Abstract
Background and Aims
It is unclear whether direct-acting antiviral (DAA) treatment-induced sustained virologic response (SVR) reduces the risk of hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV) infection. We aimed to determine the impact of DAA-induced SVR on HCC risk.
Methods
We identified 62,354 patients who initiated antiviral treatment in the Veterans Affairs (VA) national healthcare system from 1/1/1999 to 12/31/2015, including 35,871 (58%) interferon-only regimens, 4535 (7.2%) DAA+interferon regimens and 21,948 (35%) DAA-only regimens. We retrospectively followed patients until 6/15/2017 to identify incident cases of HCC. We used Cox proportional hazards regression to determine the association between SVR and HCC risk or between type of antiviral regimen (DAA-only vs DAA+interferon vs Interferon-only) and HCC risk.
Results
We identified 3271 incident cases of HCC diagnosed at least 180 days after initiation of antiviral treatment during a mean follow-up of 6.1 years. The incidence of HCC was highest in patients with cirrhosis and treatment failure (3.25 per 100 patient-years), followed by cirrhosis and SVR (1.97), no cirrhosis and treatment failure (0.87) and no cirrhosis and SVR (0.24). SVR was associated with a significantly decreased risk of HCC in multivariable models irrespective of whether the antiviral treatment was DAA-only (adjusted hazard ratio [AHR] 0.29, 95% CI 0.23–0.37), DAA+interferon (AHR 0.48, 95% CI 0.32–0.73) or interferon-only (AHR 0.32, 95% CI 0.28–0.37). Receipt of a DAA-only or DAA+interferon regimen was not associated with increased HCC risk compared to receipt of an interferon-only regimen.
Conclusions
DAA-induced SVR is associated with a 71% reduction in HCC risk. Treatment with DAAs is not associated with increased HCC risk compared to treatment with interferon.
Keywords: Liver cancer, hepatitis C treatment, prediction models
Graphical Abstract
Kaplan-Meier curves of survival free of HCC by cirrhosis and SVR status after DAA-only antiviral treatment: SVR is associated with a reduction in HCC risk both among patients with cirrhosis and those without cirrhosis.

Introduction
Eradication of hepatitis C virus (HCV) might be expected to reduce the risk of hepatocellular carcinoma (HCC) by preventing the future development of cirrhosis or by reversing early cirrhosis, a major risk factor for HCC. In addition, HCV may itself promote carcinogenesis1 such that its eradication directly decreases HCC risk. Indeed, a meta-analysis of 18 studies of interferon-based antiviral treatments for HCV, suggested that sustained virologic response (SVR) was associated with reduced risk of HCC (relative risk 0.24, 95% CI 0.18–0.31)2. Interferon-based treatments have now been replaced by direct-acting antiviral agents (DAAs). It is unclear whether the impact of SVR on HCC risk is different depending on whether SVR is achieved with interferon-based regimens or DAAs. Surprisingly, recent studies suggested little or no impact of DAA-based antiviral treatment on HCC risk and even suggested that DAAs might increase the risk of HCC recurrence3–8. However, these studies were grossly underpowered, had very limited follow-up time, studied mostly HCC recurrence rather than incidence, and did not attempt to compare those who achieved SVR as a result of DAAs to those who did not with respect to HCC risk.
We aimed to determine the extent to which eradication of HCV with DAA-based treatments was associated with reduction in the risk of HCC, and whether this association was different for SVRs achieved by DAA versus interferon-based regimens. We also aimed to determine whether receipt of DAA-based treatment as compared to interferon-based treatment was associated with HCC risk.
Methods
Data Source
The Veterans Affairs (VA) healthcare system is the largest integrated healthcare provider of HCV antiviral treatment in the United States. In 2014, 5,857,690 Veterans received care in 154 medical centers and 875 ambulatory care and community-based outpatient clinics that comprise the VA healthcare system nationally9, including 174,302 patients with known HCV infection (positive serum HCV viral load test)10.
The VA uses a single, nationwide, comprehensive electronic healthcare information network (known as the Veterans Information Systems and Technology Architecture or VistA), which consists of nearly 180 applications of clinical, financial, administrative and infrastructure needs integrated into a single, common database of all Veterans’ health information. We derived electronic data on all patients who initiated antiviral treatment in the VA system using the VA Corporate Data Warehouse (CDW), a national, continually updated repository of data from VistA developed specifically to facilitate research11. Data extracted included all patient pharmacy prescriptions, demographics, inpatient and outpatient visits, problem lists, procedures, vital signs, diagnostic tests, and laboratory tests.
The study was approved by the Institutional Review Board of the Veterans Affairs Puget Sound Healthcare System.
Study Population
We identified all HCV antiviral regimens (n=105310 regimens in 78,890 patients) initiated in the VA during 17 calendar years from 1/1/1999 to 12/31/2015. We excluded 1140 patients who had a diagnosis of HCC (ICD-9 code 155.0 or ICD-10 code C22.0 – the VA switched to ICD-10 codes on 10/01/2015) ever recorded prior to their first HCV antiviral treatment, 1100 who died within 180 days from the start-date of antiviral treatment or had fewer than 180 days of available follow-up, and 277 patients who were diagnosed with HCC within 180 days from the start-date of their antiviral treatment (including 119 who achieved SVR and 158 who did not) since these cases were very unlikely to be incident (new) cases. We excluded 8855 patients with missing SVR data and 5164 with missing genotype leaving 61,963 patients in the current analysis, including 3270 who developed HCC more than 180 days after the treatment start-date. These excluded patients were very similar to the patients included in the analysis in their baseline characteristics (Supplemental Table 1).
Antiviral Treatment Regimens (Table 1)
Table 1.
Types of HCV antiviral treatment regimens included in our study of VA patients from 1999–2015
| Regimen* | First Regimen N (%) | All Regimens N (%) | |
|---|---|---|---|
| IFN ONLY | Interferon | 2,629(4.2) | 4,256(5.1) |
| PEG | 33,242(53.3) | 41,786(50.1) | |
| DAA + IFN | Boceprevir+PEG | 3,090(5.0) | 4,815(5.8) |
| Telaprevir+PEG | 479(0.8) | 967(1.2) | |
| Simeprevir+PEG | 14(0.0) | 23(0.0) | |
| Sofosbuvir+PEG | 952(1.5) | 1,664(2.0) | |
| DAA ONLY | Sofosbuvir (±daclatasvir) | 2,786(4.5) | 3,727(4.5) |
| Sofosbuvir+Simeprevir | 1,993(3.2) | 3,219(3.9) | |
| Ledipasvir/Sofosbuvir | 12,763(20.5) | 17,369(20.8) | |
| Paritaprevir/Ritonavir/Ombitasvir/Dasabuvir | 4,406(7.1) | 5,595(6.7) |
Regimens with or without ribavirin were included together
The regimens were divided into:
Interferon only (“IFN-ONLY”) regimens: included pegylated interferon (PEG) and regular interferon with or without ribavirin but without any DAAs.
“DAA+IFN” regimens: included any DAA (NS3/4, NS5A or NS5B inhibitors) with concomitant PEG (± ribavirin)
“DAA-ONLY” regimens: included only interferon-free, DAA regimens (± ribavirin).
All VA pharmacy data are included in the CDW; dispensed drugs (rather than just prescribed drugs) were used to define antiviral treatment regimens, as we previously published12–19.
Baseline Patient Characteristics
For each HCV treatment regimen, we collected baseline data including age, sex, body mass index (BMI), HCV genotype, HCV viral load and receipt of prior antiviral treatment. We extracted relevant laboratory tests prior to treatment and recorded the value of each test closest to the treatment starting date within the preceding 6 months (except serum AFP that was recorded within 1 year). We contemplated ascertaining laboratory tests after the end of treatment but decided against that because many laboratory tests such as AST, ALT, hemoglobin and creatinine can change acutely as a result of treatment and may reflect underlying fibrosis or HCC risk less accurately. We defined HBV coinfection by positive HBV surface antigen test or viral load. We also determined the presence of cirrhosis, manifestations of decompensated cirrhosis (ascites, encephalopathy, gastroesophageal varices and hepatorenal syndrome), type 2 diabetes mellitus, alcohol use disorders, substance use disorders, HIV infection and liver transplantation based on appropriate ICD-9 or ICD-10 codes recorded at least twice prior to treatment initiation in any inpatient or outpatient encounter (these codes are shown Supplemental Table 2). These ICD-based definitions of HCC16, 20–27 as well as the definitions of cirrhosis and other comorbidities23, 25, 28–32 have been widely used and validated in studies using VA medical records.
Sustained Virologic Response (SVR)
We defined SVR as a serum HCV RNA viral load test below the lower limit of detection performed at least 12 weeks after the end of HCV treatment33.
Incident Hepatocellular Carcinoma
We identified incident cases of HCC diagnosed for the first time at least 180 days after the initiation of antiviral treatment based in ICD-9 code 155.0 or ICD-10 code C22.0 documented at least twice. The ICD-9 code-based definition of HCC using VA records has been shown to have a positive predictive value of 84–94% compared to chart extraction21, 31, 34.
Statistical Analysis
a. Association between SVR and HCC risk
We compared patients who achieved SVR to those who did not with respect to the risk of developing HCC using Cox proportional hazards regression with or without adjusting for potential confounders. We calculated time starting from 180 days after initiation of antiviral treatment since cancers diagnosed within 180 days of the treatment start-date could not possibly have been prevented by antiviral treatment and were likely present but undiagnosed at the time of antiviral treatment initiation (i.e. not truly “incident” cancers). Follow-up for HCC incidence extended until 6/15/2017 so that even the patients treated in late 2015 (i.e. the most recent in our cohort) would have substantial follow-up. Patients without incident HCC were censored at the time of death or last follow-up in the VA.
We considered using the date treatment ended or the date at which SVR was ascertained as the starting times for the time-to-event analysis, but decided against that because of the long and variable duration of the treatment and the interval from treatment end-date to ascertainment of SVR, both of which are strongly related to SVR and would therefore introduce significant bias.
In our primary analyses, we analyzed the association of SVR with HCC risk following each patient’s first antiviral treatment regimen. A significant proportion (42.7%) of patients received more than one antiviral treatment during the study period. This poses an analytical problem since patients who failed the first regimen may achieve SVR during a subsequent treatment. This would tend to slightly underestimate the magnitude of the association between “no SVR” and HCC risk, if one truly exists, by classifying some patients in the “no SVR” group who later achieved SVR. We performed two secondary analyses to further address this potential problem. Firstly, we performed analyses using the first treatment for patients who never achieved SVR and the last treatment for patients who ever achieved SVR (i.e. this included patients who ever achieved SVR in the SVR category). This tends to slightly overestimate the magnitude of the association between “no SVR” and HCC risk because almost all patients in the “no SVR” group who were retreated and finally achieved SVR would not be expected to have developed HCC otherwise they would not have been retreated. Secondly, we analyzed all treatments that each patient received clustered by patient. The intragroup correlation induced by clustering was accounted for by using robust variance estimation.
We also performed secondary analyses limited to only 2 years of follow-up because most SVRs were achieved only recently in the DAA era and have short follow-up, whereas most failures of SVR occurred in the IFN era and had much longer follow-up.
In order to determine if SVR was independently associated with HCC risk, our multivariable proportional hazards models were adjusted for the following characteristics that may be associated with both SVR and HCC risk, ascertained at the time of treatment initiation: cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, BMI, HCV genotype, HCV viral load, HIV co-infection, HBV co-infection, type 2 diabetes mellitus, alcohol use disorders, substance use disorders, body mass index, liver transplantation, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/ALT ratio, blood INR and blood hemoglobin levels. Continuous variables were categorized and modeled as dummy categorical variables. Survival analyses are stratified by the Veterans Affairs facility at which the antiviral treatment was administered.
b. Association between type of antiviral treatment regimen and HCC risk
We performed Cox proportional hazards analyses as described above except that the exposure of interest was the type of antiviral regimen (DAA-ONLY vs DAA+IFN vs IFN-ONLY) rather the SVR. The outcome was the same as in the analyses above (incident HCC occurring at least 180 days after initiation of antiviral treatment) and the same potential confounders were adjusted for in the multivariable models. We performed analyses limited to antiviral regimens initiated in years 2009–2015 to capture only the most recent IFN-only regimens (i.e. from 2009–2011) prior to the introduction of the first protease inhibitors in 2009, since HCC incidence appears to be increasing with time.
Results
Characteristics of study population
Among 62,354 patients who initiated their first antiviral regimen from 1/1/1999 to 12/31/2015, 34,370 (55.4%) achieved SVR. These antiviral treatments included 35,871 (58%) IFN-ONLY regimens, 4535 (7.3%) DAA+IFN regimens and 21,948 (35%) DAA-ONLY regimens. The distribution of different DAA regimens is shown in Table 1.
SVR rates were highest in the DAA-ONLY regimens (90.7%), followed by the DAA+IFN regimens (60.9%) and lowest in the IFN-ONLY regimens (33.4%). Patients were mostly male (96.6%), with a majority white race (55.6%) but a significant representation of other racial/ethnic groups. Mean age was 55.8 yrs and 16.8% had cirrhosis, 4.7% decompensated cirrhosis and 1.1% liver transplantation. Genotype 1 HCV infection predominated (77.4%) followed by genotype 2 (13.5%), 3 (8.3%) and 4 (0.8%).
Among “IFN-ONLY” regimens, patients who achieved SVR were more likely to have genotype 2 or 3 HCV and less likely to have diabetes, cirrhosis or markers of advanced fibrosis/cirrhosis (e.g. low platelets, low albumin), than patients who did not achieve SVR (Table 2). Among DAA-ONLY regimens, patients who achieved SVR were more likely to have genotype 1 infection and also less likely to have diabetes, cirrhosis or markers of advanced fibrosis/cirrhosis.
Table 2.
Baseline characteristics of HCV-infected patients who received their first antiviral treatment from 1999–2015 according to whether they achieved SVR
| All Patients (N=62,354) | IFN-ONLY | DAA+IFN | DAA-ONLY | ||||
|---|---|---|---|---|---|---|---|
| No SVR (n=23,883) | SVR (n=11,988) | No SVR (n= 1,772) | SVR (n= 2,763) | No SVR (n= 2,039) | SVR (n=19,909) | ||
| Age, yrs (mean [SD]) | 55.8 [7.6] | 52.4 [6.2] | 52.4 [6.8] | 57.7 [5.8] | 57.3 [6.7] | 60.7 [6.3] | 61.0 [6.7] |
| BMI, Kg/m2 (mean [SD]) | 28.2 [5.3] | 28.4 [5.2] | 28.2 [5.2] | 28.7 [5.3] | 28.3 [5.0] | 28.6 [5.9] | 27.9 [5.4] |
| Male (%) | 96.6 | 97 | 95.7 | 95.5 | 96.4 | 98.3 | 96.6 |
| Race/Ethnicity (%) | |||||||
| White, non-Hispanic | 55.6 | 52.2 | 67.5 | 50.3 | 60.8 | 52 | 52.7 |
| Black, non-Hispanic | 26.3 | 26.7 | 12.5 | 36.2 | 24.9 | 31.9 | 32.8 |
| Hispanic | 6 | 7 | 6.1 | 6 | 4.5 | 6.7 | 5 |
| Other | 1.6 | 1.6 | 1.7 | 1.4 | 1.4 | 1.8 | 1.6 |
| Declined to answer/missing | 10.5 | 12.6 | 12.2 | 6 | 8.4 | 7.6 | 7.9 |
| Genotype (%) | |||||||
| Genotype 1 | 77.4 | 80.2 | 51.2 | 99.2 | 96.6 | 73.6 | 85.7 |
| Genotype 2 | 13.5 | 10.4 | 31.3 | 0.1 | 0.8 | 13.5 | 9.3 |
| Genotype 3 | 8.3 | 8.5 | 16.6 | 0.6 | 2.2 | 12 | 4.2 |
| Genotype 4 | 0.8 | 0.9 | 0.9 | 0.2 | 0.4 | 0.9 | 0.8 |
| HCV RNA Viral load >6 million IU/mL (%) | 16.7 | 14.9 | 15.2 | 24.1 | 21.4 | 19.6 | 17.9 |
| HIV co-infection | 3.4 | 3.6 | 1.8 | 1.9 | 1.4 | 4 | 4.4 |
| HBV co-infection | 1 | 0.6 | 1 | 1.8 | 1.7 | 1 | 1.2 |
| Cirrhosis (%) | 16.8 | 13.5 | 7.8 | 27.9 | 20.4 | 36 | 22.6 |
| Decompensated Cirrhosis (%) | 4.7 | 4.1 | 2.3 | 6.5 | 3.8 | 12.7 | 5.9 |
| Liver Transplantation (%) | 1.1 | 1.1 | 0.9 | 0.1 | 0.3 | 0.9 | 1.5 |
| Diabetes (%) | 22 | 19.9 | 14.3 | 25.4 | 20.1 | 34.2 | 27.9 |
| Alcohol Use Disorder (%) | 39.3 | 36 | 36.2 | 41.1 | 40.8 | 48 | 43.7 |
| Substance Use Disorder (%) | 31.8 | 28 | 28.2 | 33.9 | 32.8 | 39.3 | 37.5 |
| Laboratory Results (mean [SD]) | |||||||
| Alpha Fetoprotein, ng/mL | 5.8 [4.1] | 6.1 [4.2] | 4.6 [3.2] | 7.9 [4.8] | 5.9 [4.0] | 7.0 [4.6] | 6.0 [4.2] |
| Hemoglobin, g/dL | 14.9 [1.5] | 15.0 [1.5] | 15.2 [1.4] | 14.9 [1.4] | 15.0 [1.4] | 14.3 [1.7] | 14.5 [1.6] |
| Platelet Count, k/μL | 192.9 [71.3] | 197.0 [71.9] | 212.0 [68.6] | 174.2 [64.4] | 188.9 [63.4] | 160.2 [74.6] | 182.1 [70.1] |
| Creatinine, mg/dL | 1.0 [0.6] | 1.0 [0.7] | 1.0 [0.4] | 1.0 [0.8] | 0.9 [0.3] | 1.0 [0.5] | 1.0 [0.5] |
| Bilirubin, g/dL | 0.7 [0.5] | 0.7 [0.5] | 0.6 [0.5] | 0.7 [0.4] | 0.6 [0.4] | 0.8 [0.7] | 0.7 [0.5] |
| Albumin g/dL | 4.0 [0.5] | 4.0 [0.4] | 4.1 [0.4] | 3.9 [0.5] | 4.0 [0.4] | 3.7 [0.6] | 3.9 [0.5] |
| INR | 1.1 [1.0] | 1.1 [0.9] | 1.1 [1.0] | 1.2 [1.3] | 1.2 [1.1] | 1.2 [1.0] | 1.2 [0.9] |
| AST/ALT | 0.9 [0.4] | 0.9 [0.4] | 0.8 [0.3] | 1.0 [0.3] | 0.8 [0.3] | 1.0 [0.4] | 1.0 [0.4] |
Compared to patients who received IFN-ONLY regimens, the patients who received DAA-ONLY or DAA±IFN regimens were older, more likely to be Black, less likely to have genotype 2 or 3 HCV and more likely to have advanced fibrosis or cirrhosis (Table 2). Also, compared to patients who received IFN-ONLY regimens, the patients who received DAA-ONLY or DAA+IFN regimens had lower mean serum platelet count and albumin level.
Association between SVR and HCC risk
We identified 3271 incident cases of HCC diagnosed more than 180 days after initiation of the first antiviral regimen in 62,354 patients during a mean follow-up of 6.1 years. HCC incidence was lower in the patients who achieved SVR (0.43 per 100 patient-years) than in treatment failures (1.15 per 100 patient-years) (Table 3).
Table 3.
Association between SVR and HCC risk among all patients and among subgroups defined by cirrhosis and antiviral regimen. (Intent to treat analysis using first treatment)
| Number of patients (%) | Patient-years | Number who developed HCC (%) | HCC per 100 patient-years | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95%CI) | ||
|---|---|---|---|---|---|---|---|
| ALL REGIMENS | No SVR | 27,694(44.4) | 230,186 | 2,629(9.5) | 1.14 | 1 | 1 |
| SVR | 34,660(55.6) | 147,988 | 642(1.9) | 0.43 | 0.36 (0.33–0.40) | 0.39 (0.35–0.43) | |
| ALL REGIMENS + CIRRHOSIS | No SVR | 4,463(42.6) | 26,221 | 851(19.1) | 3.25 | 1 | 1 |
| SVR | 6,005(57.4) | 16,511 | 326(5.4) | 1.97 | 0.57 (0.49–0.65) | 0.50 (0.43–0.59) | |
| ALL REGIMENS, NO CIRRHOSIS | No SVR | 23,231(44.8) | 203,965 | 1,778(7.7) | 0.87 | 1 | 1 |
| SVR | 28,655(55.2) | 131,478 | 316(1.1) | 0.24 | 0.29 (0.26–0.33) | 0.32 (0.28–0.37) | |
| IFN-ONLY REGIMENS | No SVR | 23,883(66.6) | 220,315 | 2,348(9.8) | 1.07 | 1 | 1 |
| SVR | 11,988(33.4) | 107,725 | 303(2.5) | 0.28 | 0.25 (0.22–0.29) | 0.32 (0.28–0.37) | |
| DAA+IFN REGIMENS | No SVR | 1,772(39.1) | 6,690 | 116(6.5) | 1.73 | 1 | 1 |
| SVR | 2,763(60.9) | 9,829 | 59(2.1) | 0.6 | 0.34 (0.24–0.46) | 0.48 (0.32–0.73) | |
| DAA-ONLY REGIMENS | No SVR | 2,039(9.3) | 3,181 | 165(8.1) | 5.19 | 1 | 1 |
| SVR | 19,909(90.7) | 30,434 | 280(1.4) | 0.92 | 0.18 (0.14–0.22) | 0.29 (0.23–0.37) |
Adjusted for cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, body mass index, HCV genotype, HCV viral load, HIV co-infection, HBV co-infection, type 2 diabetes mellitus, alcohol use disorders, substance use disorder, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/ALT ratio, blood INR and blood hemoglobin levels. The laboratory tests were categorized into quartiles and modeled as dummy categorical variables.
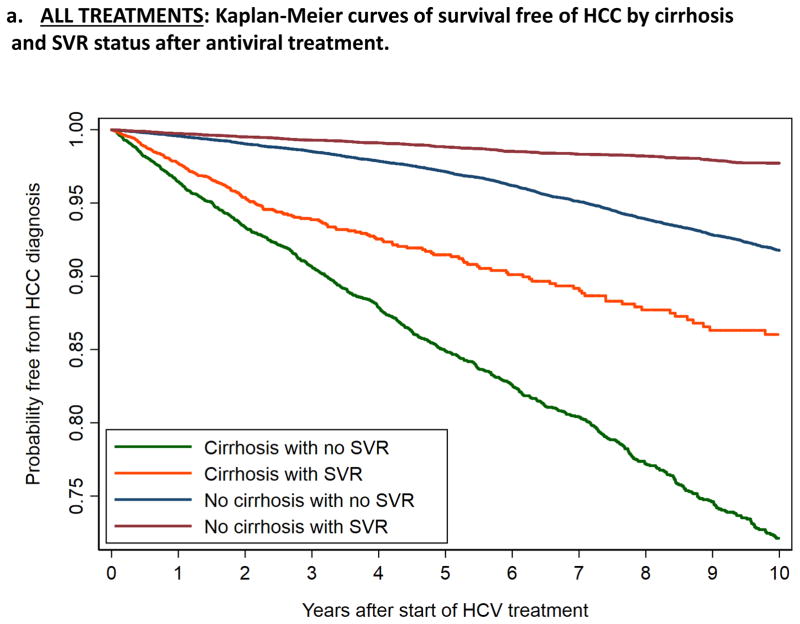
Patients who achieved SVR had lower incidence of HCC than treatment failures among both cirrhotic patients (1.97 vs 3.25 per 100 patient-years) and non-cirrhotic patients (0.24 vs 0.87 per 100 patient-years), as also illustrated by the Kaplan-Meier curves in Figure 1. The incidence of HCC was highest in patients with cirrhosis and treatment failure (3.25), followed by cirrhosis and SVR (1.97), no cirrhosis and treatment failure (0.87) and lowest in patients with no cirrhosis and SVR (0.24) (Table 3 and Figure 1a). The same pattern was observed among all patients (Figure 1a) as well as among the subgroups treated with IFN-ONLY (Figure 1b), DAA+IFN (Figure 1c) and DAA-ONLY (Figure 1d).
Figure 1.
Kaplan-Meier curves showing the development of HCC after antiviral treatment for HCV, by cirrhosis and SVR status
a. ALL TREATMENTS
b. IFN-ONLY TREATMENTS
c. DAA+IFN TREATMENTS
d. DAA-ONLY TREATMENTS
Among all patients, SVR was associated with a 61% reduction in the risk of HCC (adjusted hazard ratio [AHR] 0.39, 95% CI 0.35–0.43) in multivariable analyses adjusting for baseline confounders (Table 3). SVR was associated with a decrease in HCC risk among both patients with cirrhosis (AHR 0.50, 95% CI 0.43–0.59) and patients without cirrhosis (AHR 0.32, 95% CI 0.28–0.37).
SVR was associated with a similar and significantly decreased risk of HCC in multivariable models irrespective of whether the antiviral treatment was IFN-ONLY (AHR 0.32, 95% CI 0.28–0.37), DAA+IFN (AHR 0.48, 95% CI 0.32–0.73) or DAA-ONLY (AHR 0.29, 95% CI 0.23–0.37) (Table 3).
In secondary analyses limited to only two years of follow-up, performed because mean follow-up was inevitably shorter in the DAA-ONLY group (1.53 years) and DAA+IFN group (3.6 years) than the IFN-ONLY group (9.1 years), SVR was still associated with a significantly reduced risk of HCC irrespective of whether the antiviral treatment was IFN-ONLY (AHR 0.56, 95% CI 0.38–0.81), DAA+IFN (AHR 0.49, 95% CI 0.27–0.86) or DAA-ONLY (AHR 0.28, 95% CI 0.22–0.35) (Supplemental Table 3).
Also, in secondary analyses in which we looked at the regimen that achieved SVR or a clustered analysis of all antiviral regimens, instead of the first antiviral regimen, SVR was similarly associated with a reduction in HCC risk (Supplemental Table 4).
Association between type of antiviral treatment regimen and HCC risk
Although HCC incidence was higher after DAA-ONLY treatment (1.32 per 100 patient-years) than after DAA+IFN (1.06) or IFN-ONLY (0.81) treatment, there was no significant association between treatment regimen and HCC risk after adjusting for important confounders (Table 4). This was due to the much higher prevalence in the DAA-ONLY group of risk factors for HCC such as cirrhosis, advanced age, diabetes, low platelet count and low serum albumin. When analyzing separately patients with or without cirrhosis during period 2009–2015, there was little difference in HCC incidence by antiviral treatment group and no association between antiviral treatment group and HCC risk. Also, when limiting follow-up to 2 years, there was no association between antiviral treatment group and HCC risk (Table 4).
Table 4.
Association between type of antiviral treatment regimen (DAA-ONLY vs DAA+IFN vs IFN-ONLY) and HCC risk
| Number of patients (%) | Patient-years | Number who developed HCC (%) | HCC per 100 patient-years | Crude hazard ratio (95% CI) | Adjusted* hazard ratio (95%CI) | ||
|---|---|---|---|---|---|---|---|
| ALL PATIENTS | IFN-ONLY | 35,871(57.5) | 328,040 | 2,651(7.4) | 0.81 | 1 | 1 |
| DAA+IFN | 4,535(7.3) | 16,518 | 175(3.9) | 1.06 | 1.84 (1.56–2.17) | 1.04 (0.87–1.26) | |
| DAA-ONLY | 21,948(35.2) | 33,615 | 445(2.0) | 1.32 | 2.81 (2.44–3.23) | 1.12 (0.95–1.32) | |
| ALL PATIENTS FROM 2009–2015 | IFN-ONLY | 9,292(22.8) | 51,942 | 509(5.5) | 0.98 | 1 | 1 |
| DAA+IFN | 5,996(14.7) | 22,198 | 244(4.1) | 1.1 | 1.22 (1.04–1.44) | 0.89 (0.73–1.08) | |
| DAA-ONLY | 25,424(62.4) | 39,309 | 557(2.2) | 1.42 | 1.78 (1.51–2.09) | 0.92 (0.77–1.10) | |
| ALL PATIENTS FROM 2009–2015 LIMITED TO 2 YEARS FOLLOW-UP | IFN-ONLY | 9,292(22.8) | 18,188 | 135(1.5) | 0.74 | 1 | 1 |
| DAA+IFN | 5,996(14.7) | 11,665 | 130(2.2) | 1.11 | 1.51 (1.19–1.93) | 1.16 (0.87–1.54) | |
| DAA-ONLY | 25,424(62.4) | 38,204 | 535(2.1) | 1.4 | 1.94 (1.60–2.35) | 1.04 (0.83–1.30) | |
| ALL PATIENTS FROM 2009–2015 WITH CIRRHOSIS | IFN-ONLY | 1,491(15.3) 7,404 | 223(15.0) | 3.01 | 1 | 1 | |
| DAA+IFN | 1,617(16.6) | 5,657 | 147(9.1) | 2.6 | 0.86 (0.69–1.07) | 0.95 (0.74–1.22) | |
| DAA-ONLY | 6,626(68.1) | 10,916 | 395(6.0) | 3.62 | 1.23 (1.01–1.51) | 0.97 (0.77–1.22) | |
| ALL PATIENTS FROM 2009–2015 WITHOUT CIRRHOSIS | IFN-ONLY | 7,801(25.2) | 44,538 | 286(3.7) | 0.64 | 1 | 1 |
| DAA+IFN | 4,379(14.1) | 16,541 | 97(2.2) | 0.59 | 1.11 (0.86–1.42) | 0.90 (0.67–1.22) | |
| DAA-ONLY | 18,798(60.7) | 28,393 | 162(0.9) | 0.57 | 1.33 (1.02–1.72) | 0.98 (0.73–1.32) |
Adjusted for cirrhosis, decompensated cirrhosis, age, sex, race/ethnicity, body mass index, HCV genotype, HCV viral load, HIV co-infection, HBV co-infection, type 2 diabetes mellitus, alcohol use disorders, substance use disorder, platelet count, serum bilirubin, serum creatinine, serum albumin, serum AST/ALT ratio, blood INR and blood hemoglobin levels. The laboratory tests were categorized into quartiles and modeled as dummy categorical variables.
Discussion
Most HCV-infected patients in the United States will undergo DAA-based antiviral treatment in the next few years and the vast majority of them will achieve SVR. Our results suggest that DAA-induced SVR is associated with a 71% reduction in HCC risk (AHR 0.29, 95% CI 0.23–0.37) compared to treatment failure. The reduction in HCC risk associated with SVR was similar irrespective of whether SVR was achieved by DAA-ONLY, DAA+IFN or IFN-ONLY regimens. This suggests that eradication of HCV reduces HCC risk independently of how it is achieved. In contrast to prior reports that suggested an increased HCC risk in patients treated with DAAs3, 7, we found that receipt of DAA-ONLY antiviral treatment was not associated with increased risk of HCC when compared to receipt of IFN-ONLY antiviral treatment.
It is still unclear whether DAA-induced SVR is associated with a reduction in HCC risk. It might seem reasonable to expect that HCV eradication would reduce HCC risk by preventing or reversing cirrhosis, or by eliminating any direct carcinogenic effect of HCV. However, recent studies demonstrated little or no impact of DAA-based antiviral treatment on HCC risk and even suggested that DAAs might increase the risk of HCC recurrence3–8. A recent retrospective Spanish study reported an unexpectedly high rate of HCC recurrence (27.6% after a median follow-up of 5.7 months) following HCV antiviral treatment with DAAs in 58 patients with HCC who had achieved complete response prior to antiviral treatment3, raising concerns that DAAs may somehow promote HCC recurrence. However, a French prospective cohort study found similar risk of HCC recurrence when comparing 189 patients who received DAAs (24/189 or 12.7% with recurrence, incidence 0.73 per 100 patient/months) to 78 who did not receive DAAs (16/78 or 20.5% with recurrence, incidence 0.66/100 patient-months)4. Finally, an Italian study reported that after DAA therapy, 9/285 patients with cirrhosis without prior HCC developed new HCC during 24-week follow-up, while 17/59 patients with prior HCC had recurrence and concluded that “DAA-induced resolution of HCV infection does not seem to reduce the occurrence of HCC”5. However, these studies were grossly underpowered, had very limited follow-up time, studied mostly HCC recurrence rather than incidence, and did not attempt to compare those who achieved SVR as a result of DAAs to those who did not with respect to HCC risk.
In contrast to these studies, we found that DAA-induced SVR was associated with a 71% reduction in the risk of HCC and that a roughly similar reduction in HCC risk was associated with SVR induced by DAA-ONLY (AHR 0.29, 95% CI 0.23–0.37), DAA+IFN (AHR 0.48, 95% CI 0.32–0.73) or IFN-ONLY (AHR 0.32, 95% CI 0.28–0.37) regimens. This strongly suggests that eradication of HCV reduces the risk of HCC irrespective of the antiviral regimen used to achieve it. It is important to emphasize that our study was designed to address the impact of SVR on HCC incidence, not HCC recurrence. Hence patients with a history of HCC prior to antiviral treatment were excluded and our study offers no insight into the impact of antiviral treatment on HCC recurrence.
We observed a similar relative reduction in HCC risk associated with SVR in cirrhotic (AHR 0.34, 95% CI 0.26–0.43) and non-cirrhotic (AHR 0.29, 95% CI 0.24–0.34) patients – although the absolute reduction in HCC risk was much greater in cirrhotic (from 2.7 to 0.93 per 100 patient-years) than in non-cirrhotic patients (from 0.73 to 0.18 per 100 patient-years), as would be expected given the higher baseline HCC risk of cirrhotic patients. It is generally believed that HCC risk in HCV-infected patients increases dramatically once they develop cirrhosis, but a lower HCC risk is still present in pre-cirrhotic liver disease, especially in advanced fibrosis. The risk of HCC in non-cirrhotic HCV-infected patients may be due to the presence of occult cirrhosis at least in parts of the liver, progression from advanced fibrosis to cirrhosis during follow-up or the development of HCC in pre-cirrhotic advanced fibrosis. It is likely that in both cirrhotic and non-cirrhotic patients SVR reduces HCC risk at least in part by reversing hepatic fibrosis.
The best way to determine whether treatment with DAAs affects HCC risk is to randomize patients to treatment with DAAs vs no treatment and then follow them up for a long period of time. Such a study design would be unethical. Another possibility would be to compare patients who received DAAs as part of their routine clinical care to those who were untreated with respect to HCC risk. However, this approach is subject to considerable ascertainment bias (the untreated patients are much less likely to be diagnosed with HCC in a given time frame than the treated patients due to less screening and less contact with hepatologists and other medical providers) and selection bias (the reasons why certain patients are not treated with DAAs despite their excellent SVR rate may be difficult to determine and accurately adjust for) and confounding by indication (the indication for offering antiviral treatment may be associated with both treatment and outcome). Instead, we chose to compare patients treated with DAAs in the “current era” to patients treated with IFN in the interferon era to avoid as much as possible these sources of bias. However, this comparison could still be biased by the fact that patients with much more advanced liver disease are now candidates for DAAs who were not candidates for IFN and these patients have significantly higher HCC risk. We believe that our adjustment for baseline characteristics adequately accounted for this potential confounding. In addition, the incidence of HCC has been increasing over time25 and was much lower in the IFN era than in the DAA era. We minimized this source of bias by limiting analyses to regimens initiated from 2009–2015, as well as by adjusting for baseline characteristics that are at least partly responsible for the increasing HCC incidence over time. Any residual bias would be in favor of showing increased HCC risk in the DAA-only treated patients; it is, therefore, reassuring that no such association was found.
Our study was limited by the relatively short follow-up in the DAA-ONLY group, although we extended follow-up for HCC incidence to a time as close as possible to the time of manuscript preparation (June 2017) to maximize follow-up time. This yielded a substantial mean follow-up time of 1.53 years even in the DAA-ONLY group (with follow-up time starting at 180 days after antiviral treatment initiation). It was reassuring that very similar associations between SVR and HCC risk were observed among the DAA+IFN and the IFN-only groups that had much longer follow-up than the DAA-ONLY group. The association between SVR and HCC risk may not be causative: it is theoretically possible that unknown factors that lead to treatment failure may also lead to the future development of HCC, other than the 21 baseline characteristics that we carefully adjusted for that included known markers of advanced fibrosis/cirrhosis and risk factors for HCC. It is also possible that HCC was present but undiagnosed at the time of antiviral treatment and also led to reduced SVR. However, we excluded all HCCs that presented within 180 days of antiviral treatment initiation; in addition, the HCC cumulative incidence curves in the SVR and treatment failure groups continue to diverge for many years after treatment (Figure 1). A final limitation of the study is that the ICD-10 code for HCC (C22.0) that replaced the ICD-9 code for HCC (155.0) in October 2015 is not yet validated using VA data. However, since a single ICD-10 code directly replaced a single ICD-9 code, it is reasonable to expect a similarly high positive predictive value.
Substantial strengths of the study include the large sample size, large number of incident HCCs and long follow-up time. Baseline characteristics necessary for multivariable analyses to adjust for potential confounding were available. All patients were derived from a single, national healthcare system with fairly uniform antiviral treatment practices and guidelines across its facilities.
In conclusion, DAA-induced SVR is associated with a 71% reduction in HCC risk compared to treatment failure. Eradication of HCV is associated with a similar reduction in HCC risk irrespective of the regimen that is used to achieve eradiation. We found no evidence that treatment with DAAs was associated with increased risk of HCC compared to treatment with IFN.
Supplementary Material
Lay Summary.
Eradication of hepatitis C infection with direct-acting antiviral agents reduces the risk of liver cancer dramatically.
DAA-induced SVR is associated with a 71% reduction in HCC risk
Treatment with DAAs is not associated with increased HCC risk compared to interferon
Acknowledgments
Declaration of Funding Sources
The study was funded by a NIH/NCI grant R01CA196692 and VA CSR&D grant I01CX001156 to GNI.
Role of Funding Source
The funding source played no role in study design, collection, analysis or interpretation of data.
Abbreviations
- DAA
Direct-Acting Antiviral treatment for HCV
- HCC
Hepatocellular carcinoma
- HCV
Hepatitis C Virus
- IFN
Interferon
- PEG
pegylated interferon
Footnotes
Declaration of Personal Interests
None
Disclaimer
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Authors’ Contributions and Authorship Statement
All authors approved the final version of the manuscript
George Ioannou is the guarantor of this paper.
George Ioannou: Study concept and design, acquisition of data, statistical analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript, obtained funding.
Pamela Green: Acquisition of data, study design, analysis of data.
Kristin Berry: Study design, analysis of data, critical revision of manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–8. doi: 10.1053/j.gastro.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–37. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 3.Reig M, Marino Z, Perello C, et al. Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 2016 doi: 10.1016/j.jhep.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 4.AcsgohcEa. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 2016;65:734–40. doi: 10.1016/j.jhep.2016.05.045. stanislas.pol@aphp.fr. [DOI] [PubMed] [Google Scholar]
- 5.Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–33. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Yang JD, Aqel BA, Pungpapong S, et al. Direct acting antiviral therapy and tumor recurrence after liver transplantation for hepatitis C-associated hepatocellular carcinoma. J Hepatol. 2016;65:859–60. doi: 10.1016/j.jhep.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 7.Reig M, Boix L, Marino Z, et al. Liver Cancer Emergence Associated with Antiviral Treatment: An Immune Surveillance Failure? Semin Liver Dis. 2017;37:109–118. doi: 10.1055/s-0037-1601349. [DOI] [PubMed] [Google Scholar]
- 8.Cheung MC, Walker AJ, Hudson BE, et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J Hepatol. 2016;65:741–7. doi: 10.1016/j.jhep.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 9.National Center for Veterans Analysis and Statistics, Number of Veteran Patients by Healthcare Priority Group. [accessed 4/27/2016];FY 2000 to FY. 2014 Available at: http://www.va.gov/vetdata/Utilization.asp.
- 10.United States Department of Veterans Affairs. [Last accessed 4/27/2016];National Hepatitis C Registry Reports. 2015 Available at http://vaww.hepatitis.va.gov/data-reports/ccr-index.asp#b-1.
- 11. [Last accessed on 12/19/16];Veterans Affairs Corporate Data Warehouse. Available at: http://www.hsrd.research.va.gov/for_researchers/vinci/cdw.cfm.
- 12.Su F, Green PK, Berry K, et al. The association between race/ethnicity and the effectiveness of direct antiviral agents for hepatitis C virus infection. Hepatology. 2017;65:426–438. doi: 10.1002/hep.28901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Beste LA, Green PK, et al. Direct-acting antivirals are effective for chronic hepatitis C treatment in elderly patients: a real-world study of 17 487 patients. Eur J Gastroenterol Hepatol. 2017 doi: 10.1097/MEG.0000000000000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui JI, Williams EC, Green PK, et al. Alcohol use and hepatitis C virus treatment outcomes among patients receiving direct antiviral agents. Drug Alcohol Depend. 2016;169:101–109. doi: 10.1016/j.drugalcdep.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioannou GN, Beste LA, Chang MF, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151:457–471. e5. doi: 10.1053/j.gastro.2016.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beste LA, Green PK, Berry K, et al. Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma. J Hepatol. 2017;67:32–39. doi: 10.1016/j.jhep.2017.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hum J, Jou JH, Green PK, et al. Improvement in Glycemic Control of Type 2 Diabetes After Successful Treatment of Hepatitis C Virus. Diabetes Care. 2017 doi: 10.2337/dc17-0485. [DOI] [PubMed] [Google Scholar]
- 18.Johnson K, Green PK, Ioannou GN. Implications of HCV RNA level at week 4 of direct antiviral treatments for hepatitis C. J Viral Hepat. 2017 doi: 10.1111/jvh.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon AM, Green PK, Berry K, et al. Transformation of hepatitis C antiviral treatment in a national healthcare system following the introduction of direct antiviral agents. Aliment Pharmacol Ther. 2017;45:1201–1212. doi: 10.1111/apt.14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–82. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 21.Kramer JR, Giordano TP, Souchek J, et al. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100:56–63. doi: 10.1111/j.1572-0241.2005.40670.x. [DOI] [PubMed] [Google Scholar]
- 22.Ioannou GN, Splan MF, Weiss NS, et al. Incidence and Predictors of Hepatocellular Carcinoma in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2007;5:938–945. doi: 10.1016/j.cgh.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154:85–93. doi: 10.7326/0003-4819-154-2-201101180-00006. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou GN, Bryson CL, Weiss NS, et al. The prevalence of cirrhosis and hepatocellular carcinoma in patients with human immunodeficiency virus infection. Hepatology. 2013;57:249–57. doi: 10.1002/hep.25800. [DOI] [PubMed] [Google Scholar]
- 25.Beste LA, Leipertz SL, Green PK, et al. Trends in Burden of Cirrhosis and Hepatocellular Carcinoma by Underlying Liver Disease in US Veterans, 2001–2013. Gastroenterology. 2015;149:1471–1482. e5. doi: 10.1053/j.gastro.2015.07.056. [DOI] [PubMed] [Google Scholar]
- 26.El-Serag HB, Kramer J, Duan Z, et al. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109:1427–35. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Kanwal F, Davila JA, et al. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology. 2014;146:1249–55. e1. doi: 10.1053/j.gastro.2014.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kramer JR, Kanwal F, Richardson P, et al. Importance of patient, provider, and facility predictors of hepatitis C virus treatment in veterans: a national study. Am J Gastroenterol. 2011;106:483–91. doi: 10.1038/ajg.2010.430. [DOI] [PubMed] [Google Scholar]
- 29.Beste LA, Ioannou GN, Larson MS, et al. Predictors of early treatment discontinuation among patients with genotype 1 hepatitis C and implications for viral eradication. Clin Gastroenterol Hepatol. 2010;8:972–8. doi: 10.1016/j.cgh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Backus LI, Boothroyd DB, Phillips BR, et al. Predictors of response of US veterans to treatment for the hepatitis C virus. Hepatology. 2007;46:37–47. doi: 10.1002/hep.21662. [DOI] [PubMed] [Google Scholar]
- 31.Kanwal F, Hoang T, Kramer JR, et al. Increasing Prevalence of HCC and Cirrhosis in Patients With Chronic Hepatitis C Virus Infection. Gastroenterology. 2011;140:1182–1188. e1. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.b10. [DOI] [PubMed] [Google Scholar]
- 33.Yoshida EM, Sulkowski MS, Gane EJ, et al. Concordance of sustained virological response 4, 12, and 24 weeks post-treatment with sofosbuvir-containing regimens for hepatitis C virus. Hepatology. 2015;61:41–5. doi: 10.1002/hep.27366. [DOI] [PubMed] [Google Scholar]
- 34.Davila JA, Weston A, Smalley W, et al. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–82. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.