Abstract
Background
Patients with anorexia nervosa exhibit higher levels of behaviours typically associated with autism-spectrum disorder (ASD), but the neural basis is unclear. We sought to determine whether elevated autistic traits in women with anorexia nervosa may be reflected in cortical morphology.
Methods
We used voxel-based morphometry (VBM) to examine regional grey matter volumes in high-resolution MRI structural brain scans in women with anorexia nervosa and matched healthy controls. The Autism-spectrum Quotient (AQ) scale was used to assess autistic traits.
Results
Women with anorexia nervosa (n = 25) had higher AQ scores and lower bilateral superior temporal sulcus (STS) grey matter volumes than the control group (n = 25). The AQ scores correlated negatively with average left STS grey matter volume in women with anorexia nervosa.
Limitations
We did not control for cognitive ability and examined only women with ongoing anorexia nervosa.
Conclusion
Elevated autistic traits in women with anorexia nervosa are associated with morphometric alterations of brain areas linked to social cognition. This finding provides neurobiological support for the behavioural link between anorexia nervosa and ASD and emphasizes the importance of recognizing autistic traits in preventing and treating anorexia nervosa.
Introduction
Anorexia nervosa is a severe psychiatric disorder that predominantly affects young women.1 Although the diagnosis is defined by restricted eating and disturbed body perception,2 converging research links anorexia nervosa to a wide range of poorly understood behavioural alterations.3 Specifically, behavioural risk factors typically associated with autism-spectrum disorder (ASD) are consistently found in patients with anorexia nervosa.4 For instance, affected women typically exhibit perfectionism, particularly regarding symmetry and exactness,5 obsessive–compulsiveness, low cooperativeness, low novelty-seeking, impaired social cognition6,7 and set-shifting difficulties.8 Moreover, a large number of studies have found persistently elevated levels of autistic traits in patients with anorexia nervosa,4,7,9–13 and autistic traits and eating disorder behaviours are correlated in typically developing children.14 In fact, a relatively high proportion of women with anorexia nervosa also meet the criteria for ASD,11,15 and the prevalence of ASD is higher in populations affected by eating disorders.16
Despite the large and growing body of research showing behavioural and cognitive ASD-like traits in women with anorexia nervosa, the neural correlates of elevated autistic traits in women with anorexia nervosa are poorly understood. However, recent research links anorexia nervosa to specific alterations in social cognition typically associated with ASD, such as impaired understanding of other people’s mental states, or theory of mind (ToM).17–21 Although only a handful of studies have examined the neural basis of altered sociocognitive function in patients with anorexia nervosa,22–25 studies specifically targeting ToM processes have identified reduced activity in brain circuits associated with social cognition, 26 including the superior temporal cortex25 and temporoparietal junction (TPJ).22,23 Consistently, a relatively large number of studies have identified grey matter reductions of the temporal lobe in patients with anorexia nervosa.27–29 These findings echo findings of temporal cortex alterations in patients with ASD,30 including alterations of the superior temporal sulcus (STS) and the TPJ.31–33
In the present study, we sought to determine whether elevated autistic traits in women with anorexia nervosa may be reflected in morphometric brain alterations. Specifically, we examined focal alterations in cortical grey matter volume through voxel-based morphometry (VBM) analyses of structural brain scans. As deficit ToM is a classical symptom of ASD30 and because brain regions linked to ToM are altered in patients with anorexia nervosa22–25 and those with ASD,31–33 we hypothesized that autistic traits in women with anorexia nervosa would correlate with grey matter volume of regions associated with ToM.
Methods
Participants
Patients with anorexia nervosa aged 16–25 years were recruited consecutively from the in- and outpatient specialist Anorexia-Bulimia unit at the Queen Silvia Children’s University Hospital in Gothenburg, Sweden. At first assessment by a psychiatrist, all patients received a diagnosis of anorexia nervosa according to DSM-IV. Patients with any neurologic disorder were excluded. All healthy participants were asked to report neurologic disorders, ongoing diseases and medications using a written questionnaire, and ongoing eating disorders were assessed using the Structured Clinical Interview (SCID-I) for DSM-IV. Inclusion criteria for matched control participants were no ongoing eating disorders, no neurologic disorders, no ongoing diseases and no medication. Depressive symptoms were assessed in all patients using the Beck Depression Inventory (BDI). The Regional Ethical Review Board at the University of Gothenburg approved the study (registration number 007–14), and all participants gave written informed consent.
Assessment of autistic traits
We assessed the level of autistic traits in all participants by administering the Autism-spectrum Quotient (AQ) questionnaire.34
Magnetic resonance imaging
Structural brain scans were collected using a Philips Gyroscan 3 T Achieva scanner, software release 3.2. The scanner’s 2-channel parallel transmit was used for improved signal homogeneity over the field of view, and the participant’s head was firmly supported with cushions in the head coil (32-channel SENSE, Philips). The T1-weighted scan (3D T1–turbo field echo [TFE]) was obtained using the following parameters: flip angle 8º, echo time (TE) 4.0 ms, repetition time (TR) 8.4 ms, SENSE factor 2.7, TFE factor 240, 170 sagittal slices with a scan resolution of 1.0 × 1.0 × 1.0 mm3.
Data processing
Structural brain images were processed using the VBM8 package in SPM8 (Wellcome Trust Centre for Neuroimaging, Institute of Neurology, www.fil.ion.uncl.ac.uk/spm) including the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) in MATLAB 2014a (The MathWorks). Processing involved spatial normalization into Montreal Neurological Institute (MNI) template space using the high-dimensional diffeomorphic anatomic registration through exponentiated lie algebra (DARTEL) method with a final voxel size of 1.5 × 1.5 × 1.5 mm3; segmentation into grey matter, white matter and cerebrospinal fluid; and modulation by the nonlinear component only for volume changes during spatial normalization to identify regional differences in grey matter volume corrected for individual brain size. The spatially normalized and modulated grey matter partitions were smoothed with an isotropic 8 mm full-width at half-maximum (FWHM) Gaussian kernel. Finally, we computed an average of all participants’ normalized structural scans for visualization of the results.
Theory of mind region of interest analyses
To specifically test the hypothesis that autistic traits in women with anorexia nervosa correlate with grey matter volume of regions associated with ToM, we defined a set of independent ToM regions of interest (ROIs). Here, we used Neurosynth software (neurosynth.org) to conduct a reverse inference meta-analysis of previously published studies with the predefined search term “theory mind.” This process identified all studies indexed by the software that mentioned the search term “theory mind” at least once in the abstract, as per the default procedure. All voxels of the reverse inference map passing the default threshold of a false-discovery rate (FDR) of 0.01 were included in the set of ROIs.
Group difference analyses
We conducted a voxel-wise general linear model (GLM) analysis to identify brain regions exhibiting group differences between women with anorexia nervosa and healthy control participants. Because brain regions linked to social cognition, including temporal cortex areas such as the TPJ, mature during adolescence,35 we included age as a covariate. The results were assessed using nonparametric permutation testing clusterwise inference as implemented in the Statistical Non-Parametric Mapping toolbox (http://warwick.ac.uk/snpm), with a cluster-forming threshold of 0.005 and a family-wise error (FWE) of 0.05. Given our a priori hypothesis, we assessed the results within the predefined ROIs.
Correlation analyses
To assess associations between autistic traits and grey matter volume in women with anorexia nervosa, we extracted individual average grey matter volumes from areas exhibiting significant group differences using the MarsBaR toolbox (http://marsbar.sourceforge.net/) and computed the Pearson linear correlation coefficient between grey matter volumes and AQ scores while controlling for age.
Control analyses
We conducted a number of control analyses to examine whether any observed effects may have been due to general grey matter loss in women with anorexia nervosa rather than to social cognition processes. First, we examined total grey matter across the entire brain volume and assessed group differences as well as associations with AQ. Second, we replicated the analyses described previously within regions reliably associated with grey matter loss in patients with anorexia nervosa, as reported in a recent meta-analysis:29 namely, the hypothalamus, the left inferior parietal lobule, the right putamen and the right caudate. These regions were anatomically identified using the Automated Anatomic Labelling (AAL) system in the WFU pickatlas (www.nitrc.org/projects/wfu_pickatlas/). Since the hypothalamus is not defined in AAL, we constructed an ROI as a 10 mm sphere centred on the coordinates reported in the meta-analysis,29 converted from Talairach to MNI space using GingerAle (www.brainmap.org/ale/; resulting MNI coordinates: x, y, z = −1, −3, −16).
Whole brain analyses
We conducted 2 exploratory whole brain analyses. First, we searched for group differences between women with anorexia nervosa and healthy control participants. Second, we examined correlations between grey matter volume and AQ scores only in women with anorexia nervosa. Again, the analysis included age as a covariate and the results were assessed on the basis of nonparametric cluster-wise inference, with a cluster-forming threshold of 0.005 and cluster-based correction for multiple comparisons such that pFWE < 0.05. In addition, we reported any results passing the cluster-forming threshold of p < 0.005 located near any of the ToM or control ROIs.
Results
Demographic characteristics
A total of 37 patients were asked to participate, and 25 patients accepted. Thus, the final sample consisted of 50 female participants aged 16–25 years: 25 with anorexia nervosa and 25 healthy controls (Table 1). All patients with anorexia nervosa had a body mass index (BMI) of 17.5 kg/m2 or lower, as measured at the unit. All patients with anorexia nervosa were medically stable at the time of scanning, and 1 patient was admitted to a psychiatric ward. Twelve patients were not medicated, and the rest used the following psychoactive medications: fluoxetine (n = 6), sertraline (n = 4), olanzapine (n = 2), quetiapine (n = 1), venflaxine (n = 1), propiomazine (n = 4), lamotrigine (n = 1) and lisdexametafine (n = 1). Four patients had the binge-eating/purging type of anorexia nervosa and the rest had the restrictive type. Patients had significantly lower BMI and higher depression scores than control participants. No participant reported substance abuse, and no healthy participant reported any neurologic disorder, ongoing disease (including eating disorder) or medication use.
Table 1.
Demographic and clinical characteristics of study participants
| Group; mean ± SD | |||
|---|---|---|---|
|
|
|||
| Characteristic | Anorexia nervosa, n = 25 | Control, n = 25 | p value |
| Age, yr | 20.32 ± 2.23 | 21.28 ± 2.11 | 0.12 |
| BMI | 16.28 ± 0.93 | 21.13 ± 2.27 | < 0.001 |
| BDI | 26.88 ± 13.06 | 7.76 ± 7.33 | < 0.001 |
| Duration of illness, yr | 4.14 ± 3.54 | — | — |
BDI = Beck Depression Inventory; BMI = body mass index; SD = standard deviation.
Assessment of autistic traits
Women with anorexia nervosa had significantly higher levels of autistic traits than control participants (AQ score group mean 16.60 ± 6.73 v. 11.64 ± 6.82, p = 0.010). There was a strong positive association between AQ and BDI scores in women with anorexia nervosa (r = 0.53, p = 0.006), but not in control participants (r = 0.24, p = 0.24). The AQ score did not correlate with BMI in patients (r = 0.24, p = 0.25) or control participants (r = −0.01, p = 0.95), and there was no association between AQ and duration of illness in patients (r = −0.09, p = 0.67).
Theory of mind region of interest definition
The Neurosynth search yielded 140 studies (Appendix 1, Table S1, available at jpn.ca/170072-a1). The resulting reverse inference meta-analysis map revealed a set of ROIs consistently associated with ToM processes (pFDR < 0.01), including the bilateral superior temporal cortex extending into the TPJ, and medial frontal areas (Appendix 1, Fig. S1 and Table S2).
Theory of mind region of interest analyses
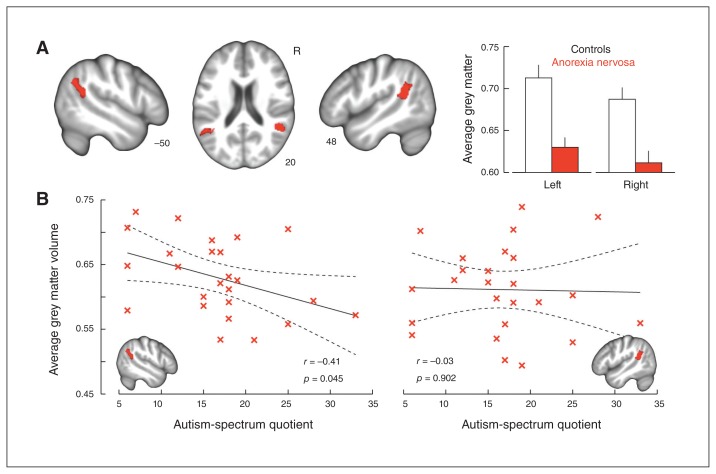
The ToM ROI analysis revealed 2 regions with significantly reduced grey matter volume in women with anorexia nervosa relative to control participants (p < 0.005, k ≥ 293): the left (MNI coordinates: x, y, z = −60.00, −55.50, 12.00; t = 3.66, 706 voxels) and right STS (MNI coordinates: x, y, z = 46.50, −43.50, 19.50; t = 3.99, 503 voxels), extending into the TPJ (Fig. 1A). No region exhibited increased grey matter volume in patients relative to control participants.
Fig. 1.
Grey matter correlates of autistic traits in women with anorexia nervosa. (A) Significant grey matter reductions within theory of mind regions of interest in women affected by anorexia nervosa compared with healthy control participants (cluster-level corrected p < 0.05). (B) Inverse association between autistic traits in women with anorexia nervosa and left, but not right, superior temporal grey matter volumes extracted from the group contrast. Coordinates refer to the Montreal Neurological Institute (MNI) atlas space, the results are displayed in neurologic convention on a group average brain, error bars indicate standard error, and the dotted lines indicate the 95% confidence bounds.
We found a negative association between AQ score and average grey matter volume extracted from the left (r = −0.41, p = 0.045), but not the right (r = −0.03, p = 0.90), STS region in women with anorexia nervosa (Fig. 1B). Post hoc analyses showed that grey matter volumes did not correlate with BMI (all p > 0.25); however, when controlling for BMI, the strength of the association between AQ score and left STS grey matter was reduced (r = −0.38, p = 0.08) whereas the right hemisphere correlation was marginally strengthened (r = −0.07, p = 0.76)
Whole brain analyses
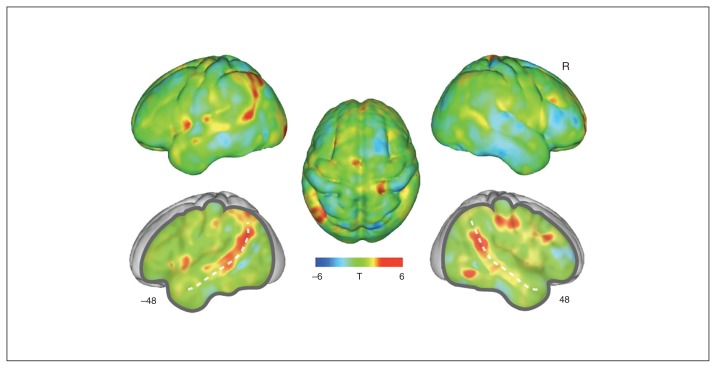
The group difference analysis did not reveal any additional significantly altered regions on the whole brain level (p < 0.005, k ≥ 2464; Fig. 2).
Fig. 2.
Whole brain grey matter reductions in women with anorexia nervosa compared with healthy control participants. The un-thresholded group difference T-map is overlaid on a group average brain. Positive values indicate voxels where control participants show increased grey matter compared with women with anorexia nervosa, and vice versa for negative values. Coordinates indicate X axis cuts to reveal deeper structures, shown in Montreal Neurological Institute (MNI) atlas space. The dotted line indicates the superior temporal sulcus. R = right hemisphere.
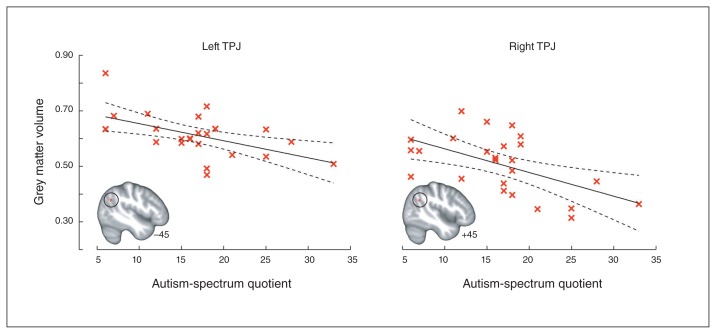
The whole brain search for correlations with AQ score did not yield any significant results at the predetermined statistical threshold (p < 0.005, k ≥ 2484). However, we found 2 clusters of voxels located near the predetermined ROIs that passed the uncorrected threshold of p < 0.005 in the left and right TPJ (left: x, y, z = −45.00, −63.00, 30.00; peak uncorrected p = 0.003, t = −3.03, 6 voxels; right: x, y, z = 45.00, −54.00, 28.50; peak uncorrected p = 0.003, t = −3.05, 7 voxels; Fig. 3).
Fig. 3.
Association between autistic traits and temporoparietal grey matter volume. Inverse association between autistic traits in women with anorexia nervosa and temporoparietal junction (TPJ) grey matter volume identified in the whole brain search (at uncorrected p < 0.005; note that these clusters did not meet the family-wise error–corrected cluster-forming threshold of k ≥ 2484). The data indicate the cluster mean grey matter volumes, coordinates refer to the Montreal Neurological Institute (MNI) atlas space, and the dotted lines indicate the 95% confidence bounds.
Control analyses
The control analysis showed that total grey matter volume did not differ significantly between women with anorexia nervosa and control participants (p = 0.38) and did not correlate with AQ score in women with anorexia nervosa (p = 0.48).
Also, the control analyses revealed significantly reduced grey matter of the right putamen (p < 0.005, k ≥ 46; x, y, z = 33.00, 3.00, 1.50; t = 3.21, 240 voxels) and the hypothalamus (p < 0.005, k ≥ 27; x, y, z = −3.00, 3.00, −9.00; t = 3.38, 82 voxels). In the left inferior parietal lobule, 2 clusters passed the cluster-forming threshold of p < 0.005, but these were too small to pass the cluster threshold of k ≥ 145 (x, y, z = −25.5, −66, 42; t = 3.08, 29 voxels, and x, y, z = −37.5, −63, 51; t = 3.45, 88 voxels). In the caudate, 2 clusters passed the cluster-forming threshold, but none were near the cluster threshold of k ≥ 44 voxels (x, y, z = 4.5, 6, −6; t = 2.84, 2 voxels, and x, y, z = 6, 21, −1.5; t = 2.72, 2 voxels).
Average grey matter extracted from these clusters did not correlate with AQ score in patients with anorexia nervosa (putamen: r = 0.26, p = 0.23; hypothalamus: r = 0.01, p = 0.98; inferior parietal cortex: r = −0.03, p = 0.88; caudate: r = −0.03, p = 0.90), and the whole brain voxel-wise search for correlations with AQ score did not identify any voxels that passed the uncorrected threshold of p < 0.005 within or near any of these ROIs (p < 0.005, k ≥ 2484).
Discussion
We searched for neuromorphometric correlates of autistic traits in women with anorexia nervosa and hypothesized that these would be found in brain regions associated with social cognition and ToM. The results confirmed our hypothesis: grey matter volume of the STS was reduced bilaterally in women with anorexia nervosa, and average left STS grey matter showed a weak but significant negative correlation with AQ scores. In addition, we found a trend toward a correlation between TPJ grey matter volume and autistic traits bilaterally.
The finding that patients with anorexia nervosa exhibited significantly higher levels of autistic traits than control participants adds to the growing body of studies observing behavioural overlaps between anorexia nervosa and ASD.4,7,10,13–15 Our results are also consistent with the extensive literature on structural brain alterations in anorexia nervosa.27,29,36–45 Specifically, previous structural brain studies found grey matter reductions of the temporal lobe in patients with anorexia nervosa,27,28 and a recent activation likelihood estimation (ALE) meta-analysis showed that the temporal lobe consistently exhibits reduced regional grey matter in patients with anorexia nervosa.29 Our results are further supported by functional MRI studies that found reduced activations of brain regions linked to social cognition, including the superior temporal cortex25 and TPJ,22,23 in patients with anorexia nervosa. Additionally, increased functional connectivity in the left angular gyrus, located near the TPJ, has been noted in patients with ongoing anorexia nervosa.46 Finally, the control analyses showed that the women with anorexia nervosa had significantly reduced grey matter of the putamen and the hypothalamus, replicating findings of robust grey matter alterations in patients with anorexia nervosa.29
Morphological brain alterations are largely ubiquitous in patients with anorexia nervosa,4,7,10,13–15 leaving the possibility that the observed association between autistic traits and grey matter volume may be a generalized effect of grey matter loss due to the illness. However, the whole brain search for associations with AQ scores did not reveal any additional areas showing a similar effect, and grey matter volume in regions known to be affected in patients with anorexia nervosa did not correlate with AQ score. Moreover, AQ score was not associated with whole brain grey matter volume. Taken together, these control analyses therefore suggest that the identified association between autistic traits and grey matter volume of social cognition areas does not reflect general loss of grey matter in women with anorexia nervosa. Instead, the results suggest that autistic traits may be specifically linked to temporal lobe grey matter morphology.
Although the literature on grey matter alterations in patients with ASD is highly inconsistent,47–49 superior temporal grey matter decreases have been observed in high-functioning individuals with ASD.50 This suggests the possibility that elevated autistic traits in patients with anorexia nervosa may share similarities with high-functioning patients with ASD. However, the majority of ASD studies have been conducted mainly with male participants, and recent findings highlight puzzling sex differences in brain structure and function in patients with ASD. Specifically, STS responses to social cues are altered in male but not in female patients with ASD,51 and bilateral superior temporal cortex volume is greater in female than in male patients with ASD and in typically developing participants.52 Notably, healthy girls exhibit a higher rate of cortical thinning of social brain regions during adolescence than boys, including thinning of the right temporal cortex and the left TPJ.35 Our findings raise the possibility that adolescent onset of anorexia nervosa may be linked to such cortical developments. However, further studies directly comparing boys and girls with anorexia nervosa are required to characterize shared and distinct alterations; this is a particularly difficult challenge given the low prevalence of anorexia nervosa in boys.
We found correlations with AQ score primarily in the left hemisphere. Alterations in social perception processes, including processing of stimuli such as biological motion, are primarily associated with right hemisphere STS alterations in patients with ASD.51,53 Indeed, the results from the meta-analysis revealed largely symmetric bilateral regions, but with a larger right (3540 voxels) than left (2530 voxels) STS area. However, the lateralization of ToM processing is mixed. For instance, a study of adults with brain damage suggests that the left TPJ is critical for functional ToM processes.54 Also, cortical TPJ thinning during adolescence is localized to the left hemisphere.35 Nevertheless, we observed bilateral STS grey matter reductions in women with anorexia nervosa and found trends toward associations between TPJ grey matter volume and autistic traits bilaterally. Any lateralization should therefore be interpreted with caution, and further studies in larger samples are required to confirm such effects.
Corroborating studies showing a link between autistic traits and depression,12 the AQ measure was strongly associated with BDI scores in patients with anorexia nervosa. This finding highlights the question of whether depression may influence autistic traits or vice versa12 and emphasizes the need for future research into the nature of the association between the 2 measurements. The finding also raises the possibility that depression could contribute to the observed morphological alterations. Indeed, subclinical depression is associated with altered grey matter volume of the temporal cortex;55 however, the opposite pattern of increased grey matter with higher depression scores was found, speaking against a confounding effect of depression on the results of the present study.
Limitations
A limitation of this work is that we did not control for cognitive ability in women with anorexia nervosa; however, cognitive ability is not generally affected in patients with this disorder56 and is unlikely to have had any substantial impact on the results. An additional limitation is that we examined only patients with ongoing anorexia nervosa; hence, low body weight and associated factors may have contributed to the observed grey matter alterations. Indeed, we found that controlling for BMI reduced the association between grey matter in the left STS and AQ scores, suggesting that body weight may play a role. Also, the broad structural grey matter abnormalities found in patients with acute anorexia nervosa generally normalizes as patients recover.57 However, 2 studies reported remaining grey matter reductions of the left angular gyrus, part of the TPJ, after weight recovery,58,59 raising the possibility that the observed alterations may be persistent. Further studies in weight-recovered women are required to establish whether this is the case. As socioemotional impairments may be perpetuated by the illness — for instance, chronic anorexia nervosa can lead to ASD-like symptoms60,61 — such studies are particularly important.
Conclusion
With its limitations in mind, this study shows that elevated autistic traits in women with anorexia nervosa are associated with morphometric alterations of brain areas linked to social cognition. This finding provides neurobiological support for the behavioural link between anorexia nervosa and ASD4,7,9–13 and raises the possibility that neurobiological risk factors linked to autistic traits may contribute to anorexia nervosa in adolescent girls. Critically, further research is required to disentangle predisposing and perpetuating features of any such effect. Finally, our results have important clinical implications: the observation that elevated autistic traits may be rooted in structural brain alterations similar to those observed in patients with ASD supports the notion that patients with anorexia nervosa may benefit from treatment schemes explicitly acknowledging ASD-like difficulties. For instance, treatment approaches used in patients with ASD, such as highly structured and concrete pedagogic methods or experimental pharmacological procedures such as oxytocin administration,62 may accelerate recovery in patients with anorexia nervosa who exhibit high levels of autistic traits.
Acknowledgements
The authors thank the participants for making this study possible. They also thank the staff at the Anorexia-Bulimia Unit, Queen Silvia Children’s Hospital for their continuous support. M. Björnsdotter was supported by the European Union Seventh Framework Program (FP7/2007-2013) under grant agreement PIOF-GA-2012-302896, The Söderström König Foundation, Linnea and Joself Carlsson’s Foundation, the Fredrik och Ingrid Thuring Foundation and O. E. och Edla Johanssons’ Foundation. L. Karjalainen was supported by the Wilhelm och Martina Lundgren Foundation. The study was supported by ALF-Västra Götaland.
References
- 1.Bulik CM, Sullivan PF, Tozzi F, et al. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–12. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) Lake St. Louis (MO): American Psychiatric Association; 2013. p. 1629. [Google Scholar]
- 3.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- 4.Oldershaw A, Treasure J, Hambrook D, et al. Is anorexia nervosa a version of autism spectrum disorders? Eur Eat Disord Rev. 2011;19:462–74. doi: 10.1002/erv.1069. [DOI] [PubMed] [Google Scholar]
- 5.Srinivasagam NM, Kaye WH, Plotnicov KH, et al. Persistent perfectionism, symmetry, and exactness after long-term recovery from anorexia nervosa. Am J Psychiatry. 1995;152:1630–4. doi: 10.1176/ajp.152.11.1630. [DOI] [PubMed] [Google Scholar]
- 6.Cassin SE, von Ranson KM. Personality and eating disorders: a decade in review. Clin Psychol Rev. 2005;25:895–916. doi: 10.1016/j.cpr.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Zucker NL, Losh M, Bulik CM, et al. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychol Bull. 2007;133:976–1006. doi: 10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]
- 8.Westwood H, Stahl D, Mandy W, et al. The set-shifting profiles of anorexia nervosa and autism spectrum disorder using the Wisconsin Card Sorting Test: a systematic review and meta-analysis. Psychol Med. 2016;46:1809–27. doi: 10.1017/S0033291716000581. [DOI] [PubMed] [Google Scholar]
- 9.Baron-Cohen S, Jaffa T, Davies S, et al. Do girls with anorexia nervosa have elevated autistic traits? Mol Autism. 2013;4:24. doi: 10.1186/2040-2392-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillberg C, Råstam M. Do some cases of anorexia nervosa reflect underlying autistic-like conditions? Behav Neurol. 1992;5:27–32. doi: 10.3233/BEN-1992-5105. [DOI] [PubMed] [Google Scholar]
- 11.Mandy W, Tchanturia K. Do women with eating disorders who have social and flexibility difficulties really have autism? A case series. Mol Autism. 2015;6:6. doi: 10.1186/2040-2392-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchanturia K, Smith E, Weineck F, et al. Exploring autistic traits in anorexia: a clinical study. Mol Autism. 2013;4:44. doi: 10.1186/2040-2392-4-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wentz E, Gillberg IC, Anckarsäter H, et al. Adolescent-onset anorexia nervosa: 18-year outcome. Br J Psychiatry. 2009;194:168–74. doi: 10.1192/bjp.bp.107.048686. [DOI] [PubMed] [Google Scholar]
- 14.Coombs E, Brosnan M, Bryant-Waugh R, et al. An investigation into the relationship between eating disorder psychopathology and autistic symptomatology in a non-clinical sample. Br J Clin Psychol. 2011;50:326–38. doi: 10.1348/014466510X524408. [DOI] [PubMed] [Google Scholar]
- 15.Anckarsäter H, Hofvander B, Billstedt E, et al. The sociocommunicative deficit subgroup in anorexia nervosa: autism spectrum disorders and neurocognition in a community-based, longitudinal study. Psychol Med. 2012;42:1957–67. doi: 10.1017/S0033291711002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huke V, Turk J, Saeidi S, et al. Autism spectrum disorders in eating disorder populations: a systematic review. Eur Eat Disord Rev. 2013;21:345–51. doi: 10.1002/erv.2244. [DOI] [PubMed] [Google Scholar]
- 17.Gillberg IC, Billstedt E, Wentz E, et al. Attention, executive functions, and mentalizing in anorexia nervosa eighteen years after onset of eating disorder. J Clin Exp Neuropsychol. 2010;32:358–65. doi: 10.1080/13803390903066857. [DOI] [PubMed] [Google Scholar]
- 18.Jewell T, Collyer H, Gardner T, et al. Attachment and mentalization and their association with child and adolescent eating pathology: a systematic review. Int J Eat Disord. 2016;49:354–73. doi: 10.1002/eat.22473. [DOI] [PubMed] [Google Scholar]
- 19.Russell TA, Schmidt U, Doherty L, et al. Aspects of social cognition in anorexia nervosa: affective and cognitive theory of mind. Psychiatry Res. 2009;168:181–5. doi: 10.1016/j.psychres.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 20.Tapajóz P, de Sampaio F, Soneira S, et al. Theory of mind and central coherence in eating disorders: two sides of the same coin? Psychiatry Res. 2013;210:1116–22. doi: 10.1016/j.psychres.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 21.Tapajóz Pereira de Sampaio F, Soneira S, Aulicino A, et al. Theory of mind in eating disorders and their relationship to clinical profile. Eur Eat Disord Rev. 2013;21:479–87. doi: 10.1002/erv.2247. [DOI] [PubMed] [Google Scholar]
- 22.McAdams CJ, Lohrenz T, Montague PR. Neural responses to kindness and malevolence differ in illness and recovery in women with anorexia nervosa. Hum Brain Mapp. 2015;36:5207–19. doi: 10.1002/hbm.23005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McAdams CJ, Krawczyk DC. Impaired neural processing of social attribution in anorexia nervosa. Psychiatry Res. 2011;194:54–63. doi: 10.1016/j.pscychresns.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 24.McAdams CJ, Krawczyk DC. Who am I? How do I look? Neural differences in self-identity in anorexia nervosa. Soc Cogn Affect Neurosci. 2014;9:12–21. doi: 10.1093/scan/nss093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schulte-Rüther M, Mainz V, Fink GR, et al. Theory of mind and the brain in anorexia nervosa: relation to treatment outcome. J Am Acad Child Adolesc Psychiatry. 2012;51:832–41.e11. doi: 10.1016/j.jaac.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- 27.Boghi A, Sterpone S, Sales S, et al. In vivo evidence of global and focal brain alterations in anorexia nervosa. Psychiatry Res Neuroimaging. 2011;192:154–9. doi: 10.1016/j.pscychresns.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 28.Suchan B, Busch M, Schulte D, et al. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res. 2010;206:63–7. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 29.Titova OE, Hjorth OC, Schiöth HB, et al. Anorexia nervosa is linked to reduced brain structure in reward and somatosensory regions: a meta-analysis of VBM studies. BMC Psychiatry. 2013;13:110. doi: 10.1186/1471-244X-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. doi: 10.1016/S0140-6736(13)61539-1. [DOI] [PubMed] [Google Scholar]
- 31.Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14:319–51. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelphrey KA, Shultz S, Hudac CM, et al. Research review: Constraining heterogeneity: the social brain and its development in autism spectrum disorder. J Child Psychol Psychiatry. 2011;52:631–44. doi: 10.1111/j.1469-7610.2010.02349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philip RCM, Dauvermann MR, Whalley HC, et al. A systematic review and meta-analysis of the fMRI investigation of autism spectrum disorders. Neurosci Biobehav Rev. 2012;36:901–42. doi: 10.1016/j.neubiorev.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 34.Baron-Cohen S, Wheelwright S, Skinner R, et al. The autism-spectrum quotient (AQ): evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J Autism Dev Disord. 2001;31:5–17. doi: 10.1023/a:1005653411471. [DOI] [PubMed] [Google Scholar]
- 35.Mutlu AK, Schneider M, Debbané M, et al. Sex differences in thickness, and folding developments throughout the cortex. Neuroimage. 2013;82:200–7. doi: 10.1016/j.neuroimage.2013.05.076. [DOI] [PubMed] [Google Scholar]
- 36.Amianto F, Caroppo P, D’Agata F, et al. Brain volumetric abnormalities in patients with anorexia and bulimia nervosa: a voxel-based morphometry study. Psychiatry Res Neuroimaging. 2013;213:210–6. doi: 10.1016/j.pscychresns.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Brooks SJ, Barker GJ, O’Daly OG, et al. Restraint of appetite and reduced regional brain volumes in anorexia nervosa: a voxel-based morphometric study. BMC Psychiatry. 2011;11:179. doi: 10.1186/1471-244X-11-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castro-Fornieles J, Bargalló N, Lázaro L, et al. A cross-sectional and follow-up voxel-based morphometric MRI study in adolescent anorexia nervosa. J Psychiatr Res. 2009;43:331–40. doi: 10.1016/j.jpsychires.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 39.D’Agata F, Caroppo P, Amianto F, et al. Brain correlates of alexithymia in eating disorders: a voxel-based morphometry study. Psychiatry Clin Neurosci. 2015;69:708–16. doi: 10.1111/pcn.12318. [DOI] [PubMed] [Google Scholar]
- 40.Frank GK, Shott ME, Hagman JO, et al. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–60. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Friederich H-C, Walther S, Bendszus M, et al. Grey matter abnormalities within cortico-limbic-striatal circuits in acute and weight-restored anorexia nervosa patients. Neuroimage. 2012;59:1106–13. doi: 10.1016/j.neuroimage.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 42.Gaudio S, Nocchi F, Franchin T, et al. Gray matter decrease distribution in the early stages of anorexia nervosa restrictive type in adolescents. Psychiatry Res Neuroimaging. 2011;191:24–30. doi: 10.1016/j.pscychresns.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 43.Lázaro L, Andrés S, Calvo A, et al. Normal gray and white matter volume after weight restoration in adolescents with anorexia nervosa. Int J Eat Disord. 2013;46:841–8. doi: 10.1002/eat.22161. [DOI] [PubMed] [Google Scholar]
- 44.Suchan B, Busch M, Schulte D, et al. Reduction of gray matter density in the extrastriate body area in women with anorexia nervosa. Behav Brain Res. 2010;206:63–7. doi: 10.1016/j.bbr.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 45.Wagner A, Greer P, Bailer UF, et al. Normal brain tissue volumes after long-term recovery in anorexia and bulimia nervosa. Biol Psychiatry. 2006;59:291–3. doi: 10.1016/j.biopsych.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 46.Boehm I, Geisler D, King JA, et al. Increased resting state functional connectivity in the fronto-parietal and default mode network in anorexia nervosa. Front Behav Neurosci. 2014;8:346. doi: 10.3389/fnbeh.2014.00346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeRamus TP, Kana RK. Anatomical likelihood estimation meta-analysis of grey and white matter anomalies in autism spectrum disorders. Neuroimage Clin. 2015;7:525–36. doi: 10.1016/j.nicl.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riddle K, Cascio CJ, Woodward ND. Brain structure in autism: a voxel-based morphometry analysis of the Autism Brain Imaging Database Exchange (ABIDE) Brain Imaging Behav. 2017;11:541–551. doi: 10.1007/s11682-016-9534-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Via E, Radua J, Cardoner N, et al. Meta-analysis of gray matter abnormalities in autism spectrum disorder: should Asperger disorder be subsumed under a broader umbrella of autistic spectrum disorder? Arch Gen Psychiatry. 2011;68:409–18. doi: 10.1001/archgenpsychiatry.2011.27. [DOI] [PubMed] [Google Scholar]
- 50.Mueller S, Keeser D, Samson AC, et al. Convergent findings of altered functional and structural brain connectivity in individuals with high functioning autism: a multimodal MRI study. PLoS ONE. 2013;8:e67329. doi: 10.1371/journal.pone.0067329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Björnsdotter M, Wang N, Pelphrey K, et al. Quantified social perception circuit activity as a neurobiological marker of ASD. JAMA Psychiatry. 2016;73:614–21. doi: 10.1001/jamapsychiatry.2016.0219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schaer M, Kochalka J, Padmanabhan A, et al. Sex differences in cortical volume and gyrification in autism. Mol Autism. 2015;6:42. doi: 10.1186/s13229-015-0035-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaiser MD, Hudac CM, Shultz S, et al. Neural signatures of autism. Proc Natl Acad Sci U S A. 2010;107:21223–8. doi: 10.1073/pnas.1010412107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Samson D, Apperly IA, Chiavarino C, et al. Left temporoparietal junction is necessary for representing someone else’s belief. Nat Neurosci. 2004;7:499–500. doi: 10.1038/nn1223. [DOI] [PubMed] [Google Scholar]
- 55.Besteher B, Gaser C, Langbein K, et al. Effects of subclinical depression, anxiety and somatization on brain structure in healthy subjects. J Affect Disord. 2017;215:111–7. doi: 10.1016/j.jad.2017.03.039. [DOI] [PubMed] [Google Scholar]
- 56.Phillipou A, Gurvich C, Castle DJ, et al. Comprehensive neurocognitive assessment of patients with anorexia nervosa. World J Psychiatry. 2015;5:404–11. doi: 10.5498/wjp.v5.i4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seitz J, Bühren K, von Polier GG, et al. Morphological changes in the brain of acutely ill and weight-recovered patients with anorexia nervosa. Z Kinder Jugendpsychiatr Psychother. 2013;42:7–18. doi: 10.1024/1422-4917/a000265. [DOI] [PubMed] [Google Scholar]
- 58.Joos A, Hartmann A, Glauche V, et al. Grey matter deficit in long-term recovered anorexia nervosa patients. Eur Eat Disord Rev. 2011;19:59–63. doi: 10.1002/erv.1060. [DOI] [PubMed] [Google Scholar]
- 59.Mainz V, Schulte-Rüther M, Fink GR, et al. Structural brain abnormalities in adolescent anorexia nervosa before and after weight recovery and associated hormonal changes. Psychosom Med. 2012;74:574–82. doi: 10.1097/PSY.0b013e31824ef10e. [DOI] [PubMed] [Google Scholar]
- 60.Treasure J, Corfield F, Cardi V. A three-phase model of the social emotional functioning in eating disorders. Eur Eat Disord Rev. 2012;20:431–8. doi: 10.1002/erv.2181. [DOI] [PubMed] [Google Scholar]
- 61.Treasure J, Schmidt U. The cognitive-interpersonal maintenance model of anorexia nervosa revisited: a summary of the evidence for cognitive, socio-emotional and interpersonal predisposing and perpetuating factors. J Eat Disord. 2013;1:13. doi: 10.1186/2050-2974-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon I, Wyk BCV, Bennett RH, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;111:1672–3. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]