Abstract
Objectives
Poor adherence to psychological treatment for insomnia is common and limits treatment gains. Very little is known about predictors of adherence among patients with chronic pain, although adherence is theorized to be more critical and more challenging for these patients. This secondary data analysis examines predictors of drop-out and therapy non-attendance in an osteoarthritis population receiving psychological treatment for insomnia and pain.
Methods
Data were analyzed from the “Lifestyles” trial, a randomized controlled trial of a six-week group cognitive-behavioral pain coping skills intervention (CBT-P), group cognitive behavioral therapy for pain and insomnia (CBT-PI), and an education only attention control group (EOC). The current analysis focuses on 122 participants randomized to CBT-PI from 6 primary care clinics. Measures of treatment acceptability, demographics, and symptoms were collected at baseline. Factor analysis was used to clarify the boundaries of these domains, and hierarchical regression was used to examine the incremental predictive power of these patient characteristics on therapy attendance.
Results
Ratings of treatment acceptability were distinct from demographic and medical variables and baseline symptoms. Treatment acceptability was significantly related to session attendance and drop-out (r’s ranging from .24–.32) and was also one of the strongest predictors of session attendance (β = .20, p < .05).
Discussion
Perceptions of treatment acceptability early in treatment represent a potentially modifiable target to enhance adherence to psychological treatment for insomnia and pain among patients with chronic pain. This work represents an important step towards understanding how to best maximize sleep treatments for this patient population.
Keywords: adherence, CBT-I, chronic pain, insomnia, sleep, treatment acceptability
Psychological treatments for insomnia are challenging. Sleep restriction, during which patients are asked to limit amount of time in bed to match total sleep time, is consistently rated as the least liked component of cognitive behavioral therapy for insomnia (CBT-I) by patients and is least likely to be utilized consistently, despite being associated with improvements in sleep and quality of life.1–3 Although sleep restriction increases homeostatic sleep pressure, leading to reduced time to fall asleep and fewer awakenings during the night, it does temporarily increase daytime sleepiness and impair objective performance.4 During therapy, patients report initial dissatisfaction with sleep restriction, followed by a shift towards acceptance and endorsement of the therapy as they begin to experience improvements in sleep functioning.4, 5 Patients who fail to fully engage in treatment (e.g., nonattendance, premature drop-out, failure to follow treatment recommendations) do not get a chance to experience the full benefits of CBT-I, including improvements in sleep, mood, and pain functioning.6, 7
Failure to attend therapy appointments and premature termination of therapy are important indicators of nonadherence in psychological treatments.8, 9 Up to 40% of patients prematurely drop out of CBT-I in clinical practice, severely limiting treatment gains.10 Consistent adherence to treatment recommendations generally falls around 47–52%.2, 11, 12 It has been hypothesized that for patients with unremitting medical conditions like chronic pain, adherence is even more critical for sleep improvement and may be even more difficult due to the wide-spread effects of pain on emotional, cognitive, and physical functioning.2, 13–16 Importantly, patients with chronic pain who arguably stand to benefit the most from CBT-I may be at greatest risk for limited engagement.17
There is little agreement as to the most important factors related to patient adherence during CBT-I, which hinders the development of interventions to promote adherence.14 Practical barriers, beliefs about treatment acceptability, and motivation are thought to be related to adherence.14, 18 More severe psychological symptoms, sleep disturbances, or medical conditions at baseline are also thought to reduce adherence,10, 14 but confidence in these findings is undermined by inconsistent definitions of adherence and a lack of theoretical frameworks to organize findings.14, 18 Moreover, the generalizability of these findings to patients with chronic pain is unclear. In theory, maladaptive pain coping strategies and dysfunctional beliefs about pain and sleep interfere with treatment recommendations, perpetuating both insomnia and pain,17 but few studies have prospectively examined predictors of engagement with sleep treatment in patients with chronic pain.
To maximize psychological treatment for insomnia for patients with chronic pain, we need a greater understanding of the relative importance of modifiable factors related to adherence. The goal of this study was to provide a comprehensive examination of predictors of treatment adherence to psychological sleep treatment in patients with chronic pain, using a conceptual model of adherence to guide hypotheses and organize findings. The study utilizes data from the “Lifestyles” trial, a double-blind, cluster-randomized, controlled trial of a six-week cognitive-behavioral pain coping skills intervention (CBT-P), cognitive behavioral therapy for pain and insomnia (CBT-PI), and an education only attention control (EOC).19, 20 All interventions were delivered in a group setting, with the goal of improving pain and sleep outcomes. The current analysis focuses on 122 participants randomized to CBT-PI from 6 Group Health primary care clinics in Washington State. It is important to note that CBT-I was delivered via a modified form of the treatment that incorporated elements of cognitive behavioral therapy for chronic pain. No CBT-I–only treatment arm was included in the study protocol because the primary study goal was to determine if an integrated treatment would improve pain and sleep outcomes relative to pain-only CBT treatment.
Adherence was examined through session attendance and drop-out. Drop-out was defined as voluntary study withdrawal prior to the 2 month post-treatment assessment (i.e., during the active treatment phase; most common reasons were failing health and no longer interested in participating). Rates of drop-out in this randomized trial were smaller than what one might expect in clinical practice. For example, drop-out rates in the current study were 2.5% from CBT-P, 6.6% from CBT-PI, and 1.0% from EOC. This is in contrast to drop-out rates of 21% among patients receiving treatment during the VA roll-out of CBT-P and approximately 24% among patients receiving treatment during the VA roll-out of CBT-I.21, 22 However, the drop-out rates in this trial are comparable to other randomized trials of CBT-I for comorbid insomnia and pain23 and to randomized trials of CBT for comorbid pain and insomnia.24 Similarly, rates of attendance were higher than what has been reported in clinical practice (at least 4 of 6 sessions were attended by 91.8% for CBT-P, 93.4% for CBT-PI, and 94.3% for EOC). Given that the samples were drawn from primary care practice and appear to be representative,25 differences in rates of adherence are most likely a function of the research protocol rather than the patient population. For example, treatment sessions were conducted in the subjects’ usual primary care clinics; the familiarity and convenient nature of the study location may have enhanced adherence. In addition, randomized trials include financial incentives, careful screening, and reminder calls, which enhance attendance. Despite the limited variance in outcomes, this work supports the development of preliminary conceptual models of adherence to sleep treatment in patients with chronic pain. In addition, the prospective, longitudinal nature of the data allowed us to test baseline predictors of treatment adherence. Although we include both drop-out and attendance, the multivariate analyses focus on attendance due to the low rates of drop-out.
Our hypotheses were informed by a conceptual framework that is based on previous research and social cognitive theory, as shown in Figure 1. Concepts from social cognitive theory are commonly used to organize research on adherence with evidence-based psychotherapy,26, 27 including insomnia treatments.15, 18, 28 These theories suggest that an individual’s actions are dependent not only on the conditions of the real world, but on their perceptions of this reality (e.g., treatment beliefs). As shown in Figure 1, individual differences among patients (e.g., demographics, baseline symptoms) are theorized to influence individual beliefs (e.g., sleep treatment preferences, acceptability) and ultimately willingness to initiate and adhere to sleep treatment.
Figure 1.
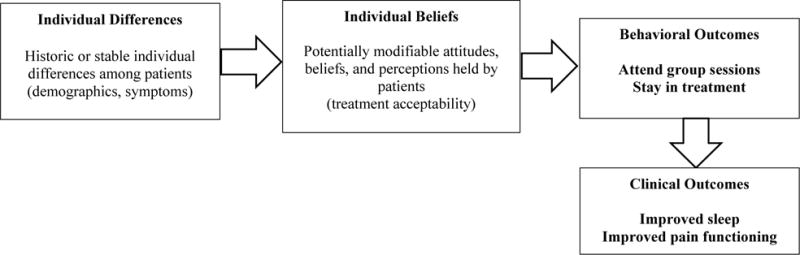
Integrated conceptual model of individual patient factors hypothesized to be related to adherence during psychological sleep treatment
We hypothesized that beliefs early in treatment would predict attendance and drop-out, such that those rating the treatment as less acceptable would be more likely to attend fewer sessions and drop-out of the trial.14, 18 Based on previous research, we also hypothesized that demographics (e.g., younger age) and higher baseline symptom levels (e.g., pain, sleep, depression/anxiety) would predict non-adherence.10, 14
More tentatively, we explored the possibility of a mediational relationship, such that demographics and baseline symptoms indirectly influence adherence via beliefs. There is very little research evidence to support this pathway,1 although social cognitive theory would predict this mediational relationship. Finally, we controlled for baseline medical variables, including chronic illness and medication use (hypnotics, opioids, non-opioid analgesics). Inclusion of these variables was exploratory as there is very little previous research,14 but it is conceivable that higher medical comorbidity and use of medications for pain or sleep would negatively influence adherence. There is some evidence that users of sleeping medications attend fewer psychological sleep treatment sessions.15
Materials and Methods
Participants
Participants were 122 paid volunteers randomized to the CBT-PI treatment arm. The trial was approved by the Group Health and University of Washington institutional review boards and ethical standards were followed. Details of the study are provided in previous publications.19, 20 Briefly, participants who were 60 years or older and had obtained care for osteoarthritis in the last 3 years were eligible if they had clinically significant insomnia (i.e., met research diagnostic criteria for insomnia)29 and pain (i.e., Grade II, III, or IV pain on the Graded Chronic Pain Scale).30 Exclusion criteria included rheumatoid arthritis, primary sleep disorders, dementia, Parkinson’s disease, cancer in the past year, and inpatient treatment for congestive heart failure within the prior 6 months. Of 3,321 potentially eligible participants who were initially screened, 367 were eligible and enrolled in the trial. Of note, the selection criteria allowed for considerable comorbidity in the sample, with 59.8% reporting chronic illness.20
Eligible participants were randomized to either an intervention group (CBT-P or CBT-PI) or an education only attention control group (EOC). The CBT-PI intervention was delivered in a classroom setting in the participants’ primary care clinic by a pair of mental health professionals (master’s level counselor and PhD psychologist) and consisted of 6 weekly 90-minute sessions involving components of cognitive behavioral therapy for pain (pain education, physical activation, goal setting, relaxation, activity pacing, guided imagery, and cognitive restructuring) and CBT-I (sleep hygiene education, stimulus control, sleep restriction, and daily sleep monitoring using sleep logs). Table 1 in Balderson et al. (2016) provides a detailed description of the content of each intervention session.31 As discussed in previous reports,20, 31 treatment arms did not differ significantly on demographics and outcomes measures at baseline, or on ratings of treatment acceptability at the end of session one and post-treatment. CBT-PI resulted in a greater clinically significant reduction in insomnia severity compared to EOC and CBT-P.
The following measures, in addition to demographics and information on current medication use, were collected at baseline prior to starting treatment, with the exception of the treatment acceptability items, which were collected at the end of the first treatment session.
Medical Comorbidity
The Charlson Index,32 a measure of medical comorbidity, was completed for all participants, with the data dichotomized into 0 vs. 1 to identify those without and those with chronic medical conditions other than osteoarthritis and insomnia.
Sleep
Insomnia Severity Index (ISI)
The ISI33 is a 7-item questionnaire designed to provide a global measure of difficulties sleeping at night and daytime impairment. This instrument has adequate psychometric properties, including concurrent validity with sleep diaries and polysomnography.33
Pittsburgh Sleep Quality Index (PSQI)
The PSQI34 was used to obtain information about both nighttime and daytime complaints over the past month. The PSQI has been shown to differentiate patients with sleep disorders from control groups.34
Flinders Fatigue Scale (FFS)
The FFS35 is a 7-item scale that measures frequency and severity of daytime fatigue associated with insomnia. It has shown good psychometric properties and is responsive to changes in sleep following CBT-I.35
Dysfunctional Beliefs and Attitudes About Sleep (DBAS)
The DBAS-1036 is a 10-item shortened version of the DBAS-16,37 a scale that assesses maladaptive sleep-related cognitions. Responses were rated on a 100-point scale and averaged for a final score. The DBAS-10 has been shown to have adequate internal reliability and differentiates normal sleepers from those with insomnia.36
Pain
Graded Chronic Pain Scale (GCPS)
Arthritis pain severity was measured using a mean of six ratings from the GCPS30 assessing arthritis pain intensity (average pain, worst pain, pain right now), and interference with usual, work, recreational, social, and family activities. The GCPS is significantly related to functional impairment due to pain.30
Arthritis Impact Measurement Scales Version 2, Short Form, Revised (AIMS2-SF), symptom subscale
The revised AIMS was designed as an expanded measure of health-related quality-of-life among people with arthritis.38, 39 The symptom subscale on the AIMS2-SF consists of three items asking about arthritis functioning and pain; item values are summed and scores are normalized to range from 0 to 10, with higher scores indicating high functioning and little pain. The AIMS2-SF has shown evidence of good reliability and validity among patients with arthritis.40
Pain Catastrophizing Scale (PCS)
The PCS41 consists of 13 items describing catastrophizing thoughts and negative feelings that individuals may experience when they are in pain. The PCS has been shown to contribute significant unique variance to the prediction of pain intensity beyond measures of depression and anxiety.41
Tampa Scale for Kinesiophobia (TSK)
This study used a shortened 10 item version of the TSK, a scale that measures fear of movement, pain, and injury.42, 43 The shortened form has shown to have good reliability and validity and is predictive of pain disability.43
Depression/Anxiety
Geriatric Depression Scale (GDS)
Participants completed the GDS.44 This scale has 30 yes-no items that are specifically designed to measure depression in older adults. Scores of 11–13 indicate mild depression while 14 or greater indicates moderate to severe depression. It has shown evidence of good psychometric properties, including convergent validity with other measures of depression.44
AIMS2-SF, affect subscale
The affect subscale of the AIMS2-SF38, 39 consists of 5 items asking about symptoms of anxiety/depression, including nervousness, sad mood, and anhedonia. Scores are normalized to range from 0 to 10, with higher scores indicating less anxiety/depression.
Treatment Acceptability
Participants completed six items as part of a treatment evaluation questionnaire adapted from prior work45 at the end of the first treatment session. In the current study, four items correlating .65 and higher were summed to create a Treatment Acceptability Scale. The following items were included in the scale: 1) Does this treatment and its rationale make sense to you?, 2) How acceptable do you consider this treatment?, 3) How suitable is this treatment for improving the quality of your life despite having osteoarthritis, and 4) How effective do you expect this treatment to be? Items were scored from 1 (not at all) to 7 (very much so). The coefficient alpha for the 4-item Treatment Acceptability Scale was .90.
Adherence
The two markers of adherence in this study include total number of CBT-PI treatment sessions attended (mean = 5.34, SD = 1.24; 5% attended 1 treatment session, 1% attended two and three sessions respectively, 8% attended 4 sessions, 19% attended 5 sessions, 66% attended 6 sessions) and drop-out prior to the 2 month post-treatment assessment (n = 8, 6.6%).
Statistical Analyses
The primary goal of current analyses was to explore adherence to psychological sleep treatment within a theoretical framework. Given that very little work has been done to develop conceptual models of adherence, we first used exploratory factor analysis to clarify the boundaries of the domains thought to be related to adherence, including sleep, pain, depression/anxiety, and treatment acceptability. It is important to demonstrate that these domains represent distinct constructs rather than manifestations of general distress (e.g., low ratings of treatment acceptability are not simply a manifestation of depression or severe insomnia). From the CBT-PI group, 113 had complete data for all the baseline measures described above and the data were submitted to exploratory principal factor analysis with varimax rotation using SAS (version 9.2). Scale level data were used for all domains, with the exception of treatment acceptability. Since the Lifestyles study only included one measure of acceptability, individual items were submitted to analysis. An orthogonal rotation was used to model these theoretically independent domains. The prior communality estimate was calculated using squared multiple correlations (SMCs). The goal in these analyses was to extract the greatest number of distinct and interpretable factors.
Next we conducted a regression analysis, including demographics, medical variables, baseline symptoms, and treatment acceptability as predictors and total CBT-PI treatment session attendance as the criterion variable among 116 participants who had complete data on these measures. Drop-out was not used as a criterion variable given extremely low rates of attrition and the resulting skewed distribution, however, we do report bivariate correlations of the predictors with drop-out.
Results
Descriptive Statistics
Table 1 presents demographic and medical variables for the participants in the CBT-PI group, as well as sleep, pain, depression/anxiety and treatment acceptability measures at baseline. Participants reported an average of five pain sites. Nearly half were identified as having comorbid chronic illness in addition to osteoarthritis and insomnia as classified by the Charlson index. Nearly a quarter reported current use of hypnotics and opioids, and 84.3% reported current use of non-opioid analgesics. In general, participants reported moderate levels of sleep disturbances and pain, with low levels of depression/anxiety. Around 30 to 40% of participants reported being above clinical cut-offs for moderate to severe insomnia and pain, whereas 10% reported being above clinical cut-offs for moderate to severe depression.
Table 1.
Characteristics of Participants Randomized to the Cognitive Behavioral Therapy for Pain and Insomnia (CBT-PI) Group in the Lifestyles Study
| Demographics, No (%) | |
| Age, mean (SD), y | 73.16 (7.99) |
| Female | 97 (79.51) |
| Caucasian | 112 (91.80) |
| Married | 62 (50.82) |
| Education > high school | 103 (84.43) |
|
| |
| Medical Variables, No (%) | |
| Charlson Index, above 0 | 57 (46.72) |
| Number of pain sites, mean (SD), No. | 5.24 (2.50) |
| Current use of hypnotics | 27 (22.31) |
| Current use of opioids | 24 (19.83) |
| Current use of non-opioid analgesics | 102 (84.30) |
|
| |
| Baseline sleep scales, mean (SD) | |
| ISI (range, 0–28 [worst]) | 11.20 (5.18) |
| ≥15 (moderate to severe), No (%) | 35 (28.69) |
| PSQI (range, 0–21 [worst]) | 9.23 (3.68) |
| FFS (range, 0–31 [worst]) | 11.44 (6.25) |
| DBAS (range, 0–100 [worst]) | 47.39 (15.59) |
|
| |
| Baseline pain scales, mean (SD) | |
| GCPS (range, 0–10 [worst]) | 4.57 (1.50) |
| ≥5 (moderate to severe), No (%) | 52 (42.62) |
| AIMS2-SF, Symptom (range, 0–10 [best]) | 6.06 (2.12) |
| PCS (range, 0–52 [worst]) | 10.34 (8.88) |
| TSK (range, 17–68 [worst]) | 37.12 (9.49) |
|
| |
| Baseline depression/anxiety scales, mean (SD) | |
| GDS (range, 0–30 [worst]) | 6.46 (5.10) |
| ≥14 (moderate to severe), No (%) | 12 (9.84) |
| AIMS2-SF, Affect (range, 0–10 [best]) | 7.51 (1.32) |
|
| |
| Treatment acceptability scale, mean (SD) | |
| Treatment Acceptability Scale (range, 4–28 [best]) | 21.14 (4.38) |
Note. n = 117–122.
ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, FFS = Flinders Fatigue Scale, DBAS = Dysfunctional Beliefs and Attitudes About Sleep, GCPS = Graded Chronic Pain Scale, AIMS2-SF = Arthritis Impact Measurement Scales Version 2 Short Form Revised, PCS = Pain Catastrophizing Scale, TSK = Tampa Scale for Kineseophobia, GDS = Geriatric Depression Scale.
The two markers of adherence, total number of CBT-PI treatment sessions attended and drop-out prior to the 2 month post-treatment assessment, were significantly correlated at r = −.48, p < .001, such that those attending fewer treatment sessions were more likely to drop out. Prior to running analyses, we investigated differences on baseline demographics and symptoms among those who dropped out and those who were retained. There were no significant differences between groups on mean scores on the measures of insomnia, pain, and anxiety/depression listed in Table 1, nor were there differences in age, sex, chronic medical illness, or medication use. Although not used in the current analyses, the study also collected a week of actigraphy at baseline and there were no significant differences in objectively measured sleep variables, including total sleep time and sleep efficiency.
Factor Structure of Treatment Acceptability, Pain, Sleep, and Depression/anxiety
Exploratory factor analyses initially revealed the presence of a large and relatively broad factor of sleep, pain, and depression/anxiety that accounted for 50% of the total variance (treatment acceptability items did not have significant loadings on this factor). In the next step, two factors representing depression/anxiety/pain/sleep and treatment acceptability were extracted. These factors accounted for 89% of the common variance. When three factors were extracted, they represented depression/anxiety/sleep, pain, and treatment acceptability and accounted for all of the common variance. When four factors were extracted, they represented treatment acceptability, pain, depression/anxiety, and sleep. Although both the three and four factor models were interpretable and well-defined, we report on the four-factor model since it defines the hypothesized domains of interest and demonstrated replicability across treatment arms (see Footnote 1).
As shown in Table 2, the Acceptability factor is defined by the four items from the Treatment Acceptability Scale. Pain is defined by scales measuring pain intensity, functioning, and dysfunctional beliefs about pain. Depression/anxiety is defined by scales measuring these symptoms. Sleep is defined by scales measuring insomnia and daytime fatigue. The four-factor structure is clear and distinct, with limited cross-loadings. We examined correlations of regression-based factor scores with age, sex, chronic medical condition, and medication usage; none of these correlations was significant, suggesting that the proposed predictors of adherence are independent constructs. Given the lack of significant correlations between treatment acceptability and demographics/symptoms, we did not pursue mediational analyses.
Table 2.
Factor Structure of Treatment Acceptability Items and Baseline Symptoms
| Scales | Factor I (Acceptability) |
Factor II (Pain) |
Factor III (Dep/Anx) |
Factor IV (Sleep) |
|---|---|---|---|---|
| Treatment made sense | .84 | −.07 | .03 | .14 |
| Treatment is acceptable | .83 | −.07 | −.02 | .12 |
| Treatment is suitable | .80 | .04 | .07 | −.08 |
| Expect will be effective | .76 | −.07 | .05 | −.12 |
| GCPS | −.17 | .68 | .10 | .12 |
| PCS | .03 | .59 | .36 | .12 |
| TKS | −.02 | .53 | .21 | .08 |
| AIMS2-SF symptoms | .03 | −.62 | −.01 | −.29 |
| GDS | −.01 | .13 | .77 | .21 |
| DBAS | .13 | .22 | .37 | .11 |
| AIMS2-SF affect | .01 | −.13 | −.69 | −.18 |
| ISI | .03 | .23 | .31 | .75 |
| PSQI | −.01 | .14 | .13 | .60 |
| FFS | .04 | .31 | .42 | .55 |
Note. n = 113. Factor loadings of |.40| and greater are highlighted.
Dep/anx = Depression/anxiety. GCPS = Graded Chronic Pain Scale, PCS = Pain Catastrophizing Scale, TSK = Tampa Scale for Kineseophobia, AIMS2-SF = Arthritis Impact Measurement Scales Version 2 Short Form Revised, GDS = Geriatric Depression Scale, DBAS = Dysfunctional Beliefs and Attitudes About Sleep, ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, and FFS = Flinders Fatigue Scale.
Predicting Adherence to Psychological Treatment for Insomnia and Pain
Prior to conducting regression analyses, we examined bivariate correlations of adherence with baseline demographics, medical variables, and with the individual scales that loaded most strongly on each factor. These scales were considered the best markers of each domain and were used in the subsequent hierarchical regression analysis (i.e., ISI for sleep, GDS for depression/anxiety, GCPS for pain). The Treatment Acceptability Scale was used as a marker of treatment acceptability in all analyses. As shown in Table 3, Treatment Acceptability and opioid use showed the highest correlations with adherence, with r’s ranging from .24 to .32.
Table 3.
Correlations of Adherence with Acceptability, Demographics, Medical Variables, and Baseline Symptoms
| Total number of sessions | 2-month drop-out | |
|---|---|---|
| Treatment Acceptability Scale | .24* | −.32* |
| Age | .13 | .05 |
| Sex | −.15 | .12 |
| Charlson index | −.02 | −.02 |
| Hypnotics | −.13 | −.13 |
| Opioids | −.24* | −.04 |
| Non-opioid analgesics | −.04 | .00 |
| ISI | −.02 | .05 |
| GDS | −.18 | .10 |
| GCPS | −.10 | .08 |
Note. n = 116.
significant at p ≤ .01.
ISI = Insomnia Severity Index, GDS = Geriatric Depression Scale, GCPS = Graded Chronic Pain Scale.
Table 4 shows the hierarchical multiple regression using treatment session attendance as a criterion variable. First, a block of demographics was entered, including age and sex. Next a block of baseline medical variables was entered, including chronic illness, hypnotic use, opioid use, and non-opioid analgesic use. Third, a block of baseline symptoms was added, including the measures of sleep, depression/anxiety, and pain. The Treatment Acceptability Scale was entered in a final step. Table 4 reports the R2 for each step, as well as the R2 change for steps 2 through 4. The standardized regression coefficients and standard error for each predictor in final model are also shown in the final columns of Table 4. In step 1, demographics did not significantly predict treatment session attendance. In step 2, demographics and medical variables significantly predicted attendance, F(6, 109) = 2.29, p = .04, with opioid use as the significant predictor (β = −.24, p < .05). In step 3, symptom scales did not add significantly to the prediction of attendance over and above demographics and medical variables. The Treatment Acceptability Scale in step 4 showed incremental predictive power, significantly predicting an additional 3% of the variance in treatment session attendance. In the final model, 17% of the variance in treatment session attendance was predicted, F(10,105) = 2.21, p < .05, with Treatment Acceptability (β = .20, p < .05) and opioids use (β = −.21, p < .05) the only significant predictors. See Footnote 2 for results of these analyses in the other treatment arms.
Table 4.
Hierarchical Multiple Regression Predicting Treatment Session Attendance
| Steps/Scales | R2 | ΔR2 | β | SE |
|---|---|---|---|---|
| 1. Demographics | .04 | |||
| Age | .12 | .01 | ||
| Sex | −.14 | .27 | ||
| 2. Medical Variables | .11* | .07 | ||
| Charlson index | .00 | .21 | ||
| Hypnotics | −.08 | .25 | ||
| Opioids | −.21* | .27 | ||
| Non-opioid analgesics | .00 | .30 | ||
| 3. Symptoms | .14 | .03 | ||
| ISI | .11 | .02 | ||
| GDS | −.18 | .02 | ||
| GCPS | .02 | .07 | ||
| 4. Treatment Acceptability Scale | .17* | .03* | .20* | .02 |
Note. n = 116.
p < .05.
ISI = Insomnia Severity Index, GDS = Geriatric Depression Scale, GCPS = Graded Chronic Pain Scale.
Discussion
This study provides a comprehensive examination of predictors of adherence to psychological treatment for insomnia and pain over the course of a randomized treatment trial among patients with chronic pain. Findings were organized and interpreted within the framework of a conceptual model of adherence informed by social cognitive theory and previous research.
Treatment Acceptability is an Independent Construct
Factor analyses suggest that treatment acceptability is a distinct construct; items referring to perceived credibility, acceptability, and initial impressions of the effectiveness of the psychological treatment for insomnia and pain hung together to form a factor that was clearly distinct from demographics and baseline symptoms. This suggests that these items are not simply reflecting general distress, but in fact represent a unique cognitive construct. Moreover, contrary to our hypotheses, we found little evidence that treatment acceptability is related to demographics and baseline symptoms. Additional research is needed to discover how opinions about acceptability are formed. The first session of the CBT-PI treatment group involved education, treatment rationale, and active treatment components (sleep hygiene, stimulus control) and it remains to be determined which of these elements may have driven perceptions of acceptability. Nevertheless, the independence of treatment acceptability from relatively stable individual differences makes treatment acceptability a promising, potentially modifiable construct that can be targeted prior to or during treatment to enhance adherence.
Treatment Acceptability Significantly Predicts Adherence Beyond Demographics, Medical Variables, and Symptoms
The overall power of our model in predicting treatment session attendance from treatment beliefs, demographics, and symptoms was modest (17% of total variance), suggesting that treatment session attendance is a fairly complex behavior to predict. More comprehensive models predicting treatment attendance could include additional individual characteristics (e.g., motivation, current mental and physical state) and environmental barriers (e.g., conflicting appointments, caregiving demands, weather). Despite the limited overall predictive power of the model, the four-item Treatment Acceptability Scale completed at the end of the first treatment session emerged as one of the strongest predictor of treatment session attendance in the CBT-PI group, predicting an additional 3% of the variance above and beyond demographics, medical variables, and symptoms. Overall the model suggests that patients who did not find the treatment credible at the end of the first session attended fewer treatment sessions over the six week treatment period. Treatment acceptability was also significantly related to drop-out (r = −.32, p < .01).
Acceptability as a predictor of adherence to sleep treatment has been demonstrated previously among patients with primary insomnia.14, 18 This study replicates this finding among patients with more complex medical comorbidities; unexpectedly, treatment beliefs emerged as a powerful predictor of engagement, above and beyond baseline symptoms, maladaptive pain coping strategies, and dysfunctional beliefs about pain and sleep. It has been suggested that preparatory work to enhance treatment acceptability prior to the start of psychological sleep treatment may reduce premature attrition.5, 18 Similarly, patients who express a positive attitude and motivation for treatment prior to starting therapy may be more likely to engage and derive greater therapeutic value.5, 18
Given the apparent importance of first impressions, efforts to increase perceived acceptability of treatment prior to the start of treatment would most likely lead to increases in behaviors that contribute to the effectiveness of psychological sleep treatments like CBT-I, although future work is needed to determine the extent to which treatment session attendance during a six session group treatment protocol contributes to symptom reduction. It is generally accepted that premature drop-out (stopping treatment prior to pre-specified number of treatment sessions during manualized treatment) is related to worse outcomes, however, future studies will also need to examine early vs. late drop-out and additional measures of adherence (e.g., homework completion, weekly adherence to treatment recommendations) when modeling the complex relationship between treatment beliefs, adherence, and effectiveness.18 The findings from this study suggest that it will be valuable to continue examining the impact of early treatment beliefs on specific measures of adherence throughout the course of treatment, as well as the impact of adherence on treatment outcomes.
The hierarchical regression also found that patients who were taking opioid medications at baseline attended fewer treatment sessions, with a predictive power similar to treatment acceptability. Given the relatively high standard error of this predictor and the fact that this finding has not been reported elsewhere, it will need to be replicated in future studies. It is possible that patients who were being treated with opioids had lower functionality and mobility in general, making it more difficult to attend treatment. Alternatively, long-term opioid use for chronic pain has been associated with reduced motivation and apathy, which may have a negative impact on treatment attendance and motivation for treatment adherence.46 In order to continue investigating this relationship, future studies of adherence will need to include patients who are on medications for sleep and pain. Interestingly, baseline pain levels were not predictive of treatment session attendance, suggesting pain intensity per se may not be a significant barrier.
Limitations and Conclusions
Given that this was a secondary data analysis and the parent trial was not designed to examine adherence, several limitations should be noted. As mentioned earlier, CBT-I was delivered via a modified form of the treatment that included pain-specific treatment components. The extent to which the adherence findings would generalize to traditional CBT-I, or to psychotherapy in general, remains to be determined. The Treatment Acceptability Scale completed at the end of the first treatment session did not emerge as a significant predictor in the other treatment arms (see Footnote 2), despite the equivalence of average baseline acceptability ratings, drop-out rates, and attendance rates across groups.31 A related limitation is that the findings are limited to group treatment; the social support inherent in this treatment modality may facilitate session attendance compared to individual therapy. Additional studies will be needed to determine if social support during treatment is a relevant domain for conceptual models of adherence.
An additional limitation is that our analyses did not control for symptom changes across sessions, so we were unable to determine if drop-out or non-attendance was influenced by changes in sleep and pain symptoms across treatment (i.e., patients stop attending because they are improving or because they are not improving). Without controlling for symptom changes over time, measures of adherence could potentially be confounded with treatment efficacy. It has been suggested that insomnia severity at baseline may moderate treatment response. Specifically, a biologically severe phenotype of insomnia, marked by short objective total sleep time at baseline, may not respond as well to psychological sleep treatments.10, 47, 48 It is possible, then, that these treatment non-responders may attend fewer treatment sessions or drop out. However, as reported earlier, there were no differences between patients who dropped out of the study and those who completed on total sleep time at baseline, as measured by sleep logs and actigraphy. In addition, it has been demonstrated that short total sleep time at baseline is related to treatment response, but not to attendance, suggesting a degree of independence between measures of adherence and symptom improvement.47
Finally, the extent to which these findings replicate in real-world clinical settings remains to be determined. As discussed earlier, drop-out rates for this study were lower than what might be expected in clinical practice, possibly due to financial incentives and a highly structured protocol. Additionally, patients may have had milder symptom profiles than those seen in clinical practice, which may limit the generalizability of findings. Similarly, as an RCT, the treatment was designed to be delivered over six consecutive weeks; however, in clinical settings there is more variability in scheduling due to patient needs and provider availability. Investigating attendance rates for treatment over a longer period of time (e.g., 6 sessions attended over 10 weeks) would most likely provide a measure of adherence and premature drop-out that more closely represents real-world conditions.
This study presents the initial development of a conceptual model for adherence to CBT-I among patients with chronic pain. It is our hope that future research will continue to build and refine this model by investigating additional measures of adherence (e.g., adherence to sleep window, extent to which patients followed individual guidelines for stimulus control and sleep restriction) and additional analytic methods (e.g., sleep logs, therapist ratings). Future research is also needed to investigate the generalizability of the model to other patient populations, particularly those with complex comorbidities, who would benefit from psychological sleep treatment.
Supplementary Material
Acknowledgments
Source of Funding: This study was supported by NIH grant R01-AG031126 (MVV, SMMc and MVK - Multiple Principal Investigators). This material is the result of work supported with resources and the use of facilities at the Minneapolis VA Health Care System, Minneapolis, MN. The views expressed in this article are those of the authors and do not reflect the official policy or position of the US Department of Veterans Affairs.
Footnotes
Conflict of Interest: For all authors, no conflicts of interest were declared.
The four factor structure was also found in the EOC and CBT-P groups, suggesting that it is robust and replicable.
We examined the generalizability of the predictive model across treatment arms. In the CBT-P group, the final model did not reach statistical significance. In the EOC group, number of treatment sessions attended was significantly predicted by pain severity as measured by the GCPS (β = −.30, p <.01).
Contributor Information
Erin Koffel, Center for Chronic Disease Outcomes Research, Minneapolis VA Health Care System; University of Minnesota Medical School.
Michael V. Vitiello, Departments of Psychiatry and Behavioral Sciences, School of Medicine, University of Washington.
Susan M. McCurry, Psychosocial and Community Health, School of Nursing, University of Washington.
Bruce Rybarczyk, Psychology Department, Virginia Commonwealth University.
Michael Von Korff, Group Health Research Institute, Seattle, Washington.
References
- 1.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24:411–7. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 2.Vincent N, Hameed H. Relation between adherence and outcome in the group treatment of insomnia. Behav Sleep Med. 2003;1:125–39. doi: 10.1207/S15402010BSM0103_1. [DOI] [PubMed] [Google Scholar]
- 3.Harvey L, Inglis SJ, Espie CA. Insomniacs’ reported use of CBT components and relationship to long-term clinical outcome. Behav Res Ther. 2002;40:75–83. doi: 10.1016/s0005-7967(01)00004-3. [DOI] [PubMed] [Google Scholar]
- 4.Kyle SD, Miller CB, Rogers Z, et al. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: Implications for the clinical management of insomnia disorder. Sleep. 2014;37:229–37. doi: 10.5665/sleep.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyle SD, Morgan K, Spiegelhalder K, et al. No pain, no gain: An exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med. 2011;12:735–47. doi: 10.1016/j.sleep.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Koffel E, Koffel J, Gehrman P. A meta-analysis of group cognitive behavioral therapy for insomnia. Sleep Med Rev. 2015;19:6–16. doi: 10.1016/j.smrv.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang NK, Lereya ST, Boulton H, et al. Nonpharmacological treatments of insomnia for long-term painful conditions: a systematic review and meta-analysis of patient-reported outcomes in randomized controlled trials. Sleep. 2015;38:1751–64. doi: 10.5665/sleep.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oldham M, Kellett S, Miles E, et al. Interventions to increase attendance at psychotherapy: a meta-analysis of randomized controlled trials. J Consult Clin Psychol. 2012;80:928–39. doi: 10.1037/a0029630. [DOI] [PubMed] [Google Scholar]
- 9.Corrigan PW, Rusch N, Ben-Zeev D, et al. The rational patient and beyond: implications for treatment adherence in people with psychiatric disabilities. Rehabil Psychol. 2014;59:85–98. doi: 10.1037/a0034935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong JC, Kuo TF, Manber R. Who is at risk for dropout from group cognitive-behavior therapy for insomnia? J Psychosom Res. 2008;64:419–425. doi: 10.1016/j.jpsychores.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McChargue DE, Sankaranarayanan J, Visovsky CG, et al. Predictors of adherence to a behavioral therapy sleep intervention during breast cancer chemotherapy. Support Care Cancer. 2012;20:245–52. doi: 10.1007/s00520-010-1060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruiter Petrov ME, Lichstein KL, Huisingh CE, et al. Predictors of adherence to a brief behavioral insomnia intervention: Daily process analysis. Behav Ther. 2014;45:430–42. doi: 10.1016/j.beth.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Matthews EE, Schmiege SJ, Cook PF, et al. Adherence to cognitive behavioral therapy for insomnia (CBTI) among women following primary breast cancer treatment: A pilot study. Behav Sleep Med. 2012;10:217–29. doi: 10.1080/15402002.2012.666220. [DOI] [PubMed] [Google Scholar]
- 14.Matthews EE, Arnedt JT, McCarthy MS, et al. Adherence to cognitive behavioral therapy for insomnia: A systematic review. Sleep Med Rev. 2013:453–64. doi: 10.1016/j.smrv.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincent N, Lewycky S, Finnegan H. Barriers to engagement in sleep restriction and stimulus control in chronic insomnia. J Consult Clin Psychol. 2008;76:820–8. doi: 10.1037/0022-006X.76.5.820. [DOI] [PubMed] [Google Scholar]
- 16.Turk DC, Rudy TE. Neglected topics in the treatment of chronic pain patients–relapse, noncompliance, and adherence enhancement. Pain. 1991;44:5–28. doi: 10.1016/0304-3959(91)90142-K. [DOI] [PubMed] [Google Scholar]
- 17.Smith MT, Huang MI, Manber R. Cognitive behavior therapy for chronic insomnia occurring within the context of medical and psychiatric disorders. Clin Psychol Rev. 2005;25:559–92. doi: 10.1016/j.cpr.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 18.Cheung JM, Bartlett DJ, Armour CL, et al. Treating insomnia: A review of patient perceptions toward treatment. Behav Sleep Med. doi: 10.1080/15402002.2014.981818. in press. [DOI] [PubMed] [Google Scholar]
- 19.Von Korff M, Vitiello MV, McCurry SM, et al. Group interventions for co-morbid insomnia and osteoarthritis pain in primary care: The lifestyles cluster randomized trial design. Contemp Clin Trials. 2012;33:759–68. doi: 10.1016/j.cct.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vitiello MV, McCurry SM, Shortreed SM, et al. Cognitive-behavioral treatment for comorbid insomnia and osteoarthritis pain in primary care: The lifestyles randomized controlled trial. J Am Geriatr Soc. 2013;61:947–56. doi: 10.1111/jgs.12275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stewart MO, Karlin BE, Murphy JL, et al. National dissemination of cognitive-behavioral therapy for chronic pain in veterans: therapist and patient-level outcomes. Clin J Pain. 2015;31:722–9. doi: 10.1097/AJP.0000000000000151. [DOI] [PubMed] [Google Scholar]
- 22.Trockel M, Karlin BE, Taylor CB, et al. Cognitive Behavioral Therapy for insomnia with Veterans: Evaluation of effectiveness and correlates of treatment outcomes. Behav Res Ther. 2014;53:41–6. doi: 10.1016/j.brat.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Smith MT, Finan PH, Buenaver LF, et al. Cognitive-behavioral therapy for insomnia in knee osteoarthritis: A randomized, double-blind, active placebo-controlled clinical trial. Arthritis Rheumatol. 2015;67:1221–33. doi: 10.1002/art.39048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang NK, Goodchild CE, Salkovskis PM. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: A pilot randomised controlled trial. Behav Res Ther. 2012;50:814–21. doi: 10.1016/j.brat.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 25.McCurry SM, Von Korff M, Vitiello MV, et al. Frequency of comorbid insomnia, pain, and depression in older adults with osteoarthritis: Predictors of enrollment in a randomized treatment trial. J Psychosom Res. 2011;71:296–9. doi: 10.1016/j.jpsychores.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montano DA, Kasprzyk D. Theory of reasoned action, theory of planned behavior, and the integrated behavioral model. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: theory, research and practice. San Francisco: John Wiley & Sons; 2008. pp. 67–96. [Google Scholar]
- 27.McAlister AL, Perry CL, Parcel GS. How individuals, environments, and health behaviors interact: Social Cognitive Theory. In: Glanz K, Rimer BK, Viswanath K, editors. Health behavior and health education: theory, research and practice. San Francisco: John Wiley & Sons; 2008. pp. 169–188. [Google Scholar]
- 28.Hebert EA, Vincent N, Lewycky S, et al. Attrition and adherence in the online treatment of chronic insomnia. Behav Sleep Med. 2010;8:141–50. doi: 10.1080/15402002.2010.487457. [DOI] [PubMed] [Google Scholar]
- 29.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 30.Von Korff M, Ormel J, Keefe FJ, et al. Grading the severity of chronic pain. Pain. 1992;50:133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 31.Balderson BH, McCurry SM, Vitiello MV, et al. Information without Implementation: A Practical Example for Developing a Best Practice Education Control Group. Behav Sleep Med. 2016;14:514–27. doi: 10.1080/15402002.2015.1036271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Gradisar M, Lack L, Richards H, et al. The Flinders Fatigue Scale: Preliminary psychometric properties and clinical sensitivity of a new scale for measuring daytime fatigue associated with insomnia. J Clin Sleep Med. 2007;3:722–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Edinger JD, Wohlgemuth WK. Psychometric comparisons of the standard and abbreviated DBAS-10 versions of the dysfunctional beliefs and attitudes about sleep questionnaire. Sleep Med. 2001;2:493–500. doi: 10.1016/s1389-9457(01)00078-8. [DOI] [PubMed] [Google Scholar]
- 37.Morin CM, Blais F, Savard J. Are changes in beliefs and attitudes about sleep related to sleep improvements in the treatment of insomnia? Behav Res Ther. 2002;40:741–752. doi: 10.1016/s0005-7967(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 38.Guillemin F, Coste J, Pouchot J, et al. The AIMS2-SF: A short form of the Arthritis Impact Measurement Scales 2. Arthritis Rheum. 1997;40:1267–74. doi: 10.1002/1529-0131(199707)40:7<1267::AID-ART11>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 39.Meenan RF, Mason JH, Anderson JJ, et al. AIMS2. The content and properties of a revised and expanded Arthritis Impact Measurement Scales Health Status Questionnaire. Arthritis Rheum. 1992;35:1–10. doi: 10.1002/art.1780350102. [DOI] [PubMed] [Google Scholar]
- 40.Ren XS, Kazis L, Meenan RF. Short-form Arthritis Impact Measurement Scales 2: Tests of reliability and validity among patients with osteoarthritis. Arthritis Care Res. 1999;12:163–71. doi: 10.1002/1529-0131(199906)12:3<163::aid-art3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 41.Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 42.Vlaeyen JW, Kole-Snijders AM, Boeren RG, et al. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–72. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 43.Moore JE, Von Korff M, Cherkin D, et al. A randomized trial of a cognitive-behavioral program for enhancing back pain self care in a primary care setting. Pain. 2000;88:145–53. doi: 10.1016/S0304-3959(00)00314-6. [DOI] [PubMed] [Google Scholar]
- 44.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 45.Morin CM. somnia: Psychological Assessment and Management. New York: Guilford Press; 1993. [Google Scholar]
- 46.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–53. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 47.Bothelius K, Kyhle K, Broman JE, et al. Initial sleep time predicts success in manual-guided cognitive behavioral therapy for insomnia. Behav Sleep Med. doi: 10.1080/15402002.2015.1007995. in press. [DOI] [PubMed] [Google Scholar]
- 48.Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17:241–54. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


