Abstract
A substantial literature suggests that abnormal cortisol reactivity may be a vulnerability for deleterious mental health outcomes, including ADHD. ADHD has been linked with difficulty in emotion regulation and increased risk of experiencing stressors, both of which may be related to psychobiological abnormalities (e.g., abnormal cortisol reactivity). Research has been mixed regarding the association between cortisol reactivity and ADHD. Therefore, the present meta-analytic review (k = 12) sought to quantify this association and review the relevant methodological issues and theoretical implications of this area of research. Overall, no effect was found between cortisol reactivity and ADHD (r = 0), although significant heterogeneity in the analyses suggested that there might be moderators of this association, if one does exist. Results highlight the importance of addressing limitations of the current literature on cortisol reactivity and ADHD and exploring additional indices of emotion regulation that may be associated with ADHD. Implications for future research efforts are discussed.
Keywords: Meta-analytic review, ADHD, Cortisol reactivity, HPA axis
Introduction
Attention-deficit hyperactivity disorder (ADHD) is a common neurodevelopmental disorder characterized by age-inappropriate overactivity, impulsivity, inattention, and disorganization (American Psychiatric Association 2013). Although ADHD has often been conceptualized as a disorder of cognitive dysregulation, research has also highlighted the potential role of emotion dysregulation, as 50–70% of youth and adults with ADHD exhibit emotion regulation deficits (Shaw et al. 2016). Prior research has posited that emotions are brief and malleable biologically based reactions that result in physiological changes (Gross 1998). It follows that emotion regulation is the process by which an individual modulates internal feeling states, emotion-related physiological processes, and motivational states to accomplish individual goals (Eisenberg and Spinrad 2004).
Etiological theories of ADHD posit that the disorder reflects underactivity in the behavioral inhibition system (BIS), which is linked with both cognitive deficits related to regulating emotions and with nonspecific physiological arousal, making this system particularly relevant for the association between stress reactivity and ADHD (King et al. 1998). The BIS controls passive avoidance and inhibition of responding that is learned under threat of punishment and no reward. Importantly, individuals with ADHD display deficits in response inhibition in the face of these types of conditions (Quay 1997). Interestingly, some research has suggested that stress reactivity (i.e., the immediate response to a potentially stressful event that also involves an individual’s perception of the event), subjective distress, and physiological arousal (e.g., measured via release of cortisol) (Goldstein 1984) may be moderated by individual differences in cognitive functioning related to the BIS (Williams et al. 2009). Specifically, executive functioning, a set of cognitive processes that allows an individual to problem solve and override pre-potent behavioral and emotional response in order to achieve a goal, is considered crucial to the occurrence and management of life stress (Williams et al. 2009). These cognitive functions are often impaired in individuals with ADHD (Willcutt et al. 2005), again making it more difficult for them to respond to stress appropriately. These connections between cognitive and emotion dysregulation have been underscored in theoretical models of ADHD (Nigg and Casey 2005) and clearly highlight the potentially important role of an abnormal stress response in this disorder. Furthermore, ADHD is associated with impairments in multiple life domains (Kessler et al. 2006) that also likely place individuals with ADHD at an increased risk of experiencing stressful life events (Combs et al. 2015). Thus, there is a clear need to further delineate the role of stress and its psychobiological correlates as they relate to ADHD in children and adults.
One potential underlying mechanism connecting emotion regulation deficits to ADHD involves the functioning of the HPA axis. Specifically, research related to the physiology of emotions has often focused on the hypothalamic–pituitary–adrenal axis (HPA axis) system because it is hypothesized to be sensitive to emotion processes and can be noninvasively measured via saliva samples of cortisol, a hormonal product of this system (Stansbury and Gunnar 1994). Cortisol is primarily regulated by centers in the central nervous system, including the hypothalamus and the limbic system, which modulate and coordinate hypothalamic activity with perceptual and cognitive inputs from other brain centers (de Kloet 1991). Specifically, the hypothalamus produces corticotropin-releasing hormone (CRH) and vasopressin, which control the production of cortisol. Because cortisol also influences emotion and cognition (Stansbury and Gunnar 1994), it may be especially relevant for etiological theories of psychological conditions that involve abnormalities in emotion regulation and cognition. It is possible that emotions may even mediate the magnitude of adrenocortical response to environmental challenges (Mason 1975).
Research has traditionally focused on two main approaches to examining HPA axis activity: basal cortisol activity (resting cortisol levels to examine alterations in the natural, diurnal pattern) and cortisol response to an environmental challenge or stressor. Basal levels of cortisol follow a daily (diurnal), circadian rhythm, modulated by the limbic system according to timing of daily activities (e.g., sleeping and eating) (Weitzman et al. 1971). Given that this general pattern of cortisol activity is well established, deviations from the pattern can be examined as a possible indication of abnormal psychological functioning and/or health problems. For example, a typical research study examining basal cortisol activity includes taking samples of cortisol concentration at multiple time points throughout the day in the absence of a stressor. Specifically, one early study examining the diurnal rhythm of cortisol in children with ADHD involved collecting two saliva samples of cortisol in the morning, one in the afternoon and one in the evening, finding that those with ADHD failed to show a normal pattern (Kaneko et al. 1993). Kaneko et al. (1993) also found that those with ADHD who had moderate-to-severe hyperactivity were more similar in their cortisol rhythm than those with mild hyperactivity. While not without limitations (e.g., absolute cortisol levels were not reported), this study has been highlighted as exemplar of the typical research done on basal cortisol secretion in ADHD and related disorders (Fairchild 2012).
Additionally, because the transmission of psychological stimuli is one of the major pathways that mediate the HPA axis stress response, examination of cortisol output after a stressor occurs has also been important to understanding normal and abnormal functioning of the HPA axis (Stansbury and Gunnar 1994). Before the stress response is initiated, first, psychological stimuli reach the limbic circuits from cerebral cortex and travel to the hypothalamus (de Kloet 1991). Then, the amygdala is thought to play a major role in initiating the stress response, while the hippocampus serves to terminate this response and return the HPA axis system to its baseline, or basal regulation (de Kloet 1991; Levine and Smuts 1977). After a stressor occurs, it usually takes approximately 10–15 min to produce a rise in cortisol levels and 20–30 min for cortisol to reach peak stress concentrations in plasma (Dickerson and Kemeny 2004). It can take much longer (several hours) for the ‘‘extra’’ cortisol to be removed from circulation (Stansbury and Gunnar 1994). Therefore, in addition to important differences in theoretical interpretations of analyzing basal activity versus stress-induced activity of the HPA axis system, there are also important methodological considerations (i.e., timing of measurements) for researchers to take into account. Typically, research examining cortisol reactivity in response to a stressor utilizes either a laboratory stressor (e.g., Trier Social Stress Test), or a naturalistic stressor (e.g., intravenous needle insertion at doctor’s visit). Similar to typical research on basal cortisol activity, these studies also normally include multiple samples of cortisol concentration (one before a stressor, and at least one post-stressor), as well as comparison groups (control and/or other psychiatric conditions). However, there is vast variability among how cortisol reactivity studies have been conducted (e.g., lack of control groups, differences in length of time post-stressor a sample is taken, differences in psychiatric comorbidity and/or lack of accounting for comorbidity), so it is difficult to provide one example of how this research is generally done (Fairchild 2012).
Moreover, research suggests that emotion regulation and HPA axis activity are causally associated, such that HPA activation and regulation both influence, and are influenced by, emotions (Stansbury and Gunnar 1994). As outlined by Stansbury and Gunnar (1994), when the stress response is activated, physiological changes begin to influence central nervous system activity. At this time, an individual is more likely to be engaged in appraisal of the emotional meaning of the event and to begin to mobilize coping and emotion regulation resources (Lazarus and Folkman 1984). This process of continued emotion appraisal and regulation may alter activity in the limbic circuits involved in the stress response (Stansbury and Gunnar 1994). For example, the individual may decide the event is not threatening, decreasing the stimulation of limbic stress, and ultimately terminating the stress response. On the other hand, if the person appraises the event as more threatening, this stimulation of limbic stress would increase. Initial cortisol effects should result in increased energy and concentration, facilitation of passive avoidance, and attention to changes in the environment (Stansbury and Gunnar 1994). This in turn may lead to emotion regulation (e.g., avoidance of threatening stimuli), and an individual might employ various strategies or behaviors in order to modulate emotions in search of the most adaptive solution. Additionally, there are other, more slowly developing neuronal effects of stress that are also important for understanding emotion regulation. Specifically, slow gene-mediated effects that influence memory and the process of integrating new information may also affect how emotion regulation experiences change future responses to stress activation (de Kloet et al. 2008). Given that an abnormal stress response may lead to deficits in emotion regulation abilities and vice versa, research on these mutual and reciprocal associations has important implications for understanding psychopathology in general, and more specifically, ADHD, because of its emotion dysregulation correlates.
Taken together, ADHD is not only a disorder of cognitive dysregulation, but also largely a problem of emotion regulation. Stress reactivity is one proposed measure of the emotion regulation construct, with changes in cortisol levels (e.g., as measured in saliva) commonly used as an index of stress reactivity. The mechanisms linking ADHD to stress reactivity abnormality may involve emotion regulation deficits, which then make it difficult to modulate physiological arousal and cause dysregulation in HPA axis reactivity, including abnormalities in the release of the stress hormone, cortisol. Determining the association between cortisol reactivity and ADHD may reveal a possible avenue by which ADHD develops and/or highlight more homogenous subgroups of ADHD that will aid in clinical understanding and treatment of the disorder. Specifically, a plausible mechanism for the development of this psychopathology or for how an individual with ADHD is affected by the disorder is via alterations in the body’s stress response system. Increased recognition of the importance of patterns of emotion dysregulation, including its psychobiological correlates, may be helpful for clarifying heterogeneity in externalizing spectrum problems, such as ADHD.
Cortisol reactivity and ADHD
Cortisol reactivity is a biomarker related to multiple psychopathologies, but there exists substantial variability in findings for ADHD. Few studies have examined cortisol reactivity in adults with ADHD, but some work has found that they display specific trends in cortisol response. For example, one study demonstrated that adults with combined presentation of ADHD (i.e., both symptoms of inattention and hyperactivity–impulsivity) displayed a blunted cortisol response, whereas adults with inattentive presentation (i.e., only inattentive symptoms) trended toward higher cortisol after stress induction (Corominas-Roso et al. 2015). Other work has shown no significant differences between cortisol reactivity in individuals with ADHD versus healthy controls (Hirvikoski et al. 2009; Lackschewitz et al. 2008). In studies of children with ADHD, it has been noted that after stress, children with combined presentation ADHD display low cortisol response while those with primarily inattentive presentation display high cortisol, when compared to healthy controls (Maldonado et al. 2009; van West et al. 2009). However, similar to the adult literature, there have been inconsistencies in findings. For example, another study found that children with inattentive presentation displayed lower cortisol responses after stress induction (Pesonen et al. 2011). Additionally, major depressive disorder has been found to be associated with increased cortisol levels (Morris et al. 2012; Stewart et al. 2013; Yehuda 2002), and some work has also found that anxiety is associated with increased cortisol secretion (Greaves-Lord et al. 2007), making the examination of comorbidities in ADHD particularly relevant in this context. While few studies have examined cortisol reactivity in adults with ADHD and comorbid internalizing disorders, children with ADHD and comorbid anxiety disorders have demonstrated an increase in cortisol release in response to stress compared to children with ADHD only (Hastings et al. 2009). Interestingly, cortisol response of the inattentive ADHD presentation found in Corominas-Roso et al. (2015) resembles the response of individuals with anxiety and depression (Abelson and Curtis 1996; Morris et al. 2012; Stewart et al. 2013; Yehuda 2002). Relatedly, ADHD is also often comorbid with externalizing psychopathology, and a previous meta-analysis found no overall association between cortisol reactivity and externalizing behavior (Alink et al. 2008), again suggesting that examining comorbidities in the association between cortisol reactivity and ADHD is critical.
Overall, there appears to be some evidence for blunted cortisol reactivity in ADHD, especially in those with a combined presentation. However, the substantial variability in these findings presents a challenge for researchers aiming to build on and extend this work, and subsequently, it becomes more difficult for clinicians to apply these results in practice (e.g., assessment and treatment). Importantly, a variety of factors likely contribute to this variability. Limitations in the research to date, such as cross-sectional designs and lack of healthy control groups, make it difficult to draw causal inferences with any certainty. Additionally, potential moderators of the association between ADHD and cortisol reactivity, such as comorbidity, sex, age, measurement index of cortisol reactivity, type of stressor, and type of ADHD measure, may also contribute to the variability of findings in the current literature.
The present study
Several studies have emerged suggesting that cortisol reactivity is associated with ADHD. However, given the variability of results, differences in study design (e.g., stress paradigms and cortisol reactivity indices), and renewed interest in understanding the psychobiological correlates of ADHD, a clear research priority is the need to address the current gap in knowledge regarding the magnitude of the association between cortisol reactivity and ADHD. In the absence of this knowledge, refinement of etiological explanations, clinical assessment, and interventions is hindered. Furthermore, without quantifying this association, the most optimal methods for examining the role of stress in ADHD will remain unclear. Therefore, the purpose of the current meta-analytic review is twofold: (1) to quantify the magnitude of the overall association between cortisol reactivity and ADHD, and (2) to provide a brief overview of the methodological issues in studies of cortisol reactivity and ADHD, as well as to discuss theoretical implications and future directions for work in this area. It is hypothesized that there will be a small, or no, overall association between cortisol reactivity and ADHD, given inconsistencies in prior work.
Methods
Sample of studies
To identify studies with relevant data (i.e., journal articles and abstracts), literature searches were conducted through February 2016 using PsycINFO, MEDLINE (PubMed), and ProQuest Dissertations and Theses databases. Search terms were combined in a pairwise fashion in order to maximize the number of potentially relevant articles returned. Terms for the outcome of interest included ADHD and related constructs (e.g., attention-deficit hyperactivity disorder, attention deficit disorder, hyperkinesis, hyperkinetic disorder, inattention, impulsivity, and hyperactivity), which were all paired with each of the following stress response-related terms: cortisol reactivity, stress reactivity, cortisol responsivity, cortisol response, stress responsivity, and stress response. The search terms could appear anywhere in the text for the literature searches conduced in MEDLINE and PsycINFO, but the search terms were required to be keywords for the literature search in ProQuest Dissertation and Theses. All included studies were published in English and from peer-reviewed journals. To further identify eligible studies, backward searches were conducted from two previous relevant quantitative reviews that examined biomarkers for ADHD and cortisol basal levels and reactivity in externalizing psychopathology (Alink et al. 2008; Scassellati et al. 2012). These searches returned 977 potential data sources.
Inclusion criteria
Studies had to meet the following criteria in order to be included in the meta-analysis. First, studies were published in the English language in a peer-reviewed journal or were a component of a dissertation or thesis. Second, studies had to examine an association between cortisol reactivity and a correlate of interest (ADHD), as well as report sufficient information so that an effect size could be calculated (e.g., means and standard deviations, d-statistics, correlations). Third, studies had to include a measure of cortisol reactivity (as opposed to any other physiological indicator of stress reactivity). Cortisol reactivity was measured as an index of an individual’s physiological response to some acute stressor, and all included studies measured this via saliva samples. Fourth, studies had to include a measure of ADHD, such as a clinical interview, behavioral ratings, or both. In some cases, studies were included if they reported a history of ADHD diagnosis (it is assumed that the individual currently has ADHD, as it is a persistent disorder) made by a professional, although few studies did this (k = 1). Fifth, studies had to include both a sample of individuals with ADHD and a non-ADHD control sample. Finally, to be included, the research had to be an empirical article, dissertation, or thesis (literature reviews, meta-analyses, and case studies were not included).
The literature search yielded a total of 977 articles. Of those, 949 studies were excluded for the following reasons: (1) not a peer-reviewed, empirical article (k = 153), (2) not in English (k = 12), (3) sample was not human (e.g., animal model used) (k = 238), (4) no cortisol measure was reported (k = 116), (5) no ADHD measure was reported (k = 408), (6) no stress paradigm/no stressor reported (e.g., no acute stressor such as Trier Social Stress Test, or medical examination) (k = 14), and (7) no results reported for the association between ADHD and cortisol reactivity (k = 8). Based on the aforementioned inclusion and exclusion criteria, a total of 28 studies were retained (e.g., individual articles). After careful examination of the methods, studies were excluded if they did not provide sufficient data to calculate an effect size (k = 16). Authors of published papers were contacted if studies did not provide sufficient data to calculate an effect size in order to maximize the number of included studies. This yielded a total of 12 studies included in the current analyses.
Incomplete and nonindependent data
When authors reported descriptive statistics, such as age and sex, by diagnostic subgroup, but not for the overall sample, overall sample statistics were calculated. When studies did not report group means and standard deviations, and lacked any other appropriate statistics for effect size conversion, figures of results were used to visually approximate means and standard errors. Standard deviations were then calculated from the standard errors, so that means and standard deviations could ultimately be used for effect size calculation. In cases where effects were not reported and insufficient data were available to calculate effects, authors were contacted in an effort to obtain the necessary information. If said information could not be obtained, these studies were excluded.
In the event that a single study included multiple ADHD subsamples (e.g., ADHD-I, ADHD-C), results were averaged to create one ADHD group and therefore could be coded as belonging to the same samples to account for nonindependence stemming from comparison of multiple proband groups to the same control group (Table 1).
Table 1.
Predicted effects with 95% CI from mixed regression analyses
| k | N | r | 95% CI
|
P | ||
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Overall effect | 12 | 1041 | 0.00 | −0.36 | 0.36 | 1.00 |
CI confidence interval, r Pearson’s correlation
Coding procedures
Variables coded include the following: study year, authors, sample type (e.g., clinical vs. community), mean age, percent male, ADHD measure (e.g., Child Behavior Check List), ADHD assessment category (e.g., interview, questionnaire, multi-method, previous diagnosis), stressor paradigm (e.g., Trier Social Stress Test, dental examination, sensory challenge), stressor type (e.g., laboratory vs. naturalistic), cortisol reactivity measure (all studies used saliva), and whether or not the sample included comorbid disorders. While a moderator analysis was not conducted due to the small k of 12 and inadequate report of these variables across studies, the authors still coded for these potential moderators to illustrate these important inconsistencies that remain relevant for future researchers to consider. All included studies and corresponding variables coded (including whether or not the moderator was reported) are found in Table 2.
Table 2.
Included studies
| References | N | ES | %male | Age | Sample type | Assessment | Presentation | Comorbidity | Stressor |
|---|---|---|---|---|---|---|---|---|---|
| Blomqvist et al. (2007) | 89 | −0.07 | 70 | 13 | Community | Questionnaire | Yes (HY) | No | Naturalistic |
| Corominas-Roso et al. (2015) | 121 | 0.17 | 56 | 35.51 | Clinical | Multi-method | Yes (IN/C) | No | Laboratory/ST |
| Hirvikoski et al. (2009) | 56 | 0.13 | 68 | 33.32 | Clinical | Multi-method | No | No | Laboratory/cognitive |
| Lackschewitz et al. (2008) | 36 | 0.40 | 44 | 35.97 | Community | Multi-method | No | Depression | Laboratory/ST |
| Lane et al. (2010) | 54 | −0.07 | 56 | 8.48 | Community | History | No | No | Laboratory/SC challenge |
| Maldonado et al. (2009) | 66 | −0.02 | 52 | 6.33 | Community | Multi-method | Yes (IN/HY/C) | No | Laboratory/ST |
| McCarthy et al. (2011) | 368 | −0.06 | 50 | 7.08 | Community | Questionnaire | No | No | Naturalistic |
| Randazzo et al. (2008) | 32 | 0.17 | 51 | 10.78 | Community | Multi-method | No | No | Laboratory/ST |
| Raz and Leykin (2015) | 49 | 0.31 | 29 | 26.62 | Community | Multi-method | No | No | Laboratory/math ability |
| Snoek et al. (2004) | 48 | 0.15 | 81 | 10.07 | Clinical | Interview | No | Anxiety | Laboratory/ST |
| van de Wiel et al. (2004) | 22 | −0.17 | 100 | 10.33 | Clinical | Multi-method | No | ODD/CD | Laboratory/ST |
| van West et al. (2009) | 100 | −0.89 | 83 | 8.64 | Clinical | Multi-method | Yes (IN/C) | No | Laboratory/ST |
ES = Effect size. Sex was coded as percent of the sample that was male. Presentation indicates if the study reported results separately by ADHD presentation. Only four out of the twelve studies did this. HY = hyperactivity–impulsivity. IN = inattention. C = combined. Comorbidity indicates if the study reported inclusion of comorbid disorders in the sample. Only three studies did this. ODD = oppositional defiant disorder. CD = conduct disorder. Stressor indicates the type and general description of stressor used to elicit stress response. ST = social threat. SC = sensory challenge. The two naturalist studies listed involved medical procedures (i.e., dental examination and catheter insertion)
Meta-analytic strategy
Effect size calculation
For each study, an effect size d (Cohen 1988) was calculated for the association between cortisol reactivity and ADHD (derived from the following: mean differences and standard deviations, standardized mean differences, and f-statistics and associated Ns and p values) and this was converted to r for reporting of results. Standardized mean differences were converted to a correlation based on procedures by Lipsey and Wilson (2001). A positive r statistic was obtained when the ADHD group had a higher mean difference of cortisol reactivity compared to controls, while a negative r statistic reflected a blunted cortisol response in the ADHD group compared to controls. Cohen’s (1988) guidelines were used to interpret effect sizes such that effects sizes of rs = 0.10 were considered ‘‘small,’’ rs = 0.24 were considered ‘‘moderate,’’ and rs = 0.37 were considered ‘‘large.’’ Again, in cases where an article did no report sufficient statistics, efforts were made to contact the corresponding author to obtain the necessary information.
Regression analyses
Mixed-effect regression analyses were employed to estimate meta-analytic effects. Analyses were conducted using the metafor package in R (Viechtbauer 2010). Ultimately, for the purposes of conducting the mixed-effect regression analyses, ds were converted to the effect size, r (Pearson’s correlation), as indicated in the R programming script used. Average effect sizes were calculated using the rma function, which fits meta-analytic random and mixed effects models, and the method was specified to be DerSimonian – Laird (1986). Further, this function provides an estimate of the effect size, as well as the 95% CI, while the predict function provides an 80% credibility interval for the overall estimate of the effect size. Reported estimates of heterogeneity include Q, a statistical test of whether between-study variance is greater than within study variance, and I2, the ratio of true heterogeneity to total variation in the observed effects. Large I2 values (range 0–100) indicated that the observed heterogeneity was not entirely attributable to sampling error and may be systematically explained by moderating variables. Specifically, a test for residual heterogeneity was conducted by the rma function, resulting in a Q-statistic (Cochran 1954). A significant test suggests the true outcomes are heterogeneous, warranting the examination of moderators. Despite this, the limited number of studies and inconsistently reported variables made it difficult to examine moderators with any meaningful interpretability. Specifically, age and sex were the only variables consistently reported across the 12 studies; however, even these were not retained for a moderator analysis because without a full distribution of age and sex, interpreting such results would be limited and unclear.
Publication bias
The potential impact of publication bias, reflecting the issue of possible ‘‘missing’’ studies, was evaluated via visual inspection of a funnel plot of observed effects against associated sampling variances. Publication bias may lead to asymmetrical funnel plots, though asymmetrical funnel plots should not be equated with publication bias (Rothstein et al. 2006). To test and adjust for publication bias, the trim and fill funnel-plot-based method (Duval and Tweedie 2000) was used for the overall random effects analyses. The basis of this method is to trim or remove the smaller studies that may be contributing to funnel plot asymmetry, use the trimmed funnel plot to estimate the ‘‘true’’ center of the funnel (i.e., the true effect size), and then replace the omitted studies and missing studies around the adjusted center. Additionally, a test of funnel plot asymmetry was conducted using Egger’s test of the intercept (Egger et al. 1997).
Outlier analysis
In order to manage potential outliers in the data, several steps were taken, including examination of standardized residuals and visual inspection of forest plots. First, the Student’s t test was used in which standardized residuals for each study were calculated according to a normal t-distribution (mean of zero, standard deviation of one). Studies were considered outliers if they had a standardized residual with an absolute value greater than three. Second, the leave-one-out analysis was used to further determine if outliers were present in the data. This method repeatedly fits the specified model, leaving one study out at a time. The potential impact of each study on the overall effect size can be examined visually through generating a forest plot. If an effect size deviates largely from the other effects when a specific study is left out, an outlier is detected. Finally, visual inspection of a forest plot sorted by the effect sizes and associated CI of studies can help detect outliers. Here, an outlier is detected if any study has a CI that does not overlap with CI from other studies.
Results
Overall effects
Cortisol reactivity and ADHD
A summary of effect size estimates, 95% CI, and associated p values for both the overall effect are presented in Table 1. Current analyses examined 12 studies that met inclusion criteria. Analyses examining the association between cortisol reactivity and ADHD revealed no overall meta-analytic effect (r = 0.00, 95% CI −0.36 to 0.36, p = 0.10), indicating no association between cortisol reactivity and ADHD. However, results revealed there was significant heterogeneity [Q (11) = 617.18, p < 0.0001, and τ2: 0.38 (SE = 0.30), I2 = 98.22%], suggesting there may still be important moderators to explore, although present limitations of the literature precluded such an analysis (Fig. 1).
Fig. 1.
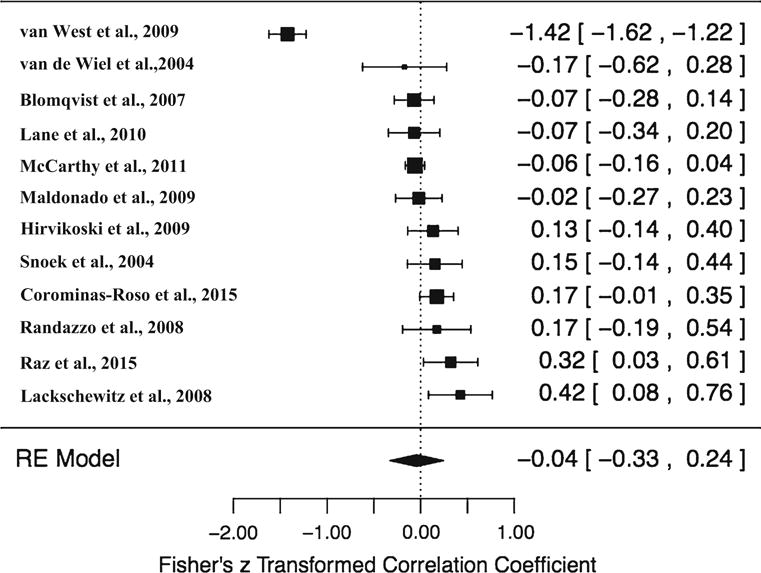
Forest plot of included studies. Note: This forest plot was derived from running a meta-regression with Fisher’s z transformed correlation coefficient so that z’s could be used for publication bias analyses. Studies sorted by effect size
Publication bias
To test and adjust for publication bias, two methods were used. First, this bias was examined with Egger’s regression test of funnel plot asymmetry, which tests for an association between effect size and standard error by regressing the standard normal deviate on the inverse of the standard error (Egger et al. 1997; Moreno et al. 2009). However, when sample size decreases and effect sizes are small, the regression method may inflate type I error rate (Sterne and Egger 2005). Results indicated that this test did not reveal significant asymmetry (z = 1.67, p = 0.10), suggesting no publication bias based on Egger’s test. Second, the Duval and Tweedie (2000) trim and fill funnel-plot-based method was used for the overall random effects analyses. Visual inspection of a funnel plot of observed effects by sampling variances reveals that effects are asymmetrically distributed (see Fig. 2). Results of this publication bias test indicated an estimated total of 5 studies missing on the left side of the distribution and that there may be significant publication bias [τ2: 0.24, I2: 94.39%; Q (16) = 285.18, p < 0.0001]. However, model results yielded an estimate of −0.24, with a 95% CI of −0.49 to 0.00, demonstrating there is not significant publication bias based on this test.
Fig. 2.
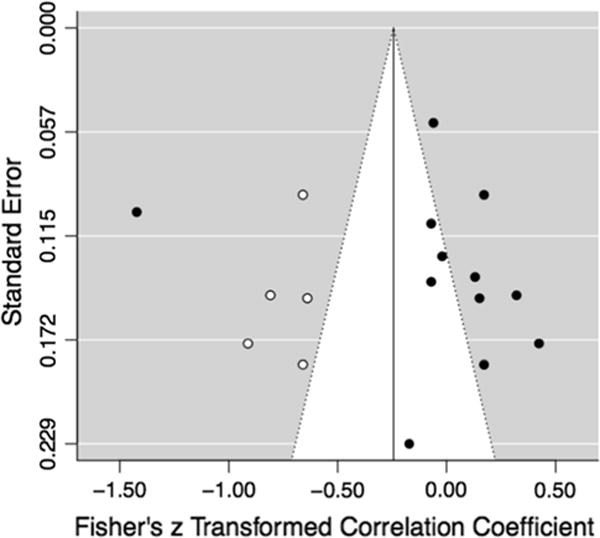
Funnel plot after correcting for publication bias. Note: Trim and fill test for publication bias indicated 5 studies missing from the left side. White dots indicate correction/inclusion of those missing studies
Outlier analysis
Three analyses were conducted to determine the presence of outliers. Visual inspection of the forest plot (see Fig. 1) sorted by effect size illustrated one potential outlier (van West et al. 2009) given that its CI was not close to overlapping with the CI of the remaining studies. Additionally, its standardized residual was greater than the absolute value of three and further suggests it may be an outlier. However, the follow-up leave-one-out analysis (see Fig. 3) illustrated that leaving this study out does not significantly change the overall effect size, and it therefore may not be a true outlier. Further, based on visual inspection of this leave-one-out forest plot, no outliers were detected, as there appeared to be no large deviations among effect sizes after omitting each study.
Fig. 3.
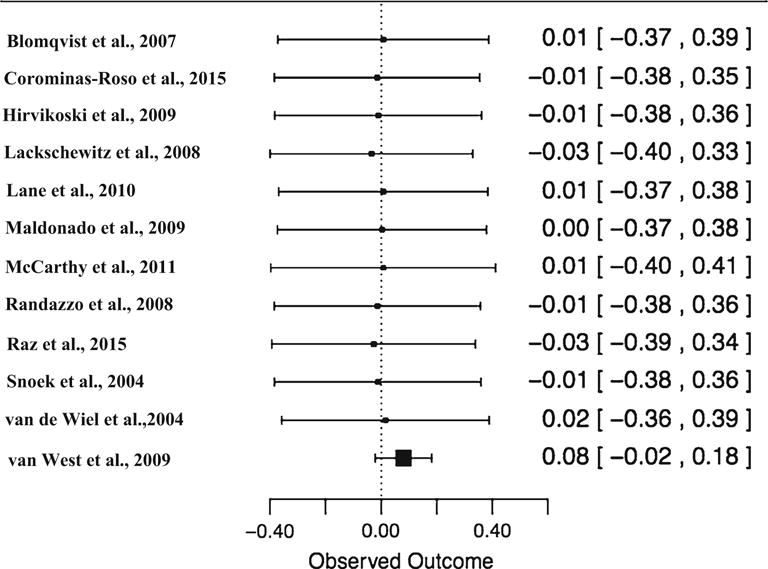
Forest plot: leave-one-out analysis. Note: Student’s t test indicated study 12 as a potential outlier, however the leave-one-out analysis indicated that when omitting study 12, and the overall effect size estimate does not result in a significant deviation
Discussion
The current study aimed to quantify the association between cortisol reactivity and ADHD. Consequently, the overall magnitude of this association was determined. Overall, it appears that there is no significant association between ADHD and cortisol reactivity. Even if the overall effect size for this association is 0.00, there may still be moderators such that cortisol reactivity and ADHD are linked positively understand some conditions and negatively under others. Notably, there was significant heterogeneity in the overall effect, indicating that approximately 98% of the variability has yet to be explained. Importantly, the small number of studies included limits this analysis and prevents a meaningful moderator analysis from being conducted. It is possible that with a larger k, at least age and sex would emerge as significant moderators, which would point to important developmental considerations in understanding the association between cortisol reactivity and ADHD.
The result of no overall effect may not be that inconsistent with the current literature. Specifically, while no previous meta-analyses have focused particularly on the association of cortisol reactivity and ADHD, the present findings are consistent with one prior meta-analysis, which included only two studies of ADHD, finding no significant association between cortisol reactivity and externalizing behavior (Alink et al. 2008). Further, the previous study found that this association was only significant in small subsets of studies that assessed disruptive behavior disorders and behaviors such as disinhibition and anger. Research on HPA axis functioning and externalizing psychopathology more broadly has suggested that children with high levels of externalizing behavior may be less sensitive to stress and less physiologically aroused (Raine 1996). Compared to individuals without externalizing psychopathology, this can result both in differences in cortisol response, as an index of physiological response to a stressor, and increased engagement in events that result in stress (e.g., engage in more outgoing, externalizing behavior, such as fighting) (Raine 1996). It may be then that more severe externalizing behaviors, such as those typical of disruptive behavior disorders, drive any observed association between cortisol reactivity and externalizing psychopathology, including ADHD. Of course, it is possible that there is still a significant association between cortisol reactivity and ADHD specifically, given that prior work has found some significant differences between those with ADHD and healthy controls. Importantly, because individuals with ADHD exhibit substantial heterogeneity in symptom presentation as well as comorbidity (e.g., externalizing vs. internalizing), and because the studies included in the present meta-analysis were limited in number and did not often differentiate among symptom presentations, it may not be entirely surprising that the overall effect based on this analysis was null. For example, it is not clear if the ADHD phenotype, as captured by these analyses, even includes those severe, externalizing behaviors (or internalizing psychopathology) that may be most relevant to this association. Furthermore, as previously noted, prior research has documented different patterns in this association based on primarily inattention symptoms versus hyperactivity–impulsivity symptoms, as well as depending upon presence of internalizing versus externalizing comorbidity. Again, the present analyses were not able to disentangle these possibilities that may be crucial to appropriately quantifying this association.
Implications and future directions
Methodological implications
Given the apparent null effect, it is important to consider the methodological implications of the present work. First, the literature to date on cortisol reactivity lacks in precision in measurement of cortisol, standardization of stressors/paradigms used (including systematic collection of samples pre- and post-stressor), and report of appropriate statistics for effect size conversion. Therefore, future work should aim to address these issues, for example, by conducting studies of reliability and validity of cortisol measures and stressors. As previously discussed, cortisol reactivity is considered a reliable and valid indicator HPA axis fluctuation. However, it is less clear how well HPA axis activity indexes emotion regulation, especially in specific disorders. In order to establish cortisol reactivity as a reliable and valid indication of emotion regulation, it would be important to more closely examine how cortisol reactivity as elicited by the types of tasks traditionally used (e.g., Trier Social Stress Test), as well as how it is associated with other measures of emotion regulation, including self-and informant report. For example, if researchers find that emotion regulation fluctuates as a result of task performance, and if this similarly corresponds with HPA axis activity as indexed by cortisol reactivity, then there would be more support for examining cortisol reactivity as a valid indicator of emotion regulation. It would also be useful for future work to conduct studies examining the stability of cortisol reactivity over time, which could help in understanding if a stressor reliably produces HPA axis changes as indexed by cortisol reactivity. In line with these suggestions, research may benefit from enhancing the quantitative models used to analyze questions of the association of cortisol reactivity and emotion regulation. Specifically, mediation analysis could serve as a more mechanistic approach, which has the potential to highlight if cortisol reactivity is indeed an indicator of emotion regulation, by statistically testing for both direct and indirect effects of a stressor on emotion regulation deficits via cortisol reactivity (or effects of stressor on cortisol reactivity via emotion regulation).
Second, it may also be important for future research to consider factors that impact the between and within-group heterogeneity in stress responsivity. It is well known that not everyone who experiences stress goes on to experience psychopathology, but at this time, it is unclear if, and how, the stress response differs within individuals across time and between individuals. Individuals may vary in stress sensitivity, such that some individuals require less stress compared to others in order to exhibit a certain stress response, which may also be associated with some forms of psychopathology. To understand why individuals vary in stress sensitivity, research should focus on potential mediators and moderators in the association of stressor and response to stressor. For example, researchers could examine how certain genetic variants or personality traits affect cortisol reactivity following a stressor. Moreover, in order for readers to draw comparisons across studies, researchers should use standardized protocols, including well-established stressors, such as the Trier Social Stress Test, take cortisol samples prior to the stressor and 10–15 min post-stressor (to test initial rise in cortisol levels) and 20–30 min post-stressor (to test peak stress levels), and provide details regarding sample descriptive (mean age of sample, percent male in sample, etc.).
Finally, there are multiple aspects of cortisol reactivity to consider that go beyond the traditional measure of change in cortisol levels from pre-to post-stressor. For example, it is possible that examining the amount of time it takes for an individual’s cortisol level to return to baseline after a stressor may better differentiate individuals with ADHD from those without ADHD. Also, as previously stated, in addition to initial neuronal effects of the stress response, slow gene-mediated neuronal effects also alter emotion regulation abilities. Therefore, gathering data at multiple time points, including collecting a cortisol sample after the individual has left the laboratory, may provide better insight into how stress responsivity relates to ADHD. Future directions may include quantifying the association of cortisol reactivity and ADHD based on multiple indices of the cortisol response.
Theoretical implications
The current findings suggest that cortisol reactivity is not associated with ADHD, and it remains unclear what might explain the significant heterogeneity that continued to emerge in the analyses. Importantly, if there is truly no association between cortisol reactivity and ADHD, despite substantial emotion regulation deficits and behavioral difficulties in the face of stress in individuals with ADHD, it may be necessary to consider alternative indices for examining emotion regulation and stress. Specifically, cortisol reactivity may not be the optimal means by which to index emotion regulation deficits because of the continued inconsistences in the cortisol reactivity literature as it relates to both externalizing (Alink et al. 2008) and internalizing psychopathology (Lopez-Duran et al. 2009). Moreover, it may be that cortisol reactivity in response to an isolated stressor reflects larger system-level functioning of HPA axis and neurobiological systems involved in emotion regulation. Future directions may include examining other psychobiological correlates (e.g., skin conductance response, heart rate variability) that are more easily and systematically measured and that can more closely map onto neurobiological systems involved in emotion regulation.
On the other hand, given that the heterogeneity in the present analyses suggests that if there is an association between ADHD and cortisol reactivity, it is not the same across all individuals with ADHD and future work continuing to examine this association may benefit from investigating additional comorbid symptoms. It may be that combining effect sizes across studies that include individuals with ADHD and co-occurring externalizing and internalizing disorders has lead to this canceling out effect demonstrated in this meta-analysis, resulting in no overall effect size. In future work, it may be fruitful to examine moderators that tap into the externalizing behavioral aspects of ADHD, especially in light of results from a previous meta-analysis which suggested there is no association between cortisol reactivity and externalizing disorders (Alink et al. 2008). Specifically, examining impulsivity or deficits in inhibition may be especially relevant in linking ADHD to externalizing problems and therefore should be included and reported on in future work. Moreover, given that externalizing problems, like ADHD, represent a heterogeneous set of behaviors, it may be important to unpack the specific behaviors in terms of whether they are proactive or reactive (Alink et al. 2008). At any rate, despite inconsistencies even within examining of externalizing psychopathology, it does seem that including such aspects of ADHD in future work would at least aid in clarifying a potential association, or even provide an explanation for why association may be found in those with internalizing problems but not externalizing, further highlighting how an overall effect could get suppressed.
Additionally, individuals with ADHD often have comorbid internalizing disorders (e.g., anxiety, depression); however, the vast majority of empirical and theoretical work has focused on externalizing comorbidity (Jacob et al. 2014; Tremblay 2000), so it is even less well understood how this may alter the association between the disorder and cortisol reactivity. A relatively underexplored possibility is that sluggish cognitive tempo (SCT) symptoms (e.g., daydreaming, mind-wandering, slow to process information, easily bored) link ADHD symptoms to emotion regulation problems. Emerging evidence of strong associations between ADHD and SCT (co-occurring in nearly 50% of cases for children and adults) (Barkley 2012, 2013) supports this future direction proposal. Like ADHD, SCT is also associated with emotion regulation deficits (Flannery et al. 2014). Specifically, SCT has been found to explain variance in predicting emotion self-regulation deficits (44.5%) over and above that explained by ADHD–inattention (1.4%) or ADHD–hyperactivity–impulsivity (7.7%) (Barkley 2012), and SCT has been significantly associated with poor emotional control, even after controlling for ADHD–inattention and hyperactivity– impulsivity (Jimenez et al. 2013). While it appears possible that cortisol reactivity may not be the most optimal measure of emotion regulation, it is possible that if it is a valid and reliable measure, those individuals with ADHD and SCT symptoms may have a distinct pattern of cortisol response to stress compared to individuals without SCT symptoms, potentially explaining some of the heterogeneity found in this study.
Limitations
There are some limitations to note of the present study. First, the current study included only 12 studies, making it difficult to confidently conclude there is a null overall effect, and making it impossible to analyze potentially important moderators (e.g., symptom presentation, comorbidity). Moreover, the inconsistency in reporting of moderators across those 12 studies broadly limits this area of research as there may be theoretically relevant processes (i.e., comorbidity, age, sex) that need to be considered along with methodological consistency issues (i.e., type of stressor paradigm used, length of time between pre- and post-collection of cortisol) in order to determine this association. Second, this speaks to an overall limitation of the literature to date on cortisol reactivity and ADHD. Specifically, although only 28 studies met initial inclusion, 16 of these were excluded because they did not report appropriate statistics for effect size conversion (e.g., pre-and post-stressor cortisol level means and standard deviations). Further, a few of the final 12 studies retained for analyses only included a figure of results with no corresponding means and standard deviations. For these studies, the author and another rater familiar with research procedures had to measure and calculate means and standard deviations from the figures. While this method of visual approximation allowed for calculation of effect sizes, it does not preclude the possibility of manual measurement error. Thus, it will be important for future work to include statistics from which effect sizes can be calculated and to include figures with the corresponding values for the calculation to occur. Finally, many studies in this area of the literature in general do not report results for subgroups or presentations of ADHD, making it difficult to examine how cortisol reactivity differs for individuals with primarily inattention versus hyperactivity–impulsivity. For example, the substantial heterogeneity in symptom presentation in ADHD makes it difficult to truly disentangle if there is an association between cortisol reactivity and ADHD. Therefore, future research could also benefit from reporting results for different subgroups of ADHD, or for symptoms dimensionally, so that it will be possible to examine potential differences in the association based on presence of inattention versus hyperactivity–impulsivity symptoms.
Conclusion
Previous work supports the notion that abnormal cortisol reactivity may be a vulnerability for physical and mental health impairments, including ADHD. However, research has been mixed regarding the role of stress in ADHD, as well as if there is an association between cortisol reactivity and this disorder. Meta-analysis results of the 12 included studies yielded no overall association between cortisol reactivity and ADHD. While this study included a small number of studies, results also revealed significant heterogeneity in effects, indicating that methodological and theoretical factors are likely both influencing differences in effects across studies. Therefore, it appears there are several priorities for future research in this area: (1) more careful accounting and examination of effects based on comorbidity patterns, (2) increased documentation of potentially important moderators (e.g., age, sex, stressor type), (3) more precise calibration of stressor and cortisol measurement approach, and (4) increased work to support reliability and validity of cortisol as an index of emotion regulation, and/or exploration of additional indices of emotion regulation that may be better suited. Finally, in light of emerging evidence that SCT symptoms, which often co-occur in those with ADHD, are associated with emotion regulation deficits as well; it seems plausible that SCT symptoms may be of relevance in untangling associations between ADHD and emotion regulation, as indexed by, but not limited to, cortisol reactivity.
Footnotes
Compliance with ethical standards
Conflict of interest The authors (omitted for blind review) report no potential conflicts of interest.
Human participation in research The authors report no human participation in research as this was a meta-analytic review research project.
Informed consent No informed consent was necessary (see point above).
References
- Abelson JL, Curtis GC. Hypothalamic–pituitary–adrenal axis activity in panic disorder: 24-hour secretion of corticotropin and cortisol. Arch Gen Psychiatry. 1996;53(4):323–331. doi: 10.1001/archpsyc.1996.01830040059010. [DOI] [PubMed] [Google Scholar]
- Alink LR, van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J, Juffer F, Koot HM. Cortisol and externalizing behavior in children and adolescents: mixed meta-analytic evidence for the inverse relation of basal cortisol and cortisol reactivity with externalizing behavior. Dev Psychobiol. 2008;50(5):427–450. doi: 10.1002/dev.20300. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [Google Scholar]
- Barkley RA. Distinguishing sluggish cognitive tempo from attention-deficit/hyperactivity disorder in adults. J Abnorm Psychol. 2012;121(4):978–990. doi: 10.1037/a0023961. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Distinguishing sluggish cognitive tempo from ADHD in children and adolescents: executive functioning, impairment, and comorbidity. J Clin Child Adolesc Psychol. 2013;42(2):161–173. doi: 10.1080/15374416.2012.734259. [DOI] [PubMed] [Google Scholar]
- Blomqvist M, Holmberg K, Lindblad F, Fernell E, Ek U, Dahllof G. Salivary cortisol levels and dental anxiety in children with attention deficit hyperactivity disorder. Eur J Oral Sci. 2007;115(1):1–6. doi: 10.1111/j.1600-0722.2007.00423.x. [DOI] [PubMed] [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Erlbaum, Hillsdale; 1988. n L. [Google Scholar]
- Combs MA, Canu WH, Broman-Fulks JJ, Rocheleau CA, Nieman DC. Perceived stress and ADHD symptoms in adults. J Atten Disord. 2015;19(5):425–434. doi: 10.1177/1087054712459558. [DOI] [PubMed] [Google Scholar]
- Corominas-Roso M, Palomar G, Ferrer R, Real A, Nogueira M, Corrales M, et al. Cortisol response to stress in adults with attention deficit hyperactivity disorder. Int J Neuropsychopharmacol. 2015;18(9):pyv027. doi: 10.1093/ijnp/pyv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Kloet ER. Brain corticosteroid receptor balance and homeostatic control. Front Neuroendocrinol. 1991;12(2):95–164. [Google Scholar]
- de Kloet ER, Karst H, Joels M. Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol. 2008;29(2):268–272. doi: 10.1016/j.yfrne.2007.10.002. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Spinrad TL. Emotion-related regulation: sharpening the definition. Child Dev. 2004;75(2):334–339. doi: 10.1111/j.1467-8624.2004.00674.x. [DOI] [PubMed] [Google Scholar]
- Fairchild G. Hypothalamic–pituitary–adrenocortical axis function in attention-deficit hyperactivity disorder. Curr Top Behav Neurosci. 2012;9:93–111. doi: 10.1007/7854_2010_101. [DOI] [PubMed] [Google Scholar]
- Flannery AJ, Becker SP, Luebbe AM. Does emotion dysregulation mediate the association between sluggish cognitive tempo and college students’ social impairment? J Atten Disord. 2014 doi: 10.1177/1087054714527794. [DOI] [PubMed] [Google Scholar]
- Goldstein L. In: Stress, appraisal, and coping. Lazarus RS, Folkman S, editors. Springer; New York: 1984. [Google Scholar]
- Greaves-Lord K, Ferdinand RF, Oldehinkel AJ, Sondeijker FE, Ormel J, Verhulst FC. Higher cortisol awakening response in young adolescents with persistent anxiety problems. Acta Psychiatr Scand. 2007;116(2):137–144. doi: 10.1111/j.1600-0447.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: an integrative review. Rev Gen Psychol. 1998;2(3):271. [Google Scholar]
- Hastings PD, Fortier I, Utendale WT, Simard LR, Robaey P. Adrenocortical functioning in boys with attention-deficit/hyperactivity disorder: examining subtypes of ADHD and associated comorbid conditions. J Abnorm Child Psychol. 2009;37(4):565–578. doi: 10.1007/s10802-008-9292-y. [DOI] [PubMed] [Google Scholar]
- Hirvikoski T, Lindholm T, Nordenström A, Nordström A-L, Lajic S. High self-perceived stress and many stressors, but normal diurnal cortisol rhythm, in adults with ADHD (attention-deficit/hyperactivity disorder) Horm Behav. 2009;55(3):418–424. doi: 10.1016/j.yhbeh.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Jacob C, Gross-Lesch S, Jans T, Geissler J, Reif A, Dempfle A, Lesch KP. Internalizing and externalizing behavior in adult ADHD. Atten Deficit Hyperact Disord. 2014;6(2):101–110. doi: 10.1007/s12402-014-0128-z. [DOI] [PubMed] [Google Scholar]
- Jimenez EAA, Ballabriga MCJ, Martin AB, Arrufat FJ, Giacobo RS. Executive functioning in children and adolscents with symptoms of sluggish cognitive tempo and ADHD. J Atten Disord. 2013;19(6):507–514. doi: 10.1177/1087054713495442. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Hoshino Y, Hashimoto S, Okano T, Kumashiro H. Hypothalamic–pituitary–adrenal axis function in children with attention-deficit hyperactivity disorder. J Autism Dev Disord. 1993;23(1):59–65. doi: 10.1007/BF01066418. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, et al. The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry. 2006;163(4):716–723. doi: 10.1176/appi.ajp.163.4.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Barkley RA, Barrett S. Attention-deficit hyperactivity disorder and the stress response. Biol Psychiatry. 1998;44(1):72–74. doi: 10.1016/S0006-3223(97)00507-6. [DOI] [PubMed] [Google Scholar]
- Lackschewitz H, Hüther G, Kröner-Herwig B. Physiological and psychological stress responses in adults with attention-deficit/hyperactivity disorder (ADHD) Psychoneuroendocrinology. 2008;33(5):612–624. doi: 10.1016/j.psyneuen.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Lane SJ, Reynolds S, Thacker L. Sensory over-responsivity and ADHD: differentiating using electrodermal responses, cortisol, and anxiety. Front Integr Neurosci. 2010 doi: 10.3389/fnint.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, appraisal, and coping. Springer; New York: 1984. [Google Scholar]
- Levine S, Smuts B. Limbic system regulation of ACTH. Acta Physiol Pol. 1977;28(15):93. [PubMed] [Google Scholar]
- Lipsey MW, Wilson D. Practical meta-analysis. Sage Publications Inc; Thousand Oaks: 2001. [Google Scholar]
- Lopez-Duran NL, Kovacs M, George CJ. Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: a meta-analysis. Psychoneuroendocrinology. 2009;34(9):1272–1283. doi: 10.1016/j.psyneuen.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado EF, Trianes MV, Cortés A, Moreno E, Escobar M. Salivary cortisol response to a psychosocial stressor on children diagnosed with attention-deficit/hyperactivity disorder: differences between diagnostic subtypes. Span J Psychol. 2009;12(02):707–714. doi: 10.1017/s1138741600002079. [DOI] [PubMed] [Google Scholar]
- Mason JW. A historical view of the stress field. J Hum Stress. 1975;1(2):22–36. doi: 10.1080/0097840X.1975.9940405. [DOI] [PubMed] [Google Scholar]
- McCarthy AM, Hanrahan K, Scott LM, Zemblidge N, Kleiber C, Zimmerman MB. Salivary cortisol responsivity to an intravenous catheter insertion in children with attention-deficit/hyperactivity disorder. J Pediatr Psychol. 2011;36(8):902–910. doi: 10.1093/jpepsy/jsr012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno SG, Sutton AJ, Ades AE, Stanley TD, Abrams KR, Peters JL, Cooper NJ. Assessment of regression-based methods to adjust for publication bias through a comprehensive simulation study. BMC Med Res Methodol. 2009;9(1):2. doi: 10.1186/1471-2288-9-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MC, Rao U, Garber J. Cortisol responses to psychosocial stress predict depression trajectories: social-evaluative threat and prior depressive episodes as moderators. J Affect Disord. 2012;143(1):223–230. doi: 10.1016/j.jad.2012.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- Pesonen A-K, Kajantie E, Jones A, Pyhälä R, Lahti J, Heinonen K, et al. Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. J Psychiatr Res. 2011;45(11):1471–1476. doi: 10.1016/j.jpsychires.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Quay HC. Inhibition and attention deficit hyperactivity disorder. J Abnorm Child Psychol. 1997;25(1):7–13. doi: 10.1023/a:1025799122529. [DOI] [PubMed] [Google Scholar]
- Raine A. Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior. Biosocial perspectives and treatment implications. Ann N Y Acad Sci. 1996;794:46–59. doi: 10.1111/j.1749-6632.1996.tb32508.x. [DOI] [PubMed] [Google Scholar]
- Randazzo WT, Dockray S, Susman EJ. The stress response in adolescents with inattentive type ADHD symptoms. Child Psychiatry Hum Dev. 2008;39(1):27–38. doi: 10.1007/s10578-007-0068-3. [DOI] [PubMed] [Google Scholar]
- Raz S, Leykin D. Psychological and cortisol reactivity to experimentally induced stress in adults with ADHD. Psychoneuroendocrinology. 2015;60:7–17. doi: 10.1016/j.psyneuen.2015.05.008. [DOI] [PubMed] [Google Scholar]
- Rothstein HR, Sutton AJ, Borenstein M, editors. Publication bias in meta-analysis: Prevention, assessment and adjustments. Wiley; England: 2006. [Google Scholar]
- Scassellati C, Bonvicini C, Faraone SV, Gennarelli M. Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatry. 2012;51(10):1003–1019. doi: 10.1016/j.jaac.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Shaw P, Stringaris A, Nigg J, Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. FOCUS. 2016;14(1):127–144. doi: 10.1176/appi.focus.140102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek H, Van Goozen SHM, Matthys W, Buitelaar JK, Van Engeland H. Stress responsivity in children with externalizing behavior disorders. Dev Psychopathol. 2004;16(2):389–406. doi: 10.1017/S0954579404044578. [DOI] [PubMed] [Google Scholar]
- Stansbury K, Gunnar MR. Adrenocortical activity and emotion regulation. Monogr Soc Res Child Dev. 1994;59(2–3):108–134. [PubMed] [Google Scholar]
- Sterne JA, Egger M. Regression methods to detect publication and other bias in meta-analysis In: Publication bias in meta-analysis: Prevention, assessment and adjustments. Wiley; New York: 2005. pp. 99–110. [Google Scholar]
- Stewart JG, Mazurka R, Bond L, Wynne-Edwards KE, Harkness KL. Rumination and impaired cortisol recovery following a social stressor in adolescent depression. J Abnorm Child Psychol. 2013;41(7):1015–1026. doi: 10.1007/s10802-013-9740-1. [DOI] [PubMed] [Google Scholar]
- Tremblay RE. The development of aggressive behaviour during childhood: what have we learned in the past century? Int J Behav Dev. 2000;24(2):129–141. doi: 10.1080/016502500383232. [DOI] [Google Scholar]
- van de Wiel NMH, van Goozen SHM, Matthys W, Snoek H, van Engeland H. Cortisol and treatment effect in children with disruptive behavior disorders: a preliminary study. J Am Acad Child Adolesc Psychiatry. 2004;43(8):1011–1018. doi: 10.1097/01.chi.0000126976.56955.43. [DOI] [PubMed] [Google Scholar]
- van West D, Claes S, Deboutte D. Differences in hypothalamic–pituitary–adrenal axis functioning among children with ADHD predominantly inattentive and combined types. Eur Child Adolesc Psychiatry. 2009;18(9):543–553. doi: 10.1007/s00787-0090011-1. [DOI] [PubMed] [Google Scholar]
- Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33(1):14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry. 2005;57(11):1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Williams PG, Suchy Y, Rau HK. Individual differences in executive functioning: implications for stress regulation. Ann Behav Med. 2009;37(2):126–140. doi: 10.1007/s12160-009-9100-0. [DOI] [PubMed] [Google Scholar]
- Yehuda R. Current status of cortisol findings in post-traumatic stress disorder. Psychiatr Clin N Am. 2002;25(2):341–368. doi: 10.1016/s0193-953x(02)00002-3. [DOI] [PubMed] [Google Scholar]


