Abstract
Objective
Given its lethality, associated stigma, and symptom burden, a lung cancer diagnosis poses a substantial challenge for patients. The goal of this study was to examine how daily hope, defined as goal-directed effort and planning to meet goals, and daily stigma were related to same- and next-day functioning in lung cancer patients receiving cancer treatment.
Methods
Fifty lung cancer patients (39 non-small cell stages IIIa–IV; 11 limited and extensive small cell) completed a baseline questionnaire and 21 daily diaries (n = 1,042) assessing hope, stigma, physical symptoms, treatment factors, and functioning. Hypotheses were tested in same and next day models with multilevel modeling.
Results
Patients who reported more daily hope reported higher social and role functioning in same and next-day models. On days that patients reported more hope than usual (compared to their own across-day average), they had higher social, role, and physical functioning; this effect did not carry into the next day. Treatment days were associated with lower social and role functioning when patients reported lower hope and associated with higher functioning when patients reported higher hope. Within-person hope was not predicted by disease symptoms. On days that patients reported more stigma than usual, they reported lower social and role functioning.
Conclusions
Hope is associated with functioning in lung cancer patients, regardless of physical symptoms from disease and treatment. Hope and stigma may therefore be appropriate interventions targets to support daily social and role functioning during lung cancer treatment.
Keywords: lung cancer, hope, quality of life, stigma, daily diary, daily assessment, goals, functioning
Introduction
Lung cancer is the second most diagnosed and the most lethal cancer in the U.S. In 2017, over 220,000 new lung cancer cases are expected, along with 155,000 deaths (Siegel, Miller, & Jemal, 2017). The majority of lung cancers are diagnosed in locally advanced or advanced disease stages. For advanced (i.e. metastatic disease), the median survival at the time this study was performed was approximately 8–12 months (Siegel, et al., 2017). While the prognosis for locally advanced disease is better, the median survival is approximately 24 months, with an approximately 75–80% disease associated mortality. Poor prognosis, coupled with high psychological distress (Zabora, BrintzenhofeSzoc, Curbow, Hooker, & Piantadosi, 2001) and physical symptoms associated with disease and treatment (Deshields, Potter, Olsen, & Liu, 2014), makes maximizing quality of life critical to treatment (Smith et al., 2012).
Quality of life refers broadly to the impact of disease and treatment on patients’ physical, social, emotional, and functional wellbeing (Cella & Tulsky, 1993). The impact of disease and treatment may best be understood from the perspective of patients’ daily lives (Schneider & Stone, 2016). Physical symptoms that are known to negatively impact quality of life can vary daily (Brown et al., 2005). The challenges patients face on days that they have medical appointments can differ from non-appointment days (Dhotre, Adams, Hebert, Bottai, & Heiney, 2016). Most importantly, much of the distress patients report from physical symptoms and treatment is related to their interference with daily plans, activities, and ability to maintain societal and familial roles (Dhotre, et al., 2016; Ellis, 2012; Maguire et al., 2013; Molassiotis, Lowe, Blackhall, & Lorigan, 2011). Identifying modifiable factors that predict variation in daily functioning may facilitate interventions that reduce the burden from lung patients experience in their daily lives. The aim of this study was to examine two such factors, hope and stigma.
Hope and stigma have both been related to aspects of quality of life in lung cancer, but have not been examined in relation to daily functioning. “Hope,” defined as a positive motivational state based on setting goals and thinking about ways to reach them (Snyder, 2002; Snyder et al., 1991; Snyder et al., 1996), has been inversely related to psychological distress and physical symptoms in lung cancer (Berendes et al., 2010). This conceptualization of hope has been applied to understand differences in psychosocial functioning in cancer (Stanton, Danoff-burg, & Huggins, 2002; Thornton et al., 2014) and recommended as an organizing framework for goal setting in palliative care (Boa, Duncan, Haraldsdottir, & Wyke, 2014). It is salient in the context of life-limiting illness because of its focus on identifying achievable actions to take to fulfill meaningful goals (Feldman et al., 2008). People with higher hope should better sustain effort towards goals such as spending time with family or friends, working as long as possible, or maintaining hobbies (Feldman, et al., 2008). Many of these goals may be difficult to pursue not only because of physical symptoms from disease, but also because of stigma. Stigma, which can encompass shame, guilt, perceived discrimination, and perceived blame from others for illness, has been associated with nihilism about treatment (Pujol, Merel, & Roth, 2016), psychological distress (Cataldo, Jahan, & Pongquan, 2012; Criswell, Owen, Thornton, & Stanton, 2016), and lower physical functioning (Cataldo & Brodsky, 2013; Cataldo, et al., 2012). Stigma may make it difficult for patients to attend social activities, heighten perceptions of being a burden to family, and lead to isolation (Cataldo, Slaughter, Jahan, Pongquan, & Hwang, 2011).
Although individual differences in hope and stigma are likely associated with functioning in people with lung cancer, there may also be important within-person variability in these experiences that may affect both same day and future day functioning. Hope has been shown to vary daily (Ouweneel, Le Blanc, Schaufeli, & van Wijhe, 2012; Steffen & Smith, 2013) and has been manipulated in experimental tasks (Snyder et al., 2005; Snyder, et al., 1996) and interventions (Cheavens, Feldman, Gum, Michael, & Snyder, 2006; Thornton, et al., 2014). Daily variations in hope reflect a person’s current goal-directed thinking (Snyder, et al., 1996). On days that patients think more about their goals and ways to meet them, they should direct more of their energy into activities that align with those goals (Ouweneel, et al., 2012) and thereby report less interference from cancer in their daily functioning. Goal-directed thinking may help patients identify actions they can take on treatment days even though their schedule is interrupted. Increased goal-directed thinking may also exert next-day effects, as patients may start their day ready to implement actions they identified the previous day to achieve personal plans and goals. Stigma has been shown to vary daily in other populations (Hatzenbuehler, Nolen-Hoeksema, & Dovidio, 2009). It may be experienced more on days that family members convey frustration about how cancer has changed their lives (Milbury, Badr, Fossella, Pisters, & Carmack, 2013) or when patients disclose their diagnosis to others and are asked if they smoked (Pujol, et al., 2016). These experiences may interfere with same day functioning, but may also deter patients from carrying out planned next-day activities.
Current Study
Given the potential cross-sectional and longitudinal influences of hope and stigma on functioning, this study examined how individual differences and within-person changes in hope and stigma were related to daily social, role, and physical functioning in patients undergoing treatment for lung cancer (non-small cell stages IIIA–IV; limited or extensive small cell). A daily assessment methodology was selected to understand the impact of disease on daily functioning (Schneider & Stone, 2016) and to test whether individual and within-person differences in hope and stigma were related to daily functioning after accounting for physical symptoms and treatment factors such as treatment type and treatment day. An exploratory aim was to determine whether within-person increases in hope protected against the expected negative effect of treatment days on functioning.
People with higher hope and lower lung cancer stigma were hypothesized to report higher daily social, role, and physical functioning in same day and next day (i.e., longitudinal) models. Within-person increases in hope and stigma were hypothesized to be associated with higher and lower (respectively) daily social, role, and physical functioning. Within-person increases in hope and stigma were hypothesized to predict functioning in longitudinal models.
Method
Participants
Eligibility criteria for recruitment included: (a) histologically or cytologically documented non-small cell lung cancer (NSCLC) stage IIIa, IIIb, or IV (7th edition staging; adenocarcinoma, large cell, squamous, or mixture of these types) or limited or extensive small cell lung cancer (SCLC); (b) 21 years of age or older; (c) Eastern Cooperative Oncology Group performance status 0–2 (0 = fully active, 2 = ambulatory and capable of all self-care, but unable to carry out any work activities); (d) no concurrent malignancy or unstable brain metastases (defined as those with midline shift, inadequately controlled seizures or requiring escalating steroid doses); (e) undergoing treatment for lung cancer specific to stage IIIa, IIIb, or IV NSCLC or limited or extensive small cell SCLC; (f) patient could provide informed consent in English.
Recruitment began in April 2014 and ended in September 2015. Patients who were eligible based on pre-screening through the clinic schedule were approached after their appointments with oncologists or at their chemotherapy or radiation appointments. Patients provided written informed consent. The University of New Mexico Health Sciences Center Institutional Review Board (#13–303) and the New Mexico Cancer Care Alliance (#1403) approved this study.
Assessment
Patients completed an initial questionnaire on a computer or on paper. As feasibility was a concern with this population, patients were given the option of how they wanted to complete the diaries (i.e., online, paper, or via telephone) and set their own time of day to complete the diaries with the investigator upon enrollment. Patients who chose to complete surveys online were emailed a link for the daily survey each day at their preferred time. Patients were encouraged to report the previous day’s diary before entering the current day if they missed a day. Time of completion and date were captured through the online system. Patients were also required to enter the date to which their responses corresponded.
Patients who completed diaries on paper were given 21 diaries that they were to date with the time of day completed and minutes taken to complete. They were provided with a self-addressed, postage-paid return envelope. Initially, patients who completed paper diaries were given a flat-rate envelope and returned all 21 diaries at once. Research staff checked in weekly with patients to assure adherence and patients had the option of receiving a reminder to do the daily survey as often as often as they felt they needed or wanted it. Halfway through the study’s data collection period, patients who completed paper diaries were given envelopes to mail each week, with the intention of improving validity of daily assessment. The primary author called phone participants at their preferred time and administered the diary. Participants were paid $30 for completing the initial questionnaire, $3 for each daily entry (up to $63 possible), $4 for each week they completed (up to $12 possible), and $6 for completing all 21 days. Participants received payment in the form of gift cards.
Measures Administered
Level-1 (i.e. Daily) Variables
Level-1 (Daily) Predictor Variables
Treatment Day
Treatment day was a binary variable (0 = no) (1= yes) indicating whether or not someone self-reported chemotherapy or radiation that day.
Daily Affect
To minimize their overlap with physical symptoms assessed (e.g., fatigue and “energetic”), valence and arousal (Larsen & Diener, 1992) were considered when selecting affect items. Positive affect was assessed with two items (“cheerful,” “happy”) from the Positive Affect and Negative Affect Schedule – Expanded Form (Watson & Clark, 1994) on a scale of 0 (not at all) to 3 (extremely). Items were averaged (α’s = 0.78 – 0.94, day 1–21). Two items (“sad,” “nervous”) from the PANAS-X and two additional items (“depressed” and “anxious”) assessed negative affect (0–3 scale). Items were averaged (α’s = 0.66 – 0.92, days 1–21).
Physical Symptoms
Seven items from the FACT-L (D. F. Cella et al., 1995) assessed daily physical symptoms (dyspnea, pain, fatigue, appetite, weakness, coughing, nausea) on a scale of 0 = not at all to 3 = very much. Items were averaged (α’s = 0.72 – 0.90, days 1–21).
Daily Hope
Daily hope was assessed using four items from State Hope Scale (Snyder, et al., 1996) “At the present time, I am trying to pursue my personal goals and plans,” “I can think of many ways to reach my current goals,” “There are ways around any problem that I am facing now,” and “At this time, I am meeting the goals that I have set for myself.” Items were responded to on a scale of 0 (definitely false) to 7 (definitely true) and averaged (α’s = 0.88 – 0.97, days 1–21).
Lung Cancer Stigma
Five items from the Shame, Social Isolation, and Discrimination subscales of the Cataldo Lung Cancer Stigma Scale were used to assess daily experience of lung cancer stigma (Cataldo, et al., 2011). These items were: “I feel guilty because I have lung cancer”, “I feel set apart, isolated from the rest of the world”, “Having lung cancer makes me feel like I’m a bad person” (all from the Shame subscale), “Some people who know have grown more distant” (Social Isolation subscale), and “Some people act as though it is my fault that I have lung cancer” (Discrimination subscale). Items were selected based on their expected potential to vary daily and face value for assessing lung cancer related stigma. Each item was rated on a 4-point Likert-type scale (0 = strongly disagree to 3 = strongly agree). Items were averaged (α’s = 0.75 – 0.91, days 1–21).
Level-1 (Daily) Dependent Variables
Social/Role Functioning
Social and role functioning was assessed with four items from the EORTQ-QLQ-30 (Aaronson et al., 1993). Items included: “Has your physical condition or medical treatment interfered with your family life today?”, “Has your physical condition or medical treatment interfered with your social life today?”, “Were you limited in pursuing your hobbies or other leisure time activities?”, and “Were you limited in pursuing your work or other daily activities?”. Items were rated on a four-point scale with 1 = not at all to 4 = very much. Subscales were transformed linearly to have a range of 0–100, with higher scores reflecting better social functioning. Internal consistency was good (α’s = 0.82 – 0.93, days 1–21). Ratings from the first day of the diary correlated with the full Role and Social Functioning subscales of the EORTC-QLQ-30 administered at baseline (r = .61, p <.001, r = .74, p <.001, respectively).
Physical Functioning
Physical functioning was assessed with one item from the EORTC-QLQ-30 (Aaronson, et al., 1993), “Did you need to stay in a bed or a chair during the day today?”, which was rated on the same four point scale used to assess social/role functioning, 1 = not at all to 4 = very much. Physical functioning was transformed linearly to have a range of 0–100, with higher scores reflecting better physical functioning. This item was selected over other items from the subscale (e.g.,“Did you have any trouble doing strenuous activities…?”) because of its daily applicability to all patients and prognostic relevance (Jones et al., 2012; Oken et al., 1982). Ratings provided on the first day of the diary correlated with the full Physical Functioning subscale of the EORTC-QLQ-30 administered at baseline (day 1: r = .64, p < .001).
Level 2 (i.e. Between-Person) Variables1
Time-Invariant Controls
Demographics
Participant sex, education, relationship status, age, ethnicity, race, income, treatment type, time since diagnosis, and smoking history were assessed at baseline.
Depression and Anxiety
The Hospital Anxiety and Depression Scale (Zigmond & Snaith, 1983) was administered at baseline to assess anxiety and depression symptoms. Each of the 14 items was answered on a four-point Likert-type scale (1 = not at all to 4 = very often; depression Cronbach’s α = .82; anxiety α = .86).
Cross-Day Averages
Physical Symptoms
A person’s average level of physical symptoms, assessed with 7 items from the FACT-Lung (Cella, et al., 1995) across the diary days, was used to estimate between-person differences in symptoms. A person’s average level of physical symptoms across the diary correlated with the full FACT-Lung administered at baseline (r = −.61, p < .001).
Hope
A person’s average level of hope across the diary, assessed with 4 items daily from the State Hope Scale (Snyder et al., 1996), was used to estimate between person differences in hope. A person’s average level of hope across the diary correlated with the full measure of the Dispositional Hope Scale (Snyder, et al., 1991) administered at baseline (r = .30, p = .038).
Lung Cancer Stigma
A person’s average level of stigma across the diary, assessed with 5 items daily from the Cataldo Lung Cancer Stigma Scale (Cataldo, et al., 2011) was used to estimate between person differences in stigma. A person’s average level of stigma across the diary correlated with the full 31-item scale administered at baseline (r = .69, p <.001).
Statistical Analyses
Data were analyzed using SPSS v. 21 (IBM, 2012). In order to be included in the analysis, participants were required to provide at least seven days (33% of all possible days) of diary data to provide estimates of next-day relations.2 The repeated (daily) data was nested within an individual. Hierarchical linear modeling (HLM) was used to address the nested structure of the data and estimate both the Level-1 (daily) and Level-2 (individual) variance. The Level-1 data included daily ratings of symptom severity, functioning, stigma, and hope. The Level-2 data included demographic information (e.g. age, cancer type and stage, treatment type), baseline quality of life, personality (e.g. neuroticism, optimism), and depression and anxiety. Level 1 (i.e., daily) predictor variables were person-mean centered. A person’s average on a daily predictor variable was included in the model as a Level-2 predictor. Level-2 predictors assessed at baseline were centered on a clinical meaningful value if available (e.g., a score of “8” representing a likely “case” of depression) or grand-mean centered. Models were estimated using restricted maximum likelihood. An autoregressive covariance structure for residual level-1 errors was used to account for within-person interdependence of the repeated outcomes.
Analysis of Fixed Effect Hypotheses
The unconditional model was estimated first to partition variance into within- and between-person variability. Intra-class correlations ranged from .64 to .69. Next, time was included as a predictor variable to help assess for reactivity to monitoring (Barta, Tennen, & Litt, 2012). To assess for whether administration method (i.e., online/phone vs. paper) affected responses, administration method was added as a predictor. Finally, the rest of the pre-specified model was estimated. Level-2 covariates (neuroticism, optimism, depression, anxiety, time since diagnosis) and level-1 covariates (daily positive and negative affect) were removed if they did change the effect of focal predictors.3 Treatment type was considered a key disease control variable because it reflects stage of disease and treatment intent and is associated with different treatment-related side effects; it was therefore retained in all models. See Appendix for an example of the reduced form equation and study variable correlations.
Results
Sixty-two eligible patients were approached, of which 56 consented to participate (90.3%). In total, 50 individuals (89.3% of those consented) provided baseline and at least seven diary entries. The analyzed sample (N = 50) was 58% female and most were married (62.0%). On average, participants were 68.7 years (SD = 8.8) of age and had 13.5 years (SD = 2.0) of education; 41% had a high school degree or lower. Participants were primarily Caucasian (80%; Native American: 6.0%; African American: 4.0%; Asian/Pacific Islander: 4.0%; Other/Declined to identify: 6.0%). A minority (14.0%) were currently smoking. Cancer details are shown in Table 1. Average days since cancer diagnosis was 153.1 (SD = 202.9). Most participants had non-small cell lung cancer (78.0%), advanced disease (stage IV NSC = 64.1%; extensive SC = 72.7%), an ECOG performance status of 1 (54.0%), were receiving only chemotherapy (52.0%).
Table 1.
Descriptive Statistics of Sample (N = 50)
| Variable | n (%) |
|---|---|
| Lung Cancer Subtype & Stage | |
| Non-small cell | 39 (78.0) |
| IIIA | 11 (28.2) |
| IIIB | 3 (7.7) |
| IV | 25 (64.1) |
| Small-cell | 11 (22.0) |
| Limited | 3 (27.3) |
| Extensive | 8 (72.7) |
| Current Treatment | |
| Chemotherapy | 26 (52.0) |
| Chemoradiotherapy | 16 (32.0) |
| Radiation | 5 (10.0) |
| Chemotherapy, radiation, and surgery | 1 (2.0) |
| Surgery and radiation | 2 (4.0) |
| ECOG Performance Status | |
| 0 | 10 (20.0) |
| 1 | 27 (54.0) |
| 2 | 13 (26.0) |
| Brain Metastases Present | 10 (20.0) |
| Whole Brain Radiotherapy | 9 (19.6) |
Note. ECOG = European Cooperative Oncology Group
Of the 1,050 potential daily diaries (50 people×21 days), 1,042 (99.2%) were completed. Forty-three participants (86%) completed 20 days or more. Twenty-eight (56%) participants completed the diaries on paper, of which 10 (35.7%) mailed weekly batches of surveys. Three participants completed the diaries on the telephone. As participants were encouraged to enter the previous day’s diary if they missed a day, we coded online diary entries on which a person entered 2 days within a single session. On such instances, both diaries entered were considered non-adherent. Using this coding, 66 (16.6%) of the online diaries were non-adherent.
There were 307 days on which participants reported receiving lung cancer treatment, representing 30.8% of diary days. Missing dependent variable data for the daily diary was under 5%. The minimum number of next day observations per participant was 12, with a maximum of 20. There did not appear to be reactivity to the diary, as day did not predict outcomes when added as a sole predictor in the null models and variance estimates remained similar.
Social/Role Functioning
Same Day Model
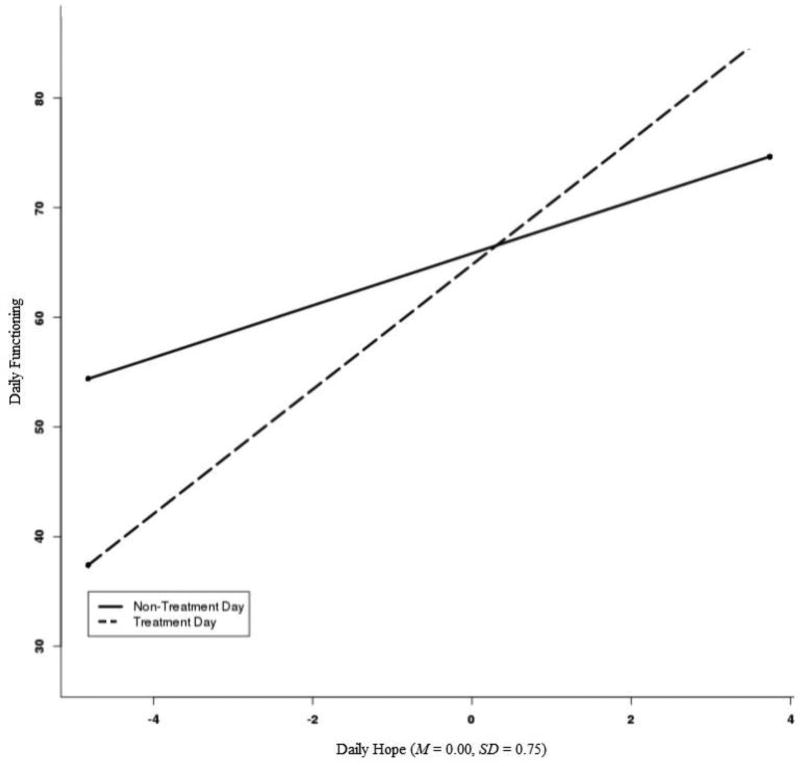
Results for variables independently associated with daily social/role functioning after adjusting for other variables appear in Table 2. Age and treatment type were not associated with daily functioning. Daily social and role functioning was associated with differences in mean levels of hope (Est. = 5.87, SE = 1.53, 95% CI = 2.78, 8.96) and physical symptoms of lung cancer (Est. = −20.79, SE = 4.40, 95% CI = −28.94, −12.64). On days that patients reported higher hope (Est. = 3.10, SE = 0.74; 95% CI = 1.66, 4.54), they reported higher functioning, whereas on days where they reported more stigma (Est. = −5.26, SE = 1.64, 95% CI = −8.48, −2.05) and more physical symptoms (Est. = −21.38, SE = 2.62, 95% CI = −26.66, −16.11), they reported lower functioning. Treatment day and daily hope interacted (Est. = 3.64, SE = 1.32, 95% CI = 1.05, 6.24; Figure 1). A region of significance test (Preacher, Curran, & Bauer, 2006) indicated that the relation between treatment day and functioning was significant at values of daily hope above 1.53 and at values of daily hope below −0.39. That is, treatment days were associated with higher social/role functioning if a patient reported daily hope approximately 2 SDs above their mean, whereas treatment days were associated with lower functioning if a patient reported daily hope half a standard deviation below their mean.4
Table 2.
Same and Next Day Models for Social/Role Functioning
| Daily Social/Role Functioning (ICC = .69) | Next Day Social/Role Functioning | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 95% CI | 95% CI | |||||||||
| Fixed Effects | Est (SE) | p | LL | UL | Est (SE) | p | LL | UL | ||
| Intercept | 64.52 | (9.18) | <.001 | 46.04 | 83.00 | 37.62 | (5.77) | <.001 | 25.78 | 49.45 |
| Day | 0.06 | (0.08) | .453 | −0.10 | 0.22 | 0.004 | (0.08) | .961 | −0.15 | 0.15 |
| Treatment day | −1.04 | (1.14) | .362 | −3.28 | 1.20 | −0.39 | (1.22) | .751 | −2.78 | 2.00 |
| Daily hope | 3.10 | (0.74) | <.001 | 1.66 | 4.54 | 1.19 | (0.69) | .084 | −0.16 | 2.54 |
| Daily stigma | −5.26 | (1.64) | .001 | −8.48 | −2.05 | −2.75 | (1.77) | .122 | −6.23 | 0.73 |
| Daily hope X treatment day | 3.64 | (1.32) | .006 | 1.05 | 6.24 | |||||
| Daily physical symptoms | −21.38 | (2.62) | <.001 | −26.66 | −16.11 | −7.36 | (2.36) | .003 | −12.09 | −2.63 |
| Daily social/role functioning | 0.41 | (0.03) | <.001 | 0.34 | 0.48 | |||||
| Average hope | 5.87 | (1.53) | <.001 | 2.78 | 8.96 | 3.58 | (0.91) | <.001 | 1.69 | 5.46 |
| Average stigma | −3.62 | (5.18) | .488 | −14.06 | 6.81 | −2.86 | (3.01) | .354 | −9.11 | 3.40 |
| Age | −0.19 | (0.24) | .433 | −0.67 | 0.29 | −0.17 | (0.13) | .234 | −0.45 | 0.12 |
| Treatment type | −5.53 | (4.36) | .211 | −14.31 | 3.24 | −3.50 | (2.55) | .184 | −8.79 | 1.79 |
| Average physical symptoms | −20.79 | (4.04) | <.001 | −28.94 | −12.64 | −11.52 | (2.44) | <.001 | −16.56 | −6.49 |
|
| ||||||||||
| Repeated | ||||||||||
|
| ||||||||||
| Variance | 154.96 | (8.27) | 139.57 | 172.05 | 190.52 | (9.92) | 172.03 | 211.00 | ||
| Correlation | 0.23 | (0.04) | 0.15 | 0.30 | −0.08 | (0.06) | −0.21 | 0.04 | ||
|
| ||||||||||
| Random Effects | ||||||||||
|
| ||||||||||
| Intercept variance | 189.51 | (43.16) | 121.28 | 296.12 | 57.85 | (20.61) | 28.78 | 116.30 | ||
| Intercept-Slope covariance | 44.30 | (37.12) | −28.45 | 117.05 | 21.29 | (18.92) | −15.79 | 58.36 | ||
| Slope | 157.59 | (61.69) | 73.17 | 339.43 | 56.50 | (32.90) | 18.05 | 176.82 | ||
Note. ICC = Intraclass correlation; Treatment type (1 = concurrent chemoradiotherapy; 0 = not in chemoradiotherapy); LL = lower limit; UL = upper limit; SE = standard error.
Figure 1. Interaction between Treatment Day and Daily Hope on Daily Social and Role Functioning.
Note. The x-axis for Hope represents the minimum and maximum values of hope observed in the data (−4.82, 3.74, respectively). A region of significance plot of the range of observed daily hope scores showed that the relation between treatment day and functioning was significant at values of daily hope above 1.53 (approximately 2 SDs above the mean of hope) and below −0.39 (approximately 0.5 SD below the mean of hope).
Next Day Model
To better understand the influence of hope and stigma on daily functioning, the same variables were added to a model that predicted next day functioning while adjusting for current day’s level of functioning. A non-significant interaction between daily hope and treatment day (p = .121) was removed. Consistent with the same day model, people with more physical symptoms (p <.001) reported lower social and role functioning, whereas people with more hope reported better functioning (p <.001; see Table 2, “Next Day Social/Role Functioning”). Daily physical symptoms predicted lower next day social/role functioning (p = .003). Daily hope trended toward higher next day functioning (p = .084).
Physical Functioning
Same Day Model
Results appear in Table 3. People who were undergoing concurrent chemoradiotherapy (Est. = −11.72, SE = 5.58, 95% CI = −22.96, −0.47) and with more physical symptoms (Est. = −33.61, SE = 5.18, 95% CI = −44.02, −23.16) reported lower physical functioning. On days people had higher hope (Est. = 3.61, SE = 0.83; 95% CI = 1.98, 5.25), they reported better physical functioning, whereas on days they reported more physical symptoms (Est. = −30.11, SE = 3.73, 95% CI = −37.63, −22.60), they reported worse physical functioning.
Table 3.
Same and Next Day Models for Physical Functioning
| Daily Physical Functioning (ICC = .64) | Next Day Physical Functioning | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| 95% CI | 95% CI | |||||||||
| Fixed Effects | Est (SE) | p | LL | UL | Est (SE) | p | LL | UL | ||
| Intercept | 87.84 | (11.75) | <.001 | 64.17 | 111.51 | 46.72 | (7.35) | <.001 | 31.70 | 61.75 |
| Day | −0.02 | (0.10) | .828 | −0.22 | 0.18 | 0.01 | (0.09) | .868 | −0.17 | 0.21 |
| Treatment day | −0.44 | (1.50) | .767 | −3.38 | 2.49 | 0.78 | (1.56) | .618 | −2.28 | 3.84 |
| Daily hope | 3.61 | (0.83) | <.001 | 1.98 | 5.25 | −1.05 | (0.86) | .224 | −2.73 | 0.64 |
| Daily stigma | −1.60 | (2.17) | .460 | −5.87 | 2.66 | −1.99 | (2.26) | .380 | −6.43 | 2.45 |
| Daily physical symptoms | −30.11 | (3.73) | <.001 | −37.63 | −22.60 | −7.81 | (2.94) | .010 | −13.72 | −1.91 |
| Physical functioning | 0.45 | (0.03) | <.001 | 0.39 | 0.52 | |||||
| Average hope | 2.73 | (1.96) | .172 | −1.23 | 6.69 | 1.66 | (1.12) | .153 | −0.67 | 3.99 |
| Average stigma | 5.17 | (6.64) | .440 | −8.21 | 18.56 | 2.10 | (3.80) | .586 | −5.78 | 9.97 |
| Age | −0.58 | (0.30) | .062 | −1.20 | 0.03 | −0.34 | (0.17) | .061 | −0.70 | 0.02 |
| Treatment type | −11.72 | (5.58) | .041 | −22.96 | −0.47 | −7.52 | (3.16) | .029 | −14.19 | −0.85 |
| Average physical symptoms | −33.61 | (5.18) | <.001 | −44.02 | −23.16 | −17.87 | (3.16) | <.001 | −24.35 | −11.40 |
|
| ||||||||||
| Repeated | ||||||||||
|
| ||||||||||
| Variance | 266.12 | (13.49) | 240.96 | 293.91 | 360.17 | (20.56) | 322.04 | 402.81 | ||
| Correlation | 0.14 | (0.04) | 0.06 | 0.22 | −0.22 | (0.05) | −0.32 | −0.11 | ||
|
| ||||||||||
| Random Effects | ||||||||||
|
| ||||||||||
| Intercept variance | 307.29 | (69.45) | 197.32 | 478.56 | 89.32 | (30.97) | 45.28 | 176.23 | ||
| Intercept-Slope covariance | −52.92 | (71.20) | −192.47 | 86.62 | −0.77 | (29.80) | −59.18 | 57.64 | ||
| Slope | 361.37 | (124.85) | 183.60 | 711.27 | 68.06 | (49.32) | 16.44 | 281.65 | ||
Note. ICC = Intraclass correlation; Treatment type (1 = concurrent chemoradiotherapy; 0 = not in chemoradiotherapy); LL = lower limit; UL = upper limit; SE = standard error.
Next Day Model
The same model used for examining same day physical functioning was used to predict next day physical functioning, while adjusting for current day physical functioning. Consistent with the same day model, people who were undergoing concurrent chemoradiotherapy (p = .029) and who had more physical symptoms (p <.001) reported lower next day functioning (Table 3, “Next Day Physical Functioning”). Daily hope was not related to next day physical functioning (p = .224). Daily physical symptoms were a significant predictor of next day functioning (p = .010).
Sensitivity Analysis
Given the variability in how diaries were completed and that participants were incentivized to complete all 21, we conducted sensitivity analysis selecting the subset of the sample that mailed batches of questionnaires weekly or completed the questionnaires online or on the phone. Multiple entries (e.g., yesterday and today’s diary) entered within a single online session were excluded as was one person who showed no within-person variability on daily assessment variables of interest in the analyses. This yielded a subset of 25 participants. Sensitivity results were consistent with main analyses (see online Supplemental Material) with the following exceptions: the interaction between treatment day and daily hope became non-significant (p =.353) and daily hope was not related to same-day physical functioning (p = .147).
Hope as a Dependent Variable
Hope was examined as a dependent variable to determine whether daily levels of hope were a function of symptoms or functioning. Depression, age, treatment type, and treatment day were co-variates. Neither daily nor average disease symptoms were related to daily hope (p’ s = .306 to .892). People with higher physical functioning did not report higher hope in same or next-day models (p’s = .163 to .263). Higher daily physical functioning was related to same day hope (Est. = 0.004, SE = 0.001, 95% CI = 0.001, 0.006), but not next day hope (p = .892). People with higher social/role functioning reported higher hope in same and next-day models (Est. = 0.05, SE = 0.01, 95% CI = 0.02, 0.07, Est. = 0.02, SE = 0.01, 95% CI = 0.01, 0.03, respectively). Higher daily social/role functioning was related to same-day hope (Est. = 0.01, SE = 0.002, 95% CI = 0.002, 0.01) and but not next-day hope (Est. = 0.003, SE =0.001, 95% CI = −0.001, 0.01).
Discussion
The goal of this study was to examine hope and stigma in relation to daily quality of life (social, role, and physical functioning) in lung cancer patients after accounting for disease and treatment factors. To our knowledge, this is the first daily assessment study of these factors in a sample predominantly composed of patients with advanced disease. Our results suggest that hope may be an important variable that provides support to social and role functioning, even after accounting for physical symptom severity. Our findings also suggest the need for continued advances in measuring lung cancer stigma and developing interventions to address it.
At the between-person level, people who reported more hope reported better social and role functioning in both same-day and next-day (longitudinal) models. Within-person increases in hope were related to higher daily physical, social, and role functioning. Although these were same-day relations, analyses accounted for third variable explanations (e.g., affect, disease symptoms), and longitudinal models indicated a trend for within-person increases in hope to predict higher social and role functioning after adjusting for the previous day’s functioning. The opposite was largely not true—although between-person increases in social and role functioning predicted higher daily hope, within-person increases were not related to hope in longitudinal models. Furthermore, physical symptoms at both the between and within-person level did not predict hope, nor did between person differences in physical functioning. Findings lend credence to the interpretation that hope supports better functioning and add to a growing literature on the importance of goals in quality of life (Goldzweig, Baider, Andritsch, Pfeffer, & Rottenberg, 2016; Goldzweig, Baider, Andritsch, & Rottenberg, 2016; Lam et al., 2016; Peh et al., 2016).
In addition to the main effects of hope, our results suggest that within-person fluctuations in hope may modify the effect of treatment days. On days that people had cancer treatment, if they reported less hope than usual, they reported lower social and role functioning, whereas they reported higher functioning if they reported hope two standard deviations above their mean. This effect was not accounted for by individual differences in expectations for treatment to cure their cancer (footnote 4). However, we did not assess whether patients were accompanied to treatment or whether they perceived greater social support on treatment days, which might help explain why treatment days could be associated with higher social functioning in particular.
Contrary to hypotheses, at the between-person level, people with higher levels of stigma did not report lower daily functioning. Within-person increases in stigma were related to lower social and role functioning. To our knowledge, no other studies have simultaneously evaluated between and within-person effects of stigma in this population. Items used to assess within-person stigma may have been more reflective of between-person differences and constrained available variance to simultaneously detect a between and within-person effect (Kraemer, Gullion, Rush, Frank, & Kupfer, 1994). The effect of within-person increases in stigma was maintained in models that adjusted for negative affect; however, this effect did not carry into the next day once the previous day’s social and role functioning was included in the model. Two of the items used to assess stigma explicitly involved a social component (e.g., “People act as though it is my fault”); the other items reflected more internalized stigma and shame. Feeling more distant from others or perceiving blame from others likely affected same-day ratings of the impact of illness on their family and social lives. Feeling shame may have decreased motivation for work or hobbies or increased self-criticism about perceived performance on these tasks, which could have affected ratings of the impact of illness on role functioning. The effect of stigma on these aspects of functioning may be strongest on the day it is experienced, but may also accumulate over time. Other designs such as event-based responding (e.g., answering questions if a person felt stigmatized) or measures of behavioral responses to stigma may better capture the effect of stigma on functioning.
Our results suggest that hope and stigma may be two appropriate intervention targets to improve daily social and role functioning in lung cancer patients. Because goal-directed effort is at the core of Snyder’s conceptualization of hope, hope lends itself to cognitive behavioral, palliative care, or supported, self-management interventions, depending on available resources and patient needs. Cognitive behavioral interventions have been developed to increase hope (Thornton, et al., 2014) and can be modified to address stigma as a barrier to goals. These interventions may help patients identify and prioritize realistic short-and long-term goals related to their social and role activities, reinforce agency for working towards goals, anticipate and effectively respond to likely barriers, including stigma, and monitor their progress. For patients without high internalized stigma (i.e., shame, guilt), stigma may only arise as a barrier to certain activities, in which case rehearsal of potential behavioral responses to it may be sufficient. For patients with high levels of shame, guilt, or perceived blame from family about lung cancer, acceptance-based approaches that teach cognitive defusion and self-compassion may be appropriate (Chambers et al., 2015).
Hope may also be addressed through palliative care interventions that focus on setting feasible, but meaningful goals to support quality of life. Redirecting patient effort toward feasible goals has been identified as a component of palliative care associated with better quality of life outcomes (Hoerger et al., 2017). However, many cancer centers do not have interdisciplinary, outpatient palliative care services (Hui, Elsayem, De La Cruz, & et al., 2010), highlighting the need for less resource intensive interventions to help patients pursue their goals. Supported, self-management may be an ideal platform for a hope-focused intervention because it is less resource intensive and utilizes goal-setting and planning to empower patients to live more effectively with disease (Grady & Gough, 2014). A supported self-management intervention could consist of meeting briefly with an interventionist to query valued social and role activities that are not being pursued, set feasible goals related to those aspects of functioning, and identify potential barriers and ways to overcome them. Stigma could be queried as a barrier to social goals, in particular, and addressed through psychoeducation and working with an interventionist on anticipating stigma experiences and generating potential responses to it. This type of intervention could be delivered by nurses, which would increase access while also positioning patients with a provider who could help them identify ways to overcome physical symptoms if they emerge as barriers to daily goals.
Limitations
Results and conclusions are limited by several factors. First, although the nested design allowed participants to serve as their own statistical control to show the influence of within-person increases or decreases in hope or stigma, these data are observational. Second, the Level-2 sample size made it difficult to detect between person differences based on lung cancer subtype, stage, or demographic variables. The majority of our sample was non-Hispanic white. Hope appears to function similarly across racial and ethnic groups (Chang & Banks, 2007), but future research should evaluate whether hope functions similarly across racial and ethnic groups in the setting of lung cancer, particularly given disparities by race, ethnicity, and socioeconomic status in its diagnosis and treatment (Williams et al., 2012). Third, for patients who completed the diaries on paper, we do not know how well they adhered to their diary reporting schedule. The daily compensation schedule, combined with encouraging patients to enter a previous day’s response prior to their current day’s if they missed a day, may have decreased adherence to the daily recall period. However, most online patients were adherent and sensitivity analyses yielded largely similar results. Fourth, assessment of reactivity to the diary was not built into the study design. It is possible that patients self-monitored (i.e., became more aware of their affect ratings or physical activity level, for example) and that this influenced their daily ratings and that response shift occurred. This concern is reduced by number of items, assessment of several domains instead of one behavior, and mixed valence of behaviors measured (e.g., staying in bed vs. “There are ways around any problem I am facing now”) (Barta, Tennen, & Litt, 2013). Patients may have missed diaries on days they felt the worst. This was addressed by asking patients to enter a missed day prior to their current day. Fifth, the sample was heterogeneous with respect to disease stage (locally advanced, advanced), therapy (chemoradiotherapy, chemotherapy), and prognosis (potentially curative, palliative).
Conclusion
Between and within-person differences in hope (i.e., goal-directed thinking) and within-person fluctuations in stigma are related to social and role functioning in patients with lung cancer, regardless of physical symptoms. Results support the use of interventions that emphasize goal-setting and address shame or guilt as barriers to social and role goals.
Supplementary Material
Acknowledgments
Laurie Steffen was supported by R25CA122061 (PI: Nancy Avis, Ph.D.).
Footnotes
Neuroticism and optimism, measured with the Big Five Personality Inventory (Benet-Martínez & John, 1998) and the Life Orientation Scale-Revised (Scheier, Carver, & Bridges, 1994), were assessed as covariates and removed from the models as they did not predict outcomes (neuroticism estimates ranged from 0.02 to 0.98, p’s = .16 to .97; optimism estimates ranged from 0.50 to 1.85, p’s = .58 to .99) and did not change the significance of predictor variables of interest. Full details are available from the authors upon request.
Although we did not require that the 7 days be consecutive, once we excluded participants without at least 7 days, all participants retained in the analysis had at least 7 consecutive days available.
Anxiety was related to lower social and role functioning (Est.same day = −1.30, SE = 0.53, p = .018; Est.next day = −0.74, SE = 0.32, p = .031). Depression was related to lower physical functioning (Est.same day = −2.21, SE = 0.70, p = .003; Est.next day = −1.32, SE = 0.41, p = .004). Daily positive affect was related to same-day social and role functioning (Est. = 4.30, SE = 0.76, p <.001) and same-day physical functioning (Est. = 4.03, SE = 1.03, p<.001). Daily negative affect was related to same-day physical functioning (Est. = −2.91, SE = 1.48, p = .049). Removing these variables from the models did not change focal predictor findings.
We conducted follow-up analysis examining patients’ expectations for cure from treatment (one item, “My expectation is that treatment will cure my cancer.”) in this model. It did not change the interaction effect observed with hope and treatment days (Est. = 3.93, SE = 1.35, p = .004) or predict functioning (Est. = −0.27, SE = 1.62, p = .870).
References
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- Barta W, Tennen H, Litt M. Measurement reactivity in diary research. In: Mehl MR, Conner TS, editors. Handbook of Research Methods for Studying Daily Life. New York, NY: Guilford Press; 2012. pp. 108–123. [Google Scholar]
- Benet-Martínez V, John OP. Los Cinco Grandes across cultures and ethnic groups: Multitrait-multimethod analyses of the Big Five in Spanish and English. Journal of Personality and Social Psychology. 1998;75(3):729–750. doi: 10.1037/0022-3514.75.3.729. [DOI] [PubMed] [Google Scholar]
- Berendes D, Keefe FJ, Somers TJ, Kothadia SM, Porter LS, Cheavens JS. Hope in the context of lung cancer: relationships of hope to symptoms and psychological distress. Journal of Pain and Symptom Management. 2010;40(2):174–182. doi: 10.1016/j.jpainsymman.2010.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boa S, Duncan EAS, Haraldsdottir E, Wyke S. Goal setting in palliative care: A structured review. Progress in Palliative Care. 2014;22(6):326–333. doi: 10.1179/1743291X14Y.0000000097. [DOI] [Google Scholar]
- Brown J, Thorpe H, Napp V, Fairlamb DJ, Gower NH, Milroy R, Peake MD. Assessment of quality of life in the supportive care setting of the big lung trial in non-small-cell lung cancer. Journal of Clinical Oncology. 2005;23(30):7417–7427. doi: 10.1200/jco.2005.09.158. [DOI] [PubMed] [Google Scholar]
- Cataldo JK, Brodsky JL. Lung cancer stigma, anxiety, depression and symptom severity. Oncology. 2013;85(1):33–40. doi: 10.1159/000350834. [DOI] [PubMed] [Google Scholar]
- Cataldo JK, Jahan TM, Pongquan VL. Lung cancer stigma, depression, and quality of life among ever and never smokers. European Journal of Oncology Nursing. 2012;16(3):264–269. doi: 10.1016/j.ejon.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo JK, Slaughter R, Jahan TM, Pongquan VL, Hwang WJ. Measuring stigma in people with lung cancer: psychometric testing of the cataldo lung cancer stigma scale. Oncology Nursing Forum. 2011;38(1):E46–54. doi: 10.1188/11.onf.e46-e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- Cella DF, Tulsky DS. Quality of life in cancer: Definition, purpose, and method of measurement. Cancer Investigation. 1993;11(3):327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- Chambers SK, Morris BA, Clutton S, Foley E, Giles L, Schofield P, Dunn J. Psychological wellness and health-related stigma: a pilot study of an acceptance-focused cognitive behavioural intervention for people with lung cancer. Eur J Cancer Care (Engl) 2015;24(1):60–70. doi: 10.1111/ecc.12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EC, Banks KH. The color and texture of hope: Some preliminary findings and implications for hope theory and counseling among diverse racial/ethnic groups. Cultural Diversity and Ethnic Minority Psychology. 2007;13(2):94–103. doi: 10.1037/1099-9809.13.2.94. [DOI] [PubMed] [Google Scholar]
- Cheavens JS, Feldman DB, Gum A, Michael ST, Snyder CR. Hope therapy in a community sample: A pilot investigation. Social Indicators Research. 2006;77(1):61–78. doi: 10.1007/s11205-005-5553-0. [DOI] [Google Scholar]
- Criswell KR, Owen JE, Thornton AA, Stanton AL. Personal responsibility, regret, and medical stigma among individuals living with lung cancer. Journal of Behavioral Medicine. 2016;39(2):241–253. doi: 10.1007/s10865-015-9686-6. [DOI] [PubMed] [Google Scholar]
- Deshields TL, Potter P, Olsen S, Liu J. The persistence of symptom burden: symptom experience and quality of life of cancer patients across one year. Supportive Care in Cancer. 2014;22(4):1089–1096. doi: 10.1007/s00520-013-2049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhotre K, Adams SA, Hebert JR, Bottai M, Heiney SP. Oncology nurses' experiences with patients who choose to discontinue cancer chemotherapy. Oncol Nurs Forum. 2016;43(5):617–623. doi: 10.1188/16.onf.617-623. [DOI] [PubMed] [Google Scholar]
- Ellis J. The impact of lung cancer on patients and carers. Chronic Respiratory Disease. 2012;9(1):39–47. doi: 10.1177/1479972311433577. [DOI] [PubMed] [Google Scholar]
- Feldman DB, Kasl-Godley J, Khouzam A, Pisca NE, Cabrera AP, Donboli M. From cure to quality of life: The shifting meaning of hope at the end of life. Oxford, England: Inter-Disciplinary Press; 2008. [Google Scholar]
- Goldzweig G, Baider L, Andritsch E, Pfeffer R, Rottenberg Y. A dialogue of depression and hope: Elderly patients diagnosed with cancer and their spousal caregivers. Journal of Cancer Education. 2016 doi: 10.1007/s13187-015-0975-0. [DOI] [PubMed] [Google Scholar]
- Grady PA, Gough LL. Self-Management: A Comprehensive Approach to Management of Chronic Conditions. American Journal of Public Health. 2014;104(8):e25–e31. doi: 10.2105/AJPH.2014.302041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Nolen-Hoeksema S, Dovidio J. How does stigma"get under the skin”? Psychological Science. 2009;20(10):1282–1289. doi: 10.1111/j.1467-9280.2009.02441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerger M, Temel JS, Jackson VA, Park ER, Pirl WF, El-Jawahri A, Greer JA. EARLY INTEGRATED PALLIATIVE CARE FOR PATIENTS WITH ADVANCED LUNG AND GASTROINTESTINAL CANCER: WHAT ARE THE KEY ELEMENTS? Paper presented at the ANNALS OF BEHAVIORAL MEDICINE 2017 [Google Scholar]
- Hui D, Elsayem A, De La Cruz M, et al. Availability and integration of palliative care at us cancer centers. JAMA. 2010;303(11):1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM. IBM SPSS Statistics for Macintosh (Version 21.0) Armonk, NY: IBM Corp; 2012. [Google Scholar]
- Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. doi: 10.1016/j.lungcan.2011.10.009. doi: http://dx.doi.org/10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Gullion CM, Rush AJ, Frank E, Kupfer DJ. Can state and trait variables be disentangled? A methodological framework for psychiatric disorders. Psychiatry Research. 1994;52(1):55–69. doi: 10.1016/0165-1781(94)90120-1. doi: http://dx.doi.org/10.1016/0165-1781(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Lam WW, Yeo W, Suen J, Ho WM, Tsang J, Soong I, Fielding R. Goal adjustment influence on psychological well-being following advanced breast cancer diagnosis. Psychooncology. 2016;25(1):58–65. doi: 10.1002/pon.3871. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Diener E. Emotion. Thousand Oaks, CA, US: Sage Publications, Inc; 1992. Promises and problems with the circumplex model of emotion; pp. 25–59. [Google Scholar]
- Maguire R, Papadopoulou C, Kotronoulas G, Simpson MF, McPhelim J, Irvine L. A systematic review of supportive care needs of people living with lung cancer. European Journal of Oncology Nursing. 2013;17(4):449–464. doi: 10.1016/j.ejon.2012.10.013. doi: https://doi.org/10.1016/j.ejon.2012.10.013. [DOI] [PubMed] [Google Scholar]
- Milbury K, Badr H, Fossella F, Pisters KM, Carmack CL. Longitudinal associations between caregiver burden and patient and spouse distress in couples coping with lung cancer. Supportive Care in Cancer. 2013;21(9):2371–2379. doi: 10.1007/s00520-013-1795-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molassiotis A, Lowe M, Blackhall F, Lorigan P. A qualitative exploration of a respiratory distress symptom cluster in lung cancer: cough, breathlessness and fatigue. Lung Cancer. 2011;71(1):94–102. doi: 10.1016/j.lungcan.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- Ouweneel E, Le Blanc PM, Schaufeli WB, van Wijhe CI. Good morning, good day: A diary study on positive emotions, hope, and work engagement. Human Relations. 2012;65(9):1129–1154. doi: 10.1177/0018726711429382. [DOI] [Google Scholar]
- Peh CX, Liu J, Bishop GD, Chan HY, Chua SM, Kua EH, Mahendran R. Emotion Regulation and Emotional Distress: The Mediating Role of Hope on Reappraisal and Anxiety/Depression in Newly Diagnosed Cancer Patients. Psychooncology. 2016 doi: 10.1002/pon.4297. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interactions in multiple linear regression, multilevel modeling, and latent curve analysis. Journal of Educational and Behavioral Statistics. 2006;31(4):437–448. doi: 10.3102/10769986031004437. [DOI] [Google Scholar]
- Pujol JL, Merel JP, Roth C. How preconceptions about lung cancer treatment interact with medical discourse for patients who accept chemotherapy? Psychooncology. 2016 doi: 10.1002/pon.4231. [DOI] [PubMed] [Google Scholar]
- Scheier MF, Carver CS, Bridges MW. Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): a reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology. 1994;67(6):1063–1078. doi: 10.1037//0022-3514.67.6.1063. [DOI] [PubMed] [Google Scholar]
- Schneider S, Stone AA. Ambulatory and diary methods can facilitate the measurement of patient-reported outcomes. Quality of Life Research. 2016;25(3):497–506. doi: 10.1007/s11136-015-1054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Temin S, Alesi ER, Abernethy AP, Balboni TA, Basch EM, Von Roenn JH. American Society of Clinical Oncology provisional clinical opinion: the integration of palliative care into standard oncology care. Journal of Clinical Oncology. 2012;30(8):880–887. doi: 10.1200/jco.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- Snyder CR. Hope theory: Rainbows in the mind. Psychological Inquiry. 2002;13(4):249–275. doi: 10.1207/S15327965PLI1304_01. [DOI] [Google Scholar]
- Snyder CR, Berg C, Woodward JT, Gum A, Rand KL, Wrobleski KK, Hackman A. Hope against the cold: individual differences in trait hope and acute pain tolerance on the cold pressor task. J Pers. 2005;73(2):287–312. doi: 10.1111/j.1467-6494.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- Snyder CR, Harris C, Anderson JR, Holleran SA, Irving LM, Sigmon ST, Harney P. The will and the ways: development and validation of an individual-differences measure of hope. Journal of Personality and Social Psychology. 1991;60(4):570–585. doi: 10.1037//0022-3514.60.4.570. [DOI] [PubMed] [Google Scholar]
- Snyder CR, Sympson SC, Ybasco FC, Borders TF, Babyak MA, Higgins RL. Development and validation of the State Hope Scale. Journal of Personality and Social Psychology. 1996;70(2):321–335. doi: 10.1037//0022-3514.70.2.321. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Danoff-burg S, Huggins ME. The first year after breast cancer diagnosis: hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11(2):93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- Steffen LE, Smith BW. The influence of between and within-person hope among emergency responders on daily affect in a stress and coping model. Journal of Research in Personality. 2013;47(6):738–747. doi: https://doi.org/10.1016/j.jrp.2013.06.008. [Google Scholar]
- Thornton LM, Cheavens JS, Heitzmann CA, Dorfman CS, Wu SM, Andersen BL. Test of mindfulness and hope components in a psychological intervention for women with cancer recurrence. Journal of Consulting and Clinical Psychology. 2014;82(6):1087–1100. doi: 10.1037/a0036959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA. The PANAS-X: Manual for the Positive and Negative Affect Schedule-Expanded Form. Ames: The University of Iowa; 1994. [Google Scholar]
- Williams DR, Kontos EZ, Viswanath K, Haas JS, Lathan CS, MacConaill LE, Ayanian JZ. Integrating multiple social statuses in health disparities research: The case of lung cancer. Health Services Research. 2012;47(3pt2):1255–1277. doi: 10.1111/j.1475-6773.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-Oncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.