Abstract
Objective
To determine the frequency of incidental acute or subacute CI (cerebral infarction) in a population-based study.
Patients and Methods
We identified 2,095 participants ages 50 to 98 in the population-based Mayo Clinic Study of Aging from October 23, 2009 to October 5, 2016 with a usable Diffusion Tensor Imaging (DTI) sequence (total scans=3,230). Acute and subacute infarcts were identified by neuroradiologists. For each participant, vascular risk factors, medications, clinical symptoms, and neurological examination near the time of the CI were abstracted from the medical record. The probable etiologic mechanism for the CI was determined.
Results
Nine CIs were identified with a frequency of 0.28% among individual MRI scans and 0.43% among unique individuals. Infarctions were detected in 0.097% of scans from participants younger than 70 years and in 0.36% of scans of those 70 years or older. Six CI’s were acute, and three were subacute. The majority of participants with infarcts were male (78%) with a mean age of 76.9 (SD: ±6.74). All were asymptomatic at the time of CI detection. The probable mechanisms of CI were small vessel (n=6), cardioembolic (n=2), and cryptogenic (n=1).
Conclusions
Acute and subacute cerebral infarcts occur as incidental findings in approximately 1 in 225 people ages 50 to 98 years, particularly in elderly males and those with vascular risk factors. As brain MRI becomes more widely used, incidentally detected acute or subacute infarcts will provide an opportunity to improve stroke prevention.
Introduction
With increased utilization of neuroimaging in clinical and research settings and improved imaging quality, incidental findings on brain MRI are becoming more common. The frequency of incidental findings has varied among studies due to differences in the study populations and specific type of findings included in each study (i.e.: tumors, aneurysms, infarcts, etc.). The differences in the MRI field strength and pulse sequences also have added to this variability. However, a meta-analysis published in 2009 reviewing 16 studies reported the prevalence of incidental findings as 2.7% on brain MRI.1 Cerebrovascular findings including infarcts were excluded from this particular meta-analysis. Studies which included cerebrovascular disease typically focused on chronic infarcts or infarcts of unspecified chronicity, and therefore, the frequency of incidental acute or subacute infarcts is not clear.2–5
Data on incidentally-found acute infarcts have mainly come from identifying areas of restricted diffusion on diffusion-weighted images on MRI in asymptomatic individuals. Through this investigative approach, the frequency of acute cerebral infarcts found incidentally has been described in both hospitalized and community-dwelling participants as 0.37% and 0%, respectively.6, 7 A higher prevalence of acute or subacute infarction has been reported in clinical series of patients with progressive cognitive impairment (1.2%)8, cerebral amyloid angiopathy (15%)9, and primary acute intracerebral hemorrhages (35%).10 The frequency in a population-based neuroimaging study has not been reported.
Our objective was to determine the frequency of incidentally-found acute and subacute infarctions in a population-based sample.
Methods
Subject Selection
Participants were enrolled in the Mayo Clinic Study of Aging (MCSA), a population-based longitudinal study including adult residents of Olmsted County, MN. The MCSA study design is described in detail elsewhere11, but briefly, the Rochester Epidemiology Project medical records linkage system12 was used to identify Olmsted County residents ages 50 to 89 years. Participants were randomly selected and stratified by sex and age. As part of the study protocol, participants without contraindications were invited to undergo multimodality imaging studies, including brain MRI at 3T. The present analyses included both cognitively impaired and unimpaired individuals who had brain MRI (Signa; GE Healthcare, Waukesha, WI) from October 23, 2009 to October 5, 2016 with a usable DTI sequence. During this time period, a fraction of participants was scanned with a DTI slice thickness of 2.5 mm which was later changed to 2.7 mm for a higher signal to noise ratio and to match the ADNI2 pulse sequence for improved resolution.13 Specifically, from January 2010 onward, 3,174 scans had 2.7 mm slices; prior to that, 56 scans had 2.5 mm slices. The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent.
Assessment of Incidental Acute/Subacute Infarcts
All MRI scans were reviewed by board-certified neuroradiologists at the time of the scan for abnormalities as part of routine clinical workflow. Pertinent findings including acute or subacute cerebral infarction were recorded in the radiology report. The radiology reports of each MRI scan performed during the study from October 23, 2009 to October 5, 2016 were then searched for key terms including “restricted diffusion”, “acute ischemia”, “acute stroke”, “acute infarct”, “subacute ischemia”, “subacute stroke”, and “subacute infarct”. Scans fitting the inclusion criteria based on the key term search were reviewed by a cerebrovascular neurologist (JGR, AAR). Acute and subacute infarctions were defined by traditional radiologic descriptions.14 Specifically, an acute infarct was considered present if the scan showed hyperintensity on DTI sequences, a correlating hypointensity on ADC, and isointense T1 and hyperintense T2/FLAIR signals. Subacute infarction (i.e. infarct up to 14 days) was defined by variable hyperintensity of DTI signal, mildly hyperintense ADC correlate, and hypointense T1 and hyperintense T2/FLAIR signals. Scans with DTI hyperintensity thought secondary to T2 shine-through (i.e. isointense on ADC correlate and hyperintense T2/FLAIR signal) were excluded.
Data including cerebrovascular risk factors (hypertension, hyperlipidemia, atrial fibrillation, diabetes mellitus, current or history of tobacco use) and medication regimen (antithrombotic, antihypertensive, cholesterol-lowering agent) were abstracted from the medical record from the clinic visit closest to the date of MRI. Per study protocol, participants or the primary care providers were notified of any abnormal findings on the MRI that were potentially clinically significant. The medical team in conjunction with the participant then determined the additional clinical evaluation. Additional evaluations for each participant with an acute or subacute infarct were reviewed, and the probable etiology of infarction was then determined. Additionally, previously described criteria and definitions were applied to infarcts attributed to small vessel disease.15 To confirm that the infarct was clinically silent, neurologic exams performed at the time of infarction recognition were also reviewed.
Results
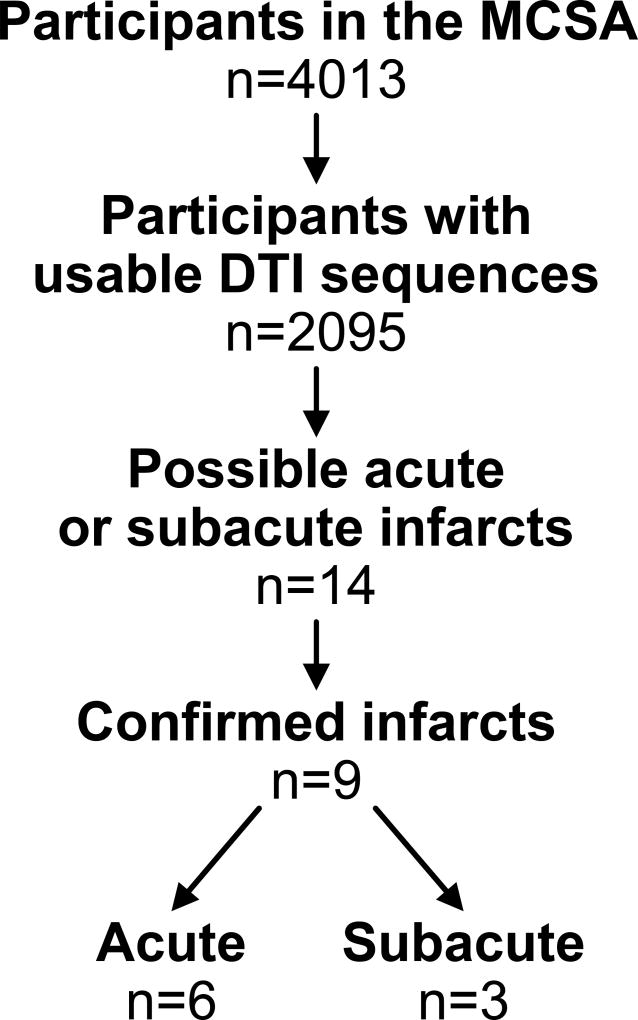
Of the 4,013 MCSA participants who completed a clinic visit from October 23, 2009 to October 5, 2016, 2,095 (52%) had at least one MRI brain scan. These 2,095 participants had a total of 3,230 MRI scans with DTI sequences during the time period specified (Table 1). Fourteen scans revealed a possible infarct with hyperintensity on DTI, but five were excluded after the review of the remaining MRI sequences was consistent with T2 shine-through. Nine acute or subacute infarctions were identified in nine unique subjects with a frequency of 0.28% among individual MRI scans (95% CI [0.13%, 0.53%]) and 0.43% among unique individuals (95% CI [0.20%, 0.81%]. Furthermore, 0.097% of scans in those younger than age 70 and 0.36% of scans in those ages 70 or older showed an infarction. Of the nine infarcts detected, six were acute and three were subacute (Figure 1). All the subjects were asymptomatic at the time of the MRI showing the infarction, and neurologic examinations performed around the time of the scan, per study protocol, were comparable to prior exams with no abnormalities attributable to the infarct. Additionally, multiple participants underwent serial MRI scans as part of the MCSA during the study period as shown in Table 1. Detected infarcts were found on the first scan in five unique individuals, on the second scan in three unique individuals, and on the third scan in one unique individual. No participants had infarcts detected on MRI at two different time points. As previously mentioned, DTI slice thickness changed during the study. Of the nine infarcts, eight were discovered on DTI with 2.7 mm slices.
Table 1.
Total number of scans per subject
| Serial Scan Number |
Number of Subjects |
Total Scans |
|---|---|---|
| 1 | 1239 | 1239 |
| 2 | 623 | 1246 |
| 3 | 192 | 576 |
| 4 | 36 | 144 |
| 5 | 5 | 25 |
| 2095 | 3230 |
Figure 1.
Flow chart for determination of acute and subacute infarcts in the Mayo Clinic Study of Aging. Of the 14 possible infarcts, 5 were excluded after review of the remaining MRI sequences was consistent with T2 shine-though.
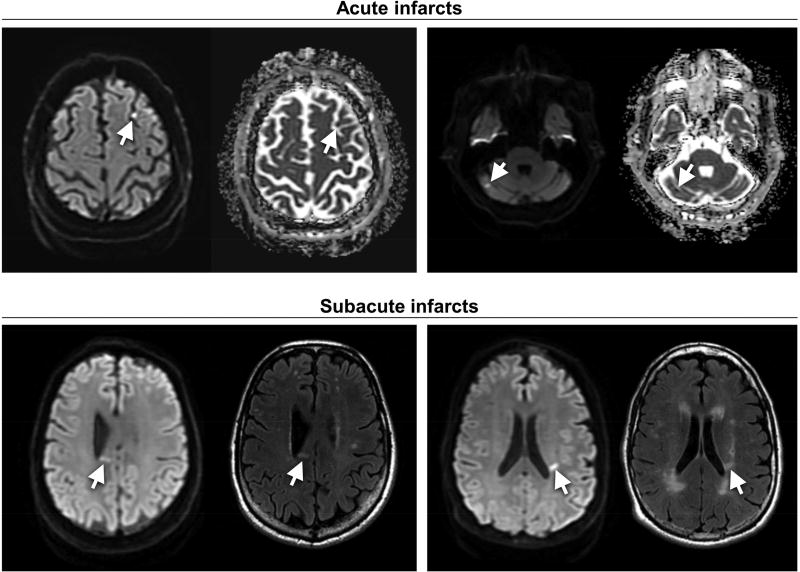
Figure 2 includes examples of acute and subacute infarcts. The median size of the infarcts was 7.1 mm in greatest diameter with a range from 5.0 mm to 10.8 mm. Six (67%) of the infarcts were located in subcortical white matter and one (11%) was located within the deep gray matter. One (11%) was cortically-based, and another (11%) was located within the cerebellum. Of the nine cases of acute or subacute infarcts, six (67%) had evidence of prior chronic infarction on MRI, and eight (89%) had evidence of chronic ischemic small vessel disease represented as white matter hyperintensity on T2 or FLAIR images. Microbleeds were noted on MRI in only one (11%) of the cases. None of the nine had a prior clinically-apparent cerebral infarction or intracerebral hemorrhage.
Figure 2.
Examples of acute and subacute infarcts indicated by arrows. Acute infarcts are demonstrated by hyperintensity on DTI and hypointensity on correlating ADC image. Subacute infarcts are demonstrated by hyperintensity on DTI and hyperintensity on correlating T2/FLAIR image.
Of the nine participants with asymptomatic acute or subacute infarcts, the majority was male (78%) with a mean age of 76.9 years (SD: ±6.74). Additionally, eight of the nine participants had at least one of the cerebrovascular risk factors collected in our study and seven of nine had two or more risk factors (Table 2). Only one participant was without a vascular risk factor. The most common risk factor present was hypertension which was seen in eight subjects. Hyperlipidemia was seen in seven subjects. Two had atrial fibrillation, and two had type 2 diabetes mellitus. Most participants had been on an antiplatelet agent (56%), lipid-lowering therapy (78%), or an antihypertensive (78%) at the time of infarction. Please see Supplemental Table for characteristics comparing participants of MCSA who did not undergo brain imaging to those with imaging.
Table 2.
Characteristics of participants with acute or subacute infarcts on MRI
| Age of Infarct |
Age | Sex | Risk Factors | Medication Regimen at the Time of Infarction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HTN | HLD | AF | DM | Smoking | Antiplatelet | Anticoagulation | Lipid- lowering agent |
Anti- HTN agent |
|||
| Acute | 74 | M | N | ASA | |||||||
| Acute | 76 | M | X | X | X | N | ASA | Warfarin | X | X | |
| Acute | 77 | F | X | X | N | X | X | ||||
| Acute | 79 | M | X | Y | X | ||||||
| Acute | 83 | M | X | X | X | N | ASA | X | X | ||
| Acute | 89 | F | X | X | X | X | Remote | Warfarin | X | X | |
| Subacute | 65 | M | X | X | N | X | X | ||||
| Subacute | 72 | M | X | X | N | ASA | X | X | |||
| Subacute | 77 | M | X | X | N | ASA-dipyridamole | X | ||||
M=male F=female X=present N=No Y=Yes
HTN: Hypertension HLD: Hyperlipidemia AF: Atrial Fibrillation DM: Diabetes Mellitus, Type 2 ASA: aspirin
The probable etiology of each infarct was determined based upon the participant’s risk factors, imaging characteristics, and results of additional clinical evaluation and then classified according to TOAST criteria; additional criteria described by Wardlaw, et al. were also applied to subcortical infarcts.15, 16 Six infarcts were categorized as secondary to small vessel disease; two were categorized as cardioembolic; and the etiology of one was undetermined. Five participants were referred for a Neurology consultation for further evaluation after the infarct was found. Medication adjustments were recommended in four of these five cases for improved stroke prevention. Two of the four participants evaluated by primary care providers alone were recommended adjustments to their medication regimen for stroke prevention.
Discussion
Our study shows that incidental acute and subacute infarcts were uncommon in a population-based study with a frequency of 0.28% among individual MRI scans and 0.43% among individual participants age 50 to 98 years. Our rate of detection of silent acute/subacute brain infarctions was comparable to that of a previous study of hospitalized patients which reported a frequency of 0.37% [6], but higher than a community-based study in which no acute/subacute infarctions were detected among 793 subjects who underwent brain MRI.6, 7 This finding may be in part due to the older age of our cohort (mean age 72.2 ± 10.2 years) and the hospital-based study (mean age 70 ±10 years) relative to the community-based study (mean age 58.4 ± 8.0) as multiple studies have shown associations between increased age and incidental stroke.3–5
Our reported frequency of incidentally-found acute or subacute ischemia was significantly lower than that described in studies involving elderly subjects with progressive cognitive impairment (1.2%)8, cerebral amyloid angiopathy (15%)9, and primary acute intracerebral hemorrhages (35%).10 Because patients with cerebral amyloid angiopathy also have a high prevalence of acute incidental ischemia, it is not surprising that some studies have shown an association between incidental acute cerebral infarction and cerebral microbleeds.17 However, only one of our subjects with incidental ischemia had evidence of microbleeds on MRI. Additionally, patients with dementia and cerebral amyloid angiopathy have a high burden of microinfarcts which may be the pathologic substrate of some of these acute ischemic lesions.17, 18 Interestingly, a study of serial MRI scans of five patients over a 16 week period with severe white matter disease revealed a higher incidence of acute, clinically-silent infarcts which overtime developed an appearance similar to leukoaraiosis, supporting the hypothesis that some of these infarcts contribute to leukoaraiosis which is known to have deleterious effects on functional status.19 Similarly, cerebral microinfarcts impair functional status due to the disruption of innumerable neuronal connections. Whether some of these acute or subacute CI become cerebral microinfarcts is unclear but with more advanced imaging modalities and consistent rating criteria for microinfarcts, this will be an important future area of research.20
In the population-based Rotterdam study, approximately 20% of participants over age 60 had a silent cerebral infarction on T2-weighted sequence. (DWI sequence was not used in that study).21 Compared to other cerebrovascular lesions, the described frequency of incidental acute or subacute clinical infarction is greater than other incidental findings such as cavernous malformations or arteriovenous malformations with previously reported prevalence of 0.16% and 0.05%, respectively.1 A more recent study which also included participants of the MCSA found a frequency of cavernous malformations (0.44%) similar to the frequency of incidentally-detected acute and subacute cerebral infarctions.22
As expected, acute or subacute incidental infarcts were associated with traditional vascular risk factors. In fact, most of our subjects had two or more risk factors. This observation is congruent with previous studies that reported that the vascular risk factors for silent cerebral infarcts are similar to those for clinical stroke and chronic small vessel disease.3, 6 Interestingly, many of the infarcts in our study occurred in participants who had already been prescribed stroke-preventative medications. Furthermore, most of our subjects with incidental acute or subacute infarcts had evidence of chronic infarcts and significant white matter disease on MRI which coincides with a prior study which found an association between history of stroke/TIA and incidentally-detected acute infarcts.8 Additionally, although participants 50 years old and greater were included in our study, the average age of incidental acute or subacute infarction was 76.9 years. Furthermore, infarctions were detected in only 0.097% of scans from participants younger than 70 years and in 0.36% of scans from participants age 70 or older. These findings further support that increasing age is a risk factor for incidental stroke.3–5 Furthermore, because stroke-like symptoms are often under-recognized or underreported but are associated with cumulative silent cerebral infarction and future risk of TIA and stroke, it may be beneficial to screen patients for such symptoms as they are at higher risk of future cerebrovascular events.23 Our results suggest that roughly 1 in 225 individuals undergoing brain MRI scanning will have an incidentally-detected acute or subacute infarction. We suspect that this number will increase as imaging becomes more advanced and more frequently used for both clinical and research purposes. As more MRI brain scans are performed for a variety of reasons, more acute or subacute infarctions will be incidentally detected and will provide practitioners and patients an opportunity to address and improve ongoing stroke prevention through risk factor modification and medication adjustments with the goal of preventing symptomatic stroke and accumulation of silent ischemic lesions which are known to have deleterious consequences on a person’s functional status.
Our study has multiple limitations. First, not all participants enrolled in the MCSA underwent MRI scan. Also, the MCSA sample population includes roughly the same number of participants in each age and sex strata. In the general population, however, there are many more middle-aged adults (e.g. 50–60 years old) than older adults (e.g. 80–90 years old). Therefore, our reported frequency of incidentally-detected cerebral infarctions may not accurately reflect that of the general population. Additionally, by using clinical radiology reports, the reporting of acute or subacute infarction may be variable due to interrater variability among neuroradiologists. Furthermore, the MRI scans were performed between October 23, 2009 and October 5, 2016 and during that period, the diffusion sequences changed. Whether this affected the detection rate is not known; however, a 0.2 mm increase in slice thickness seems unlikely to affect visual ascertainment. Also, given that the participants were asymptomatic, the etiologic mechanism underlying the infarction may be less valid since clinical symptoms can be useful in defining the subtype, especially for those of small vessel mechanism.16
Conclusions
Acute and subacute cerebral infarcts occur as incidental findings in approximately 1 in 225 people ages 50 to 98 years, and are most commonly noted in elderly males and in those with traditional vascular risk factors. As brain MRI becomes more frequently and widely used in clinical practice and research, incidentally-found acute or subacute cerebral infarction will provide an opportunity to improve stroke prevention.
Supplementary Material
Acknowledgments
This work was supported by NIH grants K76 AG057015-01 (PI: Graff-Radford) R01 NS097495 (PI: Vemuri), U01 AG06786 (PI: Petersen), P50 AG16574 (PI: Petersen), R01 AG034676 (PI: Rocca), R01 AG11378 (PI: Jack), R01 AG041851 (PIs: Jack and Knopman); the Gerald and Henrietta Rauenhorst Foundation grant, the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation, and the Elsie and Marvin Dekelboum Family Foundation, U.S.A. The funding sources were not involved in the manuscript review or approval.
MCSA Data
U01 AG006786: (Petersen) Mayo Clinic Study of Aging (MCSA)
P50 AG016574: (Petersen) Alzheimer’s Disease Research Center (ADRC)
R01 AG034676: (Rocca) Rochester Epidemiology Project (REP)12
R01 AG011378: (Jack) Evaluating and Extending Our Hypothetical Model of Alzheimer’s Biomarkers
R01 AG041851: (Jack/Knopman) Validating the New Criteria for Preclinical Alzheimer’s disease
R01 NS097495: (Vemuri) Development, Validation, and Application of an Imaging based CVD Scale
Dr. Mielke consults for Eli Lilly and Lysosomal Therapeutics, Inc. She receives research grants from the National Institute of Health/National Institute on Aging, Department of Defense, Biogen, Roche, and Lundbeck.
Dr. Graff-Radford is supported by the Mayo Clinic Myron and Jane Hanley Career Development Award in Stroke Research.
Dr. Vemuri receives research support from R01 NS097495 and P50 AG016574/P1.
Dr. Brown receives research support from the NIH
Dr. Knopman serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by Biogen, TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Dr. Jack serves on scientific advisory board for Eli Lilly & Company; receives research support from the NIH/NIA, and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation; and holds stock in Johnson & Johnson.
K. Kantarci serves on the Data Safety Monitoring Board for Takeda Global Research & Development Center, Inc.; the data monitoring boards of Pfizer and Janssen Alzheimer Immunotherapy; and is funded by the NIH (R01 AG040042 [PI], R21 NS066147 [PI], P50 AG44170/Project 2 [PI], P50 AG16574/Project 1 [PI], and R01 AG11378 [Co-I]).
Dr. Petersen serves on data monitoring committees for Pfizer, Inc., Janssen Alzheimer Immunotherapy, and is a consultant for Biogen, Roche, Inc., Merck, Inc. and Genentech, Inc.; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003), and receives research support from the National Institute of Health.
Abbreviations
- ADC
Apparent diffusion coefficient
- DTI
Diffusion Tensor Imaging
- FLAIR
fluid-attenuated inversion recovery
- MCSA
Mayo Clinic Study of Aging
- MRI
Magnetic Resonance Imaging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures/Disclosures:
Dr. Arnold Fiebelkorn, Dr. Rabinstein, Mr. Przybelski report no disclosures.
References
- 1.Morris Z, Whiteley WN, Longstreth WT, Jr, et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsushima Y, Taketomi-Takahashi A, Endo K. Prevalence of abnormal findings on brain magnetic resonance (MR) examinations in adult participants of brain docking. BMC Neurol. 2005;5:18. doi: 10.1186/1471-2377-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vermeer SE, den Heijer T, Koudstaal PJ, Oudkerk M, Hofman A, Breteler MMB. Incidence and risk factors of silent brain infarcts in the population-based Rotterdam Scan Study. Stroke. 2003;34:392–396. doi: 10.1161/01.str.0000052631.98405.15. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Longstreth WT, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurology. 2007;6:611–619. doi: 10.1016/S1474-4422(07)70170-9. [DOI] [PubMed] [Google Scholar]
- 5.Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. New England Journal of Medicine. 2007;357:1821–1828. doi: 10.1056/NEJMoa070972. [DOI] [PubMed] [Google Scholar]
- 6.Yamada K, Nagakane Y, Sasajima H, et al. Incidental acute infarcts identified on diffusion-weighted images: a university hospital-based study. AJNR Am J Neuroradiol. 2008;29:937–940. doi: 10.3174/ajnr.A1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batool S, O'Donnell M, Sharma M, et al. Incidental magnetic resonance diffusion-weighted imaging-positive lesions are rare in neurologically asymptomatic community-dwelling adults. Stroke. 2014;45:2115–2117. doi: 10.1161/STROKEAHA.114.005782. [DOI] [PubMed] [Google Scholar]
- 8.Saini M, Suministrado MS, Hilal S, et al. Prevalence and Risk Factors of Acute Incidental Infarcts. Stroke. 2015;46:2722–2727. doi: 10.1161/STROKEAHA.115.009963. [DOI] [PubMed] [Google Scholar]
- 9.Kimberly WT, Gilson A, Rost NS, et al. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2009;72:1230–1235. doi: 10.1212/01.wnl.0000345666.83318.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon RS, Burgess RE, Wing JJ, et al. Predictors of highly prevalent brain ischemia in intracerebral hemorrhage. Ann Neurol. 2012;71:199–205. doi: 10.1002/ana.22668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: Half a Century of Medical Records Linkage in a US Population. Mayo Clinic Proceedings. 2012;87:1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nadgir R, Yousem DM. Vascular Diseases of the Brain Neuroradiology : the requisites. Fourth. Philadelphia, PA: Elsevier; 2017. pp. 87–149. [Google Scholar]
- 15.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurology. 2013;12:822– 838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of Subtype of Acute Ischemic Stroke - Definitions for Use in a Multicenter Clinical-Trial. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Okamoto Y, Ihara M, Tomimoto H, Taylor Kimberly W, Greenberg SM. Silent ischemic infarcts are associated with hemorrhage burden in cerebral amyloid angiopathy. Neurology. 2010;74:93. doi: 10.1212/WNL.0b013e3181c77627. author reply 93. [DOI] [PubMed] [Google Scholar]
- 18.Smith EE, Schneider JA, Wardlaw JM, Greenberg SM. Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 2012;11:272–282. doi: 10.1016/S1474-4422(11)70307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Conklin J, Silver FL, Mikulis DJ, Mandell DM. Are acute infarcts the cause of leukoaraiosis? Brain mapping for 16 consecutive weeks. Ann Neurol. 2014;76:899–904. doi: 10.1002/ana.24285. [DOI] [PubMed] [Google Scholar]
- 20.van Veluw SJ, Shih AY, Smith EE, et al. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurology. 2017;16:730–740. doi: 10.1016/S1474-4422(17)30196-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vermeer SE, Prins ND, den Heijer T, Hofman A, Koudstaal PJ, Breteler MM. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 22.Flemming KD, Graff-Radford J, Aakre J, et al. Population-Based Prevalence of Cerebral Cavernous Malformations in Older Adults: Mayo Clinic Study of Aging. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2017.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windham BG, Griswold ME, Shibata D, Penman A, Catellier DJ, Mosley TH., Jr Covert neurological symptoms associated with silent infarcts from midlife to older age: the Atherosclerosis Risk in Communities study. Stroke. 2012;43:1218–1223. doi: 10.1161/STROKEAHA.111.643379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.