Abstract
3,4-Methylenedioxypyrovalerone (MDPV) is a common constituent of illicit bath salts products, and in vitro studies implicate monoamine transporters as mediators of its pharmacological effects. Locomotor and thermoregulatory effects of MDPV depend on ambient temperature, so the current studies aimed to gauge the involvement of dopamine (DA), norepinephrine (NE), and serotonin (5-HT) in MDPV-induced locomotor stimulation and hyperthermia in the mouse at different ambient temperatures. Mice were pretreated with the selective 5-HT-reuptake inhibitor fluoxetine (3 mg/kg), the NE-reuptake inhibitor desipramine (3 mg/kg), the DA-reuptake inhibitor bupropion (10 mg/kg), or saline, followed by 10 mg/kg MDPV while thermoregulation and locomotor activity were monitored via radiotelemetry. In other studies, mice were pretreated for three days with saline, 100 mg/kg of the tryptophan hydroxylase inhibitor para-chlorophenylalanine (p-CPA), or 100 mg/kg of the tyrosine hydroxylase inhibitor α-methyl-para-tyrosine (α-MPT) before receiving 10 mg/kg MDPV on the fourth day. All manipulations were conducted at both 20°C and 28°C ambient temperatures. MDPV increased locomotor activity under both ambient conditions and modestly increased core body temperature at 20°C; however, neither pretreatment with monoamine reuptake inhibitors nor monoamine synthesis inhibitors significantly altered these effects. At 28°C, MDPV induced a more pronounced hyperthermic effect which was attenuated by bupropion, desipramine, or fluoxetine pretreatment, but not by the monoamine synthesis inhibitors. These results suggest that MDPV may have a more complex pharmacological profile than suggested by in vitro studies, perhaps extending beyond interactions with monoamine transporters. A more thorough binding profile of MDPV at various brain recognition sites should be developed.
Keywords: MDPV, monoamines, bath salts, locomotor activity, temperature
1. Introduction1
Globally, the use of synthetic designer drugs for non-medical purposes is increasing (Carroll et al., 2012; Hill and Thomas, 2011; United Nations Office of Drugs and Crime, 2016). Often used in club/rave settings, synthetic analogs of the khat-derived cathinone are sold as legal alternatives to illicit psychostimulant drugs of abuse, such as cocaine, 3,4-methylenedioxymethamphetamine (MDMA), or methamphetamine (Ross et al., 2011; Spiller et al., 2011). Names such as bath salts or plant food are often given to products containing these synthetic cathinones, and like other psychostimulant drugs of abuse, synthetic cathinones target monoamine transporters to either (1) inhibit monoamine reuptake (similar to cocaine) or (2) stimulate the release of monoamines (similar to amphetamine) at that specific transporter (Baumann et al., 2013; Eshleman et al., 2013; Simmler et al., 2013). As a result of the abuse and toxicity associated with bath salts preparations, thirteen synthetic cathinones were classified as Schedule I controlled substances in the United States (DEA, 2011; DEA, 2014).
Early studies reported 3,4-methylenedioxypyrovalerone (MDPV) to be the synthetic cathinone most often present in the blood and urine of patients admitted to emergency departments in the United States, often citing serious adverse effects including, but not limited to, agitation, psychosis, tachycardia, and death (Borek and Holstege, 2012; Kyle et al., 2011; Murray et al., 2012; Ross et al., 2011; Spiller et al., 2011). In vitro studies suggest MDPV is purely a reuptake inhibitor with potent actions at DAT and NET and minimal activity at SERT (Baumann et al., 2013; Eshleman et al., 2013; Simmler et al., 2013). In vivo, numerous assays support the notion that MDPV is psychostimulant-like in its effects. For instance in rodents, MDPV shares discriminative stimulus effects with cocaine, methamphetamine, and MDMA (e.g., Collins et al., 2016; Fantegrossi et al., 2013; Gannon et al., 2016; Gatch et al., 2013), is readily self-administered (e.g., Aarde et al., 2013; Gannon et al., 2017a; 2017b; 2017c; Schindler et al., 2016; Watterson et al., 2014), produces conditioned place preference (Karlsson et al., 2014; Gannon et al., 2017d), and stimulates locomotor activity (e.g, Fantegrossi et al., 2013; Gannon et al., 2016; Gatch et al., 2013). Moreover, MDPV has been shown to produce ambient temperature-dependent effects on locomotor stimulation and core body temperature (Fantegrossi et al., 2013; Gannon et al., 2016). Finally, microdialysis experiments have indicated that intravenously administered MDPV increases extracellular dopamine (Schindler et al., 2016).
Despite the widespread use of MDPV, its apparent toxicity in users, and the known interaction of this compound with monoamine transporters in vitro, only sparse information is available regarding its molecular mechanism of action in vivo. Since synthetic cathinones are often used in club or rave settings, of particular interest was how these mechanisms may vary depending on the ambient temperature. Because our lab has shown that MDPV produces ambient temperature-dependent effects on locomotor activity and core body temperature, these endpoints were used to test the hypothesis that, consistent with the in vitro data, interactions with DAT and NET (but not with SERT) would mediate the in vivo actions of MDPV in mice.
2. Materials and methods
2.1. Animals
All studies were carried out in accordance with the Guide for Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Arkansas for Medical Sciences. Male NIH Swiss mice (Harlan Sprague Dawley) weighing 20–25 g on delivery were housed three animals per cage (15.24 × 25.40 × 12.70 cm3) in a temperature-controlled room in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. Room conditions were maintained at 19±2°C and 45–50% humidity, with lights set to a 12 hr light/dark cycle. Animals were fed Lab Diet rodent chow (Laboratory Rodent Diet no. 5001, PMI Feeds, St. Louis, MO) ad libitum. All test conditions used groups of five or six mice, and all mice were drug naive (with the exception of surgical anesthetics) before testing.
2.2. Radiotelemetry of thermoregulation and locomotor activity
Once anesthetized with inhaled isoflurane, mice were implanted with a cylindrical glass-encapsulated radiotelemetry probe (model ER-4000 E-Mitter; Mini Mitter, Bend, OR) as previously described (Fantegrossi et al., 2013; Gannon et al., 2016). Surgeries were carried out at least 7 days before initiation of experimental conditions, allowing time for incisions to heal and for mice to recover normal body weights. Following surgery, all implanted mice were individually housed in 15.24 × 25.40 × 12.70 cm cages and provided free access to food and water for the duration of all telemetry experiments.
Implanted transmitters produced activity- and temperature-modulated signals that were sent to a receiver (model ER-4000 Receiver; Mini Mitter) situated underneath each cage inside standard light- and sound-attenuating chambers (Model ENV-022M; Med Associates) to minimize environmental variability during tests and to allow manipulation of the ambient temperature during experimental observations. Every 5 min, the computer collected two data updates from the probes—core temperature (in °C) on one channel and locomotor counts on the other. Each chamber was equipped with a house light (to establish a photoperiod) and an exhaust fan. An ambient temperature of 20°C was maintained by the HVAC system of the room, while commercial warm air space heaters mounted outside the chambers were used to elevate the ambient temperature to 28°C. Ambient temperatures were recorded every 5 min by data loggers within the chambers (Lascar EL-USB-1; MicroDAQ, Contoocook, NH), and could also be monitored via digital thermometers attached to each chamber. After at least 60 min of baseline data collection, mice (n=5 or 6) were removed from the chambers and injected with the pretreatment compound (or saline) and returned to the home cage for the duration of the pretreatment period before administration of 10 mg/kg MDPV. After MDPV, mice were again returned to the chambers and data were collected for at least 24 hr.
2.3. Pretreatment/Dosing Regimens
2.3.1. Monoamine uptake inhibition
Pretreatments of the selective SERT inhibitor fluoxetine (3 mg/kg), the NET-preferring inhibitor desipramine (3 mg/kg), the DAT-preferring inhibitor bupropion (10 mg/kg), or saline, occurred 30 minutes before administration of 10 mg/kg MDPV. The pretreatment doses used in the present study were the largest doses we tested that were within the dosing ranges used in the literature to selectively block monoamine reuptake (e.g., Eriksson et al., 2011; Fish et al., 2004; Klevin and Koek, 1998; Shen et al., 2013; Tiradentes et al., 2014; Valentini et al., 2006). Data obtained using smaller doses of the pretreatment compounds produced no effects on any of the endpoints examined (data not shown), and larger doses of the pretreatment compounds were not used in the present study because transporter selectivity would be unlikely to be maintained. The dose of MDPV was chosen for its temperature-dependent effects on locomotor activity and thermoregulation previously documented by our lab (Fantegrossi et al., 2013).
2.3.2. Monoamine synthesis inhibition
In another set of biotelemetry studies, mice were pretreated for three days with either saline, 100 mg/kg of the tryptophan hydroxylase inhibitor para-chlorophenylalanine (p-CPA), or 100 mg/kg of the tyrosine hydroxylase inhibitor α-methyl-para-tyrosine (α-MPT) before administration of 10 mg/kg MDPV on the fourth day. A 3-day pretreatment regimen was used because pretreatment with p-CPA in this manner has been previously shown to inhibit synthesis of 5-HT, thereby decreasing stores of 5-HT (Fantegrossi et al., 2005). Although a single pretreatment with 100 mg/kg α-MPT has been shown to decrease DA and NE (Thomas et al., 2008), catecholamine repletion has been shown to occur with extended periods of time (20 days) following α-MPT treatment (e.g., Lydiard et al., 1975). For this reason, and to be consistent with the 5-HT depletion regimen, 100 mg/kg α-MPT or saline control was also administered once daily for three consecutive days.
2.4. Verification of neurochemical depletion
Mice (n=12 per group) were injected every 24 hours with either saline, 100 mg/kg of the catecholamine synthesis inhibitor α-MPT, or 100 mg/kg of the 5-HT synthesis inhibitor p-CPA for 3 consecutive days, as described in section 2.3.2 above. Twenty four hours after the third injection, mice were sacrificed by cervical dislocation followed by decapitation so brain tissue could be harvested for neurochemical analysis. Brains were rapidly removed on ice, and cortex was quickly excised by blunt dissection using curved forceps. Samples were snap frozen in liquid nitrogen and stored at −80°C until assayed. All neurochemical analyses were conducted in the Division of Neurotoxicology at the National Center for Toxicological Research under the supervision of Syed F. Ali, Ph.D. (Jefferson, AR). Cortex samples were sonicated in ice-cold 0.2 M perchloric acid and centrifuged (15.500 × g, 7 min, 4°C), and the supernatants were used to determine the content of dopamine (DA) and metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA), and of 5-HT and metabolite 5-hydroxyindoleacetic acid (5-HIAA) by high-performance liquid chromatography (HPLC-ED) with an electrochemical detector, as previously described (Pereira et al., 2012; Silva et al., 2014). The pellet was resuspended in 1 mol/L sodium hydroxide and stored at −80°C for total protein quantification by the bicinchoninic acid protein assay (Thermo Fisher Scientific, MA, Waltham, USA). The concentration of DA and its metabolites were determined by comparison with peak areas of standards and expressed in nanograms per milligram of protein.
2.5. Data Analysis
Graphical presentation of all locomotor activity and core temperature data depict mean ± standard error of the mean (SEM). Although 24 hr of core temperature and locomotor data were collected following all injections, figures are truncated at 6 hr as measures had returned to control values. Time course data for core temperature are presented as 30 min means, whereas locomotor activity data have been binned in 30 min summation averages. Significant differences from saline and between ambient temperatures were determined via two-way analysis of variance (ANOVA), followed by pairwise comparisons using the Bonferroni method. Cortical levels of DA, DOPAC, 5-HT, 5-HIAA and HVA from mice treated with saline, α-MPT or p-CPA were compared using a one-way ANOVA followed by Tukey’s HSD post-hoc test and are summarized in Supplementary Table 1.
2.6. Drugs
MDPV was synthesized as a salt by Kenner C. Rice, Ph.D. at the National Institute on Drug Abuse (Bethesda, MD). α-MPT base was purchased from Sigma-Aldrich (St. Louis, MO). and reformulated as a salt in the laboratory of Peter A. Crooks, Ph.D. at the University of Arkansas for Medical Sciences (Little Rock, AR) and provided as a generous gift to the authors. p-CPA, desipramine, bupropion, and fluoxetine were purchased as salts from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in 0.9% physiological saline, and injections were administered intraperitoneally at a volume of 0.1 cc/10 g. Saline vehicle and all other experimental supplies were obtained from standard commercial sources.
3. Results
3.1. Monoamine transporter inhibitors
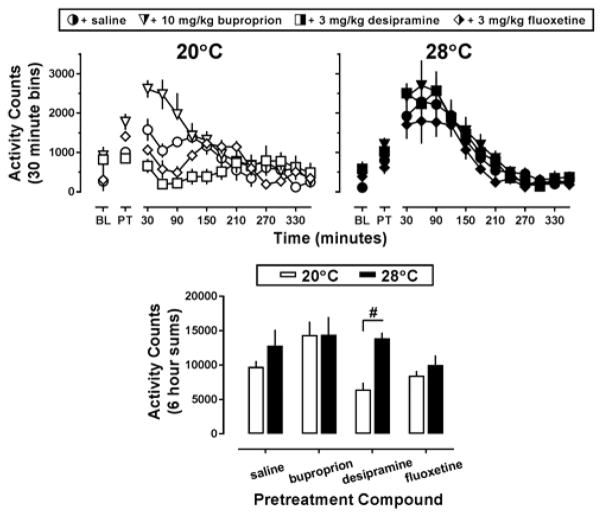
Pretreatment with saline followed by administration of MDPV increased locomotor activity from baseline levels, regardless of ambient temperature (Figure 1, top row, circles); however this effect was more pronounced at an ambient temperature of 28°C (Figure 1, top row-right panel, filled circles). Indeed, the two-way ANOVA indicated an overall effect of temperature (F[1,34]=6.85; p<0.05) and pretreatment compound (F[3,34]=3.68; p<0.05) but no interaction between the two. At an ambient temperature of 20°C, administration of 10 mg/kg bupropion alone increased locomotor activity compared to the pre-injection baseline, and this elevated activity baseline potentiated the locomotor stimulant effects of MDPV (Figure 1, top row-left panel, white triangles), while pretreatment with 3 mg/kg desipramine blunted the locomotor effects of MDPV (Figure 1, top row-left panel, white squares). Pretreatment with 3 mg/kg fluoxetine did not alter the effects of MDPV on locomotor activity (Figure 1, top row-left panel, white diamonds). Locomotor activity counts for each animal over a 6 hr period following administration of MDPV were summed and plotted as a function of ambient temperature (Figure 1, lower panel). While the time course data suggest that 10 mg/kg bupropion pretreatment potentiated the locomotor effects of MDPV while 3 mg/kg desipramine pretreatment decreased the locomotor effects of MDPV, as compared to saline pretreated animals, total locomotor counts over a 6 h period were not different from the saline treated control at the cool ambient temperature after either pretreatment drug (Figure 1, lower panel, white bars). Mice pretreated with 3 mg/kg desipramine had higher MDPV-stimulated activity counts over the course of 6 hours at 28°C than at 20°C (t=3.15; p<0.05); however, no other differences in MDPV-stimulated locomotor activity were observed in the other pretreatment groups between the two ambient temperatures.
Figure 1.
Top panel – Locomotor effects of 10 mg/kg MDPV following saline (circles), 10 mg/kg bupropion (triangles), 3 mg/kg desipramine (squares), or 3 mg/kg fluoxetine (diamonds) pretreatment at an ambient temperature of 20°C (left panel, white symbols) or 28°C (right panel, black symbols) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data collected before injection, while ‘PT’ denotes data during the 30 min pretreatment. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean activity counts calculated in 30 min bins. Lower panel – Locomotor effects of MDPV following pretreatment with saline or a monoamine reuptake inhibitor at an ambient temperature of 20°C (white bars) or 28°C (black bars). Abscissa: ‘SAL,’ ‘Bupropion,’ ‘Desipramine,’ and ‘Fluoxetine’ represent data following pretreatments as described above. Ordinate: mean total activity elicited by MDPV, recorded over 6 hr. Number sign indicates significant difference between ambient temperature conditions, p<0.05.
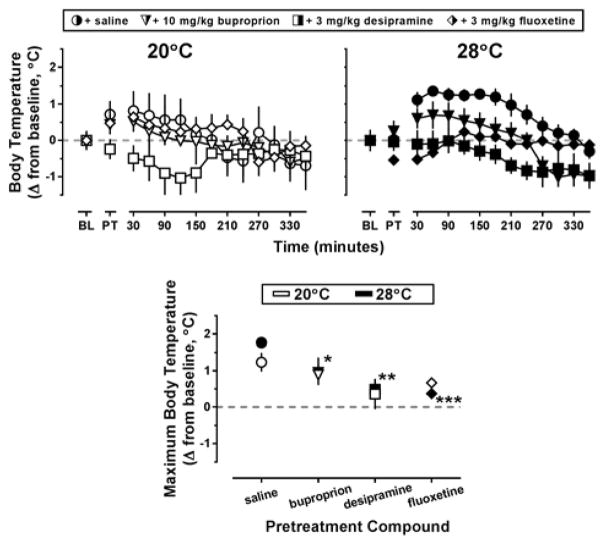
Pretreatment with saline followed by administration of MDPV increased core temperatures from baseline levels, regardless of ambient temperature; however increases in core temperature lasted longer at the warm ambient temperature (Figure 2, top row, circles). Results of the two-way ANOVA indicated a main effect of pretreatment compound (F[3,43]=8.11; p<0.001) but not ambient temperature on changes in body temperature. At an ambient temperature of 20°C, increases in core temperature were also observed in mice pretreated with 3 mg/kg fluoxetine and subsequently administered 10 mg/kg MDPV (Figure 2, top row-left panel, white diamonds); however at an ambient temperature of 28°C, this same pretreatment regimen resulted in only a transient decrease in core temperature (Figure 2, top row-left panel, black diamonds). Similar decreases in body temperature were also observed in mice pretreated with 3 mg/kg desipramine and subsequently administered 10 mg/kg MDPV, though this effect was not ambient temperature-dependent (Figure 2, top row, white squares). To compare these temperature data, the maximum core temperature for each animal over 6 hr following MDPV administration was determined, and the mean difference between this highest temperature obtained and baseline temperature was plotted (Figure 2, lower panel). Although the time course data suggest 3 mg/kg desipramine pretreatment blocked the slight hyperthermia observed following administration of MDPV at 20°C, the maximum change in body temperature recorded for desipramine pretreated mice was not significantly different from that observed in the saline controls (p=0.08). At 28°C, the time course data suggest bupropion, desipramine, and fluoxetine all block MDPV-induced hyperthermia. Indeed, the maximum change in body temperature recorded for bupropion (t=2.25, p<0.05), desipramine (t=3.53, p<0.01), and fluoxetine (t=4.04, p<0.001) pretreated mice was significantly different from that observed in the saline controls (Figure 2, lower panel, black symbols).
Figure 2.
Top panels – Thermoregulatory effects of 10 mg/kg MDPV following saline (circles), 10 mg/kg bupropion (triangles), 3 mg/kg desipramine (squares), or 3 mg/kg fluoxetine (diamonds) pretreatment at an ambient temperature of 20°C (left panel, white symbols) or 28°C (right panel, black symbols) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data collected before injection, while ‘PT’ denotes data during the 30 min pretreatment. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean changes in core temperature from baseline values, analyzed in 30 min bins (± SEM). Lower panel – Thermoregulatory effects of MDPV following pretreatment with saline or a monoamine reuptake inhibitor at an ambient temperature of 20°C (white bars) or 28°C (black bars). Abscissa: ‘SAL,’ ‘Bupropion,’ ‘Desipramine,’ and ‘Fluoxetine’ represent data following pretreatments as described above. Ordinate: mean maximum change in core temperature from baseline values elicited by MDPV injection over 6 hr. Asterisks indicate significant difference from saline controls, p<0.05.
3.2. Monoamine synthesis inhibitors
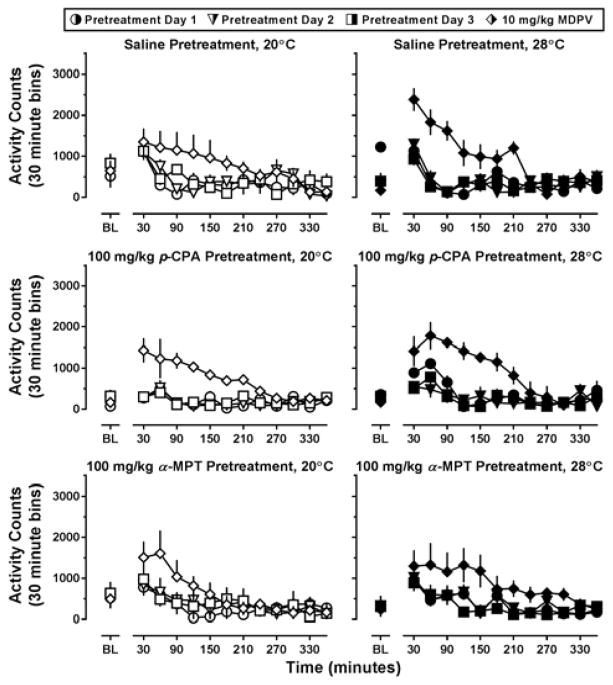
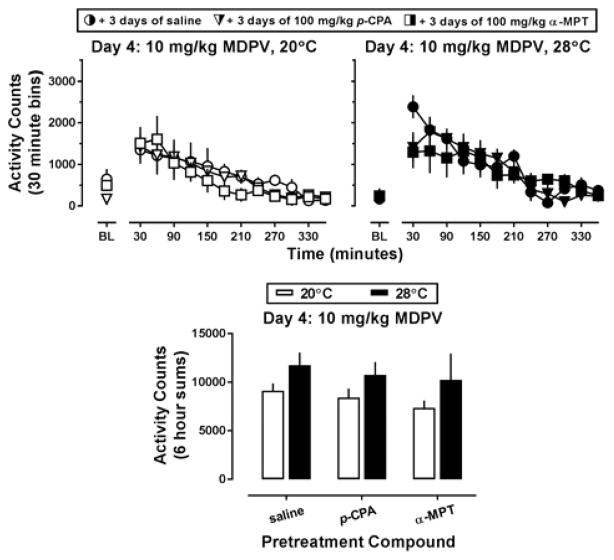
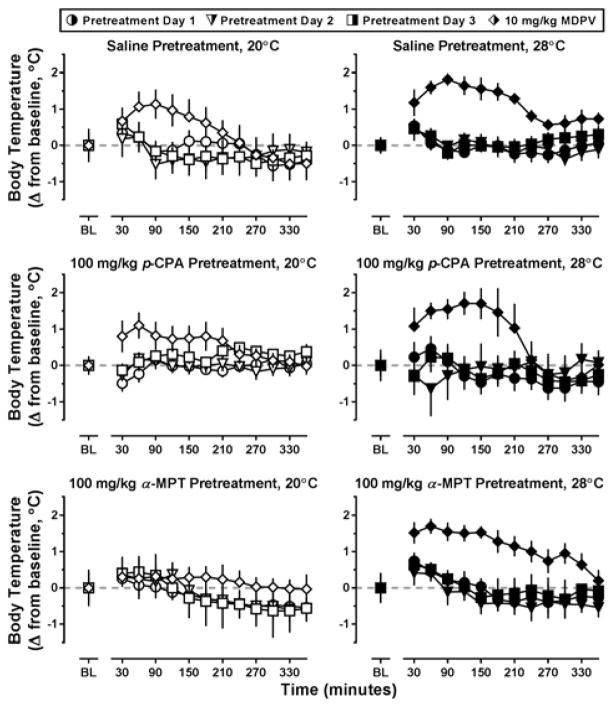
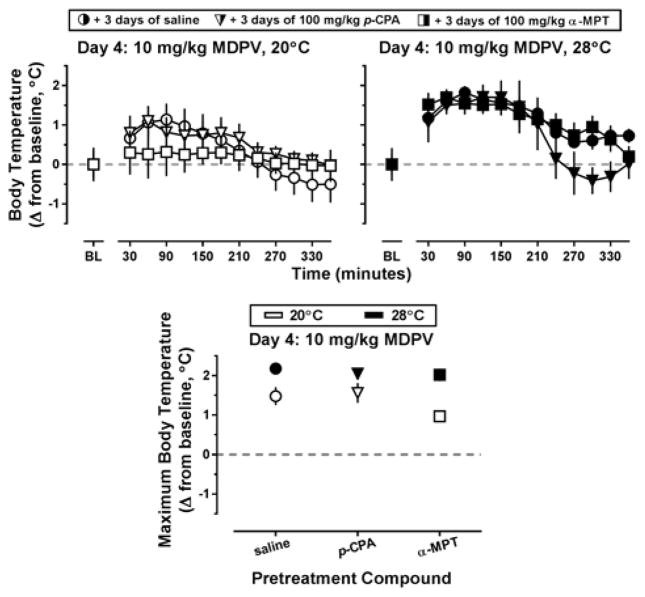
During the 3-day pretreatment interval with p-CPA or α-MPT, no systematic variability in locomotor activity was observed between groups as compared to saline-treated controls (Figure 3, all panels; circles, triangles, and squares, respectively). As such, locomotor data obtained on Day 4 (i.e., the day 10 mg/kg MDPV was administered) were compared among the groups directly (Figure 4). MDPV increased locomotor activity in all pretreatment groups, and this effect appeared more pronounced at an ambient temperature of 28°C (Figure 4, top row). Indeed, a main effect of ambient temperature (F[1,25]=4.78; p<0.05) was detected using two way ANOVA on the activity counts collected over the 6 hours following MDPV administration (Figure 4, lower panel). Although ambient temperature altered MDPV-stimulated locomotor activity, this was not dependent on pretreatment, and no differences in locomotor activity were observed between the saline, p-CPA, or α-MPT treated groups of mice, even though cortex levels of 5-HT and 5-HIAA had been decreased (by 72.79 ± 4.91% and 88.67 ± 1.93%, respectively) in the p-CPA pretreated mice and DA and DOPAC had been reduced (by 62.04 ± 8.22% and 46.60 ± 7.61%, respectively) in the α-MPT pretreated mice as compared to saline controls (Supplementary Table 1).
Figure 3.
Locomotor effects of three days of saline (top row), 100 mg/kg p-CPA (middle row), or 100 mg/kg α-MPT (bottom row) pretreatment, then of 10 mg/kg MDPV on day 4 (diamonds, all rows) at an ambient temperature of 20°C (left panels) or 28°C (right panels) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data before injection. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean activity counts calculated in 30 min bins.
Figure 4.
Top panels – Locomotor effects of 10 mg/kg MDPV when preceded by 3 days of pretreatment with saline (circles), 100 mg/kg p-CPA (triangles), or 100 mg/kg α-MPT (squares) at an ambient temperature of 20°C (left panel, white symbols) or 28°C (right panel, black symbols) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data before injection. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean activity counts calculated in 30 min bins. Lower panel – Locomotor effects of MDPV following pretreatment with saline or a monoamine synthesis inhibitor at an ambient temperature of 20°C (white bars) or 28°C (black bars). Abscissa: ‘SAL,’ ‘p-CPA,’ and ‘α-MPT’ represent MDPV challenge data in mice pretreated as described above. Ordinate: mean total activity recorded across the 6 hours immediately following MDPV administration.
Similar to the locomotor data, no systematic variability in thermoregulation was observed between saline, p-CPA or α-MPT groups during the three pretreatment days, regardless of ambient temperature (Figure 5, all panels; circles, triangles, and squares, respectively). As such, core temperature data obtained on Day 4 was directly compared among pretreatment groups in Figure 6. Three days of pretreatment with p-CPA did not alter MDPV-induced increases in body temperature at either ambient temperature (triangles), whereas pretreatment with α-MPT blocked increases in core body temperature at an ambient temperature of 20°C but not at 28°C (squares). To compare core temperature of these α-MPT pretreated mice to saline controls, maximum core temperature for each animal over 6 hr following MDPV administration was determined, and the mean difference between this highest temperature obtained and baseline temperature was plotted (Figure 6, lower panel). The two-way ANOVA detected a main effect of ambient temperature (F[1,25]=31.1; p<0.0001) on change in core body temperature, and increases in body temperature were significantly greater at 28°C than 20°C for mice pretreated with saline (t=3.11; p<0.05) or α-MPT (t=4.46; p<0.001).
Figure 5.
Thermoregulatory effects of three days of saline (top row), 100 mg/kg p-CPA (middle row), or 100 mg/kg α-MPT (bottom row) pretreatment, then of 10 mg/kg MDPV on day 4 (diamonds, all rows) at an ambient temperature of 20°C (left panels) or 28°C (right panels) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data before injection. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean change in core temperature from baseline values, calculated in 30 min bins.
Figure 6.
Top panels – Thermoregulatory effects of 10 mg/kg MDPV when preceded by 3 days of pretreatment with saline (circles), 100 mg/kg p-CPA (triangles), or 100 mg/kg α-MPT (squares) at an ambient temperature of 20°C (left panel, white symbols) or 28°C (right panel, black symbols) in NIH Swiss mice (n=5–6). Abscissa: ‘BL’ denotes 30 min baseline data before injection. Numbers refer to time, in minutes, following MDPV administration. Ordinate: mean change in core temperature from baseline values, calculated in 30 min bins. Lower panel – Maximum change in core temperature elicited by MDPV following pretreatment with saline or a monoamine synthesis inhibitor at an ambient temperature of 20°C (white symbols) or 28°C (black symbols). Abscissa: ‘SAL,’ ‘p-CPA,’ and ‘α-MPT’ represent MDPV challenge data in mice pretreated as described above. Ordinate: mean maximum change in core temperature recorded across the 6 hours immediately following MDPV administration. Number signs indicate significant difference between ambient temperature conditions, # = p<0.05; ### = p<0.001.
4. Discussion
The studies reported here include the first evaluation of the role of monoamine reuptake inhibition or synthesis inhibition on the locomotor stimulant and hyperthermic effects of bath salts constituent MDPV. Previously, we examined the role of ambient temperature on the locomotor stimulant and the thermoregulatory effects of MDPV and demonstrated that 10 mg/kg MDPV induced locomotor stimulation at both 20 and 28°C – although this effect was more pronounced at the warmer ambient temperature, and this higher ambient temperature also resulted in MDPV-induced increases in body temperature (Fantegrossi et al., 2013). The present findings replicate these previous results, and because interaction with monoamine transporters has been suggested as the mechanism of action of MDPV, the present studies aimed to link monoamine transporter inhibition or monoamine content to these ambient temperature-dependent effects.
Thus, in order to systematically assess the role of each of the three monoamine transporters, the first set of experiments used radiotelemetry to simultaneously monitor locomotor activity and core temperature changes in response to MDPV administration 30 minutes following pretreatment with the selective 5-HT reuptake inhibitor fluoxetine (3 mg/kg), the selective NE reuptake inhibitor desipramine (3 mg/kg), the DA reuptake inhibitor bupropion (10 mg/kg), or saline. Interestingly, these studies found that inhibition of monoamine reuptake had no significant effects on MDPV-stimulated locomotor activity at either the cool or warm ambient temperature. Under both ambient conditions, pretreatment with the DA reuptake inhibitor bupropion was sufficient to increase locomotor activity even before MDPV was administered. Although MDPV-elicited locomotor stimulation is likely due, at least in part, to increases in DA in the synapse, because bupropion pretreatment (alone) resulted in increased locomotor activity (under both ambient conditions), the mechanism of MDPV-induced hyperactivity could not be determined by these experiments. Perhaps the locomotor stimulant effects of MDPV are due to fluctuations in synaptic DA sufficient to saturate any post-synaptic receptors, thereby creating a “ceiling” effect on the observable activity. Further studies involving selective antagonists for post-synaptic DA, NE and 5-HT receptors should be conducted to test this hypothesis. It is also possible that the inability of the monoamine reuptake inhibitors to alter MDPV-stimulated locomotor activity may be because this effect is not regulated by monoamines and is instead regulated by non-monoaminergic systems within the CNS. Caffeine, for example, increases locomotor activity via antagonism of adenosine receptors (Finn and Holtzman, 1987); however, the interaction of MDPV with adenosine receptors (or other potential sites) has not yet been determined, and it is important to note that the downstream actions of caffeine involve DA (e.g., Ferré 2016). Thus, the supposition that MDPV-induced locomotor stimulation is DA independent seems improbable.
While locomotor activity at 28°C was not altered by pretreatment with monoamine reuptake inhibitors, pretreatment with bupropion, desipramine, or fluoxetine blocked the hyperthermia elicited by MDPV at this ambient temperature, implicating DA, NE, and 5-HT in MDPV-induced hyperthermia. Interestingly, the effects of fluoxetine pretreatment on MDPV-induced hyperthermia were significant, despite in vitro studies suggesting that MDPV has minimal affinity for SERT (Baumann et al., 2013; Simmler et al., 2013); however, the role of ambient temperature was not considered in these in vitro experiments. As such, it is possible that thermoregulatory responses may be differentially regulated depending on ambient temperature. Similarly, there is a fairly pronounced inconsistency in the degree of selectivity exhibited by MDPV among the monoamine transporters across different reports based on in vitro experiments, with one set of studies reporting an 800-fold selectivity of MDPV for DAT over SERT (Baumann et al, 2013) and another reporting a much less dramatic selectivity of ~100-fold (Eshleman et al., 2013). It may be the case that MDPV interacts with SERT in vivo to a greater degree than would be predicted based upon in vitro studies, and the presently demonstrated capacity of fluoxetine to attenuate MDPV-elicited hyperthermia at high ambient temperatures is consistent with this notion.
In the second set of experiments, mice received 3 days of pretreatment with the 5-HT synthesis inhibitor p-CPA (100 mg/kg), the DA and NE synthesis inhibitor α-MPT (100 mg/kg), or equivolume saline, followed by 10 mg/kg MDPV on the fourth day. Thus, at the time of MDPV administration, mice treated with p-CPA were depleted of cortical 5-HT by approximately 75% and its primary acidic metabolite 5-HIAA was reduced by almost 90%, while mice treated with α-MPT were depleted of cortical DA by roughly 60% and its primary acidic metabolite DOPAC was similarly reduced by approximately 50%. But like the data obtained in the first set of experiments, we discovered monoamine synthesis inhibition had no significant effects on locomotor activity regardless of ambient temperature. Because stores of DA within the cortex were only decreased by approximately 60%, it is possible that the remaining 40% of the basal DA level was sufficient to mediate the locomotor stimulant effects of MDPV. Furthermore, it is important to note that different observed effects are mediated by different brain regions, and there is no reason to assume that the measured decreases in monoamine content within the cortex due to pretreatment with either p-CPA or α-MPT are uniform throughout the brain. As it pertains to the present studies, locomotor stimulant effects of traditional psychostimulants are mediated by midbrain dopaminergic circuits (Uhl et al., 2002), while thermoregulation is related to serotonergic neurons within the hypothalamus (Lin et al., 1983). As such, in future experiments, it will be imperative to collect and analyze tissue from animals treated with monoamine synthesis inhibitors to quantify monoamine content across multiple brain regions, particularly those most relevant to in vivo endpoints of interest.
While pretreatment with monoamine synthesis inhibitors did not alter locomotor activity, pretreatment with the catecholamine synthesis inhibitor α-MPT blocked hyperthermia elicited by MDPV at the cool ambient temperature, implicating DA and/or NE in the MDPV-induced changes in core temperature. However, decreased monoaminergic stores did not affect MDPV-induced hyperthermia observed in a warm environment. The effects of increased ambient temperature on thermoregulation are complex, and become even more so when drugs which challenge thermoregulation (like MDPV) are administered. It may be the case that increased environmental stress imposed by the higher ambient temperature overwhelms the otherwise protective effects of α-MPT treatment against MDPV-elicited hyperthermia. Future studies should be designed to better understand the mechanisms by which increased ambient temperature contributes to MDPV-induced hyperthermic effects, which appear to be resistant to treatment with drugs targeting monoamines. As use of MDPV and related cathinone-derived compounds continues to increase in settings with high ambient temperatures (clubs, raves, festivals, etc.) it will become critical for patient care to devise effective treatments for acute toxicities elicited by MDPV in these challenging settings.
5. Conclusions
Taken collectively, it appears the mechanisms mediating the biological actions of MDPV may be more complicated than suggested by in vitro experiments, and ambient temperature may be a critical factor in the regulation of both locomotor and thermoregulatory responses to MDPV. A more thorough binding profile of MDPV at various brain recognition sites should be developed to gain further insight on the complex relationship between ambient temperature and mechanism of action. Given that synthetic cathinones are common club/rave drugs, further in vivo and in vitro studies with MDPV and structurally-related bath salts constituents should consider the role of ambient temperature in their findings, and temperature should be carefully controlled to whatever extent possible.
Supplementary Material
Levels of monoamines and metabolites measured in cortical samples collected from MDPV-naïve mice 24 hours following three consecutive days of saline, 100 mg/kg α-MPT, or 100 mg/kg p-CPA pretreatment (n=6 per pretreatment).
Highlights.
MDPV elicits ambient temperature-dependent effects on locomotor activity and thermoregulation.
Pretreatment with a DAT, NET, or SERT selective uptake inhibitor did not attenuate MDPV-induced locomotor stimulation at 20°C or 28°C.
Pretreatment with a DAT, NET, or SERT selective uptake inhibitor attenuated MDPV-induced hyperthermia only at 28°C.
Treatment with a-MPT significantly decreased dopamine and its major metabolite in the cortex, but did not blunt the effects of MDPV.
Treatment with p-CPA significantly decreased serotonin and its major metabolite in the cortex, but did not attenuate the effects of MDPV.
Footnotes
Abbreviations: 3,4-methylenedioxypyrovalerone (MDPV), 3,4-methylenedioxymethcathinone (methylone), 4-methylmethcathinone (mephedrone), Drug Enforcement Administration (DEA), dopamine (DA), norepinephrine (NE), serotonin (5-HT), dopamine transporter (DAT), norepinephrine transporter (NET), serotonin transporter (SERT), para-chlorophenylalanine (p-CPA), α-methyl-para-tyrosine (α-MPT), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA), analysis of variance (ANOVA), standard error of the mean (SEM)
Funding and disclosure: This research was conducted as part of the doctoral dissertation of BMG. These studies were supported in part by the University of Arkansas for Medical Sciences Center for Translational Neuroscience [Grant GM110702], the University of Arkansas for Medical Sciences Translational Research Institute [Grant RR029884], and the National Institutes of Health National Institute on Drug Abuse [Grant DA039195]; a training grant from the National Institutes of Health [T32 DA022981]; and the Intramural Research Programs of the National Institutes of Health. The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aarde SM, Huang PK, Creehan KM, Dickerson TJ, Taffe MA. The novel recreational drug 3,4-methylenedioxypyrovalerone (MDPV) is a potent psychomotor stimulant: self-administration and locomotor activity in rats. Neuropharmacology. 2013;71:130–40. doi: 10.1016/j.neuropharm.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Partilla JS, Lehner KR, Thorndike EB, Hoffman AF, Holy M, et al. Powerful cocaine-like actions of 3,4-methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive ‘bath salts’ products. Neuropsychopharmacology. 2013;38:552–562. doi: 10.1038/npp.2012.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borek HA, Holstege CP. Hyperthermia and multiorgan failure after abuse of ‘bath salts’ containing 3,4-methylenedioxypyrovalerone. Ann Emerg Med. 2012;60:103–105. doi: 10.1016/j.annemergmed.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Carroll FI, Lewin AH, Mascarella SW, Seltzman HH, Reddy PA. Designer drugs: a medicinal chemistry perspective. Ann N Y Acad Sci. 2012;1248:18–38. doi: 10.1111/j.1749-6632.2011.06199.x. [DOI] [PubMed] [Google Scholar]
- Collins GT, Abbott M, Galindo K, Rush EL, Rice KC, France CP. Discriminative Stimulus Effects of Binary Drug Mixtures: Studies with Cocaine, MDPV, and Caffeine. J Pharmacol Exp Ther. 2016;359:1–10. doi: 10.1124/jpet.116.234252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration Department of Justice. Schedules of controlled substances: temporary placement of three synthetic cathinones into Schedule I. Final Order. Fed Regist. 2011;76:65371–65375. [PubMed] [Google Scholar]
- Drug Enforcement Administration Department of Justice. Schedules of controlled substances: temporary placement of 10 synthetic cathinones into Schedule I. Final Order. Fed Regist. 2014;79:12938–12943. [PubMed] [Google Scholar]
- Eriksson O, Långström B, Josephsson R. Assessment of receptor occupancy-over-time of two dopamine transporter inhibitors by [(11)C]CIT and target controlled infusion. Ups J Med Sci. 2011;116:100–106. doi: 10.3109/03009734.2011.563878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshleman AJ, Wolfrum KM, Hatfield MG, Johnson RA, Murphy KV, Janowsky A. Substituted methcathinones differ in transporter and receptor interactions. Biochem Pharmacol. 2013;85:1803–1815. doi: 10.1016/j.bcp.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Gannon BM, Zimmerman SM, Rice KC. In vivo effects of abused ‘bath salt’ constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice: drug discrimination, thermoregulation, and locomotor activity. Neuropsychopharmacology. 2013;38:563–573. doi: 10.1038/npp.2012.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Kiessel CL, De la Garza RII, Woods JH. Serotonin synthesis inhibition reveals distinct mechanisms of action for MDMA and its enantiomers in the mouse. Psychopharmacology (Berl) 2005;181:529–536. doi: 10.1007/s00213-005-0005-8. [DOI] [PubMed] [Google Scholar]
- Ferré S. Mechanisms of the psychostimulant effects of caffeine: implications for substance use disorders. Psychopharmacology (Berl) 2016;233:1963–1973. doi: 10.1007/s00213-016-4212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn IB, Holtzman SG. Pharmacologic specificity of tolerance to caffeine-induced stimulation of locomotor activity. Psychopharmacology (Berl) 1987;93:428–434. doi: 10.1007/BF00207230. [DOI] [PubMed] [Google Scholar]
- Fish EW, Faccidomo S, Gupta S, Miczek KA. Anxiolytic-like effects of escitalopram, citalopram, and R-citalopram in maternally separated mouse pups. J Pharmacol Exp Ther. 2004;308:474–480. doi: 10.1124/jpet.103.058206. [DOI] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Rice KC, Collins GT. Individual Differences in the Relative Reinforcing Effects of 3,4-Methylenedioxypyrovalerone under Fixed and Progressive Ratio Schedules of Reinforcement in Rats. J Pharmacol Exp Ther. 2017a;361:181–189. doi: 10.1124/jpet.116.239376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Galindo KI, Mesmin MP, Rice KC, Collins GT. Reinforcing Effects of Binary Mixtures of Common bath salts Constituents: Studies with 3,4-Methylenedioxypyrovalerone (MDPV), 3,4-Methylenedioxymethcathinone (Methylone), and Caffeine in Rats. Neuropsychopharmacology. 2017b doi: 10.1038/npp.2017.141. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Rice KC, Collins GT. Reinforcing effects of abused “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and α-pyrrolidinopentiophenone (α-PVP) and their enantiomers. Behav Pharmacol. 2017c doi: 10.1097/FBP.0000000000000315. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Russell LN, Modi MS, Rice KC, Fantegrossi WE. Reinforcing, locomotor, and appetitive stimulus effects of orally self-administered bath salt constituent 3,4-methylenedioxypyrovalerone (MDPV) in mice. Drug Alcohol Depend. 2017d doi: 10.1016/j.drugalcdep.2017.06.031. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon BM, Williamson A, Suzuki M, Rice KC, Fantegrossi WE. Stereoselective effects of abused "bath salt" constituent 3,4-Methylenedioxypyrovalerone in mice: drug discrimination, locomotor activity, and thermoregulation. J Pharmacol Exp Ther. 2016;356:615–623. doi: 10.1124/jpet.115.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatch MB, Taylor CM, Forster MJ. Locomotor stimulant and discriminative stimulus effects of ‘bath salt’ cathinones. Behav Pharmacol. 2013;24:437–447. doi: 10.1097/FBP.0b013e328364166d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SL, Thomas SH. Clinical toxicology of newer recreational drugs. Clin Toxicol (Phila) 2011;49:705–719. doi: 10.3109/15563650.2011.615318. [DOI] [PubMed] [Google Scholar]
- Karlsson L, Andersson M, Kronstrand R, Kugelberg FC. Mephedrone, Methylone and 3,4-Methylenedioxypyrovalerone (MDPV) Induce Conditioned Place Preference in Mice. Basic Clin Pharmacol Toxicol. 2014;115:411–416. doi: 10.1111/bcpt.12253. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Koek W. Discriminative stimulus properties of cocaine: enhancement by monoamine reuptake blockers. J Pharmacol Exp Ther. 1998;284:1015–1025. [PubMed] [Google Scholar]
- Kyle PB, Iverson RB, Gajagowni RG, Spencer L. Illicit bath salts: not for bathing. J Miss State Med Assoc. 2011;52:375–377. [PubMed] [Google Scholar]
- Lin MT, Wu JJ, Tsay BL. Serotonergic mechanisms in the hypothalamus mediate thermoregulatory responses in rats. Naunyn Schmiedebergs Arch Pharmacol. 1983;322:271–8. doi: 10.1007/BF00508342. [DOI] [PubMed] [Google Scholar]
- Lydiard RB, Fossom LH, Sparber SB. Postnatal elevation of brain tyrosine hydroxylase activity, without concurrent increases in steady-state catecholamine levels, resulting from dl-alpha-methylparatyrosine administration during embryonic development. J Pharmacol Exp Ther. 1975;194:27–36. [PubMed] [Google Scholar]
- Murray BL, Murphy CM, Beuhler MC. Death following recreational use of designer drug “bath salts” containing 3,4-Methylenedioxypyrovalerone (MDPV) J Med Toxicol. 2012;8:69–75. doi: 10.1007/s13181-011-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira FC, Cunha-Oliveira T, Viana SD, Travassos AS, Nunes S, Silva C, et al. Disruption of striatal glutamatergic/GABAergic homeostasis following acute methamphetamine in mice. Neurotoxicol Teratol. 2012;34(5):522–529. doi: 10.1016/j.ntt.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Ross EA, Watson M, Goldberger B. “Bath salts” intoxication. N Engl J Med. 2011;365:967–968. doi: 10.1056/NEJMc1107097. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Thorndike EB, Goldberg SR, Lehner KR, Cozzi NV, Brandt SD, et al. Reinforcing and neurochemical effects of the “bath salts” constituents 3,4-methylenedioxypyrovalerone (MDPV) and 3,4-methylenedioxy-N-methylcathinone (methylone) in male rats. Psychopharmacology (Berl) 2016;233:1981–90. doi: 10.1007/s00213-015-4057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Tsuruda PR, Smith JA, Obedencio GP, Martin WJ. Relative contributions of norepinephrine and serotonin transporters to antinociceptive synergy between monoamine reuptake inhibitors and morphine in the rat formalin model. PLoS One. 2013;8:e74891. doi: 10.1371/journal.pone.0074891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva CD, Neves AF, Dias AI, Freitas HJ, Mendes SM, Pita I, et al. A single neurotoxic dose of methamphetamine induces a long-lasting depressive-like behaviour in mice. Neurotox Res. 2014;25:295–304. doi: 10.1007/s12640-013-9423-2. [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2013;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49:499–505. doi: 10.3109/15563650.2011.590812. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Francescutti-Verbeem DM, Kuhn DM. The newly synthesized pool of dopamine determines the severity of methamphetamine-induced neurotoxicity. J Neurochem. 2008;105:605–16. doi: 10.1111/j.1471-4159.2007.05155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiradentes RV, Pires JG, Silva NF, Ramage AG, Santuzzi CH, Futuro Neto HA. Effects of acute administration of selective serotonin reuptake inhibitors on sympathetic nerve activity. Braz J Med Biol Res. 2014;47:554–559. doi: 10.1590/1414-431X20143698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Hall FS, Sora I. Cocaine, reward, movement and monoamine transporters. Mol Psychiatry. 2002;7:21–6. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime. World Drug Report 2016. United Nations publication; 2016. Sales No. E.16.XI.7. [Google Scholar]
- Valentini V, Cacciapaglia F, Frau R, Di Chiara G. Differential alpha-mediated inhibition of dopamine and noradrenaline release in the parietal and occipital cortex following noradrenaline transporter blockade. J Neurochem. 2006;98:113–121. doi: 10.1111/j.1471-4159.2006.03851.x. [DOI] [PubMed] [Google Scholar]
- Watterson LR, Kufahl PR, Nemirovsky NE, Sewalia K, Grabenauer M, Thomas BF, et al. Potent rewarding and reinforcing effects of the synthetic cathinone 3,4-methylenedioxypyrovalerone (MDPV) Addict Biol. 2014;19:165–74. doi: 10.1111/j.1369-1600.2012.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Levels of monoamines and metabolites measured in cortical samples collected from MDPV-naïve mice 24 hours following three consecutive days of saline, 100 mg/kg α-MPT, or 100 mg/kg p-CPA pretreatment (n=6 per pretreatment).