Abstract
Neuron-Glia crosstalk is essential for efficient synaptic communication, cell growth and differentiation, neuronal activity, neurotransmitter recycling, and brain immune response. The master regulators of this neuron-glia communication are connexin containing Gap Junctions (GJs) and Hemichannels (HCs) as well as pannexin HCs. However, the role of these channels under pathological conditions, especially in infectious diseases is still in exploratory stages. Human Immunodeficiency Virus-1 (HIV) is one such infectious agent that takes advantage of the host intercellular communication systems, GJs and HCs, to exacerbate viral pathogenesis in the brain in spite of the antiretroviral therapy effectively controlling viral replication in the periphery. Although most infectious agents lead to total “shutdown” of gap junctional communication in parenchymal cells, HIV infection maintains and “hijacks” GJs and HCs to enable few infected cells to spread toxic intracellular agents to neighboring uninfected cells aggravating viral neuropathology even in the absence of viral replication. In this mini-review, we present a comprehensive overview of the role of GJs and HCs in augmenting HIV neuropathogenesis.
Keywords: HIV, Gap junction, Hemichannel, AIDS, Reservoirs
Introduction
After more than three decades of Human Immunodeficiency Virus (HIV)/Acquired Immunodeficiency Syndrome (AIDS) epidemic, the scientific community is still struggling to eradicate the virus from the 36 million HIV-infected individuals. The introduction of antiretroviral therapy (ART) has increased the life expectancy of HIV-infected individuals, but we are still far from a cure [1]. In the current ART scenario, the prevalence of HIV-Associated Neurocognitive Disorders (HANDs) in 50–60% of HIV-infected individuals has become a major public health concern [2]. HIV invades the brain early after primary infection and continues to harbor establishing viral reservoirs, despite effective ART and suppression of viral replication in the periphery [3]. Although the extent of HIV infection in the Central Nervous System (CNS) is fairly limited (perivascular macrophages, microglia, and astrocytes), the magnitude of neuropathogenesis observed does not correlate with the viral replication. Instead, it correlates with unidentified amplification systems used by HIV to spread toxicity and apoptosis. We propose that one of the amplification mechanisms employed by HIV is mediated by Gap Junctions (GJs) and Hemichannels (HCs) between HIV-infected cells surviving in the presence of ART and neighboring uninfected brain cells. In this review, we will elaborate on the role of Connexin (Cx) and Pannexin (Panx) containing GJ channels and HCs in HIV neuropathogenesis.
Gap junctions and hemichannels
Gap junctions are the only communication system in multicellular organisms that allows direct exchange of intracellular metabolites and electrical signals between the connected cells. Mammalian GJs are composed of hexameric assemblies of specific membrane proteins (Connexins, Cxs) from adjacent cells that juxtapose each other to form a channel creating a direct exchange hub between the two cells [4]. GJs refer to clusters of these channels in plasma membrane microdomains that result from head-to-head docking of two HCs, each contributed by one participating cell. Unopposed HCs (uHCs) may also open on the cell surface, where they serve to establish communication between the cytoplasm and the extracellular space. Other than Cxs, uHCs may also be composed of another family of proteins, Pannexins (Panxs). In contrast to Cxs, Panxs have only been documented to form uHCs [5]. GJs and uHCs have an internal pore diameter of 12 nm rotation, and allow bi-directional flow of ions (Ca2+), second messengers (ATP, cAMP, inositol triphosphate, IP3), and small molecules (small RNA, neurotransmitters, glutamate) across the connected cells or between the cytoplasm and the extracellular matrix [4]. Although uHCs are typically closed during the resting state, once open, uHCs release NAD+, glutamate, ATP, and ions into the extracellular milieu [6].
Historically, it has been believed that inflammation reduces Cx expression and GJ-mediated communication in parenchymal cells [7]. Consequently, several viral and bacterial infections lead to downregulation of Cx expression and GJ-mediated communication [8]. In astrocytes, GJs and uHCs are reciprocally regulated at the primary site of inflammation, where GJ-mediated communication is reduced while uHC activity is enhanced during acute stages [9]. Enhanced uHC activity has been documented in several cellular stress events such as hypoxia [10], oxidative stress [11], and inflammation [12]. Since the opening of astroglial uHCs has been known to assist in the release of ATP, glutamate, and NAD+ into the extracellular space, astrocytic dysfunction and enhanced uHC activity would compromise the trophic and metabolic support to neurons enhancing neuronal vulnerability. However, the mechanisms by which GJs and uHCs contribute to disease pathology are still unknown.
Connexin and Pannexin expression and their role in the CNS
The brain is composed of neurons, astrocytes, oligodendrocytes, microglia, ependymal cells and endothelial cells. All of these are connected and coordinated by GJs and uHCs. Table 1 briefly represents the expression of Cx and Panx proteins as well as their role in several of these brain cells types before discussing their involvement in HIV infection and NeuroAIDS.
Table 1.
Expression of Cx and Panx proteins and their role in CNS cells.
| Cell type | Connexin/Pannexin expression | Functions | |
|---|---|---|---|
| Astrocytes | Cx26[13], Cx30[14], Cx43[14] | Cx GJs | Adult neurogenesis[15], Glutamate transport[16], Glucose trafficking[17], Regulation of K+[18] and Na+[19] concentration |
| Panx1[20] | Cx/Panx uHCs | Neurotoxicity[21] | |
| Neurons | Cx36[14], Cx45[22], Cx50[23], Cx57[24] | Cx GJs | Electric and metabolic coupling[25], Spatial memory[26] |
| Panx1[20], Panx2[20] | CX/Panx uHCs | Electrical coupling[20], Neuronal development, adult neurogenesis[27], Synaptic plasticity[28] | |
| Microglia | Cx32[29], Cx36[30], Cx43[31] | Cx GJs | Do not express GJs under resting conditions |
| Panx1[32] | Cx/Panx uHCs | Glutamate release[29], Neurotoxicity[33], Inflammation[34] | |
| Oligodendrocytes | Cx29[35], Cx32[35], Cx45[36], Cx47[35] | Cx GJs | Maintenance of ionic homeostasis[37], Myelination[38] |
| Panx1[39] | Cx/Panx uHCs | Formation of uHCs has not been reported | |
Role of gap junctions and hemichannels during HIV infection
Only recently, several laboratories including ours have demonstrated that GJs and uHCs play a significant role in HIV life cycle as well as in the pathogenesis of NeuroAIDS. In the following section, we have given a brief introduction of HIV pathogenesis before discussing in detail the role of GJs and uHCs in HIV pathogenesis.
HIV pathogenesis: General introduction
Since its first clinical observation, HIV/AIDS has claimed more than 35 million lives worldwide. Although ART has been highly effective in suppressing systemic HIV replication, persistence of HIV in latent “viral reservoirs” in the human body still poses a challenge for the complete elimination of the virus from the infected individuals [3, 40]. It has been speculated that the human brain acts as a latent reservoir for HIV, as several antiretrovirals have poor penetration across the blood-brain barrier (BBB) [40, 41].
HIV enters the brain via Trojan horse mechanism, hiding inside the blood-borne monocytes [42]. Once inside the brain, HIV-infected monocytes/macrophages promote neuroinflammation by secreting viral proteins and proinflammatory cytokines and chemokines which aid in further recruitment of uninfected and HIV-infected cells into the brain [43]. The cell types harboring HIV infection in the brain are perivascular macrophages, microglia, and a small population of astrocytes (approximately 5%) [44, 45]. Since astrocytes are the most abundant brain cell type, even limited HIV infection in these cells correspond to a significant viral reservoir [40]. Moreover, amplification of HIV neuropathogenesis by intercellular communication systems involving GJs and uHCs exacerbate the pathology of CNS HIV infection several folds.
Role of Panx1 uHCs in HIV infection and replication
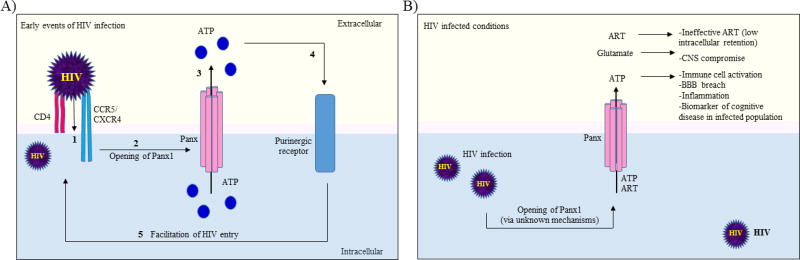
Recent work on the role of Panx1 uHCs and purinergic receptors during HIV infection has revealed novel and exciting results [46–48]. HIV infection in CD4+ T-lymphocytes and peripheral blood mononuclear cells leads to opening of Panx1 uHCs and release of ATP [48]. Extracellular ATP then activates purinergic receptors (P2Y) and allows downstream signaling cascade involving Proline-rich tyrosine kinase-2 (Pyk2), and membrane depolarization to facilitate the early steps of HIV infection [49]. Interestingly, Panx1 uHC activity has also been found to be indispensable for HIV replication in CD4+ T-lymphocytes [48]. We propose that HIV-induced Panx1 uHC opening results in increased intracellular Ca2+ levels and rearrangement of the actin cytoskeleton that assists in successful fusion of HIV virions with the host cell membrane (Figure 1A) [46, 47].
Figure 1. Role of Panx1 uHCs in HIV neuropathogenesis.
(A) Binding of HIV to host CD4 receptor and CCR5/CXCR4 co-receptors in CD4+ T-lymphocytes and monocytes (1) leads to opening of Panx1 uHCs (2). As a result, ATP is released through the Panx1 uHCs (3), which then activates purinergic receptors (4). Downstream signaling through purinergic receptors leads to membrane depolarization and actin rearrangement which facilitates the entry of HIV virions into the cells (5). (B) At later stages, ATP released from HIV infected cells via Panx1 uHCs also leads to inflammation and contributes to BBB disruption. We propose that circulating ATP levels may be considered as a biomarker for cognitive disease in the HIV-infected population. Furthermore, as a consequence of Panx1 uHC opening, there is efflux of ART from the cells leading to a lower intracellular concentration of antiretrovirals which may be ineffective against the virus. Glutamate, which is also released from the Panx1 uHCs is a prime mediator of CNS compromise. Hence, it is imperative to decipher the role of Panx1 uHCs in HIV neuropathogenesis.
The role of Panx1 uHCs in NeuroAIDS is further strengthened by the observation that pharmacological inhibition of Panx1 uHCs by probenecid has been shown to reduce the efflux of antiretrovirals from the infected cells (Figure 1B). More importantly, probenecid has been utilized extensively with Tenofovir and Zidovudine during the early HIV epidemic to increase their antiviral effects [50]. Thus, Panx1 uHCs play a crucial role in HIV infection, viral replication, and maintenance of clinically effective concentration of antiretrovirals in the infected cells.
Maintenance of CNS GJ communication during HIV infection
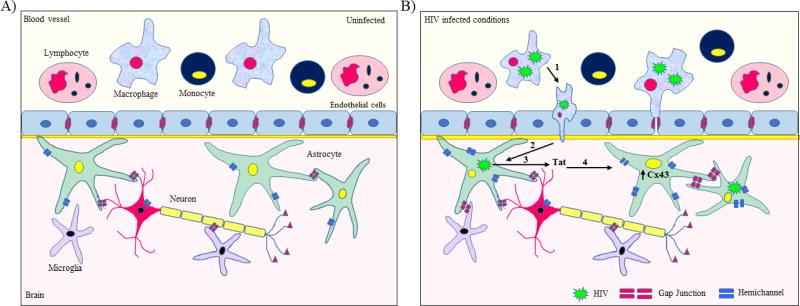
As mentioned in the preceding section, inflammation decreases Cx43 expression; however, we have observed that during HIV infection, expression of Cx43 is enhanced in HIV-infected human fetal astrocytes (HIV-p24-positive) [51]. This results in the maintenance of GJ-mediated communication which contributes to bystander apoptosis of uninfected astrocytes by transferring toxic/apoptotic signals from few HIV-infected astrocytes to the uninfected cells (Figure 2). Cx43 expression was also found to be elevated in astrocytes in post-mortem brain tissue from individuals with HIV-Encephalitis (HIV-E) as compared to CNS tissue sections from uninfected individuals [52].
Figure 2. HIV infection increases Cx43 expression in human astrocytes.
(A) During physiological conditions, Cx containing GJs mediate neuron-glia crosstalk and coordinate several essential cellular functions. (B) HIV infection leads to upregulation of Cx expression and maintenance of GJ-mediated communication. During HIV infection, BBB disruption leads to infiltration of infected monocytes and macrophages into the brain (1). As HIV infects resident brain cells such as astrocytes and microglia (2), infected cells release several proinflammatory cytokines and HIV protein, Tat (3). HIV infection, as well as HIV-Tat exposure, leads to upregulation of Cx43 expression in astrocytes. HIV infection also leads to opening of Cx43 uHCs in astrocytes. Although HIV infection in astrocytes is restricted, diffusion of toxic metabolites from HIV-infected cells to uninfected cells contributes in amplification of HIV neuropathogenesis
Our studies have demonstrated that HIV-Tat, the transactivating protein of HIV, is responsible for the maintenance of GJ-mediated communication during HIV infection [52]. HIV-Tat exposure leads to enhanced expression of Cx43 at both mRNA and protein levels by directly binding to the Cx43 promoter [52]. Since the current antiretroviral treatments have no effect on HIV-Tat production, which continues even in the absence of viral replication [53], it is imperative to consider the role of HIV-Tat in amplification of HIV neuropathogenesis via GJ channels.
Gap junctions, hemichannels, and astrocyte/neuronal compromise
As mentioned earlier, GJs contribute to the amplification of HIV neuropathogenesis by enabling the diffusion of toxic metabolites from few HIV-infected astrocytes (HIV-p24-positive cells) to surrounding uninfected astrocytes leading to apoptosis of uninfected cells [51, 54]. We have also observed mitochondrial dysfunction in HIV-infected astrocytes that lead to an uncontrolled release of cytochrome c by a Ca2+ and IP3-mediated mechanism [55]. We have proposed that IP3 and Ca2+ present in HIV-infected astrocytes diffuse, via GJ channels, into the uninfected cells leading to their apoptosis since blocking either GJ crosstalk or cytochrome c/IP3 signaling abolished bystander apoptosis [55]. However, HIV-infected astrocytes do not succumb to apoptosis even after direct intracellular microinjection of cytochrome c, which suggests unique HIV-mediated mechanisms of cellular protection that possibly contribute to the establishment of viral reservoirs [55]. These HIV-mediated mechanisms of infected cell survival are under active investigation in our laboratory.
HIV infection also induces the opening of astroglial Cx43 uHCs enhancing the secretion of Dickkopf-1 (DKK1) protein, a soluble Wnt pathway inhibitor, which leads to the compromise of neuronal processes [56]. The role of astroglial uHCs in HIV neuropathogenesis is further strengthened by observations of increased DKK1 expression in astrocytes and endothelial cells in brain tissue sections from HIV-E cases [56]. Enhanced DKK1 levels have already been linked to amyloid-β-mediated synaptic loss [57]. Since compromised neuronal processes and synaptic loss subsequently lead to cognitive impairment, uHCs may be contributing to cognitive decline in HAND patients by mediating DKK1 release. In conclusion, HIV successfully “hijacks” GJs and uHCs to promote infection, invasion of the virus into the CNS, and neurotoxicity. GJs and uHCs participate in the survival of HIV reservoirs and play a fundamental role in the viral neuropathogenesis observed in at least half of the HIV-infected population.
Discussion
The success of ART has transformed HIV/AIDS into a chronic disease with immune reconstitution and activation, ART-associated toxicities, accelerated aging, and persistence of the virus in latent reservoirs such as the brain. The exploitation of the host intercellular communication systems (especially GJs and uHCs) by HIV has allowed the virus to disseminate infection and associated inflammation leading to aggravated HIV neuropathogenesis [58]. Comprehensive evaluation of the functions of GJs and uHCs as well as other modalities of cell-cell communication (tunneling nanotubes and exosomes) is required to gain deeper insights into HIV pathology and development of NeuroAIDS.
The involvement of Panx1 uHCs and purinergic receptors in HIV life cycle has not been fully explored. Along with adenosine, ADP, and ATP, purinergic receptors and Panx1 uHCs are important regulators of various cellular events, and unlocking their critical relationship could hold the key to understanding several pathological conditions. The deeper understanding of these mechanisms will provide a unique opportunity towards the discovery of novel therapeutics for prevention and cure of HIV and NeuroAIDS.
Highlights.
HIV hijacks gap junctions and hemichannels to enhance viral neuropathogenesis.
Gap junctions contribute to bystander apoptosis of uninfected astrocytes.
Gap junctions disrupt blood-brain barrier facilitating HIV neuroinvasion.
Pannexin1 hemichannels play a crucial role in HIV infection and replication.
Acknowledgments
We would like to thank Public Health Research Institute (PHRI) imaging facility. This work was supported by the National Institute of Mental Health grant, MH096625, National Institute of Neurological Disorders and Stroke grant, NS105584, and PHRI funding to E.A.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Teeraananchai S, Kerr SJ, Amin J, Ruxrungtham K, Law MG. Life expectancy of HIV-positive people after starting combination antiretroviral therapy: a meta-analysis. HIV medicine. 2017;18:256–266. doi: 10.1111/hiv.12421. [DOI] [PubMed] [Google Scholar]
- 2.Sacktor N, Skolasky RL, Seaberg E, Munro C, Becker JT, Martin E, Ragin A, Levine A, Miller E. Prevalence of HIV-associated neurocognitive disorders in the Multicenter AIDS Cohort Study. Neurology. 2016;86:334–340. doi: 10.1212/WNL.0000000000002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013;8:165–169. doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saez JC, Contreras JE, Bukauskas FF, Retamal MA, Bennett MV. Gap junction hemichannels in astrocytes of the CNS. Acta Physiol Scand. 2003;179:9–22. doi: 10.1046/j.1365-201X.2003.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sosinsky GE, Boassa D, Dermietzel R, Duffy HS, Laird DW, MacVicar B, Naus CC, Penuela S, Scemes E, Spray DC, Thompson RJ, Zhao HB, Dahl G. Pannexin channels are not gap junction hemichannels. Channels (Austin) 2011;5:193–197. doi: 10.4161/chan.5.3.15765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spray DC, Ye ZC, Ransom BR. Functional connexin "hemichannels": a critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 7.Rouach N, Avignone E, Meme W, Koulakoff A, Venance L, Blomstrand F, Giaume C. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 8.Eugenin EA. Role of connexin/pannexin containing channels in infectious diseases. FEBS letters. 2014;588:1389–1395. doi: 10.1016/j.febslet.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karpuk N, Burkovetskaya M, Fritz T, Angle A, Kielian T. Neuroinflammation leads to region-dependent alterations in astrocyte gap junction communication and hemichannel activity. J Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantseva MV, Kokarovtseva L, Perez Velazquez JL. Ischemia-induced brain damage depends on specific gap-junctional coupling. J Cereb Blood Flow Metab. 2002;22:453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci U S A. 2006;103:4475–4480. doi: 10.1073/pnas.0511118103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duffy HS, John GR, Lee SC, Brosnan CF, Spray DC. Reciprocal regulation of the junctional proteins claudin-1 and connexin43 by interleukin-1beta in primary human fetal astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:RC114. doi: 10.1523/JNEUROSCI.20-23-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, Yasumura T, Rash JE. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- 14.Rash JE, Yasumura T, Dudek FE, Nagy JI. Cell-specific expression of connexins and evidence of restricted gap junctional coupling between glial cells and between neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:1983–2000. doi: 10.1523/JNEUROSCI.21-06-01983.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liebmann M, Stahr A, Guenther M, Witte OW, Frahm C. Astrocytic Cx43 and Cx30 differentially modulate adult neurogenesis in mice. Neurosci Lett. 2013;545:40–45. doi: 10.1016/j.neulet.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 17.Giaume C, Tabernero A, Medina JM. Metabolic trafficking through astrocytic gap junctions. Glia. 1997;21:114–123. [PubMed] [Google Scholar]
- 18.Huguet G, Joglekar A, Messi LM, Buckalew R, Wong S, Terman D. Neuroprotective Role of Gap Junctions in a Neuron Astrocyte Network Model. Biophys J. 2016;111:452–462. doi: 10.1016/j.bpj.2016.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rose CR, Ransom BR. Gap junctions equalize intracellular Na+ concentration in astrocytes. Glia. 1997;20:299–307. doi: 10.1002/(sici)1098-1136(199708)20:4<299::aid-glia3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci U S A. 2003;100:13644–13649. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orellana JA, Froger N, Ezan P, Jiang JX, Bennett MV, Naus CC, Giaume C, Saez JC. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxeiner S, Kruger O, Schilling K, Traub O, Urschel S, Willecke K. Spatiotemporal transcription of connexin45 during brain development results in neuronal expression in adult mice. Neuroscience. 2003;119:689–700. doi: 10.1016/s0306-4522(03)00077-0. [DOI] [PubMed] [Google Scholar]
- 23.O'Brien JJ, Li W, Pan F, Keung J, O'Brien J, Massey SC. Coupling between A-type horizontal cells is mediated by connexin 50 gap junctions in the rabbit retina. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:11624–11636. doi: 10.1523/JNEUROSCI.2296-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hombach S, Janssen-Bienhold U, Sohl G, Schubert T, Bussow H, Ott T, Weiler R, Willecke K. Functional expression of connexin57 in horizontal cells of the mouse retina. Eur J Neurosci. 2004;19:2633–2640. doi: 10.1111/j.0953-816X.2004.03360.x. [DOI] [PubMed] [Google Scholar]
- 25.Rash JE, Staines WA, Yasumura T, Patel D, Furman CS, Stelmack GL, Nagy JI. Immunogold evidence that neuronal gap junctions in adult rat brain and spinal cord contain connexin-36 but not connexin-32 or connexin-43. Proc Natl Acad Sci U S A. 2000;97:7573–7578. doi: 10.1073/pnas.97.13.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen K, Fuchs EC, Jaschonek H, Bannerman DM, Monyer H. Gap junctions between interneurons are required for normal spatial coding in the hippocampus and short-term spatial memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:6542–6552. doi: 10.1523/JNEUROSCI.6512-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swayne LA, Bennett SA. Connexins and pannexins in neuronal development and adult neurogenesis. BMC Cell Biol. 2016;17(Suppl 1):10. doi: 10.1186/s12860-016-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prochnow N, Abdulazim A, Kurtenbach S, Wildforster V, Dvoriantchikova G, Hanske J, Petrasch-Parwez E, Shestopalov VI, Dermietzel R, Manahan-Vaughan D, Zoidl G. Pannexin1 stabilizes synaptic plasticity and is needed for learning. PLoS One. 2012;7:e51767. doi: 10.1371/journal.pone.0051767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- 30.Parenti R, Campisi A, Vanella A, Cicirata F. Immunocytochemical and RT-PCR analysis of connexin36 in cultures of mammalian glial cells. Arch Ital Biol. 2002;140:101–108. [PubMed] [Google Scholar]
- 31.Eugenin EA, Eckardt D, Theis M, Willecke K, Bennett MV, Saez JC. Microglia at brain stab wounds express connexin 43 and in vitro form functional gap junctions after treatment with interferon-gamma and tumor necrosis factor-alpha. Proc Natl Acad Sci U S A. 2001;98:4190–4195. doi: 10.1073/pnas.051634298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saez PJ, Shoji KF, Retamal MA, Harcha PA, Ramirez G, Jiang JX, von Bernhardi R, Saez JC. ATP is required and advances cytokine-induced gap junction formation in microglia in vitro. Mediators Inflamm. 2013;2013:216402. doi: 10.1155/2013/216402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umebayashi D, Natsume A, Takeuchi H, Hara M, Nishimura Y, Fukuyama R, Sumiyoshi N, Wakabayashi T. Blockade of gap junction hemichannel protects secondary spinal cord injury from activated microglia-mediated glutamate exitoneurotoxicity. J Neurotrauma. 2014;31:1967–1974. doi: 10.1089/neu.2013.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meme W, Calvo CF, Froger N, Ezan P, Amigou E, Koulakoff A, Giaume C. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- 35.Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS. Unique distributions of the gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia. 2004;47:346–357. doi: 10.1002/glia.20043. [DOI] [PubMed] [Google Scholar]
- 36.Dermietzel R, Farooq M, Kessler JA, Althaus H, Hertzberg EL, Spray DC. Oligodendrocytes express gap junction proteins connexin32 and connexin45. Glia. 1997;20:101–114. [PubMed] [Google Scholar]
- 37.Kamasawa N, Sik A, Morita M, Yasumura T, Davidson KG, Nagy JI, Rash JE. Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: implications for ionic homeostasis and potassium siphoning. Neuroscience. 2005;136:65–86. doi: 10.1016/j.neuroscience.2005.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL. Connexins are critical for normal myelination in the CNS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5963–5973. doi: 10.1523/JNEUROSCI.23-13-05963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–120. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Li GH, Henderson L, Nath A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr HIV Res. 2016;14:373–381. doi: 10.2174/1570162x14666161006121455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decloedt EH, Rosenkranz B, Maartens G, Joska J. Central nervous system penetration of antiretroviral drugs: pharmacokinetic, pharmacodynamic and pharmacogenomic considerations. Clin Pharmacokinet. 2015;54:581–598. doi: 10.1007/s40262-015-0257-3. [DOI] [PubMed] [Google Scholar]
- 42.Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. [DOI] [PubMed] [Google Scholar]
- 43.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiley CA, Achim CL, Christopherson C, Kidane Y, Kwok S, Masliah E, Mellors J, Radhakrishnan L, Wang G, Soontornniyomkij V. HIV mediates a productive infection of the brain. Aids. 1999;13:2055–2059. doi: 10.1097/00002030-199910220-00007. [DOI] [PubMed] [Google Scholar]
- 45.Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12:442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hazleton JE, Berman JW, Eugenin EA. Purinergic receptors are required for HIV-1 infection of primary human macrophages. J Immunol. 2012;188:4488–4495. doi: 10.4049/jimmunol.1102482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Velasquez S, Eugenin EA. Role of Pannexin-1 hemichannels and purinergic receptors in the pathogenesis of human diseases. Front Physiol. 2014;5:96. doi: 10.3389/fphys.2014.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orellana JA, Velasquez S, Williams DW, Saez JC, Berman JW, Eugenin EA. Pannexin1 hemichannels are critical for HIV infection of human primary CD4+ T lymphocytes. J Leukoc Biol. 2013;94:399–407. doi: 10.1189/jlb.0512249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seror C, Melki MT, Subra F, Raza SQ, Bras M, Saidi H, Nardacci R, Voisin L, Paoletti A, Law F, Martins I, Amendola A, Abdul-Sater AA, Ciccosanti F, Delelis O, Niedergang F, Thierry S, Said-Sadier N, Lamaze C, Metivier D, Estaquier J, Fimia GM, Falasca L, Casetti R, Modjtahedi N, Kanellopoulos J, Mouscadet JF, Ojcius DM, Piacentini M, Gougeon ML, Kroemer G, Perfettini JL. Extracellular ATP acts on P2Y2 purinergic receptors to facilitate HIV-1 infection. J Exp Med. 2011;208:1823–1834. doi: 10.1084/jem.20101805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Massarella JW, Nazareno LA, Passe S, Min B. The effect of probenecid on the pharmacokinetics of zalcitabine in HIV-positive patients. Pharm Res. 1996;13:449–452. doi: 10.1023/a:1016009029536. [DOI] [PubMed] [Google Scholar]
- 51.Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Berman JW, Carvallo L, Buckner CM, Luers A, Prevedel L, Bennett MV, Eugenin EA. HIV-tat alters Connexin43 expression and trafficking in human astrocytes: role in NeuroAIDS. J Neuroinflammation. 2016;13:54. doi: 10.1186/s12974-016-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bachani M, Sacktor N, McArthur JC, Nath A, Rumbaugh J. Detection of anti-tat antibodies in CSF of individuals with HIV-associated neurocognitive disorders. Journal of neurovirology. 2013;19:82–88. doi: 10.1007/s13365-012-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eugenin EA, Clements JE, Zink MC, Berman JW. Human immunodeficiency virus infection of human astrocytes disrupts blood-brain barrier integrity by a gap junction-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9456–9465. doi: 10.1523/JNEUROSCI.1460-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eugenin EA, Berman JW. Cytochrome C dysregulation induced by HIV infection of astrocytes results in bystander apoptosis of uninfected astrocytes by an IP3 and calcium-dependent mechanism. J Neurochem. 2013;127:644–651. doi: 10.1111/jnc.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orellana JA, Saez JC, Bennett MV, Berman JW, Morgello S, Eugenin EA. HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem. 2014;128:752–763. doi: 10.1111/jnc.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malik S, Eugenin EA. Mechanisms of HIV Neuropathogenesis: Role of Cellular Communication Systems. Curr HIV Res. 2016;14:400–411. doi: 10.2174/1570162x14666160324124558. [DOI] [PMC free article] [PubMed] [Google Scholar]