Abstract
The neurobiological mechanisms that underlie the resistance of drug cue associations to extinction in drug addiction remain unknown. Fear extinction critically depends on the ventromedial prefrontal cortex (VMPFC). Here we tested if this same region plays a role in extinction of non-fear, drug and pleasant cue associations. Eighteen chronic cocaine users and 15 matched controls completed three functional MRI scans. Participants first learned to associate an abstract cue (the conditioned stimulus, CS) with a drug-related (CSD+) or pleasant (CSP+) image. Extinction immediately followed where each CS was repeatedly presented without the corresponding image. Participants underwent a second identical session 24 h later to assess retention of extinction learning. Results showed that like fear extinction, non-fear based extinction relies on the VMPFC. However, extinction-related changes in the VMPFC differed by cue valence and diagnosis. In controls, VMPFC activation to the CSD+ (which was unpleasant for participants) gradually increased as in fear extinction, while it decreased to the CSP+, consistent with a more general role of the VMPFC in flexible value updating. Supporting a specific role in extinction retention, we further observed a cross-day association between VMPFC activation and skin conductance, a classic index of conditioned responses. Finally, cocaine users showed VMPFC abnormalities for both CSs, which in the case of the CSD+, correlated with craving. These data suggest a global deficit in extinction learning in this group that may hinder extinction-based treatment efforts. More broadly, these data show the VMPFC, when functionally intact, supports extinction learning in diverse contexts in humans.
Keywords: cocaine, craving, reward, extinction, functional magnetic resonance imaging, ventromedial prefrontal cortex
Introduction
Extinction is the process by which conditioned responses to an otherwise neutral cue (a conditioned stimulus, CS) that has acquired affective properties after being paired with an arousing event (an unconditioned stimulus, US) gradually diminish when the cue is no longer reinforced (Bouton, 2004; Quirk and Mueller, 2008). The predominant view is that extinction does not eliminate the CS–US association; rather, it leads to a lessening in the conditioned response by creating a new (CS–no–US) association that competes for expression, leaving memory vulnerable to recovery of the conditioned response (Quirk and Mueller, 2008). Extinction has been extensively studied in humans and non-human animals in the domain of fear learning, e.g., using electric shock (Milad et al., 2005; Phelps et al., 2004), aversive sounds (Neumann and Waters, 2006), and even monetary loss (Schlund et al., 2015). However, less is known about extinction in the appetitive domain, and while animal work suggests similarities in the neurobiological mechanisms of extinction of, e.g., drug seeking and fear (Peters et al., 2009), the mechanism of non-fear based extinction in humans remains unknown.
Addiction is characterized by continued drug seeking and use despite reduced pleasure derived from the drug and catastrophic health and social consequences. This behavior is assumed to be at least partly driven by a learning process in which cues associated with drug consumption acquire excessive and persistent salience, perpetuating drug seeking. The persistence of drug seeking despite negative consequences and a reduction in the drug’s rewarding effects suggests that addicted individuals may have diminished ability to form and/or maintain new associations for cues that were previously, although no longer, predictive of drug rewards (e.g., learning that the drug or drug-associated cues are no longer as valuable). This is also predicted by the neural circuitry that supports extinction learning, which overlaps extensively with that directly impacted by addictive substances and addiction (Goldstein and Volkow, 2011), potentially rendering this process especially vulnerable in this population.
Substantial work in the fear domain demonstrates a central role for the ventromedial prefrontal cortex (VMPFC) in the formation, retention, and later retrieval of extinction learning (Milad and Quirk, 2012; Quirk and Mueller, 2008). In humans, VMPFC activity increases during fear extinction (Milad et al., 2007) and extinction retrieval (Kalisch et al., 2006; Phelps et al., 2004), and both neural activity (Phelps et al., 2004) and cortical thickness (Hartley et al., 2011; Milad et al., 2005) in this region correlate with psychophysiological indices of extinction success [e.g., lowered skin conductance response (SCR) to the CS]. Beyond fear extinction, the VMPFC along with the striatum form what is known as the ‘brain’s valuation system’, a set of regions that represent (and possibly update) value in a domain-general manner (Bartra et al., 2013). In addiction, the VMPFC (Kober et al., 2015) and striatum (Kuhn and Gallinat, 2011) are implicated in the experience of craving, a motivational state often triggered by drug-associated cues that can promote drug seeking.
This more general role of the VMPFC in valuation and craving suggests the VMPFC may also be a candidate region involved in extinction of non-fear-based and secondary reinforcers, including drug-related and appetitive cues. However, while the effect of extinction-based therapy on drug-cue reactivity has just begun to be examined (Prisciandaro et al., 2013; Vollstadt-Klein et al., 2011), no studies to date have investigated the role of the VMPFC in extinction learning itself in human addiction. Such an investigation has important implications for the potential utility of extinction-based therapies for addiction, and for the basic neuroscientific understanding of non-fear-based extinction more generally.
Modeled after classical fear-conditioning studies, here we examined the neural correlates of extinction learning for drug and pleasant cue associations in non-treatment seeking, chronic cocaine users and socio-demographically matched healthy non-drug users in a 2-day functional magnetic resonance imaging (fMRI) study. The study comprised an acquisition phase, where participants learned to associate an abstract cue with a drug-related (CSD+) or pleasant (CSP+) image, and two extinction phases (the latter for assessing the retention of extinction from day 1), where the abstract cues were repeatedly presented without the corresponding images. Throughout, we collected SCR and blood-oxygen-level-dependent (BOLD) response, time-locked to the presentation of the abstract cue, as indices of the conditioned response. We hypothesized that the VMPFC and striatum would exhibit parametric changes across the learning phases as participants form new, affectively neutral, associations with the CSD+ and CSP+. We expected cocaine users to show abnormalities in these regions for both cues.
Materials and Methods
Participants
Participants were native English speakers recruited from the community through advertisements and by word of mouth who provided written informed consent to participate in accordance with the local institutional review board. Participants were chronic cocaine users and healthy individuals with no history of drug or psychiatric illness. To minimize the influence of factors other than those related to cocaine addiction, the groups were selected to match on multiple socio-demographic characteristics and cigarette smoking status (see Table 1). All participants were asked to complete study procedures on two separate days (psychophysiological measures and fMRI; see below). The final sample consisted of 18 cocaine users and 15 healthy controls. G*Power 3.1.9.2 (Faul et al., 2009) was used to determine whether this sample was sufficiently powered. Given a 2 (group) × 3 (learning phase) mixed design, 80% desired power, α error probability=0.05, and a within-between subject interaction of a large effect size (Cohen’s d, henceforth referred to simply as d, of 0.8), it was determined that N=12 participants would be needed. For a medium effect size (d=0.5), N=28 participants would be needed. Thus, our sample of N=33 was sufficiently powered for effect sizes of d≥0.5.
Table 1.
Demographic and drug use characteristics of the study sample.
| Test | Control (n=15) | Cocaine Users (n=18) | |
|---|---|---|---|
| Demographics | |||
| Age (years) | t31 = 0.3 | 45.3 ± 1.6 | 45.8 ± 1.1 |
| Sex (male / female) | χ2 = 0.6 | 13 / 2 | 17/ 1 |
| Race (African-American/Caucasian/Hispanic) | χ2 = 0.8 | 10 / 2 / 3 | 12 / 4 / 2 |
| Education (years) | t31 = 1.7 | 13.3 ± 0.4 | 12.6 ± 0.3 |
| Verbal IQ: Wide Range Achievement Test III - Reading Scale | t31 = 0.7 | 94.0 ± 4.2 | 90.0 ± 3.5 |
| Nonverbal IQ: Wechsler Abbreviated Scale of Intelligence – Matrix Reasoning Scale | t31 = 0.5 | 8.4 ± 0.9 | 8.9 ± 0.7 |
| State Depression: Beck Depression Inventory II a | Z = 1.7 | 3.2 ± 1.3 | 7.6 ± 2.2 |
| Socioeconomic Status: Hollingshead Index a | t28 = 1.3 | 32.9 ± 3.4 | 28.9 ± 2.4 |
| Handedness (laterality quotient) | t31 = 0.5 | 0.7 ± 0.2 | 0.8 ± 0.2 |
| Drug Use | |||
| Cigarette smokers (current or past / nonsmokers) | χ2 = 3.5 | 6 / 9 | 13 / 5 |
| Daily cigarettes (current smokers: n=5/11) | Z = 1.0 | 4.8 ± 1.7 | 8.5 ± 2.3 |
| Alcohol use lifetime (years) (n=10/11) | t19 = 0.8 | 20.0 ± 3.1 | 22.8 ± 2.2 |
| Cocaine use lifetime (years) | -- | -- | 17.8 ± 1.6 |
| Duration of current abstinence/time since last cocaine use (days) b | -- | -- | 13.9 ± 7.4 |
| Days/week of cocaine use during the past 30 days | -- | -- | 2.6 ± 0.6 |
| Cocaine urine status (positive / negative): day 1 | day 2 | -- | -- | 6 / 12 | 4 / 14 |
| Withdrawal symptoms: 18-item CSSA (0–126): day 1 | day 2 c | -- | -- | 22.2 ± 3.6 | 11.8 ± 2.1 |
| Cocaine craving: 5-item Questionnaire (0–45): day 1 | day 2 d | -- | -- | 20.1 ± 3.1 |15.5 ± 3.5 |
Data missing for one control and two cocaine users;
Data missing for two cocaine users;
day 1 vs. day 2 (t17 = 3.9, P=0.001);
day 1 vs. day 2 (t17 = 2.2, P=0.04);
Abbreviations: CSSA, Cocaine Selective Severity Assessment Scale;
Values are frequencies or means ± standard error of the mean (SEM).
All participants were in good health and not currently taking medication. Drug use and psychiatric histories were ascertained by a comprehensive clinical interview administered by trained research staff with extensive experience evaluating drug addicted populations, consisting of the Structured Clinical Interview for DSM-IV Axis I Disorders [research version (First et al., 1996; Ventura et al., 1998)] and the Addiction Severity Index (McLellan et al., 1992). Exclusion criteria for both groups were: (A) history of head trauma, neurological disease, or loss of consciousness >30 min; (B) abnormal vital signs; (C) history of major medical conditions; (D) history of major psychiatric disorders (other than substance use disorders in the cocaine group, and nicotine use disorder in both groups); (E) positive urine pregnancy test in females; (F) contraindications to MRI; and (G) except for cocaine in the cocaine user group, positive urine screens for psychoactive drugs or their metabolites.
Cocaine users were non-treatment seeking individuals who reported an average lifetime history of 17 years of cocaine use, 2 days/week of cocaine use in the past 30 days, and some cocaine use within the past 4 months (see Table 1 for detailed drug use information). Participants identified cocaine as their primary drug of choice, meeting criteria for cocaine dependence (n=17) or abuse (n=1) [in early full (n=2) or partial (n=1) remission]. Current comorbid disorders included alcohol dependence (n=2) and marijuana abuse (n=2); one participant also met criteria for a current depressive episode. Thirteen cocaine users and 6 controls were cigarette smokers. Six participants tested positive for cocaine on day 1 (indicating use ≤72 h) and 4 tested positive for cocaine on day 2. Controls tested negative for all drugs on both study days. Apart from number of days since last use (Z=1.87, P=0.06), cocaine urine positive and cocaine urine negative participants (based on day 1 status) did not differ in their clinical profile (i.e., they did not differ in any of the drug use variables listed in Table 1; P>0.086). Nevertheless, because the passage of time from last use (day 1 to day 2) was associated with a reduction in craving and withdrawal (Table 1) and because there is some clinical and preclinical evidence to suggest that recent cocaine exposure impacts learning (McCracken and Grace, 2013; Schoenbaum et al., 2004; Spronk et al., 2016), we tested if cocaine urine status modified any of the observed diagnostic group effects.
Study Procedures & fMRI Task
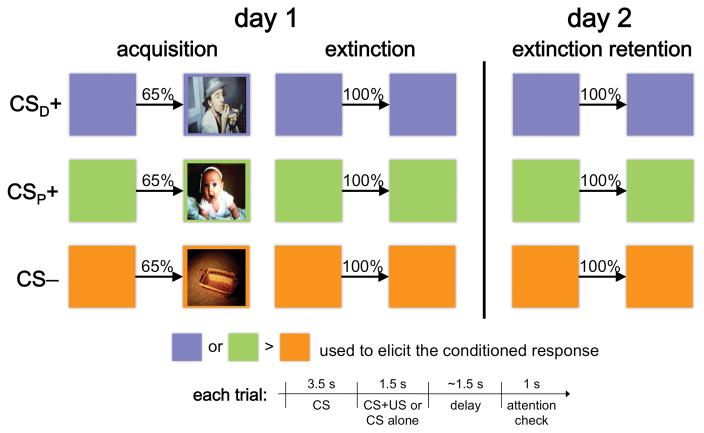
All participants completed three fMRI sessions conducted over two consecutive days (99 total sessions; Figure 1). To ensure comparable experiences between sessions and across the two study days, and to control the time in-between, participants were overnighted in the laboratory. On day 1, after a period of acclimation to the scanner environment, participants first learned to associate a colored square, the CS, with different types of “USs”. This created three cue types: CSD+ (CS paired with a drug-related image of a person smoking crack), CSP+ (CS paired with a pleasant image of a smiling baby), and CS− (CS paired with a neutral image of an unadorned wooden basket). The acquisition phase consisted of 13 paired trials in which each CS presentation co-terminated with presentation of the corresponding US and 7 unpaired trials in which each CS was presented without the US, for a total of 20 trials per cue type (60 total). Participants were instructed to “try and figure out which color predicted which image.” Day 1 extinction immediately followed, and began with 2 paired trials followed by 20 unpaired trials for each cue type (66 total). Participants completed another, identical extinction session ~24 h later but without the paired reminder trials. Trial order was pseudo-randomized in all sessions and CS color assignment was counterbalanced across participants. SCR and fMRI data were acquired throughout. Participants earned $25/session (max $75).
Figure 1.
Study overview and conditioning paradigm. While in the MRI scanner and over the course of three scanning sessions, subjects learned to associate a cue (colored square), the CS, with a drug-related (CSD+), affectively pleasant (CSP+), or neutral (CS-) image. Following two reinforced presentations of the CS for each CS type (not shown), extinction training immediately followed acquisition, where the CS was presented repeatedly without the paired image. A second extinction training session took place 24 h later. A typical paired trial consisted of presentation of the CS for 3.5 s, followed by presentation of the US (the corresponding image) inside the CS for 1.5 s, a variable ~1.5 s fixation screen, and a 1 s screen requiring a non-contingent button press indicating whether the US appeared or not on that trial. Following another variable ~1.5 s fixation screen, the next trial began. Unpaired trials were identical to the paired trials with the exception that the CS remained on the screen for the entire 5 s.
Participants also completed two-alternative forced choice tasks and provided subjective ratings for the task stimuli (see Supporting Information). No SCR or fMRI data were collected during these tasks. These data confirmed that, as expected based on our prior work in independent samples of cocaine addicted and control subjects (Moeller et al., 2010; Moeller et al., 2009; Moeller et al., 2013), participants found the drug-related image as least pleasant (and chose to view it least often) and the affectively pleasant image as most pleasant (and chose to view it most often). Controls additionally rated the drug-related image as more unpleasant (and chose to view it less often) than cocaine users (Figure S1A–B).
SCR Acquisition & Analysis
Skin conductance was acquired with shielded Ag-AgCl electrodes (AD Instruments, Inc.) attached to the second and big toes of the left foot. The electrode cables were grounded through an RF filter panel. Data were continuously recorded at 200 samples/s. Offline data analysis was performed in Matlab. The continuous data were low-pass filtered (1 Hz) and then divided into epochs. As in previous studies, the SCR amplitude on each trial was computed as the peak amplitude in the 0.5 to 4.5 s time window following CS onset minus the average amplitude in the 0.5 s prior to CS onset. Thus, SCR to the CSD+, CSP+, and CS− reflected changes in skin conductance level beyond changes in this measure produced by the preceding trial or task phase.
After square-root transformation, the data were analyzed in a 2 (cue type: drug, pleasant) × 3 (learning phase: acquisition, day 1 extinction, day 2 extinction) × 2 (group: cocaine users, controls) mixed analysis of variance (ANOVA) on the differential SCR values (CSD+ versus CS− and CSP+ versus CS−). Considering that we expected across- as well as within-session learning, the ANOVA was restricted to all unpaired trials during acquisition and the last half of all extinction trials on day 1 and day 2. Due to artifacts in the SCR signal, the subsample with complete SCR data for all three learning phases consisted of n=11 cocaine users and n=6 controls. Hence, our SCR analyses comparing the diagnostic groups across the learning phases were only powered to detect large effects (specifically, d≥0.68).
Image Acquisition & Analysis
Functional images were acquired with a 4T Varian/Siemens MRI scanner using a coronal T2*-weighted single shot gradient-echo EPI sequence (TE/TR=20/1600 ms, 3.125×3.125 mm2 in-plane resolution, 4 mm slice thickness, 1 mm gap, 33 coronal slices, 20 cm FOV, 64×64 matrix size, 90°-flip angle, 200 kHz bandwidth with ramp sampling). Image processing and analyses were performed in SPM8 (Wellcome Trust Centre for Neuroimaging, London UK). The data were first realigned, co-registered, and spatially normalized to a standard EPI template in the Montreal Neurological Institute (MNI) frame, resulting in a final voxel size of 3×3×3 mm. Criteria for acceptable motion were ≤2 mm translation or ≤2 degrees rotation in any direction. The data were spatially smoothed with an 8 mm full-width-at-half-maximum Gaussian kernel.
Three random-effects general linear models (GLMs) were specified for each participant, corresponding to acquisition, day 1 extinction, and day 2 extinction, each with a session-specific intercept, the 6 motion parameters as regressors of no interest, and all task conditions convolved with a canonical HRF and high-pass filter (cut-off frequency: 1/1500 s). The GLM for acquisition included trial onsets for each CS (CSD+, CSP+, and CS−), separately for paired and unpaired trials (6 conditions in total), modeled as epochs with duration equal to the length of CS presentation (3.5 s or 5 s; i.e., terminating when the US was presented). The GLMs for day 1 and day 2 extinction each included trial onsets for each CS (all unpaired trials, epoch duration=5 s). The GLM for day 1 extinction additionally included a fourth condition for the “reminder trials”. Beta maps were computed for each participant for each CS and learning phase.
Given that previous studies have identified a circuit centered on the VMPFC in extinction learning, and the VMPFC and striatum in the representation and updating of values including those for drug cues, we focused on these two regions, although exploratory whole-brain and targeted control region analyses were also performed (see Supporting Information). As with SCR, we conducted 2 (cue type) × 3 (learning phase) × 2 (group) mixed ANOVAs on the differential BOLD responses (CSD+ versus CS− and CSP+ versus CS−), our neural measure of the conditioned response, extracted as average beta estimates from unbiased regions of interest (ROIs). The full sample of N=33 participants was included in the fMRI analyses.
The striatum (entire caudate and putamen) ROI was anatomically defined in PickAtlas (ANSIR Laboratory; http://fmri.wfubmc.edu/software/PickAtlas). The VMPFC was defined as a 12-mm radius sphere centered on the coordinates reported in (Phelps et al., 2004) after transformation to MNI space (Talairach: x=±2, y=38, z=−3; MNI: x=±3, y=42, z=−12). The term VMPFC is used to describe a large, heterogeneous region of the medial prefrontal cortex that spans parts of the anterior cingulate cortex (anterior to the genu of the corpus callosum) and the medial orbitofrontal cortex, encompassing Brodmann areas (BAs) 25, ventral portions of 24 and 32, medial portion of 11, and ventral and medial portions of 10 (Mackey and Petrides, 2014). The particular aspect of the VMPFC included in our ROI is the medial portion of BA 11 and the ventral portion of BA 10 (see inset in Figure 2), which has been linked to emotion regulation including the use of extinction strategies (Diekhof et al., 2011). In the anterior-posterior direction, our VMPFC ROI falls centrally, touching on posterior aspects traditionally linked to the representation of negative affect and anterior aspects representing positive affect (Grabenhorst and Rolls, 2011; Myers-Schulz and Koenigs, 2012). Notably, our ROI almost fully overlaps with the VMPFC locus identified in (Bartra et al., 2013) to represent value in diverse contexts. An initial analysis that included laterality as a factor revealed differential responses in the left vs. right VMPFC ROIs. Therefore, the ANOVAs reported were performed on averaged left and right side activation values for the striatum but not the VMPFC.
Figure 2.
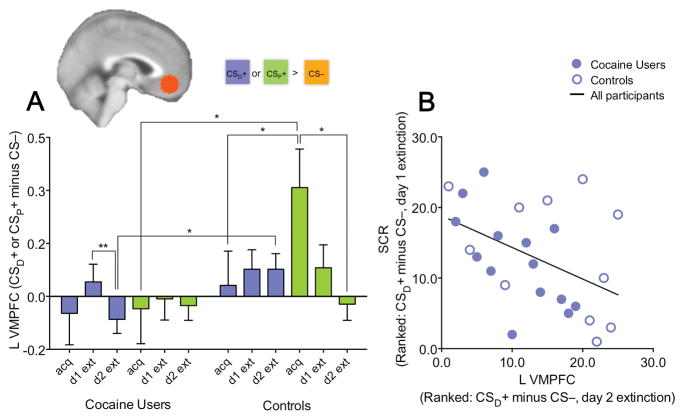
Modulation of left VMPFC activation by learning phase is valence-specific and correlates with psychophysiological response to the conditioned stimulus (CSD+) paired with a drug cue. (A) Plot shows left VMPFC activation for each learning phase (acquisition, day 1 extinction, day 2 extinction) as a function of cue-type [CSD+ (cue paired with drug-related image) and CSP+ (cue paired with pleasant image), both relative to the CS− (cue paired with neutral image)] and diagnostic group (cocaine users, controls). There was a 3-way interaction such that left VMPFC activation decreased in response to the CSP+ but increased in response to the CSD+ with extinction training in controls but not cocaine-addicted participants. (B) Left VMPFC activation on day 2 extinction correlated with the success of day 1 extinction as indexed by reductions in skin conductance response (SCR) to the CSD+ relative to the CS− across subjects, pointing to a role of this region in the recall of extinction learning. The overall pattern of results in the right VMPFC, as well as the correlation between right VMPFC activation and SCR, was similar albeit weaker (see Results). +P≤0.10, *P≤0.05, **P≤0.01.
See also Figures S3–S5 and Tables S1–S2.
Results
Psychophysiological and Self-Reported Measures
The 2 (cue type) × 3 (learning phase) × 2 (group) mixed ANOVA revealed a reduction in SCR (to the CSD+ and CSP+ relative to the CS−) over of the learning phases (F2, 30=2.71, P=0.08, d=0.85), an effect that reached significance for the linear contrast (acquisition>day 1 extinction>day 2 extinction: F=5.93, P=0.028, d=1.26; Figure S2), but no significant diagnostic group, cue type, or interaction effects (F<0.77, P>0.47, d<0.45). In addition, there were no differences between the groups in attention or subjective ratings/choice for the CSs (see Supporting Information and Figure S1C–D).
Neural Correlates of Extinction Learning for Drug and Pleasant Cue Associations
We hypothesized that, paralleling the SCR data, the striatum and VMPFC would show similar progressive increased or decreased activation over the learning phases as tested with 2×3×2 mixed ANOVAs on the differential BOLD responses (CSD+ and CSP+, both relative to the CS−) in each ROI, followed by linear contrasts specifically testing for this progression.
The main finding we observed was a diagnostic group main effect (F1, 31=5.44, P=0.026, d=0.84) and a cue type × learning phase interaction in the left VMPFC (F1.37, 42.44=4.05, P=0.039, d=0.72), which were both qualified by a significant cue type × learning phase × group interaction (F2, 62=4.21, P=0.019, d=0.74; all other effects, P>0.31, d<0.37). A similar albeit statistically weaker pattern was observed in the right VMPFC (group main effect: F1, 31=4.47, P=0.043, d=0.76; cue type × learning phase interaction: F1.27, 39.47=3.07, P=0.054, d=0.63; cue type × learning phase × group interaction: F2, 62=1.94, P=0.15, d=0.50). All other effects in the right VMPFC were non-significant (P>0.32, d<0.38). The three-way interaction in the left VMPFC was explained by differences over the learning phases in response to the CSD+ versus CSP+ in controls but not cocaine users (Figure 2A). As in fear extinction studies, in controls, VMPFC activation was higher during extinction for the CSD+ (which was rated as unpleasant). However, it was lower during extinction for the CSP+ (which was rated as pleasant; cue × learning phase interaction in controls: F1.32, 18.48=5.00, P=0.029, cue × learning phase linear effect: P=0.021). In contrast, in cocaine users, there was no such shift as extinction progressed (cue × learning phase interaction in the cocaine group: F1.44, 24.45=0.72, P=0.49). See the Supporting Information for preliminary data showing that these VMPFC findings do not appear to be specific to the abstract image cues used in the present study but rather extend to alternate USs (i.e., gain of real money).
As in (Phelps et al., 2004), we also tested the cross-day association between SCR and VMPFC activity. The success of extinction learning on day 1, as indexed by a reduction in SCR (average SCR over the last half of day 1 extinction) to the CSD+ versus CS−, correlated with the magnitude of left VMPFC activation to the CSD+ versus CS− during day 2 extinction (n=25, RS=−0.45, P=0.025; Figure 2B) and neither group alone drove this effect. In cocaine users (n=14), this relationship was RS=−0.66, while in controls (n=11) it was RS=−0.44. The relationship between SCR and right VMPFC activation was similar (RS=−0.39, P=0.053; cocaine users: RS=−0.57, controls: RS=−0.41), altogether showing the VMPFC might have a specific role in the retrieval of extinction learning for drug cues as previously found for fear.
Finally, supporting a role for the striatum in extinction learning, but not supporting our hypothesis of group differences, we observed a significant cue × learning phase interaction in this region (F2, 62=7.97, P=0.001, d=1.01) and no significant diagnostic group or additional interaction effects (all F<0.61, P>0.54, d<0.28). Similar to findings in the VMPFC, the two-way interaction was explained by higher striatum activation to the CSD+ but lower activation to the CSP+ during extinction relative to acquisition (cue × learning phase linear effect: P=0.003; Figure 3). There was no significant correlation with SCR for either day 1 or day 2 extinction (RS<0.22, P>0.31).
Figure 3.
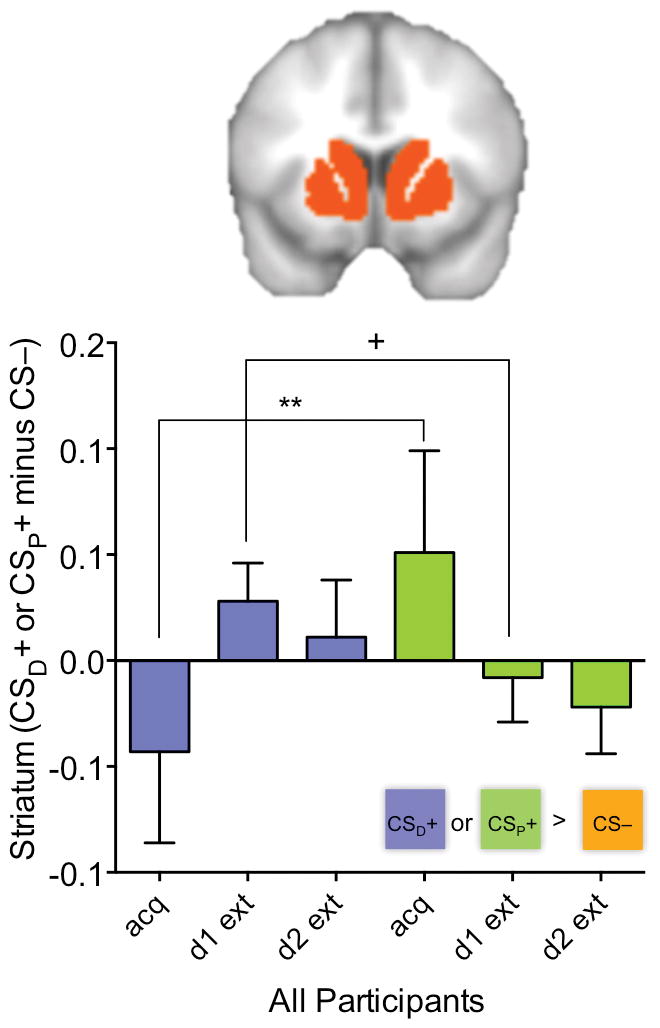
Modulation of striatum activation by learning phase is valence-specific across participants. (A) Plot shows average left and right striatum activation for each learning phase (acquisition, day 1 extinction, day 2 extinction) as a function of cue-type [CSD+ (cue paired with drug-related image) and CSP+ (cue paired with pleasant image), both relative to the CS− (cue paired with neutral image)] across controls and cocaine users. There was a 2-way interaction such that, across participants, striatum activation decreased in response to the CSP+ but increased in response to the CSD+ with extinction training. +P≤0.10, *P≤0.05, **P≤0.01. See also Figures S3–S4 and Tables S1–S2.
See Tables S1–S2 and Figures S3–S4 for results of whole-brain analyses and the Supporting Information for control region analyses. In addition to providing independent support for our ROI findings, the whole-brain analyses showed additional involvement of the amygdala and parahippocampal gyrus, among other regions such as the inferior frontal gyrus and sensory cortices, during extinction learning, and as expected, no significant differential task modulation or task condition by diagnostic group interactions in our negative control (auditory cortex) region. Finally, because a subset (n=6 on day 1 and n=4 on day 2) of participants tested positive for cocaine, indicating recent (≤72 h) exposure to the drug, we also tested whether cocaine urine status had any bearing on our main results. The linear learning phase main effect on SCR, the cue type × learning phase × group interaction in the left VMPFC, and the cue type × learning phase interaction in the striatum all remained significant when we excluded the n=4 participants who were cocaine positive on both study days (P<0.038). SCR and activation in the two ROIs also did not differ by cocaine urine status (positive/negative) at acquisition and day 1 extinction (n=6 vs. n=12, respectively; P>0.066) or day 2 extinction (n=4 vs. n=14, respectively; P>0.084). These control analyses suggest that the effects of recent cocaine use are not likely to have confounded those of diagnosis.
Relationship to Craving
Given its role in cue-induced craving, we tested whether VMPFC activation during extinction correlated with participants’ current and past 24 h desire for cocaine, including that triggered by drug-related cues (total craving score; Table 1). Those cocaine users with higher VMPFC activation to the CSD+ versus CS− during day 1 extinction (i.e., who looked more like controls) reported a greater reduction in craving on day 2 relative to day 1 (R=−0.49, P=0.04; Figure 4), as driven by the relationship to day 2 craving (R=−0.53, P=0.02; day 1 craving: R=−0.26, P=0.29).
Figure 4.
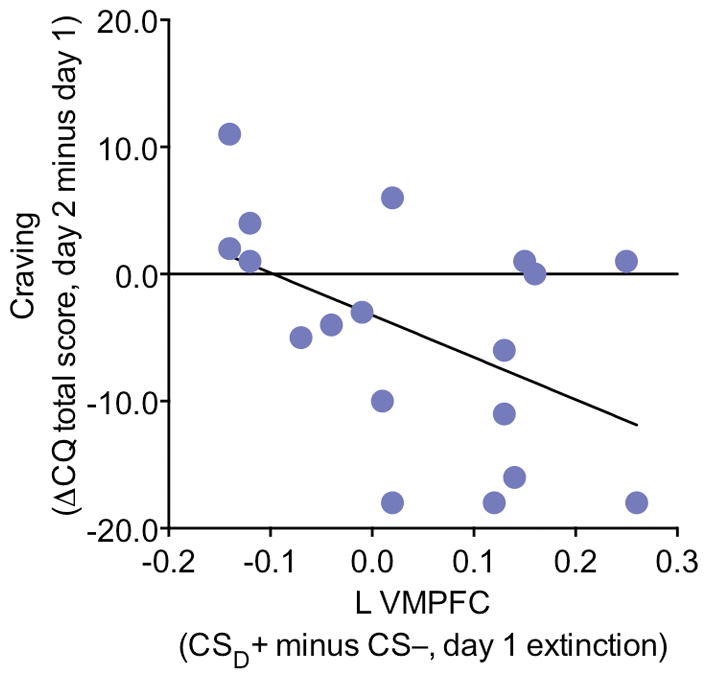
VMPFC activation during day 1 extinction correlates with a reduction in craving from day 1 to day 2. Left VMPFC activation to the CSD+ relative to the CS− on day 1 extinction correlated with a reduction in craving from day 1 to day 2, showing that participants who were more successful at modulating activation in this region on day 1 extinction in response to the drug-relevant cue experienced less severe drug cravings a day later.
Discussion
Extinction of fear critically depends on the VMPFC (Hartley et al., 2011; Milad et al., 2005; Milad et al., 2007; Phelps et al., 2004). Here we show that this same region plays a role also in extinction of drug cue associations. During extinction learning, VMPFC response to the CSD+ (cue associated with the drug-related image) increased, while psychophysiological arousal decreased. These two measures were correlated such that participants who showed the most success in extinguishing arousal responses to the CSD+ on day 1 also showed the greatest VMPFC increases to the CSD+ on day 2, presumably when extinction learning from day 1 is recalled. While a similar (increased) VMPFC activation is observed in fear extinction studies (Milad et al., 2007; Phelps et al., 2004; Schiller et al., 2013), VMPFC response to the CSP+ (cue associated with the affectively pleasant image) and an alternate appetitive CS (cue associated with monetary gain; see Supporting Information) instead decreased during extinction learning, consistent with a value updating process. Finally, while controls showed these distinct VMPFC response profiles, cocaine-addicted individuals, who manifest deficits in the VMPFC, did not, suggesting that the VMPFC, when intact, supports extinction learning in diverse contexts including of drug cue and pleasant associations.
That activation in the VMPFC might reflect a shift from a more valenced state (unpleasant as for the CSD+, or pleasant as for the CSP+) to a less valenced or neutral state follows from a large body of work showing that the VMPFC represents the value of a wide range of (appetitive and aversive) stimuli to guide behavior (Bartra et al., 2013). While this more general role of the VMPFC in extinction has been previously hypothesized (Schiller and Delgado, 2010; Schiller et al., 2008), direct empirical support has been limited as neuroimaging studies have almost exclusively focused on fear extinction. Animal work shows that the infralimbic cortex (the rodent homologue of the VMPFC) is involved in extinction of both appetitive and aversive CSs (Peters et al., 2009). In these studies inactivation of the infralimbic cortex impairs extinction as well as extinction recall for CSs that during acquisition predicted shocks [e.g., (Sierra-Mercado et al., 2011)], but seems to facilitate extinction for CSs that during acquisition predicted appetitive reinforcers [e.g., sugar (Mendoza et al., 2015)]. While additional studies are clearly needed to determine if the same VMPFC-mediated mechanism underlies extinction for all types of CSs, we speculate that the VMPFC stores the current value of the CS, likely in concert with other regions which themselves represent specific features of the CS (e.g., the amygdala in the case of aversive CSs, the striatum in the case of appetitive CSs).
In a previous fear extinction study (Phelps et al., 2004), reduction in SCR during extinction correlated with VMPFC activation a day later, suggesting the VMPFC is specifically involved in the retrieval of extinction learning. We saw this same relationship here: lower SCR during day 1 extinction correlated with higher VMPFC activation during day 2 extinction. This relationship to SCR is supported by evidence that the VMPFC, unlike a more dorsal medial region of the prefrontal cortex typically implicated in the expression of conditioned associations (namely the dorsal anterior cingulate), granger causally drives changes in skin conductance (Zhang et al., 2014). Despite differences in the activity profiles of the VMPFC for the CSD+ versus the CSP+, however, for both CSs, SCR decreased during extinction relative to acquisition. This cue-insensitive SCR pattern is not surprising given that SCR indexes arousal, and is consistent with that observed in extinction learning studies of food- and shock-paired CSs (Andreatta and Pauli, 2015). Importantly, despite eliciting similar SCRs, valence ratings clearly differentiated the CSs as appetitive or aversive in this prior study. Thus, as indices of the conditioned response, VMPFC BOLD and SCR might represent partly distinct aspects of the CS (valence versus arousal).
Perhaps most strikingly, while controls showed parametric VMPFC changes over the learning phases, which were modulated by CS type, chronic cocaine users did not. This was the case for both CSs, pointing to a generalized VMPFC abnormality in this group. Speaking to the clinical relevance of this finding, cocaine users who were more successful at modulating their VMPFC response to the CSD+ during day 1 extinction (i.e., who looked more like controls) reported greater reductions in craving 24 h later. While these data predict that cocaine-addicted individuals with greater VMPFC impairments may be particularly vulnerable in real-world situations involving drug cues, the SCR data did not reveal differences from controls. We used SCR as a passive measure of the conditioned response consistent with a large body of literature. However, SCR is a noisy measurement in the MRI environment (indeed, there was substantial data loss that may have reduced our power to detect group differences in SCR if these differences existed). Alternatively, however, these neural differences might exist outside overt SCR differences. In studies of anxiety disorders, for example, reproducible VMPFC impairments during fear extinction (Milad et al., 2008; Milad et al., 2009) and its later retrieval (Milad et al., 2013) are observed in the absence of abnormalities in SCR. That is, VMPFC activation might be more sensitive in detecting differences from health, although this possibility remains an open empirical question. To directly test this possibility, future studies could incorporate trial-by-trial expectancy ratings as explicit measures of learning and additional physiological indices of arousal (e.g., pupil dilation) and valence [e.g., startle potentiation/attenuation (Andreatta and Pauli, 2015)].
Contrary to expectations, the groups differed only in the VMPFC. Activation in the striatum mirrored that of the VMPFC, increasing in response to the CSD+ and decreasing in response to the CSP+ as extinction progressed, but there were no differences between cocaine users and controls. This coordinated pattern of activation in the VMPFC and striatum, another central node within the valuation system, further supports a value-sensitive account of extinction learning. But while we and others consistently find structural and functional impairments in stimulant users in the VMPFC (Alia-Klein et al., 2011; Ersche et al., 2013; Konova et al., 2012; Parvaz et al., 2012), that in some cases persist long after drug use ceases (Tanabe et al., 2009), a similar consensus finding regarding the striatum has been difficult to ascertain from the human neuroimaging literature, with a bulk of studies showing intact or even enhanced striatum function [see (Balodis et al., 2012; Konova and Goldstein, 2015) for a detailed discussion on this topic]. Thus, the specific contribution of the striatum to extinction learning in drug addiction requires further study.
Given the theoretical impetus for extinction-based therapy in addiction (Taylor et al., 2009), it is important to consider how our VMPFC findings might inform treatment development, considering at least two potentially meaningful aims: enhancing VMPFC function or “bypassing” it. For the former, the indirect dopamine agonist methylphenidate has been shown to enhance extinction and its retention, possibly via local effects in the infralimbic cortex (Abraham et al., 2012; Luo et al., 2015). This pharmacological approach may be particularly well-suited for cocaine-addicted individuals as prior studies in this population show that methylphenidate bolsters VMPFC function on both emotionally salient (Goldstein et al., 2010; Volkow et al., 2010) and emotionally neutral (Li et al., 2010; Moeller et al., 2014) tasks. Methylphenidate is also shown to modulate resting functional connectivity with the VMPFC (Konova et al., 2013). For the latter aim (decreasing reliance on the VMPFC), post-retrieval extinction, which interferes with memory reconsolidation, may offer a more efficacious method for targeting drug-related associations (Auber et al., 2013; Hutton-Bedbrook and McNally, 2013). Indeed, post-retrieval extinction is shown to be effective at reducing reinstatement and/or renewal of drug-, sugar-, and fear-related associations in rodents and humans (Clem and Huganir, 2010; Flavell et al., 2011; Monfils et al., 2009; Rao-Ruiz et al., 2011; Sartor and Aston-Jones, 2013; Schiller et al., 2010; Xue et al., 2012). Some of this procedure’s success is attributed to its relative independence of the VMPFC (Schiller et al., 2013), and may therefore represent a viable alternative for disorders with compromises in this region such as addiction.
In summary, a hallmark feature of drug addiction is an inability to discontinue drug seeking and use despite reduced pleasure derived from the drug and a range of negative consequences including the foregoing of other potentially rewarding outcomes. Here we show that this inability may stem from a VMPFC-mediated impairment in forming and maintaining new associations for stimuli that were previously, although no longer, predictive of both drug and non-drug related outcomes. As this impairment may hinder the success of extinction-based therapies for addiction, future work could aim to concomitantly bolster VMPFC function and/or develop treatments that minimize reliance on this region.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (1F32DA039648 to ABK; 5F32DA033088 to MAP; 1K01DA037452 to SJM; and 5R21DA020626, 2R21DA034954-01, and 5R01DA023579 to RZG) and the Netherlands Organization for Scientific Research (Rubicon 446-14-015 to AZ). The authors would like to thank Thomas Maloney, Daniel Carrero, and Xufeng Han for help with data collection and analysis, and Patricia A. Woicik for help with participant recruitment. The authors would also like to thank Elizabeth A. Phelps and Candace M. Raio for helpful discussions on the study design and early versions of the manuscript.
Footnotes
Author contributions
MAP, MRD, NA-K, and RZG were responsible for the study concept and design. ABK and MAP contributed to the acquisition of behavioral and imaging data. ABK, MAP, and VB performed statistical and imaging data analyses. ABK drafted the manuscript. MAP, AZ, SJM, MRD and RZG provided critical revision of the manuscript for important intellectual content. All authors critically reviewed content and approved final version for journal submission.
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
References
- Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learning & memory. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alia-Klein N, Parvaz MA, Woicik PA, Konova AB, Maloney T, Shumay E, Wang R, Telang F, Biegon A, Wang GJ, Fowler JS, Tomasi D, Volkow ND, Goldstein RZ. Gene x disease interaction on orbitofrontal gray matter in cocaine addiction. Archives of general psychiatry. 2011;68:283–294. doi: 10.1001/archgenpsychiatry.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreatta M, Pauli P. Appetitive vs. Aversive conditioning in humans. Front Behav Neurosci. 2015;9:128. doi: 10.3389/fnbeh.2015.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auber A, Tedesco V, Jones CE, Monfils MH, Chiamulera C. Post-retrieval extinction as reconsolidation interference: methodological issues or boundary conditions? Psychopharmacology. 2013;226:631–647. doi: 10.1007/s00213-013-3004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Attending to striatal ups and downs in addictions. Biological psychiatry. 2012;72:e25–26. doi: 10.1016/j.biopsych.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning & memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekhof EK, Geier K, Falkai P, Gruber O. Fear is only as deep as the mind allows: a coordinate-based meta-analysis of neuroimaging studies on the regulation of negative affect. Neuroimage. 2011;58:275–285. doi: 10.1016/j.neuroimage.2011.05.073. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Williams GB, Robbins TW, Bullmore ET. Meta-analysis of structural brain abnormalities associated with stimulant drug dependence and neuroimaging of addiction vulnerability and resilience. Current opinion in neurobiology. 2013;23:615–624. doi: 10.1016/j.conb.2013.02.017. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0) Biometrics Research Department, New York State Psychiatric Institute; New York: 1996. [Google Scholar]
- Flavell CR, Barber DJ, Lee JL. Behavioural memory reconsolidation of food and fear memories. Nature communications. 2011;2:504. doi: 10.1038/ncomms1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nature reviews Neuroscience. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Woicik PA, Maloney T, Tomasi D, Alia-Klein N, Shan J, Honorio J, Samaras D, Wang R, Telang F, Wang GJ, Volkow ND. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16667–16672. doi: 10.1073/pnas.1011455107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci. 2011;15:56–67. doi: 10.1016/j.tics.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain structure correlates of individual differences in the acquisition and inhibition of conditioned fear. Cerebral cortex. 2011;21:1954–1962. doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton-Bedbrook K, McNally GP. The promises and pitfalls of retrieval-extinction procedures in preventing relapse to drug seeking. Frontiers in psychiatry. 2013;4:14. doi: 10.3389/fpsyt.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:9503–9511. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kober H, Lacadie CM, Wexler BE, Malison RT, Sinha R, Potenza MN. Brain Activity During Cocaine Craving and Gambling Urges: An fMRI Study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Goldstein RZ. Addiction and Addiction Treatment. The Wiley Handbook on the Cognitive Neuroscience of Addiction. 2015:109. [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Parvaz MA, Alia-Klein N, Volkow ND, Goldstein RZ. Structural and behavioral correlates of abnormal encoding of money value in the sensorimotor striatum in cocaine addiction. The European journal of neuroscience. 2012;36:2979–2988. doi: 10.1111/j.1460-9568.2012.08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. 2013;70:857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33:1318–1326. doi: 10.1111/j.1460-9568.2010.07590.x. [DOI] [PubMed] [Google Scholar]
- Li CS, Morgan PT, Matuskey D, Abdelghany O, Luo X, Chang JL, Rounsaville BJ, Ding YS, Malison RT. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14455–14459. doi: 10.1073/pnas.1002467107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo YX, Xue YX, Liu JF, Shi HS, Jian M, Han Y, Zhu WL, Bao YP, Wu P, Ding ZB, Shen HW, Shi J, Shaham Y, Lu L. A novel UCS memory retrieval-extinction procedure to inhibit relapse to drug seeking. Nature communications. 2015;6:7675. doi: 10.1038/ncomms8675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey S, Petrides M. Architecture and morphology of the human ventromedial prefrontal cortex. Eur J Neurosci. 2014;40:2777–2796. doi: 10.1111/ejn.12654. [DOI] [PubMed] [Google Scholar]
- McCracken CB, Grace AA. Persistent cocaine-induced reversal learning deficits are associated with altered limbic cortico-striatal local field potential synchronization. J Neurosci. 2013;33:17469–17482. doi: 10.1523/JNEUROSCI.1440-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Sanio C, Chaudhri N. Inactivating the infralimbic but not prelimbic medial prefrontal cortex facilitates the extinction of appetitive Pavlovian conditioning in Long-Evans rats. Neurobiology of learning and memory. 2015;118:198–208. doi: 10.1016/j.nlm.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. quiz 554. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. Journal of psychiatric research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10706–10711. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annual review of psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Honorio J, Tomasi D, Parvaz MA, Woicik PA, Volkow ND, Goldstein RZ. Methylphenidate enhances executive function and optimizes prefrontal function in both health and cocaine addiction. Cerebral cortex. 2014;24:643–653. doi: 10.1093/cercor/bhs345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Alia-Klein N, Woicik PA, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Impaired insight in cocaine addiction: laboratory evidence and effects on cocaine-seeking behaviour. Brain. 2010;133:1484–1493. doi: 10.1093/brain/awq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Parvaz MA, Shumay E, Beebe-Wang N, Konova AB, Alia-Klein N, Volkow ND, Goldstein RZ. Gene x abstinence effects on drug cue reactivity in addiction: multimodal evidence. J Neurosci. 2013;33:10027–10036. doi: 10.1523/JNEUROSCI.0695-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann DL, Waters AM. The use of an unpleasant sound as an unconditional stimulus in a human aversive Pavlovian conditioning procedure. Biological psychology. 2006;73:175–185. doi: 10.1016/j.biopsycho.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Konova AB, Tomasi D, Volkow ND, Goldstein RZ. Structural integrity of the prefrontal cortex modulates electrocortical sensitivity to reward. Journal of cognitive neuroscience. 2012;24:1560–1570. doi: 10.1162/jocn_a_00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Prisciandaro JJ, Myrick H, Henderson S, McRae-Clark AL, Santa Ana EJ, Saladin ME, Brady KT. Impact of DCS-facilitated cue exposure therapy on brain activation to cocaine cues in cocaine dependence. Drug and alcohol dependence. 2013;132:195–201. doi: 10.1016/j.drugalcdep.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao-Ruiz P, Rotaru DC, van der Loo RJ, Mansvelder HD, Stiedl O, Smit AB, Spijker S. Retrieval-specific endocytosis of GluA2-AMPARs underlies adaptive reconsolidation of contextual fear. Nature neuroscience. 2011;14:1302–1308. doi: 10.1038/nn.2907. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Post-Retrieval Extinction Attenuates Cocaine Memories. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends in cognitive sciences. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Kanen JW, LeDoux JE, Monfils MH, Phelps EA. Extinction during reconsolidation of threat memory diminishes prefrontal cortex involvement. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20040–20045. doi: 10.1073/pnas.1320322110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlund MW, Brewer AT, Richman DM, Magee SK, Dymond S. Not so bad: avoidance and aversive discounting modulate threat appraisal in anterior cingulate and medial prefrontal cortex. Front Behav Neurosci. 2015;9:142. doi: 10.3389/fnbeh.2015.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spronk DB, Van der Schaaf ME, Cools R, De Bruijn ER, Franke B, van Wel JH, Ramaekers JG, Verkes RJ. Acute effects of cocaine and cannabis on reversal learning as a function of COMT and DRD2 genotype. Psychopharmacology (Berl) 2016;233:199–211. doi: 10.1007/s00213-015-4141-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, Banich M. Medial Orbitofrontal Cortex Gray Matter Is Reduced in Abstinent Substance-Dependent Individuals. Biol Psychiat. 2009;65:160–164. doi: 10.1016/j.biopsych.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JR, Olausson P, Quinn JJ, Torregrossa MM. Targeting extinction and reconsolidation mechanisms to combat the impact of drug cues on addiction. Neuropharmacology. 2009;56(Suppl 1):186–195. doi: 10.1016/j.neuropharm.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P) Psychiatry Res. 1998;79:163–173. doi: 10.1016/s0165-1781(98)00038-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Telang F, Fowler JS, Pradhan K, Jayne M, Logan J, Goldstein RZ, Alia-Klein N, Wong C. Methylphenidate attenuates limbic brain inhibition after cocaine-cues exposure in cocaine abusers. PloS one. 2010;5:e11509. doi: 10.1371/journal.pone.0011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstadt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Buhler M, von der Goltz C, Hermann D, Mann K, Kiefer F. Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: a randomized trial. Biological psychiatry. 2011;69:1060–1066. doi: 10.1016/j.biopsych.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L. A memory retrieval-extinction procedure to prevent drug craving and relapse. Science. 2012;336:241–245. doi: 10.1126/science.1215070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Ide JS, Luo X, Farr OM, Li CS. Ventromedial prefrontal cortex and the regulation of physiological arousal. Soc Cogn Affect Neurosci. 2014;9:900–908. doi: 10.1093/scan/nst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.