Abstract
Background
Abnormal lipid profiles have been associated with gestational diabetes mellitus (GDM), but studies with longitudinal measures of lipids throughout pregnancy are sparse. We aimed to characterize the longitudinal changes in lipid profiles throughout pregnancy and prospectively examine the associations of plasma lipid levels with risk of GDM.
Methods
This is a nested case-control study including 107 GDM cases and 214 matched non-GDM controls from participants in the NICHD Fetal Growth Studies-Singleton Cohort. Blood samples were longitudinally collected at gestational weeks 10–14, 15–26 (fasting sample), 23–31 and 33–39. Plasma concentrations of triglycerides, total cholesterol, and high-density lipoprotein cholesterol (HDL-C) were measured by enzymatic assays. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald's formula.
Results
Plasma levels of triglycerides, total cholesterol, and LDL-C increased as pregnancy progressed. At gestational weeks 10–14, the adjusted odds ratios (ORs) of GDM comparing the highest versus lowest quartile were 3.15 (95% confidence interval [CI] 1.38–7.15; P for trend = 0.002) for triglycerides and 0.44 (95% CI 0.18–1.09; P for trend = 0.045) for HDL-C. At gestational weeks 15–26, the corresponding ORs were 6.57 (95% CI 2.25–19.17; P for trend = 0.001) for triglycerides and 0.23 (95% CI 0.08–0.63; P for trend = 0.005) for HDL-C. We observed no significant associations for total cholesterol and LDL-C levels with risk of GDM.
Conclusions
Higher plasma triglyceride and lower HDL-C concentrations in early and mid-pregnancy were significantly associated with greater risk of GDM. Total cholesterol and LDL-C levels during pregnancy were not significantly associated with GDM risk.
The significant finding (s) of the study
Higher triglyceride and lower high-density lipoprotein cholesterol concentrations in early and mid-pregnancy are significantly related to greater risk of gestational diabetes mellitus. Total and low-density lipoprotein cholesterol levels during pregnancy were not associated with gestational diabetes risk.
This study adds
This study is based on longitudinal assessments of plasma lipid concentrations across pregnancy in a multiracial pregnancy cohort, which is uniquely suited to address temporal associations of plasma lipids in early and mid-pregnancy with the development of subsequent gestational diabetes.
Keywords: gestational diabetes, lipids, triglycerides, cholesterol, longitudinal, pregnancy
INTRODUCTION
Gestational diabetes mellitus (GDM), defined as glucose intolerance with onset or first recognition during pregnancy, is the most common metabolic condition during pregnancy.1 In the United States, it complicates approximately 9% of all pregnancies2 and its prevalence is on the rise.3, 4 GDM is associated with short- and long-term adverse outcomes for both the mothers and their children.1, 5 During pregnancy, a continuous supply of nutrients, regardless of intermittent maternal food consumption, is required for the growing fetus. As a result, pregnant women normally experience physiological changes in carbohydrate and lipid metabolism.6, 7 Alterations of maternal lipid metabolism may be related to adverse pregnancy and neonatal outcomes.8, 9 However, previous studies on the associations of circulating lipid patterns during pregnancy with GDM risk have yielded mixed findings.10–19 Most previous studies were designed as a cross-sectional comparison between women with GDM and those with normal pregnancies, with the majority using blood samples collected in the late second trimester or even the third trimester when GDM could have already been diagnosed.10 Therefore, such studies lack insights into the temporal relations between lipid disturbance and GDM incidence. Several prospective cohort studies have assessed circulating lipids at a certain time point in early pregnancy in relation to subsequent risk of GDM. However, the associations of longitudinal trends of circulating lipids throughout pregnancy with risk of GDM remain poorly understood.
The primary objective of this study was to prospectively examine the associations of maternal plasma lipids in the first and second trimesters with subsequent risk of GDM. In addition, we aimed to evaluate the longitudinal trend of plasma lipids across different trimesters in pregnancy.
METHODS
The original cohort
This is a nested case-control study among participants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Fetal Growth Studies-Singleton Cohort, which is a multicenter prospective cohort study conducted by the NICHD between 2009 and 2013.20 One of the secondary objectives was to collect blood samples throughout pregnancy for an etiologic study of GDM and related complications. The study was approved by the institutional review boards of all participating institutions. All participants signed written informed consents.
In the NICHD Fetal Growth Studies-Singleton Cohort, 2802 pregnant women representing four race-ethnic groups, aged 18–40 years, who had a singleton pregnancy and a pre-pregnancy body mass index (BMI) ranging from 19–45 kg/m2, were recruited from 12 clinical centers across the USA. Women with HIV or major chronic conditions such as pre-pregnancy hypertension, pre-pregnancy diabetes, cancer, psychiatric, renal or autoimmune diseases were excluded. Women were followed up from enrollment until delivery using sonograms, anthropometric measurements and questionnaires at each visit. Maternal venous blood samples were longitudinally collected during four selected study visits, which were scheduled at 8–13, 16–22, 24–29 and 34–37 weeks of gestation during pregnancy, of which the second visit blood specimen was a fasting sample. In order to obtain weekly biomarker data, the participants were randomized to different follow-up schedules within each study visit time window. A few participants returned late for the originally scheduled visit, therefore the actual gestational weeks at blood collection went slightly beyond the planned time windows, ranging at 10–14, 15–26, 23–31 and 33–39 weeks respectively. Plasma samples were processed immediately after collection and stored at −80 °C until being assayed.
Selection of cases and controls in this nested case-control study
In this study, we included all GDM cases (n=107) identified from the NICHD Fetal Growth Studies-Singleton Cohort. GDM was diagnosed by the 3-h oral glucose tolerance test (OGTT) according to the American College of Obstetrics and Gynecologists criteria.21 A total of 214 non-GDM controls were randomly selected and matched 2:1 to cases on age (± 2 years), race/ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, Asian/Pacific Islander) and gestational age at blood collection (± 2 weeks).
Exposure variables
Maternal plasma total cholesterol, high-density lipoprotein cholesterol (HDL-C), and triglycerides were measured by enzymatic assays using Roche COBAS 6000 Chemistry Analyzer (Roche Diagnostics, Indianapolis, IN). Analytical inter-assay coefficients of variation for total cholesterol, HDL-C, and triglycerides were 2.2%, 3.2% and 2.3%, respectively. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald's formula:22 LDL-C = total cholesterol - HDL-C - triglycerides/5. All values of plasma lipid levels were expressed in mg/dl. All assays were performed at the University of Minnesota Advanced Research and Diagnostics Laboratory (Minneapolis, MN), without knowledge of GDM status. For the first two visits prior to GDM diagnosis, levels of plasma lipids were measured among all GDM cases and controls. For the two visits during or after GDM diagnosis, plasma lipid measurements were performed among all cases and one of the matched controls.
Covariates
Information on maternal demographic, lifestyle, and medical characteristics was collected from questionnaires or retrieved from medical records. We included a priori selected covariates that are conventional risk factors for GDM: family history of diabetes and pre-pregnancy body mass index (BMI) calculated from measured height and self-reported pre-pregnancy weight. To obtain conservative risk estimates, we also included two matching factors that were only matched within a certain range (i.e., maternal age and gestational age at blood collection). Race/ethnicity was exactly matched between GDM cases and controls and was not included in the multivariable model. In addition, we did not include maternal smoking status in the multivariable model, because according to the original cohort design,20 non-obese women who smoked in the 6 months prior to pregnancy were not eligible for the cohort and among obese participants, only 5 smoked in the 6 months prior to pregnancy.
Statistical analysis
Descriptive statistics were reported as mean ± standard deviation (SD) for continuous variables or frequencies for categorical variables. Comparisons of participant characteristics between GDM cases and controls were performed by mixed-effect linear regression models for continuous variables and binomial/multinomial logistic regression with generalized estimating equations for binary/multilevel categorical variables, accounting for matched case-control pairs.
To assess the association of each plasma lipid variable with risk of GDM, women were grouped according to quartiles based on the distribution of the lipid variable among the controls, with the lowest quartile as the referent group. To ensure that biomarker measurements preceded the diagnosis of GDM, we excluded one GDM case at gestational weeks 10–14 and five GDM cases at gestational weeks 15–26 from the final analysis, since their blood samples were collected after the diagnosis of GDM. We calculated odds ratios (ORs) of GDM and 95% confidence intervals (CIs) using multivariable conditional logistic regression models. We adjusted for age (continuous), gestational age at blood collection (continuous), family history of diabetes (yes/no), and pre-pregnancy BMI (<25.0, 25.0–25.9, 30.0–34.9, 35.0–44.9 kg/m2). Tests for linear trends in the ORs across quartiles of plasma lipids were conducted by assigning a median value for each quartile and fitting them as a continuous variable in the model.
We performed interaction tests with multiplicative terms to evaluate whether the associations of maternal plasma lipids with GDM risk were modified by pre-pregnancy body weight status and family history of diabetes. To visualize the longitudinal changes of plasma lipids levels throughout pregnancy in GDM cases and controls, mean levels and standard errors (SE) of each biomarker were plotted against gestational-age intervals of 2–3 weeks. Comparisons of the longitudinal changes between GDM cases and controls were performed using mixed-effect linear regression models accounting for matched case-control pairs.
All statistical analyses were performed using the SAS software version 9.4 (SAS Institute, Cary, NC) program. A P-value < 0.05 was considered statistically significant.
RESULTS
Participant characteristics among GDM cases and matched controls in this study have been described in a previous report.23 In brief, compared to controls, women who developed GDM had greater pre-pregnancy BMI and were more likely to have a family history of diabetes. No statistical differences were observed in terms of education, type of health insurance, marital status, parity, smoking, and alcoholic beverage consumption (Supplementary Table 1).
Women who developed GDM had higher plasma levels of triglycerides, but lower levels of HDL-C (Figure 1). There was no significant difference in total cholesterol or LDL-cholesterol levels. Plasma levels of triglycerides and HDL-C at both 10–14 and 15–26 weeks of gestation were significantly associated with subsequent risk of GDM (Table 1). In the multivariable model, the adjusted ORs of GDM for plasma lipids across increasing quartiles were 1.00 (reference), 0.95, 1.92, and 3.15 (P for trend = 0.002) for triglycerides and 1.00 (reference), 0.61, 0.46 and 0.44 (P for trend = 0.045) for HDL-C at gestational weeks 10–14. At gestational weeks 15–26, the corresponding ORs were 1.00 (reference), 2.92, 5.32, and 6.57 (P for trend = 0.001) for triglycerides and 1.00 (reference), 0.97, 0.73 and 0.23 (P for trend = 0.005) for HDL-C. Additional adjustment for other lipid fractions modestly attenuated the observed associations. For instance, at gestational weeks 10–14, the ORs of GDM comparing the highest versus lowest quartile were 2.70 (1.15–6.35) for triglycerides and 0.71 (0.28–1.81) for HDL-C after additional adjustment for other lipid fractions. At gestational weeks 15–26, the corresponding ORs were 6.75 (2.06–22.1) for triglycerides and 0.49 (0.15–1.59) for HDL-C. We observed no significant associations for plasma total cholesterol and LDL-C levels with risk of GDM. No significant effect modification by pre-pregnancy body weight status or family history of diabetes was observed (data not shown).
Figure 1. Mean plasma levels of (A) total cholesterol, (B) triglycerides, (C) HDL-C, and (D) LDL-C among women with gestational diabetes (n = 107) and their matched controls (n = 214) at gestational weeks 10–14 and 15–26.
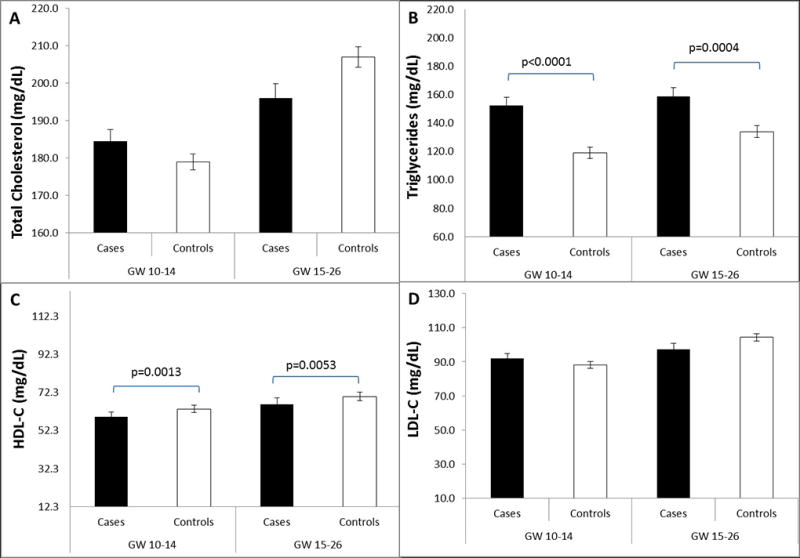
HDL-C denotes HDL cholesterol; LDL-C, LDL cholesterol; GW, gestational weeks.
P values for case-control comparisons were obtained by mixed-effect linear regression models accounting for matched case-control pairs at gestational weeks 10–14 and 15–26, respectively.
Table 1.
Risk of gestational diabetes according to quartiles of plasma lipid concentrations at gestational weeks 10–14 and 15–26, the NICHD Fetal Growth Studies-Singleton Cohort
| GDM Case n | Control n | Crude model | Multivariable model* | |
|---|---|---|---|---|
| Gestational weeks 10–14† | ||||
| Total cholesterol, mg/dL | ||||
| Q1: 109.0–158.0‡ | 21 | 53 | 1.00 | 1.00 |
| Q2: 159.0–179.0 | 25 | 54 | 1.16 (0.59, 2.28) | 1.23 (0.59, 2.56) |
| Q3: 180.0–199.0 | 34 | 52 | 1.83 (0.92, 3.64) | 2.31 (1.06, 5.03) |
| Q4: 200.0–289.0 | 20 | 53 | 1.00 (0.49, 2.05) | 0.96 (0.44, 2.11) |
| P-for-trend | 0.792 | 0.791 | ||
| HDL–C, mg/dL | ||||
| Q1: 18–54.9 | 40 | 53 | 1.00 | 1.00 |
| Q2: 55.1–63.6 | 26 | 53 | 0.63 (0.34, 1.15) | 0.61 (0.31, 1.19) |
| Q3: 63.7–72.6 | 16 | 53 | 0.37 (0.17, 0.79) | 0.46 (0.20, 1.07) |
| Q4: 73.2–125.4 | 18 | 53 | 0.42 (0.20, 0.87) | 0.44 (0.18, 1.09) |
| P-for-trend | 0.011 | 0.045 | ||
| LDL–C, mg/dL | ||||
| Q1: 1.4–70.4 | 23 | 53 | 1.00 | 1.00 |
| Q2: 70.5–88.2 | 25 | 53 | 1.10 (0.56, 2.16) | 1.43 (0.66, 3.09) |
| Q3: 88.4–105.4 | 28 | 53 | 1.26 (0.66, 2.43) | 1.63 (0.79, 3.33) |
| Q4: 105.9–170.3 | 24 | 53 | 1.04 (0.54, 2.01) | 1.05 (0.49, 2.23) |
| P-for-trend | 0.829 | 0.726 | ||
| Triglycerides, mg/dL | ||||
| Q1: 56.0–93.0 | 14 | 53 | 1.00 | 1.00 |
| Q2: 94.0–119.0 | 15 | 54 | 0.96 (0.43, 2.17) | 0.95 (0.38, 2.34) |
| Q3: 120.0–155.0 | 25 | 53 | 1.70 (0.79, 3.64) | 1.92 (0.82, 4.49) |
| Q4: 157.0–389.0 | 46 | 52 | 3.20 (1.54, 6.66) | 3.15 (1.38, 7.15) |
| P-for-trend | <0.001 | 0.002 | ||
| Gestational weeks 15–26† | ||||
| Total cholesterol, mg/dL | ||||
| Q1: 118.0–177.0 | 24 | 54 | 1.00 | 1.00 |
| Q2: 178.0–207.0 | 35 | 53 | 1.31 (0.68, 2.51) | 1.28 (0.62, 2.67) |
| Q3: 209.0–230.0 | 17 | 55 | 0.61 (0.29, 1.26) | 0.63 (0.28, 1.39) |
| Q4: 231.0–358.0 | 17 | 51 | 0.66 (0.31, 1.40) | 0.78 (0.35, 1.77) |
| P-for-trend | 0.099 | 0.299 | ||
| HDL–C, mg/dL | ||||
| Q1: 12.3–58.1 | 32 | 54 | 1.00 | 1.00 |
| Q2: 58.3–70.1 | 28 | 53 | 0.84 (0.42, 1.67) | 0.97 (0.45, 2.10) |
| Q3: 70.2–82.7 | 24 | 53 | 0.73 (0.34, 1.53) | 0.73 (0.30, 1.77) |
| Q4: 83.0–124.3 | 9 | 53 | 0.25 (0.10, 0.59) | 0.23 (0.08, 0.63) |
| P-for-trend | 0.002 | 0.005 | ||
| LDL–C, mg/dL | ||||
| Q1: 11.6–82.7 | 24 | 54 | 1.00 | 1.00 |
| Q2: 82.9–104.4 | 31 | 53 | 1.23 (0.61, 2.50) | 1.12 (0.50, 2.48) |
| Q3: 104.4–125.4 | 18 | 53 | 0.80 (0.39, 1.66) | 0.87 (0.39, 1.93) |
| Q4: 125.9–214.4 | 20 | 53 | 0.78 (0.38, 1.59) | 0.81 (0.37, 1.76) |
| P-for-trend | 0.326 | 0.486 | ||
| Triglycerides, mg/dL | ||||
| Q1: 64.0–104.0 | 9 | 57 | 1.00 | 1.00 |
| Q2: 105.0–134.0 | 17 | 50 | 1.89 (0.76, 4.73) | 2.92 (0.98, 8.75) |
| Q3: 136.0–170.0 | 32 | 53 | 3.53 (1.52, 8.23) | 5.32 (1.84, 15.39) |
| Q4: 171.0–378.0 | 35 | 53 | 3.94 (1.68, 9.25) | 6.57 (2.25, 19.17) |
| P-for-trend | 0.001 | 0.001 |
LDL-C, LDL cholesterol (mg/dL); HDL-C, HDL cholesterol (mg/dL); Q, quartile.
Multivariable conditional logistic regression models were used to estimate odds ratios (ORs) of GDM and 95% confidence intervals (CIs).
Adjusted for maternal age (years), gestational age at blood collection (weeks), parity, family history of diabetes (yes/no), and pre-pregnancy body mass index (<24.9, 25.0–25.9, 30.0–34.9, 35.0–44.9 kg/m2).
Timing of blood sample collection preceded the diagnosis of gestational diabetes.
Range of biomarker concentrations within each quartile among non-gestational diabetes controls.
In the analyses of longitudinal trends of plasma lipids throughout pregnancy, we observed a consistently lower level of HDL-C in GDM cases compared to controls, with significant differences at gestational weeks 13–15, 20–23, 24–27 and 32–35. For triglycerides, GDM cases showed a higher level on average than controls in the first and second trimesters, but there was no significant difference afterward (Figure 2). Longitudinal changes of total cholesterol and LDL-C were not significantly different between GDM cases and controls across pregnancy.
Figure 2. Mean plasma levels (± SE) of total cholesterol (A), HDL-C (B), LDL-C (C), and triglycerides (D) according to gestational-age intervals among women with gestational diabetes (squares, solid line) and their matched controls (circles, dashed line).
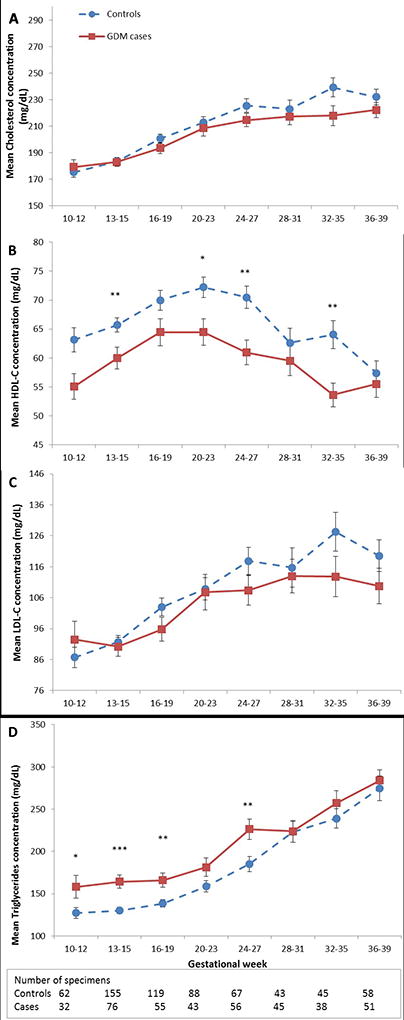
HDL-C denotes HDL cholesterol; LDL-C, LDL cholesterol.
*P <0.05; **P <0.01; ***P <0.001 for case-control comparisons obtained by mixed-effect linear regression models accounting for matched case-control pairs at each gestational-age interval.
DISCUSSION
Among women who were longitudinally followed up from early pregnancy until delivery, we observed a positive association between plasma triglyceride levels and subsequent risk of GDM and an inverse association between plasma HDL-C levels and risk of GDM. Comparing the longitudinal trends in plasma lipid levels throughout pregnancy in women who developed GDM with those who did not, we found that plasma HDL-C levels in GDM cases were consistently lower from early to late pregnancy and that plasma triglyceride levels were higher among GDM cases in the first and second trimesters than controls.
To our knowledge, only two previous studies have profiled longitudinal changes of circulating lipid levels during pregnancy in women with and without GDM.11, 12 However, both prior studies were based on a small sample size (one with 9 and the other with 12 GDM cases), potentially compromising the statistical power required to identify significant associations between plasma lipids and GDM risk. The study by Montelongo et al,11 including only 9 GDM cases and 12 healthy controls, observed a significantly lower level of HDL-C in GDM cases than controls without finding significant difference in triglyceride levels between cases and controls. Paradisi et al enrolled 50 high risk pregnant women, of whom 12 developed GDM.12 A significantly greater risk of GDM was found in association with higher levels of triglycerides in the second trimester and LDL cholesterol in the third trimester, while there were no significant associations between plasma lipid levels and risk of GDM during the first trimester.12
Our findings on the prospective associations of plasma lipid levels in the first and second trimesters with risk of GDM were consistent with some, but not all previous prospective studies. Similar to our results, Enquobarie et al13 observed a positive association between triglyceride levels at on average 13 weeks of gestation and risk of developing GDM. However, they did not find a significant association for other lipids. Savvidou et al14 and Makgoba et al15 found that women who developed GDM had higher levels of triglycerides and lower levels of HDL-C in the first trimester. Zhou et al16 reported that lower HDL-C levels and higher triglyceride levels at 20 weeks of gestation were associated with the development of GDM. In a previous study among obese pregnant women only, there was no significant associations for lipids in the first and early second trimesters with GDM.17 In our subgroup analysis with obese women, we found a positive association between plasma triglyceride levels at gestational weeks 10–14 with subsequent risk of GDM (data not shown). Other studies, for example, Sanchez-Vera et al18 and Nolan et al19 reported higher levels of triglycerides in women who developed GDM than controls in the univariate analysis but the association was not significant in multivariable analysis. The difference in these study findings may be, at least partly, due to heterogeneity in the study design and study methods, such as differences in gestational age at blood collection, fasting status, and diagnostic criteria for GDM, inconsistent or inadequate control for confounding, and variations in population characteristics. Consistent with our findings, a recent meta-analysis showed that women who developed GDM, compared with those without insulin resistance during pregnancy, had higher triglycerides across all three trimesters of pregnancy.10 HDL-C levels were significantly lower in women with GDM compared with women with normal pregnancy, but this difference was not observed in the first trimester.10 More longitudinal studies with a large sample size are warranted to establish the role of lipids metabolism in the development of GDM across populations.
The observed associations of plasma lipids with GDM in this study are biologically plausible, although precise mechanisms remain to be elucidated. During pregnancy, there are significant physiological changes in glucose and lipid metabolism. Alteration of lipids and lipoproteins could be a result of increased maternal hormone levels and other maternal factors such as pre-pregnancy BMI and gestational weight gain.24–27 Diareme et al reported that the rate of changes in individual plasma lipids varied during normal pregnancy, with triglycerides showing the largest increase and HDL-C the smallest.28 Similar to previous reports,24 we observed an initial slow slope in the increase of triglyceride levels in the first trimester, followed by a large increase towards the second trimester and doubled levels in the third trimester, although not all triglyceride levels in our study were measured in fasting samples. Human data illustrate that excessive triglyceride accumulation within skeletal muscles is linked with decreased insulin sensitivity,29 which in turn may contribute to the development of GDM.
This study has several strengths. It is nested in a prospective cohort with longitudinal assessments of maternal plasma lipid levels throughout pregnancy, which not only allows assessment of the temporal relation between lipid levels and the development of GDM, but also provides a unique opportunity to profile the longitudinal, gestational changes in lipids among GDM cases and matched controls. Compared to previous longitudinal studies on circulating lipids and GDM,11, 12 this study was relatively larger in sample size and better powered to detect the associations. This study was conducted in a multiracial cohort, which may help establish generalizability of our findings. In addition, a fasting sample was taken during the study visit at 15–26 weeks of gestation in this study, which reduces potential influence of food intake on plasma lipid levels.
We acknowledge that there are several limitations. First, we were unable to collect fasting samples for study visits other than 15–26 weeks of gestation, given the feasibility issues and that it is particularly difficult women to fast during pregnancy. As a result, the longitudinal trends of plasma lipid levels during pregnancy in this study should be interpreted with caution. However, fasting status is unlikely to have appreciable influence on the observed association. Several large population-based studies have determined that fasting times show little association with lipid subclass levels30, 31 and fasting has little impact on the overall association with clinical outcomes.32, 33 Recently, a joint consensus statement from several European Societies even recommended routine use of non-fasting blood samples for the assessment of plasma lipid profiles, and emphasized that non-fasting and fasting blood lipids should be complementary.34 Second, the prospective and longitudinal nature of this study will reduce, but could not fully exclude, the possibility of reverse causation. It should also be noted that by design, this study excluded women with major chronic conditions such as diabetes and cardiovascular diseases from the enrollment, which further lowers the possibility of reverse causation. Further investigation on the causal role of plasma lipids in GDM development is needed, especially given that a recent study35 showed an inverse association of genetically elevated LDL-C, HDL-C, and triglycerides with type 2 diabetes risk. Third, although we have considered major confounders in the analysis, we could not rule out the possibility of potential residual confounding. For instance, maternal adiposity is a shared risk factor for lipid disorders and GDM; although we adjusted for pre-pregnancy BMI, there may still be residual confounding from maternal adiposity.
In conclusion, in a longitudinal study of pregnant women of multi race/ethnicity, we observed that higher plasma triglyceride and lower HDL-C concentrations in early and mid-pregnancy are significantly related to greater subsequent risk of GDM. Future research is warranted to investigate the interplays of dyslipidemia with biomarkers in other pathways in the etiology of GDM.
Supplementary Material
Acknowledgments
We thank all the research teams at all participating clinical centers, including Christina Care Health Systems; University of California, Irvine; Long Beach Memorial Medical Center; Northwestern University; Medical University of South Carolina; Columbia University; New York Hospital Queens; St Peters’ University Hospital; University of Alabama at Birmingham; Women and Infants Hospital of Rhode Island; Fountain Valley Regional Hospital and Medical Center; and Tufts University. We also acknowledge the C-TASC Corporation that provided data coordination and the Department of Laboratory Medicine and Pathology, University of Minnesota that provided laboratory resources essential to test blood samples for biomarkers in this study.
Funding. This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development intramural funding and included American Recovery and Reinvestment Act funding via contract numbers HHSN275200800013C, HHSN275200800002I, HHSN27500006, HHSN275200800003IC, HHSN275200800014C, HHSN275200800012C, HHSN275200800028C, and HHSN275201000009C, and HHSN275201000001Z.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this work were reported.
Disclosure: None declared.
References
- 1.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27:S88–90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 2.DeSisto CL, Kim SY, Sharma AJ. Prevalence estimates of gestational diabetes mellitus in the United States, Pregnancy Risk Assessment Monitoring System (PRAMS), 2007–2010. Preventing chronic disease. 2014;11:E104. doi: 10.5888/pcd11.130415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141–6. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y, Zhang C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: a Global Perspective. Current diabetes reports. 2016;16:7. doi: 10.1007/s11892-015-0699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet. 2009;373:1773–9. doi: 10.1016/S0140-6736(09)60731-5. [DOI] [PubMed] [Google Scholar]
- 6.Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71:1256S–61S. doi: 10.1093/ajcn/71.5.1256s. [DOI] [PubMed] [Google Scholar]
- 7.Alvarez JJ, Montelongo A, Iglesias A, Lasuncion MA, Herrera E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. Journal of lipid research. 1996;37:299–308. [PubMed] [Google Scholar]
- 8.Herrera E, Ortega-Senovilla H. Disturbances in lipid metabolism in diabetic pregnancy - Are these the cause of the problem? Best Pract Res Clin Endocrinol Metab. 2010;24:515–25. doi: 10.1016/j.beem.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Barrett HL, Dekker Nitert M, McIntyre HD, Callaway LK. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37:1484–93. doi: 10.2337/dc13-1934. [DOI] [PubMed] [Google Scholar]
- 10.Ryckman KK, Spracklen CN, Smith CJ, Robinson JG, Saftlas AF. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG. 2015;122:643–51. doi: 10.1111/1471-0528.13261. [DOI] [PubMed] [Google Scholar]
- 11.Montelongo A, Lasuncion MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–9. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 12.Paradisi G, Ianniello F, Tomei C, et al. Longitudinal changes of adiponectin, carbohydrate and lipid metabolism in pregnant women at high risk for gestational diabetes. Gynecol Endocrinol. 2010;26:539–45. doi: 10.3109/09513591003632084. [DOI] [PubMed] [Google Scholar]
- 13.Enquobahrie DA, Williams MA, Qiu C, Luthy DA. Early pregnancy lipid concentrations and the risk of gestational diabetes mellitus. Diabetes research and clinical practice. 2005;70:134–42. doi: 10.1016/j.diabres.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Savvidou M, Nelson SM, Makgoba M, Messow CM, Sattar N, Nicolaides K. First-trimester prediction of gestational diabetes mellitus: examining the potential of combining maternal characteristics and laboratory measures. Diabetes. 2010;59:3017–22. doi: 10.2337/db10-0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makgoba M, Nelson SM, Savvidou M, Messow CM, Nicolaides K, Sattar N. First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus. Diabetes Care. 2011;34:1091–3. doi: 10.2337/dc10-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Zhao X, Wang Z, Hu Y. Combination of lipids and uric acid in mid-second trimester can be used to predict adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2012;25:2633–8. doi: 10.3109/14767058.2012.704447. [DOI] [PubMed] [Google Scholar]
- 17.Maitland RA, Seed PT, Briley AL, et al. Prediction of gestational diabetes in obese pregnant women from the UK Pregnancies Better Eating and Activity (UPBEAT) pilot trial. Diabet Med. 2014;31:963–70. doi: 10.1111/dme.12482. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Vera I, Bonet B, Viana M, et al. Changes in plasma lipids and increased low-density lipoprotein susceptibility to oxidation in pregnancies complicated by gestational diabetes: consequences of obesity. Metabolism. 2007;56:1527–33. doi: 10.1016/j.metabol.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Nolan CJ, Riley SF, Sheedy MT, Walstab JE, Beischer NA. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care. 1995;18:1550–6. doi: 10.2337/diacare.18.12.1550. [DOI] [PubMed] [Google Scholar]
- 20.Buck Louis GM, Grewal J, Albert PS, et al. Racial/ethnic standards for fetal growth: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2015;213:449 e1–49 e41. doi: 10.1016/j.ajog.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.American College of Obstetricians and Gynecologists Committee on Practice Bulletins--Obstetrics. ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–38. [PubMed] [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Zhu Y, Mendola P, Albert PS, et al. Insulin-like growth factor axis and gestational diabetes: A longitudinal study in a multiracial cohort. Diabetes. 2016 doi: 10.2337/db16-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mankuta D, Elami-Suzin M, Elhayani A, Vinker S. Lipid profile in consecutive pregnancies. Lipids Health Dis. 2010;9:58. doi: 10.1186/1476-511X-9-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farias DR, Franco-Sena AB, Vilela A, Lepsch J, Mendes RH, Kac G. Lipid changes throughout pregnancy according to pre-pregnancy BMI: results from a prospective cohort. BJOG. 2016;123:570–8. doi: 10.1111/1471-0528.13293. [DOI] [PubMed] [Google Scholar]
- 26.Scifres CM, Catov JM, Simhan HN. The impact of maternal obesity and gestational weight gain on early and mid-pregnancy lipid profiles. Obesity (Silver Spring) 2014;22:932–8. doi: 10.1002/oby.20576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bozkurt L, Gobl CS, Hormayer AT, Luger A, Pacini G, Kautzky-Willer A. The impact of preconceptional obesity on trajectories of maternal lipids during gestation. Sci Rep. 2016;6:29971. doi: 10.1038/srep29971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diareme M, Karkalousos P, Theodoropoulos G, Strouzas S, Lazanas N. Lipid profile of healthy women during normal pregnancy. Journal of Medical Biochemistry. 2009;28:152–60. [Google Scholar]
- 29.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24:933–41. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 30.Sidhu D, Naugler C. Fasting time and lipid levels in a community-based population: a cross-sectional study. Arch Intern Med. 2012;172:1707–10. doi: 10.1001/archinternmed.2012.3708. [DOI] [PubMed] [Google Scholar]
- 31.Steiner MJ, Skinner AC, Perrin EM. Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study. Pediatrics. 2011;128:463–70. doi: 10.1542/peds.2011-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langsted A, Freiberg JJ, Nordestgaard BG. Fasting and nonfasting lipid levels: influence of normal food intake on lipids, lipoproteins, apolipoproteins, and cardiovascular risk prediction. Circulation. 2008;118:2047–56. doi: 10.1161/CIRCULATIONAHA.108.804146. [DOI] [PubMed] [Google Scholar]
- 33.Doran B, Guo Y, Xu J, et al. Prognostic value of fasting versus nonfasting low-density lipoprotein cholesterol levels on long-term mortality: insight from the National Health and Nutrition Examination Survey III (NHANES-III) Circulation. 2014;130:546–53. doi: 10.1161/CIRCULATIONAHA.114.010001. [DOI] [PubMed] [Google Scholar]
- 34.Nordestgaard BG, Langsted A, Mora S, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cut-points-a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Eur Heart J. 2016;37:1944–58. doi: 10.1093/eurheartj/ehw152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White J, Swerdlow DI, Preiss D, et al. Association of Lipid Fractions With Risks for Coronary Artery Disease and Diabetes. JAMA Cardiol. 2016;1:692–9. doi: 10.1001/jamacardio.2016.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


