Abstract
Background
Electroencephalography (EEG) has clinical and prognostic importance after cardiac arrest (CA). Recently, interest in quantitative EEG (qEEG) analysis has grown. The qualitative effects of sedation on EEG are well known, but potentially confounding effects of sedatives on qEEG after anoxic injury are poorly characterized. We hypothesize that sedation increases suppression ratio (SR) and decreases alpha/delta ratio (ADR) and amplitude-integrated EEG (aEEG), and that the magnitude of sedation effects will be associated with outcome.
Methods
We routinely monitor comatose post-arrest patients with EEG for 48-72h. We included comatose EEG-monitored patients after CA who had protocolized daily sedation interruptions. We used Persyst v12 to quantify qEEG parameters and calculated medians for 10min immediately prior to sedation interruption and for the last 5min of interruption. We used paired t-tests to determine whether qEEG parameters changed with sedation cessation, and logistic regression to determine whether these changes predicted functional recovery or survival at discharge.
Results
78 subjects were included (median age 56, 65% male). Interruptions occurred a median duration of 34 hours post-arrest and lasted a median duration of 60min. Prior to interruption, higher aEEG predicted survival, while lower SR predicted both survival and favorable outcome. During interruption, SR decreased (p <0.001), aEEG increased (p=0.002), and ADR did not change. Larger decreases in SR predicted decreased survival (OR=1.04 per percent change; 95% CI 1.00–1.09).
Conclusion
Higher aEEG and lower SR predict survival after CA. Sedation alters aEEG and SR, but importantly does not appear to affect the relationship between these parameter values and outcome.
Introduction
Cardiac arrest (CA) affects over 500,000 Americans annually.1 Most patients with return of spontaneous circulation are comatose on hospital arrival. For these patients, sequelae of ischemic brain injury are the most common cause of morbidity and mortality.2 Electroencephalography (EEG) has clinical and prognostic importance in this population. In addition to assessing reactivity to external stimuli, EEG is helpful to detect seizures and can guide therapeutic decision making.3–5 EEG interpretation may be qualitative or quantitative (qEEG), but interest in qEEG analysis has recently grown. Continuous or reactive patterns predict favorable recovery, while patterns such as burst suppression and attenuation, with qEEG analogues of suppression ratio (SR) and amplitude-integrated EEG (aEEG), are known predictors of poor outcomes.5–8
Sedation and analgesia use is almost ubiquitous in post cardiac arrest care.9–11 In healthy individuals and non-brain injured patients, it is known that sedative and anesthetic administration can cause burst suppression and generalized slowing of EEG, however few studies describe sedation effects on EEG in patients with severe global ischemic brain injury.12 In particular, quantitative effects of sedation and analgesia on EEG are unknown in the post-cardiac arrest population, and sedation may be an important confounder in clinical prognostication using EEG.5 Coma after global ischemic brain injury is associated with functional reduction of thalamo-cortical connectivity.2, 13, 14 In patients with some preservation of cortical function, sedation may further reduce connectivity, increasing EEG suppression and altering EEG component frequencies. Specifically, sedation would decrease EEG amplitude and alpha/delta ratio (ADR) and increase suppression ratio (SR).
In this study, we describe sedation-induced changes in qEEG of post-cardiac arrest patients, and we explore the association of response to sedation with functional recovery. We hypothesize that sedation would: 1) significantly increase SR and decrease both ADR and EEG amplitude, and 2) the magnitude of sedation effects on qEEG will be associated with outcome at hospital discharge.
Methods
The University of Pittsburgh Institutional Review Board approved all aspects of this observational study with a waiver of informed consent for a minimal risk intervention.
Prior to performing this observational cohort study, we implemented a quality improvement (QI) project to systematically interrupt sedation at least daily in all comatose post-arrest patients. This intervention is consistent with institutional sedation practice for general intensive care.10, 11 Clinical contraindications to sedation interruptions included cases using sedation to suppress seizure activity, patients with significant hemodynamic instability or severe hypoxia, or patients with ongoing neuromuscular blockade. Treating clinicians determined the duration of sedation interruptions, restarting sedation for agitation, ventilator dyssynchrony, or worsening hemodynamic instability.
We included comatose patients being monitored with EEG after cardiac arrest. We excluded subjects who had a clinical contraindication to sedation interruption, a traumatic etiology of arrest, were pregnant, a prisoner or had comfort measures only as their goal of care. We also excluded sedation interruptions lasting less than 10 minutes. We prospectively screened and enrolled subjects from June 2015 and February 2017 (Figure 1). To increase our sample size, we also generated a retrospective cohort including sedation interruptions performed between February 2015 and January 2016 by retrospectively examining electronic medical records to include any interruptions which were not formally recorded as the QI was being implemented. Bedside nurses recorded sedation interruption start and stop times in the electronic medical record. We collected data for up to 5 days following cardiac arrest, but only included data from each patient’s first sedation interruption in our analysis.
Figure 1.
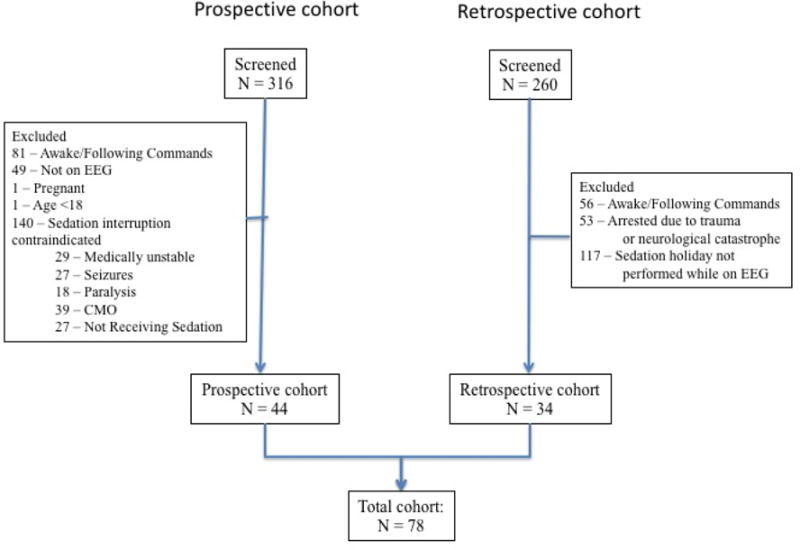
Summary of subject screening.
Abbreviations: CMO – Comfort measures only; EEG - Electroencephalography
Our institution routinely monitors EEG continuously after cardiac arrest for 2 to 3 days (during active targeted temperature management) or until death or awakening, whichever occurs first. We archive all continuous EEG recordings as part of the electronic medical record. We applied 22 gold-plated cup electrodes to the scalp in the standard 10-20 International System of Electrode Placement. Data was recorded using XLTech Natus Neuroworks digital video/EEG systems (Natus Medical, Pleasanton, CA). We used Persyst v12 (Persyst Development Co., Prescott, AZ) to generate qEEG data including SR, amplitude-integrated EEG (aEEG), and ADR. The software calculates SR by dividing each lead’s data into 10 second epochs and determines the percentage of the total duration of each epoch that is “suppressed” (defined as ≥0.5 seconds of <3μV amplitude).15–18 aEEG is a summary measure of the the amplitude characteristics of a filtered, rectified peak-to-peak measure of the EEG signal in 1 second epochs. ADR is calculated by dividing the band-pass filtered spectral power in the alpha frequency range (8-13Hz) by the band-pass filtered spectral in the delta frequency range (1-4Hz) within a 2-minute running average.15–18 We used Persyst’s algorithm for automated artifact reduction to reduce the contribution of physiological and electrode artifact. For each parameter, we averaged data across all leads of the standard 10-20 monitoring montage.
We calculated the median value of each parameter for the 10 minutes immediately prior to sedation interruption (this was termed “pre” data) and for the last 5 minutes of interruption (termed “post” data). We then calculated the difference between these two values to determine the change pre to post. If sedation was not restarted, we calculated post values 12 hours after sedation discontinuation. In addition to qEEG measures, our other outcomes of interest were survival to hospital discharge and functionally favorable recovery at discharge, which we determined based on discharge disposition. Patients discharged home or to acute rehabilitation were considered to have a functionally favorable outcome at discharge, while functionally unfavorable recoveries were discharged to a skilled nursing facility, long-term acute care facility, hospice or death. This method of determination was used because we have previously demonstrated that this correlates with long-term outcome.19, 20
We summarized population characteristics and outcomes using descriptive statistics. We used paired t-tests to determine whether qEEG parameters changed from pre to post, and used unadjusted logistic regression to determine whether pre, post or the difference from pre to post in any qEEG parameter predicted neurological outcome at hospital discharge. Because of our small sample size, we did build adjusted models. We performed a post hoc analysis to test for for significant difference between data collected prospectively and retrospectively. We performed all analyses using STATA v 14.2 (StataCorp, College Station, TX). We considered a P value <0.05 to be statistically significant for all analyses.
Results
Of 316 prospectively screened patients, 44 met inclusion and exclusion criteria, while 34 of 260 patients screened retrospectively met criteria, providing 78 total records for analysis (Figure 1). Reasons for exclusions in the prospective cohort were primarily contraindications to sedation interruption (52% of exclusions: comfort measures only care (14% of exclusions), hemodynamically instability (11% of exclusions), no sedation administered (10% of exclusions), sedation used for seizure control (10% of exclusions), and continuous neuromuscular blockade (7% of exclusions (Figure 1). Most patients were treated after out-of-hospital cardiac arrest (n=63, 81%) (Table 1). Median age was 56 (SD 17), 27 (35%) were female, and 26 (33%) had a known shockable primary rhythm of arrest. PCAC IV illness severity was present in 42% of the cohort. The choice of sedation varied, and often more than one drug was used for each subject (propofol n=60, fentanyl n=36, midazolam n=10, dexmedetomidine n=3, and ketamine n=2). The median duration of sedation interruption was 60 minutes (range 12 to 720 min), and the median length of time from arrest to interruption was 33.5 (IQR 18 to 43) hours. Average core temperature at the start of interruption was 36.1 °C (IQR 35.6-36.7). Overall, 38 (49%) subjects survived to hospital discharge and 29 (37%) subjects had a favorable functional outcome at hospital discharge. Of the 40 subjects that died, 35 died secondary to brain death or withdrawal of treatment secondary to poor neurologic prognosis. Median hospital LOS was 7 (IQR 4 to 13) days.
Table 1.
Baseline characteristics and outcomes.
| Characteristic | Overall cohort (n = 78) |
|---|---|
| Age, years | 56 ± 17 |
| Female sex | 27 (35) |
| Out-of-hospital arrest | 63 (81) |
| Witnessed arrest | 57 (73) |
| Bystander CPR | 30 (38) |
| Initial rhythm | |
| VT/VF | 26 (33) |
| PEA | 23 (29) |
| Asystole | 14 (18) |
| Unknown | 15 (19) |
| Pittsburgh Cardiac Arrest Category | |
| II | 27 (35) |
| III | 10 (13) |
| IV | 33 (42) |
| Unable+ | 8 (10) |
| Cardiac catheterization | 25 (32) |
| Survived | 38 (49) |
| Favorable outcome | 29 (37) |
Data are presented as mean ± standard deviation or raw number with corresponding percentages.
Pittsburgh Cardiac Arrest Category cannot be assigned when the neurological exam is confounded by neuromuscular blockade, overdose or severe metabolic disarray.
Pre-interruption, higher aEEG values predicted survival (OR = 1.09; 95% confidence interval (CI) 1.01 – 1.09), and higher SR values predicted decreased survival (OR = 0.97; 0.95 – 0.99) as well as an unfavorable functional outcome (OR = 0.97; 0.94 – 0.99) (Table 3). Baseline ADR was not predictive of outcome. During sedation interruption, SR decreased (median change 0; IQR: −7 to 0, p = <0.001), aEEG increased (1.1; 0.09 to 4.0, p = 0.002), but ADR had minimal change (Table 2). Larger decreases in SR were associated with decreased survival to hospital discharge (OR = 1.04; 95% CI 1.00 – 1.09) (Table 3, Figure 2). The magnitude of change in aEEG and ADR did not differ by outcome.
Table 3.
Association of quantitative electroencephalographic (qEEG) parameters with survival and favorable functional outcome at hospital discharge
| Parameter | Pre-interruption value | Post-interruption value | Change from pre to post |
|---|---|---|---|
| Survival | |||
| aEEG (uV) | 1.09 (1.01 – 1.19) | 1.06 (0.99 – 1.28) | 0.98 (0.91 – 1.05) |
| SR (%) | 0.97 (0.95 – 0.99) | 0.96 (0.93 – 0.99) | 1.04 (1.00 – 1.09) |
| ADR | 1.07 (0.46 – 2.49) | 1.09 (0.53 – 2.24) | 1.07 (0.41 – 2.80) |
| Favorable Outcome | |||
| aEEG (uV) | 1.06 (0.99 – 1.14) | 1.03 (0.96 – 1.09) | 0.96 (0.88 – 1.04) |
| SR (%) | 0.97 (0.94 – 0.99) | 0.96 (0.93 – 1.00) | 1.05 (0.99 – 1.12) |
| ADR | 0.61 (0.24 – 1.58) | 0.83 (0.39 – 1.78) | 1.31 (0.48 – 3.55) |
Data are presented as unadjusted odds ratios with associated 95% confidence intervals
Table 2.
Quantitative electroencephalographic (qEEG) characteristics before and after sedation interruption
| Parameter | Pre-interruption | Post-interruption | Change | P value for change |
|---|---|---|---|---|
| aEEG (uV) | 6.6 [2.7 – 10.4] | 9.3 [4.1 – 14.8] | 1.1 [0.09 – 4.0] | 0.002 |
| SR (%) | 0 [0 – 20] | 0 [0 – 8] | 0 [−7 – 0] | <0.001 |
| ADR | 0.60 [0.41 – 0.97] | 0.72 [0.41 – 1.15] | 0.03 [−0.08 – 0.19] | 0.1 |
Data are presented as medians with interquartile ranges. P values are for the paired t-test that post-interruption values equal pre-interruption values.
Note: Although the distributions of the pre- and post-interruption data are not normally distributed, the differences are normal, meeting the assumption of the parametric t-test. Median SR values were zero due to several subjects’ EEGs having a baseline reactivity above the suppression cutoff threshold (see discussion).
Figure 2.
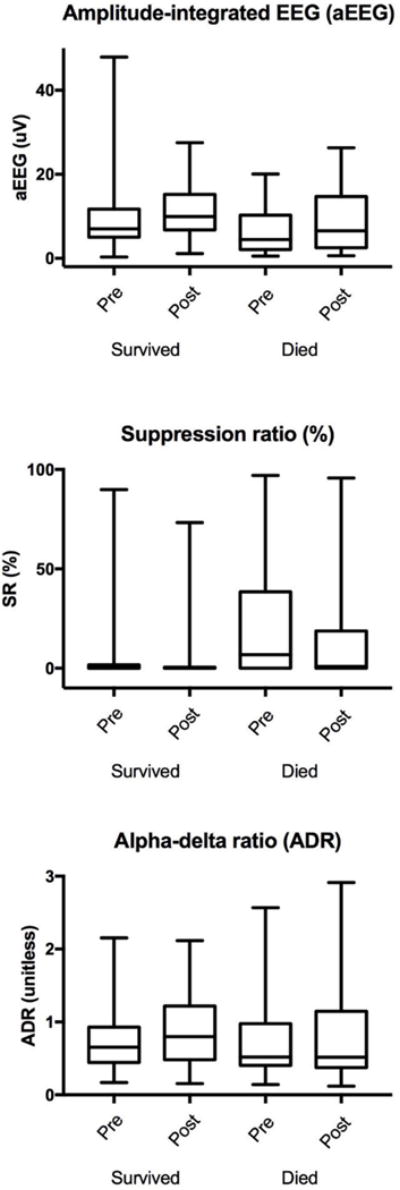
aEEG, SR, and ADR displayed as box plots with pre and post sedation interruption values in subjects that survived vs. died.
Discussion
In this study we described how sedative agents affect qEEG parameters in the cardiac arrest population. We found that while baseline SR and aEEG are affected by sedation and these baseline parameters predict outcome, the use of sedation does not affect the relationship between these parameter values and outcome. Importantly, these data suggest that future studies should report and account for the effects of concomitant sedation. While absolute qEEG measures predicted outcome at hospital discharge, magnitude of these changes with and without sedation had relatively little association with outcomes at hospital discharge. Lastly, our data suggest that in the future qEEG analysis has the potential to be one of many tools, alongside previously established methods, to aid in prognostication after cardiac arrest.
In prior work, a continuous normal voltage pattern (defined as continuous cortical activity above a threshold voltage) predicts good outcome with reported positive predictive values of 91% and 94%.21, 22 These are consistent with our findings that higher aEEG is predictive of survival to discharge (Table 3). However, prior studies grouped subjects into qualitative categories (e.g. flat, low voltage, continuous, suppression burst) in order to predict outcome. Our data extends prior work by reporting quantitative values. Quantitative EEG avoids the need to subjectively categorize EEG, increasing granularity of classification and improving reproducibility and statistical power. The changes in aEEG with sedation (Table 2) also may affect categorization of patients into the ‘flat’ or ‘low voltage’ pattern groups in prior studies.21, 22 Our study also demonstrates that brief periods of aEEG data (in this case 5 to 10 minutes) have the potential to sufficiently glean prognostic information, without the need for prolonged monitoring.
Changes in SR occur with various types of brain injury including cardiac arrest.23–25 Our finding that higher SR predicts worse outcomes is largely consistent a previous study in which baseline SR was significantly higher in post-arrest patients who were dead or vegetative at hospital discharge.25 Difference between outcome groups was largest during the first 4 hours of monitoring, but persisted for ≥70 hours. In our study, the median duration to sedation interruption following arrest was 33.5 hours, potentially increasing the discriminatory power of this predictor. In another cohort, SR ≥ 48 predicted poor outcome with a likelihood ratio of 12.7.23 This assessment was performed during neuromuscular blockade to reduce electromyographic artifact, but our results indicate neuromuscular blockade may not be necessary to determine a meaningful SR.
Interestingly, we found that SR change during sedation interruption was larger in non-survivors. One possible explanation for this is that severely injured brains are more susceptible to sedation, leading to larger change in SR when sedatives are stopped. Another possibility can be explained by the natural history of SR trajectory over time. Several of our subjects, many of whom survived, had reactive EEGs that remained above the threshold amplitude, and therefore had a baseline SR of zero (Table 2; Figure 2). In a recent longitudinal analysis using group-based trajectory modeling, we recently reported that SR falls into four distinct down-sloping trajectories that convey strong prognostic significance.9 Two of these trajectories demonstrate a steep decline of SR over time, indicating that the decrease in SR we observed during sedation interruption and its associated prognostic significance may occur irrespective of sedation use and represent the natural history of disease, rather than a causal relationship. Importantly, our data suggest that sedation may not be an important confounder to control for when associating EEG with long term outcomes.
Finally, we examined ADR, which we would expect to be decreased in post-cardiac arrest brain injury.26 In contrast to Soholm et al., we found that ADR was not associated with survival or functional outcome.27 Those authors also found that rhythmic delta activity (RDA) predicted survival. RDA is seen in many disease states such as stroke and metabolic derangements, yet overall, studies have shown that this is not an ominous prognostic sign in EEG.28 Our study looked at quantitative averages of component frequencies at discrete time epochs, which does not discriminate between background delta activity and RDA, each of which have different physiological meanings that likely affect outcome.29 While our findings should be confirmed by further studies, they suggest that for the purpose of prognostication, qEEG analysis may be better suited for amplitude-based parameters as compared to those that rely on frequencies. It is also important to note that Soholm et. al reported the unknown effect of sedation as a potential limitation. Our results suggest that sedative agents do not alter the component frequencies of cEEG.
Limitations of our study include the relatively small sample size of prospective data. Given that we found multiple significant associations in our analysis, we feel that it is unlikely that our study was underpowered. However, the small sample size did preclude development of adjusted models, as well as the ability to stratify the relatively heterogeneous population and various sedation regimens. This risks the possibility that both measured and unmeasured confounders bias the associations we found. In part, our small sample size resulted from a majority of screened subjects being excluded from analysis. Most of the reasons for exclusion are typical for the overall post-arrest patient population (e.g. post-anoxic seizures, neuromuscular blockade, etc), but these exclusions do limit the generalizability of our findings beyond the subgroup of post-arrest patients that are appropriate for sedation interruptions. Specifically, it may be that, at a population level, patients in whom sedation is never interrupted are systematically sicker or otherwise different than those in whom sedation can be safely interrupted. We developed a retrospective cohort to help increase our sample size, but this may introduce a source of unmeasured heterogeneity within our cohort. Since there were no systematic differences between the two cohorts, we believe this is not a major source of bias.
We recognize that our results should be confirmed by further studies prior to utilizing qEEG as a prognostic tool alongside previously established methods, however we were able to report novel findings regarding the overall effect of sedative agents on EEG following cardiac arrest which will be useful for further research studies. We also acknowledge that occasionally, patients initially recover good neurological function but later die of non-neurological causes (e.g. infection, comorbid conditions). In our study, this occurred in 5 subjects. Some studies classify these patients based on best attained neurological status during hospital stay, but we chose to classify outcome at discharge.21 This could have an effect on our results, as subjects that otherwise would have otherwise been analyzed as ‘favorable outcome’ ended up being analyzed with the poor outcome group. Finally, patient-centered outcome data like long-term functionally favorable survival were not available for analysis. Instead, as a surrogate measure we analyzed discharge disposition from the hospital, we have previously demonstrated predicts long-term survival.19,20 Ongoing efforts are needed to translate short-term neuroprognostication outcomes research to long-term recovery after cardiac arrest.
Conclusions
In comatose survivors of cardiac arrest, higher aEEG and lower SR values predict survival in comatose survivors of cardiac arrest. Thus far, sedation has remained a key confounding factor in cEEG research. We found that sedation alters aEEG and SR values, suggesting this should be accounted for in future studies. With the exception of suppression ratio, the qEEG changes attributable to sedation do not appear to be associated with clinical outcome.
Acknowledgments
Dr. Elmer’s research is funded by the National Institutes of Health through grants 5K12HL109068 and 1K23NS097629.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement: These sponsors had no role in the study design, collection, analysis or interpretation of data. The contents of the manuscript are solely the responsibility of the author. The authors have no other disclosures or potential conflicts of interest to report.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All aspects of of this observational study were approved with a waiver of informed consent for a minimal risk intervention; IRB PRO15030099.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elmer J, Callaway CW. The Brain after Cardiac Arrest. Semin Neurol. 2017;37:19–24. doi: 10.1055/s-0036-1597833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friberg H, Westhall E, Rosen I, Rundgren M, Nielsen N, Cronberg T. Clinical review: Continuous and simplified electroencephalography to monitor brain recovery after cardiac arrest. Crit Care. 2013;17:233. doi: 10.1186/cc12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandroni C, Cariou A, Cavallaro F, Cronberg T, Friberg H, Hoedemaekers C, et al. Prognostication in comatose survivors of cardiac arrest: an advisory statement from the European Resuscitation Council and the European Society of Intensive Care Medicine. Resuscitation. 2014;85:1779–89. doi: 10.1016/j.resuscitation.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Amorim E, Rittenberger JC, Zheng JJ, Westover MB, Baldwin ME, Callaway CW, et al. Continuous EEG monitoring enhances multimodal outcome prediction in hypoxic-ischemic brain injury. Resuscitation. 2016;109:121–6. doi: 10.1016/j.resuscitation.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fugate JE, Wijdicks EF, Mandrekar J, Claassen DO, Manno EM, White RD, et al. Predictors of neurologic outcome in hypothermia after cardiac arrest. Ann Neurol. 2010;68:907–14. doi: 10.1002/ana.22133. [DOI] [PubMed] [Google Scholar]
- 8.Cloostermans MC, van Meulen FB, Eertman CJ, Hom HW, van Putten MJ. Continuous electroencephalography monitoring for early prediction of neurological outcome in postanoxic patients after cardiac arrest: a prospective cohort study. Crit Care Med. 2012;40:2867–75. doi: 10.1097/CCM.0b013e31825b94f0. [DOI] [PubMed] [Google Scholar]
- 9.Elmer J, Gianakas JJ, Rittenberger JC, Baldwin ME, Faro J, Plummer C, et al. Group-Based Trajectory Modeling of Suppression Ratio After Cardiac Arrest. Neurocrit Care. 2016;25:415–23. doi: 10.1007/s12028-016-0263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schweickert WD, Gehlbach BK, Pohlman AS, Hall JB, Kress JP. Daily interruption of sedative infusions and complications of critical illness in mechanically ventilated patients. Crit Care Med. 2004;32:1272–6. doi: 10.1097/01.ccm.0000127263.54807.79. [DOI] [PubMed] [Google Scholar]
- 11.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 12.San-juan D, Chiappa KH, Cole AJ. Propofol and the electroencephalogram. Clin Neurophysiol. 2010;121:998–1006. doi: 10.1016/j.clinph.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Greer DM. Mechanisms of injury in hypoxic-ischemic encephalopathy: implications to therapy. Semin Neurol. 2006;26:373–9. doi: 10.1055/s-2006-948317. [DOI] [PubMed] [Google Scholar]
- 14.Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26:5–13. doi: 10.3233/NRE-2010-0531. [DOI] [PubMed] [Google Scholar]
- 15.Maynard D, Prior PF, Scott DF. Device for continuous monitoring of cerebral activity in resuscitated patients. Br Med J. 1969;4:545–6. doi: 10.1136/bmj.4.5682.545-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maynard D, Prior PF, Scott DF. A continuous monitoring device for cerebral activity. Electroencephalogr Clin Neurophysiol. 1969;27:672–3. doi: 10.1016/0013-4694(69)91265-6. [DOI] [PubMed] [Google Scholar]
- 17.Prior PF, Maynard D, Scott DF. A new device for continuous monitoring of cerebral activity: its use following cerebral anoxia. Electroencephalogr Clin Neurophysiol. 1970;28:423–4. [PubMed] [Google Scholar]
- 18.Prior PF, Maynard DE, Sheaff PC, Simpson BR, Strunin L, Weaver EJ, et al. Monitoring cerebral function: clinical experience with new device for continuous recording of electrical activity of brain. Br Med J. 1971;2:736–8. doi: 10.1136/bmj.2.5764.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raina KD, Rittenberger JC, Holm MB, Callaway CW. Functional Outcomes: One Year after a Cardiac Arrest. Biomed Res Int. 2015;2015:283608. doi: 10.1155/2015/283608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittenberger JC, Raina K, Holm MB, Kim YJ, Callaway CW. Association between Cerebral Performance Category, Modified Rankin Scale, and discharge disposition after cardiac arrest. Resuscitation. 2011;82:1036–40. doi: 10.1016/j.resuscitation.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rundgren M, Westhall E, Cronberg T, Rosen I, Friberg H. Continuous amplitude-integrated electroencephalogram predicts outcome in hypothermia-treated cardiac arrest patients. Crit Care Med. 2010;38:1838–44. doi: 10.1097/CCM.0b013e3181eaa1e7. [DOI] [PubMed] [Google Scholar]
- 22.Oh SH, Park KN, Kim YM, Kim HJ, Youn CS, Kim SH, et al. The prognostic value of continuous amplitude-integrated electroencephalogram applied immediately after return of spontaneous circulation in therapeutic hypothermia-treated cardiac arrest patients. Resuscitation. 2013;84:200–5. doi: 10.1016/j.resuscitation.2012.09.031. [DOI] [PubMed] [Google Scholar]
- 23.Seder DB, Fraser GL, Robbins T, Libby L, Riker RR. The bispectral index and suppression ratio are very early predictors of neurological outcome during therapeutic hypothermia after cardiac arrest. Intensive Care Med. 2010;36:281–8. doi: 10.1007/s00134-009-1691-1. [DOI] [PubMed] [Google Scholar]
- 24.Fabregas N, Gambus PL, Valero R, Carrero EJ, Salvador L, Zavala E, et al. Can bispectral index monitoring predict recovery of consciousness in patients with severe brain injury? Anesthesiology. 2004;101:43–51. doi: 10.1097/00000542-200407000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Selig C, Riegger C, Dirks B, Pawlik M, Seyfried T, Klingler W. Bispectral index (BIS) and suppression ratio (SR) as an early predictor of unfavourable neurological outcome after cardiac arrest. Resuscitation. 2014;85:221–6. doi: 10.1016/j.resuscitation.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Schomer AC, Hanafy K. Neuromonitoring in the ICU. Int Anesthesiol Clin. 2015;53:107–22. doi: 10.1097/AIA.0000000000000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soholm H, Kjaer TW, Kjaergaard J, Cronberg T, Bro-Jeppesen J, Lippert FK, et al. Prognostic value of electroencephalography (EEG) after out-of-hospital cardiac arrest in successfully resuscitated patients used in daily clinical practice. Resuscitation. 2014;85:1580–5. doi: 10.1016/j.resuscitation.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 28.Dericioglu N, Khasiyev F, Arsava EM, Topcuoglu MA. Frontal Intermittent Rhythmic Delta Activity (FIRDA) in the Neurological Intensive Care. Clin EEG Neurosci. 2017 doi: 10.1177/1550059416688108. 1550059416688108. [DOI] [PubMed] [Google Scholar]
- 29.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s Standardized Critical Care EEG Terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]


