymic if both scores were below threshold.
Participants were excluded from this study if they were unable to give informed consent (e.g., severe mania or psychosis or unable to speak English); had a pre-existing cardiac condition, autoimmune illness, or inflammatory illness; were taking any anti-inflammatory, anti-platelet, anti-lipidemic, anti-hypertensive or hypoglycemic agents (including insulin and metformin); had an infectious disease within the past 14 days; had any contraindications for MRI (e.g., cardiac pacemaker, metal implant in the body); had a health condition or physiologic impairment that prohibited intense exercise; or had any neurologic abnormalities or severe cognitive impairment (e.g., autism).
Anthropometric data
We calculated BMI as weight in kilograms divided by height in square metres. We calculated BMI percentile and BMI z-score based on age and sex for participants under the age of 20 years using standardized growth charts from the Centres for Disease Control and Prevention (CDC; www.cdc.gov/healthyweight/assessing/bmi/). Weight measurements were adjusted as follows: −1.3 kg if the participant was wearing long pants and a long-sleeved shirt, −1.1 kg if wearing short pants or short sleeves, and −0.9 kg if wearing both short sleeves and short pants. Waist circumference was measured using a standard measuring tape.
Image acquisition and processing
We collected MRI data with a 3 T Philips Achieva system using body coil transmission and an 8-channel head receiver coil. Structural images were acquired via T1-weighted high-resolution fast-field echo imaging with the following parameters: repetition time (TR) 9.5 ms, echo time (TE) 2.3 ms, inversion time (TI) 1400 ms, spatial resolution 0.94 × 1.17 × 1.2 mm, 256 × 164 × 140 matrix, flip angle 8°, scan duration 8 minutes 56 seconds. Three-dimensional T1-weighted images quantified grey matter and white matter using a single slab Fast Field Echo sequence with the following parameters: 140 slices, TE 2.3 ms, TR 9.5 ms and flip angle 8°, field of view 240 × 191 mm2, acquisition time 8 minutes 56 seconds.
Two independent raters blinded to diagnosis (A.H.I. and A.W.M.) visually inspected T1-weighted images of all participants for motion and image artifacts before preprocessing. A score between 0 and 3 was given for each T1 image with respect to quality. Raters were trained to score based on overall image quality (i.e., graininess, contrast between white matter and grey matter, and number of artifacts due to excessive movement while in the scanner). In cases where there were incongruent scores between raters, images were inspected a second time and consensus was reached following discussion. We used the Cohen κ test to measure the level of agreement between visual inspection ratings.37 T1-weighted images with a score of 3 (i.e., poor quality) were excluded from the data set before being processed in Free-Surfer.38 In total, we excluded 6 T1 images from the data set (2 in the BD group and 4 in the control group).
T1-weighted images for individual participants were processed into surface-based structural data using the automated reconstruction function in the image analysis suite FreeSurfer version 5.3.0 (http://surfer.nmr.mgh.harvard.edu/).39 In brief, the automated preprocessing included intensity normalization,40 registration to Montreal Neurological Institute (MNI) space and automated skull stripping.41 Automated parcellation38,42 proceeded with cortical surface reconstruction, including generation of binary white matter masks in 2 hemispheres, which were used to produce a tessellated mesh of the white matter surface, smoothed to remove voxel-based effects and corrected for topological defects.43 White matter and pial surfaces were then extracted and spherically inflated to be registered to a canonical template.44 Finally, using a parcellation algorithm, the individual brains were mapped to the Desikan–Killiany probabilistic cortical atlas45 based on anatomic landmarks and cortical geometry to assign gyral labels of interest.38 Individual participants’ cortical thickness, surface area and volume were then extracted directly from FreeSurfer. Automated segmentation of subcortical volumes occurred independently in a separate stream and involved nonlinear registration to the MNI305 probabilistic atlas to label the various subcortical structures.42
We created ROI volumes by summing individual gyral labels from the Desikan–Killiany atlas.45 The PFC included the rostral and caudal middle frontal, caudal anterior cingulate, and superior frontal gyral labels. The OFC consisted of medial and lateral orbitofrontal gyral labels. The FL was defined as all cortical labels anterior to the central sulcus, including the superior frontal, rostral and caudal middle frontal regions; pars opercularis; pars triangularis; pars orbitalis; and lateral, medial orbitofrontal, precentral, paracentral and frontal poles. Despite overlapping with the PFC and OFC, the FL was considered separately for reference to prior literature.17,24 We calculated mean cortical thickness for each cortical ROI using the surface area of the component regions as a weighting factor. For example, mean OFC thickness = [(lateral orbitofrontal area × lateral orbitofrontal thickness) + (medial orbitofrontal area × medial orbitofrontal thickness)] ÷ (lateral + medial orbitofrontal area).
Statistical analysis
We assessed normality of all continuous variables using the Shapiro–Wilks test. Nonparametric tests were used for nonnormally distributed variables. Continuous variables were compared between groups using independent-samples t tests and Mann–Whitney U tests. We examined group differences in categorical variables using χ2 tests. Effect sizes were reported as Cohen d for continuous variables and Cramer V for categorical variables. The general linear models (GLMs) included the ROIs as the outcome variable, diagnosis as the fixed factor, BMI as the continuous predictor variable of interest, and age and sex as covariates; we included intracranial volume (ICV) as an additional covariate if ROI volume was the outcome variable. When testing the interaction between diagnosis and BMI, the GLM included a diagnosis × BMI interaction term. We used η2, which measures the proportion of the total variance in a dependent variable that is explained by a given independent variable, 46 to measure the effect size of independent variables in the GLMs. Sensitivity analyses further controlled for medication, psychiatric comorbidities and systolic blood pressure.
We tested medication effects for 4 different types of medication: lithium, second-generation antipsychotics, stimulants and antidepressants (selective serotonin reuptake inhibitors [SSRIs] and non-SSRIs). For comorbidity, we tested 4 separate diagnosis effects: attention-deficit/hyperactivity disorder (ADHD), anxiety, substance use disorder (SUD) and oppositional defiant disorder (ODD). In these models, the medications/comorbidities were categorical variables, and we tested their interaction with BMI while including main effects in the model. We controlled for systolic blood pressure in the GLMs as an additional continuous covariate. Secondary analyses using waist circumference instead of BMI were also conducted. We considered results to be significant at p < 0.05. All statistical tests were performed using SPSS software version 22 (IBM Inc.). The Benjamini–Hochberg false discovery rate (FDR) method was used to correct for multiple comparisons.47
We calculated residualized ROI volume/thickness by running a linear regression with the outcome variable (i.e., ROI volume/thickness) as the dependent variable and only the covariates (i.e., age, sex, ICV) as the independent variables and saving the unstandardized residuals. Similarly, residualized BMI was calculated by running linear regression with BMI as the dependent variable and only the covariates (i.e., age, sex, ICV) as the independent variables and saving the unstandardized residuals. Residualized ROI versus residualized BMI plots were rendered to show fit lines without effect of nuisance covariates.
To conduct the whole-brain exploratory analyses, we used the QDEC (query, design, estimate, contrast) tool included in the FreeSurfer package.48 We elected to use a surface-based smoothing with a full-width at half-maximum of 15 mm based on previous literature.49 We performed vertex to vertex contrasts of cortical volume for controls versus patients with BD. For this comparison, we generated an average normal control surface, and volume data from each participant were mapped to this average surface and smoothed. Finally, each contrast was entered into an analysis of covariance GLM design matrix, which included diagnosis, ICV, sex and age as covariates and BMI as our continuous variable of interest. Results were thresholded at a surface-wide (i.e., primary) threshold of p < 0.05. We corrected for multiple comparisons with permutation testing with Monte Carlo simulation and cluster analysis, as implemented in Free-Surfer 5.3. In light of prior findings regarding sex differences in the neuroanatomy of BD among adults,50 we conducted an exploratory sex × diagnosis × BMI interaction for clusters identified in our whole brain diagnosis × BMI analyses.
Results
Demographic and clinical characteristics between groups
We included 40 adolescents with BD and 48 controls in our analyses. Compared with controls, participants with BD were older (p = 0.001); had a higher BMI (p = 0.001), increased waist circumference (p = 0.002) and more psychiatric comorbidities (all p < 0.05); and were more likely to have a family history of psychiatric illness (p = 0.003). Participants with BD did not differ from controls on other baseline characteristics (Table 1).
Table 1.
Demographic and clinical characteristics of study participants
| Group; mean ± SD or no. (%) | |||||
|---|---|---|---|---|---|
|
|
|||||
| Characteristic | BD (n = 40) | Control (n = 48) | Statistical test | Effect size | p value |
| Age, yr | 17.03 ± 1.44 | 15.85 ± 1.74 | U = 572.5 | d = 0.739 | 0.001 |
| Female sex | 24 (60) | 25 (52) | χ2 = 0.554 | V = 0.079 | 0.46 |
| Socioeconomic status | 52.35 ± 12.38 | 52.98 ± 10.31 | t = −0.26 | d = 0.051 | 0.80 |
| BD subtype | |||||
| BD-I | 14 (35) | — | — | — | — |
| BD-II | 14 (35) | — | — | — | — |
| BD-NOS | 12 (30) | — | — | — | — |
| Waist circumference | 82.15 ± 9.48 | 74.62 ± 6.97 | U = 317 | d = 0.905 | 0.001 |
| Adjusted BMI | 24.44 ± 4.32 | 21.22 ± 2.98 | U = 470 | d = 0.868 | < 0.001 |
| Resting systolic BP | 112.68 ± 12.84 | 109.85 ± 17.13 | t = 0.86 | d = 0.187 | 0.40 |
| Resting diastolic BP | 68.58 ± 7.81 | 68.07 ± 10.00 | t = 0.26 | d = 0.057 | 0.80 |
| Lifetime tobacco use | 6 (25) | 0 (0) | χ2 = 4.70 | V = 0.389 | 0.030 |
| Lifetime comorbid diagnoses | |||||
| ADHD | 17 (44) | 5 (10) | χ2 = 11.78 | V = 0.372 | 0.001 |
| Anxiety | 29 (73) | 2 (4) | χ2 = 43.88 | V = 0.710 | < 0.001 |
| SUD | 9 (23) | 0 (0) | χ2 = 12.03 | V = 0.370 | < 0.001 |
| ODD | 12 (30) | 0 (0) | χ2 = 16.67 | V = 0.435 | < 0.001 |
| CD | 3 (8) | 0 (0) | χ2 = 3.73 | V = 0.206 | 0.05 |
| Family history* | |||||
| Mania/hypomania | 22 (55) | — | — | — | — |
| Depression | 27 (68) | 9 (19) | χ2 = 21.45 | V = 0.494 | < 0.001 |
| Anxiety | 22 (55) | 9 (19) | χ2 = 12.57 | V = 0.378 | < 0.001 |
| ADHD | 9 (23) | 1 (2) | χ2 = 9.03 | V = 0.320 | 0.003 |
| Medications | |||||
| Second-generation antipsychotics | 21 (53) | — | — | — | — |
| Lithium | 8 (20) | — | — | — | — |
| SSRIs | 11(28) | — | — | — | — |
| Non-SSRI antidepressants | 4 (10) | — | — | — | — |
| Stimulants | 9 (23) | — | — | — | — |
ADHD = attention-deficit/hyperactivity disorder; BD = bipolar disorder; BMI = body mass index; BP = blood pressure; CD = conduct disorder; NOS = not otherwise specified; ODD = oppositional defiant disorder; SD = standard deviation; SSRI = selective serotonin reuptake inhibitor; SUD = substance use disorder.
First- or second-degree relatives.
Clinical characteristics
In the BD sample, there were 6 (15%) hypomanic, 11 (27.5%) depressed, 9 (22.5%) mixed and 14 (35%) euthymic individuals. The average mania score was 10.46 ± 9.76 and the average depression score was 15.89 ± 12.37. The average duration of illness was 2.35 ± 1.96 years.
Main effect of BMI on ROIs in the whole sample
In the entire sample, BMI was negatively correlated with mean FL (η2p = 0.078, pFDR = 0.006), PFC (η2p = 0.086, pFDR = 0.009) and OFC (η2p = 0.107, pFDR = 0.009) cortical thickness after controlling for covariates. No other regions were significant (all pFDR > 0.62. The effects of BMI on each ROI are reported in Table 2.
Table 2.
Main effect of body mass index on region of interest measures in the whole sample
| Region of interest | η2 | t | p value | pFDR |
|---|---|---|---|---|
| FL volume | 0.004 | −0.60 | 0.55 | 0.55 |
| PFC volume | 0.012 | −0.988 | 0.33 | 0.62 |
| OFC volume | 0.007 | −0.74 | 0.46 | 0.62 |
| Hippocampus volume | 0.002 | 0.39 | 0.70 | 0.70 |
| Amygdala volume | 0.020 | 1.31 | 0.19 | 0.62 |
| FL Thickness | 0.078 | −2.67 | 0.009 | 0.009 |
| PFC Thickness | 0.086 | −2.81 | 0.006 | 0.009 |
| OFC Thickness | 0.107 | −3.17 | 0.002 | 0.006 |
FDR = false discovery rate; FL = frontal lobe; OFC = orbitofrontal cortex; PFC = prefrontal cortex.
Interaction between diagnosis and BMI
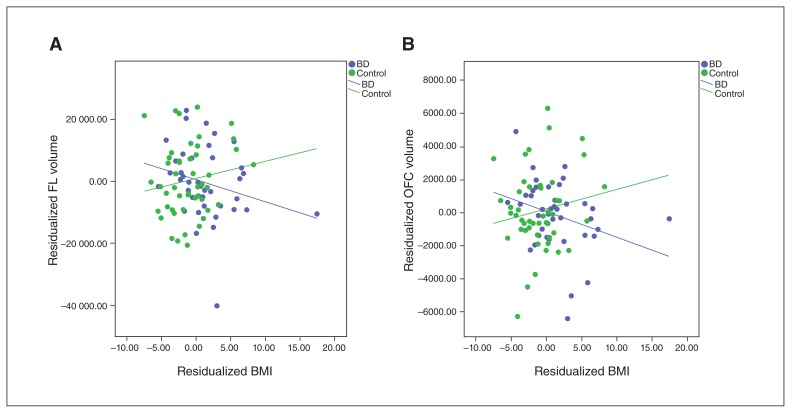
The diagnosis × BMI interaction effects on ROI measures are presented in Table 3. The interaction effect was significant for FL volume (η2p = 0.062, pFDR = 0.023), indicating that the effect of BMI was different for participants with BD than controls; OFC volume showed a nonsignificant trend toward an interaction effect (η2p = 0.072, pFDR = 0.056). No other regions had significant interaction effects (pFDR > 0.106). Figure 1A and B present corrected scatterplots of residualized BMI versus residualized FL and OFC volumes, respectively. Appendix 1, Figure S2a and b, available at jpn.ca/170041-a1, shows scatterplots of raw BMI versus raw FL and OFC volumes, respectively.
Table 3.
Effect of diagnosis × body mass index interaction on region of interest measures
| Region of interest | η2 | t | p value | pFDR |
|---|---|---|---|---|
| FL volume | 0.062 | −2.32 | 0.023 | 0.023 |
| PFC volume | 0.026 | −1.48 | 0.14 | 0.28 |
| OFC volume | 0.072 | −2.50 | 0.014 | 0.06 |
| Hippocampus volume | 0.007 | 0.77 | 0.45 | 0.60 |
| Amygdala volume | 0.001 | −0.26 | 0.80 | 0.80 |
| FL Thickness | 0.034 | −1.69 | 0.10 | 0.11 |
| PFC Thickness | 0.032 | −1.64 | 0.11 | 0.11 |
| OFC Thickness | 0.040 | −1.86 | 0.07 | 0.11 |
FDR = false discovery rate; FL = frontal lobe; OFC = orbitofrontal cortex; PFC = prefrontal cortex.
Fig. 1.
Diagnosis × body mass index (BMI) interaction on frontal lobe (FL) volume and orbitofrontal cortex (OFC) volume. (A) Residualized BMI versus residualized FL volume. (B) Residualized BMI versus residualized OFC volume.
Main effect of BMI on ROI measures within the BD group
In the BD group, BMI was negatively correlated with FL (η2p = 0.137, pFDR = 0.022), PFC (η2p= 0.154, pFDR = 0.015) and OFC (η2p = 0.239, pFDR = 0.006) cortical thickness and showed a trend toward negative correlation for FL (η2p = 0.081, pFDR = 0.087) and OFC (η2p = 0.149, pFDR = 0.072) volume. There were no significant correlations between BMI and any of the ROIs in the control group (pFDR > 0.144). Simple main effects of BMI on ROI measures for patients with BD and controls are presented in Table 4 and Table 5, respectively.
Table 4.
Main effect of body mass index on region of interest measures in adolescents with bipolar disorder
| Region of interest | η2 | t | p value | pFDR |
|---|---|---|---|---|
| FL volume | 0.081 | −1.76 | 0.09 | 0.09 |
| PFC volume | 0.044 | −1.27 | 0.21 | 0.43 |
| OFC volume | 0.149 | −2.49 | 0.018 | 0.07 |
| Hippocampus volume | 0.015 | 0.73 | 0.47 | 0.47 |
| Amygdala volume | 0.016 | 0.75 | 0.46 | 0.47 |
| FL Thickness | 0.137 | −2.39 | 0.022 | 0.022 |
| PFC Thickness | 0.154 | −2.56 | 0.010 | 0.015 |
| OFC Thickness | 0.239 | −3.36 | 0.002 | 0.006 |
FDR = false discovery rate; FL = frontal lobe, OFC = orbitofrontal cortex, PFC = prefrontal cortex.
Table 5.
Main effect of body mass index on region of interest measures in psychiatrically healthy controls
| Region of interest | η2 | t | p value | pFDR |
|---|---|---|---|---|
| FL volume | 0.049 | 1.49 | 0.14 | 0.14 |
| PFC volume | 0.014 | 0.78 | 0.44 | 0.59 |
| OFC volume | 0.041 | 1.36 | 0.18 | 0.59 |
| Hippocampus volume | 0.003 | −0.36 | 0.72 | 0.72 |
| Amygdala volume | 0.017 | 0.87 | 0.39 | 0.59 |
| FL Thickness | 0.001 | 0.25 | 0.80 | 0.99 |
| PFC Thickness | 0.000 | 0.02 | 0.99 | 0.99 |
| OFC Thickness | 0.002 | −0.26 | 0.80 | 0.99 |
FDR = false discovery rate; FL = frontal lobe, OFC = orbitofrontal cortex, PFC = prefrontal cortex.
Additional covariate analyses within the BD subgroup
To understand potential effects of medication and comorbidity in the regions where BMI effects differed between patients with BD and controls (i.e., FL and OFC volume), univariate GLMs testing the interaction between BMI and the aforementioned covariates were conducted within the BD group. As control analyses were exploratory, they were not corrected for multiple comparisons.
None of the classes of medication interacted with the BMI effect (all p > 0.17). The ADHD × BMI interaction effect was significant for FL (p = 0.042) and OFC (p = 0.011) volumes. Appendix 1, Figure S1, shows corrected scatterplots of the interaction indicating BMI effects on FL and OFC volumes for both ADHD and non-ADHD groups, with the largest effect of BMI for those with ADHD. No other interactions were found (all p > 0.14).
We controlled for systolic blood pressure and found that the diagnosis × BMI interaction remained significant for frontal lobe volume (p = 0.026).
Whole brain vertex-wise analysis
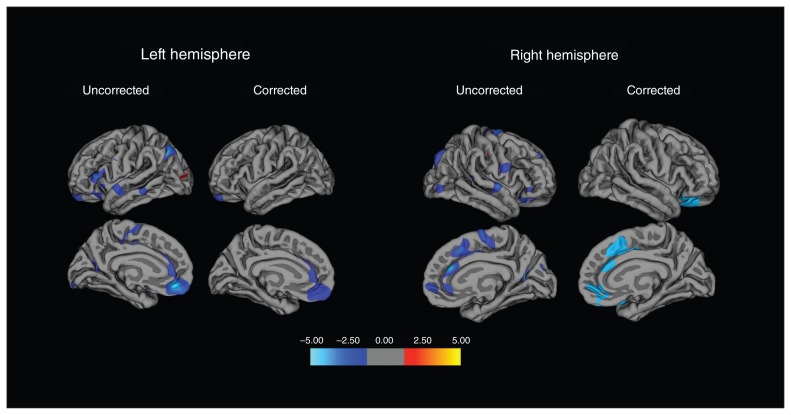
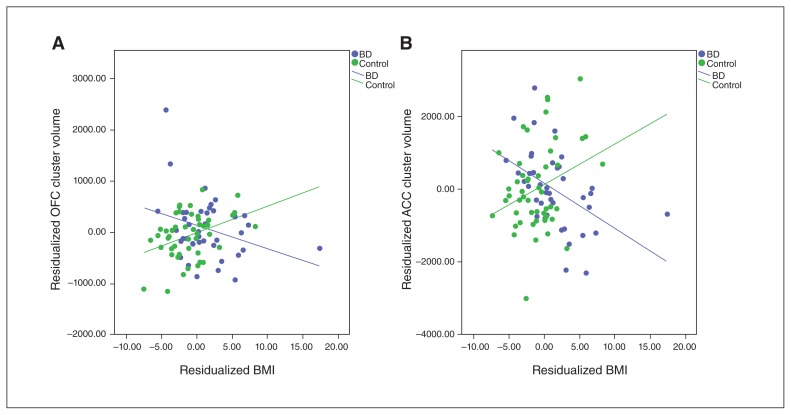
Whole brain analysis found significant interaction effects (i.e., diagnosis × BMI) in 2 clusters (Fig. 2). There was 1 cluster in the left hemisphere with a peak vertex in the medial OFC (cluster size 1544.41 mm2, cluster-wise p = 0.027, MNI coordinates: x, y, z = −7.8, 40.3, −15.8), extending to the rostral anterior cingulate cortex (ACC) as well as a small portion of the superior frontal gyrus. There was also a cluster in the right hemisphere with a peak vertex in the caudal ACC (cluster size 3197.35 mm2, cluster-wise p = 0.0001, Talairach coordinates: x, y, z = 13.3, 31.6, 17.7), extending to the superior frontal, rostral ACC, and both the lateral and medial OFC. Figure 3A and B show corrected scatter plots of the interaction indicating a greater negative correlation between BMI and volume in those clusters in patients with BD than in controls.
Fig. 2.
Whole brain vertex-wise analysis (bipolar disorder > control) of body mass index–cortical volume correlations. The colour scale indicates −log-p. Significant clusters were found within the left hemisphere, with peak vertex in the medial orbitofrontal cortex (OFC), encompassing the caudal anterior cingulate cortex (ACC), rostral ACC and superior frontal gyrus, as well as in the right hemisphere, with peak vertex in the caudal ACC, encompassing the superior frontal gyrus, rostral ACC, and medial and lateral OFC.
Fig. 3.
Diagnosis × body mass index (BMI) interaction clusters in left hemisphere (LH) and right hemisphere (RH). (A) Residualized BMI versus residualized orbitofrontal cortex cluster volume in the LH. (B) Residualized BMI versus residualized anterior cingulate cortex cluster volume in the RH. ACC = anterior cingulate corted; OFC = orbitofrontal cortex.
We found diagnosis × BMI × sex interactions in the medial OFC (p < 0.001) as well as in the caudal ACC (p < 0.001). In both clusters, the association between BMI and cortical volume was significantly more negative among male than female adolescents in the BD group; in contrast, this association was not observed in the control group.
Secondary analyses using waist circumference
There was a significant diagnosis × waist circumference interaction effect on OFC volume (p = 0.047). Within the BD group, waist circumference was negatively associated with OFC thickness (p = 0.027) and volume (p = 0.048). There were no significant associations between waist circumference and any of the ROIs in the control sample. There were no significant diagnosis × waist circumference interactions in whole brain analyses of either cortical thickness or volume. Overall, these findings converge with, but do not fully reflect, the BMI findings.
Interrater reliability statistics on T1 visual inspection ratings
Interrater reliability was κ = 0.68, indicating a moderate level of agreement.
Discussion
This study examined the association between BMI and brain structure in adolescents with and without BD. Our results show a significant diagnosis × BMI interaction effect for FL volume. Within-group analyses identified significant covariate-adjusted negative correlations between BMI and FL, PFC and OFC cortical thickness in the BD group but not the control group. Sensitivity analysis indicated an ADHD × BMI interaction effect on FL and OFC volumes in the BD group. Subsequent within-BD group analyses showed that there were stronger negative correlations between BMI and FL and OFC volumes in participants with BD with than without comorbid ADHD. Exploratory whole brain analyses showed that there were significant diagnosis × BMI interaction effects in clusters predominantly encompassing the ACC and the OFC. Specifically, the negative correlation between BMI and the volumes within those clusters were greater in participants with BD than in controls. These findings may point to a potential neurobiological mechanism linking obesity and poor health outcomes observed in patients with BD and may inform novel treatment approaches.
ROI analyses
Consistent with the study in adult BD,31 elevated BMI in our adolescent BD sample was associated with FL grey matter volume reductions. However, in contrast to the adult study, we did not observe any negative correlations between BMI and any of the subcortical limbic ROIs (i.e., amygdala or hippocampus). The present findings suggest that BMI may be implicated in prior findings of reduced FL volumes in youth with BD.51,52
There was a minor discrepancy between the significant diagnosis × BMI interaction for FL volume, contrasting solely with a main effect of BMI for FL thickness. However, there was a meaningful negative association between BMI and both FL volume and FL thickness in patients with BD and a minimal positive association between BMI and both FL volume and FL thickness in controls. The fact that 1 contrast yielded a significant interaction is based on modest statistical differences; the interaction for FL thickness was nearly significant (pFDR = 0.11). Although this modest discrepancy may be explained by different drivers of cortical volume versus thickness (e.g., different neurodevelopmental trajectories53 and/or genetics54), in our view the 2 dependent variables yielded an overall convergent pattern.
The etiopathological factors that underlie the significant interaction based on diagnosis are as yet uncertain. This study did not replicate prior findings of a negative association between BMI and brain volumes in non-BD youth in the general population.17 This inconsistency may relate to the fact that our control sample was healthier than those in prior population studies, both in terms of BMI and mental health. Out of the 48 control participants, only 4 (8.3%) met the BMI cut-off to be considered overweight or obese (as per CDC guidelines on BMI for age and sex percentiles), which is far lower than the prevalence in the general population (30%).55 Moreover, the National Comorbidity Survey has reported that nearly 1 in 4 adolescents in the United States meet the criteria for a mental disorder with severe impairment.56 In contrast, only 10% of our control sample had ADHD and 4% had an anxiety disorder. One can speculate that the lower-than-population rate of overweight/obesity combined with the lower-than-population rate of psychopathology in our control sample may have collectively contributed to the null findings in this group.
Finally, our sensitivity analyses confirmed that the observed diagnosis × BMI interaction for frontal lobe volume is not explained by systolic blood pressure. Although we speculate that mechanisms other than traditional cardiovascular risk factors explain the present finding, future studies integrating measures such as blood glucose, lipids and leptin are warranted.
Whole brain analysis
The whole brain vertex-wise analysis of BMI–volume correlations between groups resolved 2 large clusters — 1 with a peak in the ACC and 1 with a peak in the OFC — both encompassing large regions of the caudal and rostral ACC. Convergent with our ROI findings, BMI–volume correlations in those regions were more negative in patients with BD than in controls. Consistent with previous findings, the ACC is another structure that may be associated with the neuropathology of BD. Most notably, 1 study found reduced subgenual ACC volumes following the onset of BD in at-risk youth.57 This finding would imply that neurostructural changes take place after the onset of BD symptoms. Taken together with previous evidence that suggests that obese youth with BD experience the onset of BD at an earlier age,15 we can speculate that elevated BMI and its corresponding reduction in ACC volume could be a consequence of BD onset and therefore could be associated with the pathophysiology of BD. However, future longitudinal studies would be needed to confirm this hypothesis.
Furthermore, numerous fMRI studies have shown abnormal activity in the ACC in both emotional and cognitive domains across varying mood states in individuals with BD.58,59 Given that the ACC plays an integral role in regulating emotion and cognition in those with BD, its negative correlation with BMI in adolescents with BD is a finding with potential clinical applications. Intervention studies are warranted to determine whether optimization of BMI yields volumetric normalization and, in turn, improvements in emotional regulation among adolescents with BD.
Exploratory analysis of diagnosis × BMI × sex interactions within the aforementioned clusters revealed that there were within-group differences in the association between BMI and cortical volume. These results imply that the association between BMI and brain structure is moderated by sex in adolescents with BD but not in controls. Although there is currently no literature examining sex differences in the association between BMI and brain structure in individuals with BD, a recent review of neuroimaging studies of adults with BD reports evidence for sex-related structural differences within the limbic and prefrontal regions.50 Future studies examining sex × BMI interaction on clinical characteristics of BD are warranted.
ADHD × BMI interaction in patients with BD
Our results showed that the effect of BMI in predicting FL and OFC volumes in adolescents with BD differs on the basis of ADHD diagnosis. We observed a stronger negative correlation in the adolescents with both BD and ADHD than in the BD-only and the control groups. Present findings converge in part with those of prior ADHD neuroimaging studies. A meta-analysis reported reduced total brain volume in patients with ADHD compared with psychiatrically healthy controls.60 One study specifically found cortical thinning in frontal regions and the cingulate cortex in children with ADHD.61 Similar to those with BD, adolescents with ADHD are also more likely to be overweight or obese.62 Part of this association may be explained by individuals with ADHD eating impulsively possibly being more likely to binge-eat.63
Association between obesity and brain structure in patients with BD
It is likely that the association between BMI and brain structure is bidirectional, especially in the context of BD (Appendix 1, Fig. S3). Disinhibition and heightened feeding behaviour, subserved by FL and OFC volume reduction, can lead to increased BMI. Additionally, reduced volume in the OFC, a region involved in emotion regulation and reward circuitry, is associated with depressed mood,64 which in itself is correlated with overeating and decreased energy expenditure.65 Conversely, the resulting obese phenotype may be causing these reductions in the aforementioned brain regions via biochemical mechanisms, such as increased inflammatory cytokines, decreased peripheral brain-derived neurotrophic factor levels, disrupted cortisol levels and abnormal leptin signalling,66,67 all of which have been linked to both BMI and brain structure.17
Limitations
A number of study limitations warrant comment. First, the cross-sectional and observational design precludes inferences regarding the directionality of the observed associations. Second, the study did not include peripheral biomarkers or neurocognitive testing, measures that could have helped inform our understanding of mechanisms underlying the link between brain structure and obesity in individuals with BD. Third, BMI does not distinguish between fat mass and other tissue types (e.g., lean mass), between visceral and subcutaneous fat masses, or between different anthropometric fat distributions.17 Reliance on BMI rather than more nuanced measures of adiposity may have influenced the findings. Fourth, despite covarying for medications, the possibility of residual psychotropic medication effects remains.68–71 Fifth, the present cross-sectional data do not allow for analyses regarding the effect of duration of elevated BMI. Finally, the range of BMI was narrower in the control sample than the BD sample, which may explain in part the lack of negative correlation between BMI and ROIs in the control participants.
Conclusion
To our knowledge, this was the first study to examine the association between BMI and neurostructural measures in adolescents with BD. Findings from this study suggest that there are unique neurostructural correlates of elevated BMI in adolescents with BD that are not observed in controls. In particular, BMI was associated with reduced FL and OFC volumes in adolescents with BD. Prospective studies examining the direction of the observed associations and the effect of BMI optimization on brain structure in patients with BD are warranted. In the interim, our findings add to a growing literature that highlights the relevance of brain–body links in individuals with BD and other psychiatric conditions.
Acknowledgements
This study was funded by the Ontario Mental Health Foundation and the Canadian Institutes of Health Research [CIHR MOP 136947]. The authors thank MRI technologists Ruby Endre and Garry Detzler for their unwavering support with scanning participants and data collection.
References
- 1.Wang PW, Sachs GS, Zarate CA, et al. Overweight and obesity in bipolar disorders. J Psychiatr Res. 2006;40:762–4. doi: 10.1016/j.jpsychires.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 2.McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry. 2002;63:207–13. doi: 10.4088/jcp.v63n0306. [DOI] [PubMed] [Google Scholar]
- 3.Fiedorowicz JG, Palagummi NM, Forman-Hoffman VL, et al. Elevated prevalence of obesity, metabolic syndrome, and cardiovascular risk factors in bipolar disorder. Ann Clin Psychiatry. 2008;20:131–7. doi: 10.1080/10401230802177722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldstein BI, Liu SM, Zivkovic N, et al. The burden of obesity among adults with bipolar disorder in the United States. Bipolar Disord. 2011;13:387–95. doi: 10.1111/j.1399-5618.2011.00932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldstein BI, Blanco C, He JP, et al. Correlates of overweight and obesity among adolescents with bipolar disorder in the National Comorbidity Survey-Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2016;55:1020–6. doi: 10.1016/j.jaac.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro J, Mindra S, Timmins V, et al. Controlled study of obesity among adolescents with bipolar disorder. J Child Adolesc Psychopharmacol. 2016;27:95–100. doi: 10.1089/cap.2015.0154. [DOI] [PubMed] [Google Scholar]
- 7.Tsuang MT, Woolson RF, Fleming JA. Premature deaths in schizophrenia and affective disorders. An analysis of survival curves and variables affecting the shortened survival. Arch Gen Psychiatry. 1980;37:979–83. doi: 10.1001/archpsyc.1980.01780220017001. [DOI] [PubMed] [Google Scholar]
- 8.Kilbourne AM, Cornelius JR, Han X, et al. Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord. 2004;6:368–73. doi: 10.1111/j.1399-5618.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 9.Osby U, Brandt L, Correia N, et al. Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry. 2001;58:844–50. doi: 10.1001/archpsyc.58.9.844. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein BI, Schaffer A, Wang S, et al. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J Clin Psychiatry. 2015;76:163–9. doi: 10.4088/JCP.14m09300. [DOI] [PubMed] [Google Scholar]
- 11.Goldstein BI, Carnethon MR, Matthews KA, et al. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2015;132:965–86. doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- 12.Fagiolini A, Kupfer DJ, Houck PR, et al. Obesity as a correlate of outcome in patients with bipolar I disorder. Am J Psychiatry. 2003;160:112–7. doi: 10.1176/appi.ajp.160.1.112. [DOI] [PubMed] [Google Scholar]
- 13.Gomes FA, Kauer-Sant’Anna M, Magalhaes PV, et al. Obesity is associated with previous suicide attempts in bipolar disorder. Acta Neuropsychiatr. 2010;22:63–7. doi: 10.1111/j.1601-5215.2010.00452.x. [DOI] [PubMed] [Google Scholar]
- 14.Silveira LE, Kozicky JM, Muralidharan K, et al. Neurocognitive functioning in overweight and obese patients with bipolar disorder: data from the Systematic Treatment Optimization Program for Early Mania (STOP-EM) Can J Psychiatry. 2014;59:639–48. doi: 10.1177/070674371405901205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein BI, Birmaher B, Axelson DA, et al. Preliminary findings regarding overweight and obesity in pediatric bipolar disorder. J Clin Psychiat. 2008;69:1953–9. doi: 10.4088/jcp.v69n1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naiberg MR, Newton DF, Collins JE, et al. Impulsivity is associated with blood pressure and waist circumference among adolescents with bipolar disorder. J Psychiatr Res. 2016;83:230–9. doi: 10.1016/j.jpsychires.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Willette AA, Kapogiannis D. Does the brain shrink as the waist expands? Ageing Res Rev. 2015;20:86–97. doi: 10.1016/j.arr.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alosco ML, Stanek KM, Galioto R, et al. Body mass index and brain structure in healthy children and adolescents. Int J Neurosci. 2014;124:49–55. doi: 10.3109/00207454.2013.817408. [DOI] [PubMed] [Google Scholar]
- 19.Yokum S, Ng J, Stice E. Relation of regional gray and white matter volumes to current BMI and future increases in BMI: a prospective MRI study. Int J Obes. 2012;36:656–64. doi: 10.1038/ijo.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yau PL, Kang EH, Javier DC, et al. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity (Silver Spring) 2014;22:1865–71. doi: 10.1002/oby.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ross N, Yau PL, Convit A. Obesity, fitness, and brain integrity in adolescence. Appetite. 2015;93:44–50. doi: 10.1016/j.appet.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ou X, Andres A, Pivik RT, et al. Brain gray and white matter differences in healthy normal weight and obese children. J Magn Reson Imaging. 2015;42:1205–13. doi: 10.1002/jmri.24912. [DOI] [PubMed] [Google Scholar]
- 23.Bauer CC, Moreno B, Gonzalez-Santos L, et al. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: a magnetic resonance imaging study in Mexican children. Pediatr Obes. 2015;10:196–204. doi: 10.1111/ijpo.241. [DOI] [PubMed] [Google Scholar]
- 24.Taki Y, Kinomura S, Sato K, et al. Relationship between body mass index and gray matter volume in 1,428 healthy individuals. Obesity (Silver Spring) 2008;16:119–24. doi: 10.1038/oby.2007.4. [DOI] [PubMed] [Google Scholar]
- 25.Pfeifer JC, Welge J, Strakowski SM, et al. Meta-analysis of amygdala volumes in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:1289–98. doi: 10.1097/CHI.0b013e318185d299. [DOI] [PubMed] [Google Scholar]
- 26.Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry. 2005;162:1256–65. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 27.Dickstein DP, Milham MP, Nugent AC, et al. Frontotemporal alterations in pediatric bipolar disorder: results of a voxel-based morphometry study. Arch Gen Psychiatry. 2005;62:734–41. doi: 10.1001/archpsyc.62.7.734. [DOI] [PubMed] [Google Scholar]
- 28.Selvaraj S, Arnone D, Job D, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–45. doi: 10.1111/j.1399-5618.2012.01000.x. [DOI] [PubMed] [Google Scholar]
- 29.Hanford LC, Nazarov A, Hall GB, et al. Cortical thickness in bipolar disorder: a systematic review. Bipolar Disord. 2016;18:4–18. doi: 10.1111/bdi.12362. [DOI] [PubMed] [Google Scholar]
- 30.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–7. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Bond DJ, Ha TH, Lang DJ, et al. Body mass index-related regional gray and white matter volume reductions in first-episode mania patients. Biol Psychiatry. 2014;76:138–45. doi: 10.1016/j.biopsych.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 32.Bond DJ, Lang DJ, Noronha MM, et al. The association of elevated body mass index with reduced brain volumes in first-episode mania. Biol Psychiatry. 2011;70:381–7. doi: 10.1016/j.biopsych.2011.02.025. [DOI] [PubMed] [Google Scholar]
- 33.Axelson D, Birmaher B, Strober M, et al. Phenomenology of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:1139–48. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 35.Axelson D, Birmaher BJ, Brent D, et al. A preliminary study of the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children mania rating scale for children and adolescents. J Child Adolesc Psychopharmacol. 2003;13:463–70. doi: 10.1089/104454603322724850. [DOI] [PubMed] [Google Scholar]
- 36.Chambers WJ, Puig-Antich J, Hirsch M, et al. The assessment of affective disorders in children and adolescents by semistructured interview. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version. Arch Gen Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- 37.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb) 2012;22:276–82. [PMC free article] [PubMed] [Google Scholar]
- 38.Fischl B, van der Kouwe A, Destrieux C, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 39.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 40.Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- 41.Segonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 43.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- 44.Fischl B, Sereno MI, Tootell RB, et al. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Richardson JT. Eta squared and partial eta squared as measures of effect size in educational research. Educ Res Rev. 2011;6:135–47. [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 48.Fischl B. FreeSurfer. Neuroimage. 2012;62:774–81. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe C, Ekman CJ, Sellgren C, et al. Cortical thickness, volume and surface area in patients with bipolar disorder types I and II. J Psychiatry Neurosci. 2016;41:240–50. doi: 10.1503/jpn.150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jogia J, Dima D, Frangou S. Sex differences in bipolar disorder: a review of neuroimaging findings and new evidence. Bipolar Disord. 2012;14:461–71. doi: 10.1111/j.1399-5618.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Larson MP, DelBello MP, Zimmerman ME, et al. Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry. 2002;52:93–100. doi: 10.1016/s0006-3223(02)01350-1. [DOI] [PubMed] [Google Scholar]
- 52.Najt P, Nicoletti M, Chen HH, et al. Anatomical measurements of the orbitofrontal cortex in child and adolescent patients with bipolar disorder. Neurosci Lett. 2007;413:183–6. doi: 10.1016/j.neulet.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fjell AM, Grydeland H, Krogsrud SK, et al. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci U S A. 2015;112:15462–7. doi: 10.1073/pnas.1508831112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winkler AM, Kochunov P, Blangero J, et al. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–46. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–5. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 56.Merikangas KR, He JP, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–9. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gogtay N, Ordonez A, Herman DH, et al. Dynamic mapping of cortical development before and after the onset of pediatric bipolar illness. J Child Psychol Psychiatry. 2007;48:852–62. doi: 10.1111/j.1469-7610.2007.01747.x. [DOI] [PubMed] [Google Scholar]
- 58.Strakowski SM, Adler CM, Holland SK, et al. A preliminary FMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology. 2004;29:1734–40. doi: 10.1038/sj.npp.1300492. [DOI] [PubMed] [Google Scholar]
- 59.Strakowski SM, Adler CM, Almeida J, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–25. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valera EM, Faraone SV, Murray KE, et al. Meta-analysis of structural imaging findings in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:1361–9. doi: 10.1016/j.biopsych.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 61.Qiu MG, Ye Z, Li QY, et al. Changes of brain structure and function in ADHD children. Brain Topogr. 2011;24:243–52. doi: 10.1007/s10548-010-0168-4. [DOI] [PubMed] [Google Scholar]
- 62.Fliers EA, Buitelaar JK, Maras A, et al. ADHD is a risk factor for overweight and obesity in children. J Dev Behav Pediatr. 2013;34:566–74. doi: 10.1097/DBP.0b013e3182a50a67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pagoto SL, Curtin C, Lemon SC, et al. Association between adult attention deficit/hyperactivity disorder and obesity in the US population. Obesity. 2009;17:539–44. doi: 10.1038/oby.2008.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–25. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Macht M. How emotions affect eating: a five-way model. Appetite. 2008;50:1–11. doi: 10.1016/j.appet.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 66.Marsland AL, Gianaros PJ, Abramowitch SM, et al. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol Psychiatry. 2008;64:484–90. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willette AA, Bendlin BB, McLaren DG, et al. Age-related changes in neural volume and microstructure associated with interleukin-6 are ameliorated by a calorie-restricted diet in old rhesus monkeys. Neuroimage. 2010;51:987–94. doi: 10.1016/j.neuroimage.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bak M, Fransen A, Janssen J, et al. Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE. 2014;9:e94112. doi: 10.1371/journal.pone.0094112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Almandil NB, Liu Y, Murray ML, et al. Weight gain and other metabolic adverse effects associated with atypical antipsychotic treatment of children and adolescents: a systematic review and meta-analysis. Paediatr Drugs. 2013;15:139–50. doi: 10.1007/s40272-013-0016-6. [DOI] [PubMed] [Google Scholar]
- 70.Hafeman DM, Chang KD, Garrett AS, et al. Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord. 2012;14:375–410. doi: 10.1111/j.1399-5618.2012.01023.x. [DOI] [PubMed] [Google Scholar]
- 71.Atmaca M, Ozdemir H, Cetinkaya S, et al. Cingulate gyrus volumetry in drug free bipolar patients and patients treated with valproate or valproate and quetiapine. J Psychiatr Res. 2007;41:821–7. doi: 10.1016/j.jpsychires.2006.07.006. [DOI] [PubMed] [Google Scholar]