Abstract
Chronic use of drugs of abuse results in neurochemical, morphological and behavioral plasticity that underlies the emergence of compulsive drug seeking and vulnerability to relapse during periods of attempted abstinence. Identifying and reversing addiction-relevant plasticity is seen as a potential point of pharmacotherapeutic intervention in drug-addicted individuals. Despite considerable advances in our understanding of the actions of drugs of abuse in the brain, this information has thus far yielded few novel treatment options addicted individuals. MicroRNAs are small noncoding RNAs that can each regulate the translation of hundreds to thousands of messenger RNAs. The highly pleiotropic nature of miRNAs has focused attention on their contribution to addiction-relevant structural and functional plasticity in the brain and their potential utility as targets for medications development. In this review, we discuss the roles of miRNAs in synaptic plasticity underlying the development of addiction and then briefly discuss the possibility of using circulating miRNA as biomarkers for addiction.
Keywords: Addiction, alcohol, circulating RNA, epigenetics, microRNA, neuroplasticity, opiates, psychostimulants, synaptic plasticity
Drug addiction is a problem with enormous costs at the individual, familial and societal levels (Volkow & Skolnick 2012). The current state of treatment for substance use disorders leaves much to be desired. There are currently just 12 FDA-approved medications for any substance use disorder: seven therapeutics are approved for smoking cessation, three for opiate dependence and two for alcohol dependence. Furthermore, of these 12 medications, 8 are considered maintenance or replacement therapies that decrease craving for the drug by mimicking the effects of the drug and as a consequence do not necessarily address the root cause of the disorder (Mattson & Lynch 2013). There are currently no FDA-approved treatments for illicit psychostimulant addiction.
Chronic drug use results in maladaptive neuroplasticity that underlies development of compulsive drug seeking and vulnerability to relapse, even after protracted abstinence (Kalivas & Volkow 2005). Targeting this plasticity is seen as a potential point of pharmacotherapeutic intervention (Kalivas & Volkow 2011). The transition from recreational to compulsive drug use is a hallmark trait in the development of addiction, and understanding the underlying neurobiology of this transition has received a great deal of attention (Kenny 2007). Extensive experimental efforts have shown that dopaminergic and glutamatergic plasticity within the striatum following extended access to cocaine self-administration contributes to escalation of cocaine intake and development of compulsive-like drug seeking behavior (Chen et al. 2013; Everitt & Robbins 2016; Kenny 2007). Specifically, corticostriatal glutamatergic projections provide ‘top-down’ control over drug seeking and modulate initial motivation to seek the drug. During the development of addiction, the prefrontal cortex becomes hypoactive, and disrupted glutamate homeostasis primes this system to show hyperactive responses to drug-associated cues (Chen et al. 2013). This is also evidenced in human imaging data that show decreased prefrontal-cortical control in response to adverse outcomes (hypofrontality) yet increased subcortical sensitivity to reward (Vaquero et al. 2017). Initially, drug reward is mediated by the nucleus accumbens (NAc) subregion of ventral striatum (Caine et al. 1995). However, the dorsal striatum (DS) is thought to play a progressively greater role as drug-seeking transitions from casual to compulsive (Zapata et al. 2010).
A great deal of attention has been paid to examining synaptic plasticity at multiple levels. Protein modification and trafficking induces structural and functional neuroplasticity within single dendritic spines. For example, relapse to each cocaine, nicotine and heroin causes enlargement of dendritic spine heads, increased AMPA/NMDA (N-methyl-D-aspartate) receptor ratio and activation of matrix metalloproteinase (MMP) activity (Gipson et al. 2013a,b; Kourrich et al. 2007; Shen et al. 2011; Smith et al. 2014). Local protein synthesis in synapses also regulates relapse following incubation of craving, as inhibition of local translation reduced calcium-permeable α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor currents and metabotropic glutamate receptor (mGluR)-mediated plasticity (Scheyer et al. 2014). In addition to extensive experimentation examining protein translation, trafficking and activity, genetic and epigenetic regulation of mRNA expression receives a great deal of attention (Heller et al. 2016; Robison & Nestler 2011; Wright et al. 2015). However, a relatively novel mechanism for bridging the gap between nuclear epigenetic regulation of transcription and synapse-level regulation of translation exists in microRNAs (Kenny 2014).
MicroRNAs are highly conserved small (~21–23 nucleotides) noncoding RNAs that bind to complementary regions of 3′ untranslated regions (UTRs) in target mRNAs to regulate their stability and translation (Lagos-Quintana et al. 2001; Lai 2002; Lau et al. 2001; Vasudevan et al. 2007). There are over 500 identified human genes encoding miRNA, and each miRNA is able to affect hundreds to thousands of protein-coding genes. Comparative analysis of humans, mouse, rat and dog genomes showed a high level of conservation of both miRNA and their complementary regulatory sites within mRNA 3′ UTRs and estimates that between 20% and 50% of protein-coding genes are targeted by miRNA (Lewis et al. 2005; Ouellet et al. 2006; Xie et al. 2005). Furthermore, many miRNAs expressed only in specific cell types can often localize to within specific compartments in these cells. The highly pleiotropic nature of miRNAs has received a great deal of attention for their potential as novel pharmacotherapeutic targets in a plethora of disorders in all bodily systems. In this review, we will briefly examine the broad roles of miRNAs in synaptic plasticity, then specifically in drug-induced plasticity, and finally we will discuss novel insights into circulating miRNAs as biomarkers for neuropsychiatric disease.
miRNA biogenesis, localization and degradation
MicroRNA are transcribed by RNA polymerase II and processed into a ~70 nucleotide stem-loop structure, known as the pre-miRNA, which is then processed by Drosha and exported from the nucleus by a exportin–RanGTP complex. In the cytoplasm the pre-miRNA is further processed by an enzyme called Dicer. This processing step includes unwinding of the duplex into single stranded mature miRNAs (one 5P-miRNA and one 3P-miRNA), and one strand is incorporated with Dicer into an RNA-induced silencing complex (RISC; Fig. 1). A primary essential component of the RISC is a family of RNA binding proteins called Argonautes. Of the four members of the Argonaute family, Argonaute 2 (Ago2) is unique in that it is able to control mRNA stability by identifying sites of perfect mRNA–miRNA complementarity and degrading mRNAs (Liu et al. 2004; Song et al. 2004). While Ago2 possesses endonucleolytic activity, numerous studies show that miRNAs very often repress translation in an Ago2-dependent manner without degradation of the mRNA, usually through complementarity between nucleotides 2 and 7 of the mature miRNAs, known as the ‘seed region’, and the 3′ untranslated region of the targeted transcript.
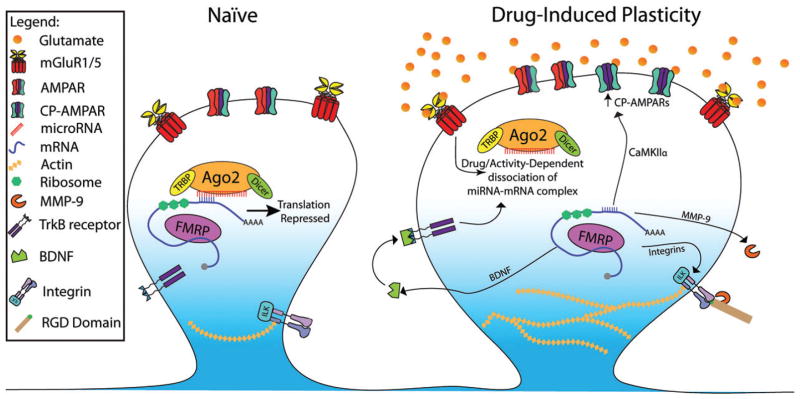
Figure 1. Potential mechanisms by which cocaine-induced changes in miRNA expression or function affect synaptic plasticity.
In drug-naive animals (left), an RISC is targeted to specific mRNA based on complementarity between its microRNA and the 3′ UTR of mRNA. This interaction suppresses translation of the mRNA. In animals repeatedly exposed to drugs of abuse, microRNAs that target pro-plasticity proteins may become downregulated, or synaptic stimulation may cause dissociation of the miRNA–mRNA complex. A number of plasticity-related proteins have been shown to undergo activity-dependent translation that is regulated by microRNAs. Examples showed above are (1) BDNF expression is acutely upregulated in the accumbens in response to cocaine, possibly because of downregulation of miR-495 and disinhibition of BDNF translation. Brain-derived neurotrophic factor is then able to signal through its receptor, TrkB, to stimulate dendritic spine expansion and synaptic plasticity (Bastle et al. 2017). (2) CaMKIIa is similarly targeted by miR-495, and its expression is upregulated in response to acute cocaine (Bastle et al. 2017). CaMKII may phosphorylate serine-845 of GluA1 AMPA receptors, driving calcium-permeable receptors into the synapse. (3) In addition to drug-induced miRNA downregulation, strong neural activity may cause dissociation of miRNA–mRNA complexes. Group 1 mGluR stimulation occurs with cue-induced reinstatement of cocaine seeking (Smith et al., 2017), and causes dissociation of miR-132 from MMP-9 mRNA (Jasinska et al. 2015). Matrix metalloproteinase-9 is an extracellular protease that signals through integrins to stimulate spine enlargement and insertion of CP-AMPARs (Smith et al. 2015). (4) Integrins are direct targets of miR-124 and miR-132 and are upregulated in response to cocaine (Chandrasekar & Dreyer 2011; Wiggins et al. 2011). Integrins can signal through multiple kinases (e.g. integrin-linked kinase) to phosphorylate cofilin and stimulate actin polymerization and dendritic spine enlargement. It is important to note that while this depicts miRNA downregulated by cocaine, this is often region or miRNA-specific, and many other miRNA are upregulated, suppressing their own gene networks. Acronyms: CaMKII, calmodulin-dependent kinase 2; CP-AMPAR, calcium permeable AMPA receptor; RGD, arginine–glycine–aspartic acid; TRBP, TAR RNA binding protein.
Once the mature miRNA has been generated, there are multiple possible fates. A large number of miRNAs exist in the cytoplasm of neuronal cell bodies, often associated with complexes called processing bodies (PBs) that are known to serve as sites for mRNA storage and degradation (Leung 2015). Ago2 phosphorylation at serine-387, mediated by p38 mitogen-activated protein kinase (MAPK) or AKT, stimulates translocation of the RISC complex to PBs and increases translational repression (Horman et al. 2013; Zeng et al. 2008). In addition to PBs, miRNAs frequently localize in processes known as stress granules (SGs), which appear transiently in the cytoplasm in response stressful conditions and contain pools of translationally stalled mRNAs. In the absence of miR-NAs following knockout of Dicer, Ago2 still shows enrichment in PBs, but not SGs, indicating that miRNAs are a crucial component of SGs but not PBs (Leung 2015). miRNAs also play a key role in regulation of local protein synthesis in response to extra- or intracellular signals and can be trafficked to specific subcellular compartments by RNA binding proteins such as the fragile X mental retardation protein (FMRP), as FMRP association with miR-125b and miR-132 is required for their effects on synaptic morphology (Edbauer et al. 2010). Recent evidence has showed that miRNA can also be packaged into exosomes for extracellular secretion to affect neighboring neurons or other tissues through the circulatory system. This is a novel mechanism of action for miRNA that this review will only briefly discuss.
miRNAs in synaptic plasticity
Synaptic plasticity is broadly defined as the ability of synapses to strengthen or weaken over time in response to the amount of stimulation they receive. This plasticity can be divided into two categories: structural and functional plasticity. Structural plasticity refers to events that fundamentally change the number or structure of neurons, such as neurogenesis, neurite outgrowth and enlargement or shrinking of dendritic spines (Robinson & Kolb 2004). Functional plasticity refers to changes in the electrophysiological synaptic strength or chemical signaling occurring within neurons, such as changes in AMPA/NMDA receptor ratio or the slope of field potentials in long-term potentiation (LTP) or depression (Sweatt 2016). miRNAs have been heavily implicated in both forms of synaptic plasticity, and many specific brain-enriched miRNA are tightly temporally controlled following stimulation paradigms that elicit LTP or depression, leading to the hypothesis that miRNA coordinate changes in protein expression underlying different phases of these changes (Ye et al. 2016). For the purposes of this review we will focus on miRNAs that have established roles in both general synaptic physiology and drug-induced plasticity.
One of the most studied miRNA in the context of synaptic plasticity has been miR-132, a cyclic-adenosine 5′-phosphate (cAMP) response element binding (CREB) induced miRNA that is enriched in neurons (Nudelman et al. 2010). Neuronal stimulation upregulates miR-132 in a CREB-dependent manner (Nudelman et al. 2010). In cultured cortical neurons miR-132 is required for CREB-mediated dendritic growth and spine formation, as well as for the neurotrophic effects of brain-derived neurotrophic factor (BDNF; Fig. 1) (Magill et al. 2010; Vo et al. 2005). Brain-derived neurotrophic factor itself exerts its effects through the TrkB and p75 receptors and can affect various intracellular signaling cascades such as the phsophoinositide-3-kinase (PI3K), phospholipase-C (PLC), MAPK, as well as CREB (Chen et al. 2012; Li et al. 2012; Numakawa et al. 2009). Furthermore, glucocorticoids suppress miR-132 expression and the cellular effects of BDNF (Kawashima et al. 2010). Consistently, inhibiting miR-132 signaling causes inhibition of ocular dominance plasticity in vivo in mice following monocular deprivation (Mellios et al. 2011). In depth examination of this ocular dominance plasticity supported a role for miR-132 in dendritic spine head development; i.e. miR-132 inhibition led to more immature filopodia and less mushroom-type spine heads. In human patients, miR-132 has been implicated in disorders of hyperexcitability through examination of patients with intractable temporal lobe epilepsy, as tissue surgically removed from the temporal lobe contained increased miR-132 and phosphorylated CREB (Guo et al. 2014).
Several interesting targets of miR-132 have been implicated in its role in synaptic plasticity. Most recently, miR-132 has been shown to regulate the morphology of dendritic spines in the hippocampus through direct targeting of mRNA encoding MMP-9 (Fig. 1), an extracellular matrix-remodeling enzyme that is critical for long-term memory as well as addiction (Nagy et al. 2006; Smith et al. 2014; Wright et al. 2003). Matrix metalloproteinase-9 mRNA is part of an FMRP complex at synapses and undergoes local translation in response to neuronal activity (Dziembowska et al. 2012; Janusz et al. 2013), and MMP-9 expression and activity are upregulated in FMR1 knockout mice (Dziembowska et al. 2013). miR-132 seems to be involved in the regulation of this activity-dependent translation, as application of the mGluR1/5 agonist 3,5-dihydroxyphenylglycine increases miR-132 in a free messenger ribonucleoprotein complex, suggesting it has dissociated from MMP-9 mRNA and released translational repression (Jasinska et al. 2015).
A second miRNA in the same family as miR-132 is miR-212. The two are encoded by the same intron of a noncoding gene and are typically coexpressed. Furthermore, they have identical seed regions and thus have similar predicted targets (Tognini & Pizzorusso 2012). Many studies have examined the role of miR-132/212 together in synaptic plasticity. Cre-mediated deletion of both miRNA results in reduction in dendritic length and complexity in granule cell neurons in the hippocampus (Magill et al. 2010), and a constitutive double knockout shares this phenotype, as well as reduced synaptic transmission and enhanced theta burst-induced LTP (Remenyi et al. 2013).
In addition to miR-132/212, a handful of other miRNA have well-established roles in synaptic plasticity (Ye et al. 2016). miR-124 is a brain-enriched miRNA that is heavily implicated in neuronal development by acting as a molecular switch encouraging mRNA splicing of brain-specific mRNA (Makeyev et al. 2007). In the adult brain, miR-124 remains one of the most highly expressed miRNA and has established roles in neuronal physiology and plasticity (Chandrasekar & Dreyer 2009; Rajasethupathy et al. 2009). Serotonin (5HT), a neuromodulatory transmitter that is important for memory, rapidly downregulates miR-124 through MAPK signaling. This downregulation of miR-124 was required for induction of CREB activity and signaling and LTP. Contrarily, artificially enhancing miR-124 activity reduced excitory postsynaptic potential slope following electrical stimulation inducing long-term facilitation in Aplysia (Rajasethupathy et al. 2009). miR-124 targets the 3′UTR of a protein that has been implicated in human learning and memory called intelligence quotient motif containing GTPase activating protein 1 (IQGAP1), and a single nucleotide polymorphism that deletes the miR-124 binding site is associated with better task performance (Yang et al. 2014). In the hippocampus of IQGAP1 knockout mice, surface expression of the NR2A NMDA receptor subunit is decreased and NMDA receptor-mediated extracellular-signal related kinase (ERK) signaling is impaired, as well as LTP and contextual fear conditioning (Gao et al. 2011). miR-124 also affects local translation of GluA2 AMPA receptor subunits in the hippocampal cultures by direct targeting of the GluA2 3′UTR, and overexpression of miR-124 downregulates GluA2 expression by approximately 30% (Ho et al. 2014). GluA2 is the calcium-gating subunit of AMPA receptors, and insertion of GluA2-lacking receptors is a mechanism of increasing synaptic strength. Thus, suppression of GluA2 translation by miR-124 may play a role in LTP by promoting formation of GluA2-lacking AMPA receptors.
In a triple-transgenic mouse model of Alzheimer’s disease in which presinilin-1 (PS1), Tau and amyloid beta precursor protein are altered, miR-181 expression is increased in the hippocampus in an age-dependent manner and functions to decrease expression of the deacetylase sirtuin-1 as well as the immediate early gene c-Fos. Sirtuin-1 has been implicated in plasticity a number of times and is also targeted by miR-132 (Gao et al. 2010; Rodriguez-Ortiz et al. 2014; Zhang et al. 2014). A loss-of-function mutation in SIRT1 protein in mice causes upregulation of miR-134 and downregulation of CREB and BDNF. Furthermore, these mutant mice show decreased synaptophysin immunoreactivity in the hippocampus, indicating decreased synapse number (Gao et al. 2010).
miRNA in drug addiction
Cocaine
Cocaine exerts its effects primarily through blockade of the dopamine transporter, thus increasing synaptic dopamine transmission (Bozarth & Wise 1986). Subsequent increases in dopamine receptor stimulation in the ventral striatum mediate the acute rewarding effects of the drug (Giros et al. 1996). The striatum is composed primarily of medium spiny neurons (MSNs; ~90–95% of neurons) (Graybiel 1991). These cells can be broadly subcategorized by their genotypic expression patterns and neuroanatomical projections. One population of MSNs coexpresses the Gs or Golf coupled D1 receptor and prodynorphin, and project to the substantia nigra pars reticulate (SNpr) and internal portion of the globus pallidus. This is called a ‘direct pathway’, because the MSNs innervate neurons that directly exit the basal ganglia. A second population of MSNs coexpresses the Gi-coupled D2 receptor and enkephalin, and project to the external portion of the globus pallidus, then to the subthalamic nucleus and finally to the internal globus pallidus (Hersch et al. 1995; Yung et al. 1996). This is called the indirect pathway because of its polysynaptic path out of the basal ganglia. The stimulatory nature of the direct pathway and the inhibitory nature of the indirect pathway over locomotion, and also over reward-related behaviors, provide the basic mechanism by which the balance of these two circuits modulates the flow of information through the basal ganglia (Schmidt 1995).
Increases in cAMP production by D1 receptor stimulation causes activation of downstream protein kinases such as protein kinase A, which in turn phosphorylates CREB at serine-133 to exert effects on gene expression (Kano et al. 1995). Infusion of cAMP analogs directly into the NAc causes an increase in CREB phosphorylation and cocaine self-administration, and shifted the cocaine dose–response curve to the right, indicating that CREB activity may decrease the reinforcing properties of cocaine (Self et al. 1998). In 2010 our lab produced data showing that extended (6 h/day) access to cocaine self-administration produced an increase in CREB phosphorylation in the DS and that this effect was amplified by overexpression of miR-212. Consistent with CREB activation attenuating the reinforcing effects of cocaine, miR-212 overexpression reduced cocaine self-administration selectively in animals with extended access to the drug, but not restricted (1 h/day) access (Hollander et al. 2010). This effect was mediated by miR-212 suppression of SPRED1, an endogenous inhibitor of Raf1. This increased Raf1 activity and induced sensitization of adenylyl cyclase activity and increased cAMP production, further leading to increased PKA activity and finally CREB phosphorylation. Conversely, we also showed that inhibition of miR-212 via infusion of a locked nucleic acid (LNA) antisense sequence against the full mature miR-212 augmented cocaine self-administration, showing that miR-212 can bidirectionally control compulsive-like cocaine intake under extended access conditions (Hollander et al. 2010). Interestingly, a recent report has showed that both miR-212 and miR-132 are increased in the striatum following short access to cocaine and that this effect is apparently after 10 days of cocaine self-administration and is also persistent through at least 10 days of abstinence (Sadakierska-Chudy et al. 2017).
Further support for miR-212 in addiction has been shown by two recent publications by Quinn et al. Initially, they showed that miR-212 is downregulated in the dorsomedial striatum (DMS) of addiction-prone rats, but not of addiction-resistant rats. It is notable that it was the only miRNA that was down-regulated in these rats, while both miR-101b and miR-431 were upregulated. The effect of miR-212 was specific to the DMS and was not apparent in the dorsolateral striatum (DLS). Interestingly, miR-212 family member miR-132 was upregulated specifically in the DLS, but not the DMS striatum of the same addiction-prone rats. The effects of miR-101b and miR-431 were not subregion-specific, as they occurred in both areas (Quinn et al. 2015). These data support the work by Hollander et al., showing that increased miR-212 in the DS leads to increased cocaine intake (Hollander et al. 2010). In a follow-up study, Quinn et al. then examined the effects of extinction training and reinstatement testing on microRNA expression in both the dorsal and ventral striatum. They found that following reinstatement, addiction-prone rats had increased levels of miR-101b, miR-137, miR-212 and miR-132 in the NAshell, and miR-137 in the DLS. Furthermore, the decrease in miR-212 in DMS of addiction prone relative to resilient animals was still apparent following reinstatement, indicating it may be a long-lasting change induced by cocaine exposure (Quinn et al. 2017).
A second interesting target for miR-212 in the striatum is the epigenetic regulator methyl CpG binding protein 2 (MeCP2). Mutation of the MeCP2 gene is the primary cause of Rett syndrome (Bienvenu et al. 2000) and has been the focus of many studies examining its role in learning, memory and executive function (Moretti et al. 2006). MeCP2-null mice show decreased tyrosine hydroxylase (TH) immunore-activity and dopaminergic tone in the striatum (Panayotis et al. 2011). Furthermore, MeCP2 is broadly implicated in addiction, as repeated cocaine injections increase its expression in the DS, among other regions (Cassel et al. 2006). MeCP2 acts as a transcriptional repressor through recruitment of histone deacetylases to methylated DNA segments (Bird 2002). However, more recently its function as a transcriptional activator has come to light, partly through CREB interactions (Chahrour et al. 2008). Our lab has showed that MeCP2 can repress expression of miR-212. Interestingly, MeCP2 is upregulated in animals with extended access to cocaine compared with restricted access rats, and knock down of striatal MeCP2 decreases the reinforcing value of the drug (Im et al. 2010). Following extended access to cocaine self-administration, MeCP2 knockdown results in exacerbated miR-212 upregulation, and increased miR-212 signaling is thought to explain the decreased cocaine intake observed in rats after MeCP2 knockdown (Im et al. 2010). Intriguingly, MeCP2 itself is a target for miR-212. This suggests that MeCP2 and miR-212 homeostatically regulate expression of each other. Viola et al. (2016) showed that MeCP2 is upregulated following cocaine conditioned place preference (CPP), and this is accompanied by a decrease in miR-212 expression (Viola et al. 2016).
Most recently, Bastle et al. uncovered an important role for the striatal-enriched miR-495 in the NAc as an important regulator of motivation for cocaine intake (Bastle et al. 2017). The authors showed that this miRNA is quickly downregulated in response to an acute injection of cocaine. Using luciferase reporter assays, they showed that miR-495 targets mRNA encoding BDNF, CaMKIIa and Arc, three genes previously known to be important for addiction (Anderson et al. 2008; Berglind et al. 2009; Hearing et al. 2011). Then they verified that these proteins are upregulated by an acute injection of cocaine, at the same time point that miR-495 is downregulated, indicating that suppression of the miRNA by cocaine alleviated inhibition of protein translation. Over-expression of miR-495 attenuated acute cocaine-induced upregulation of BDNF and CaMKIIa mRNA and reduced motivation for cocaine during progressive ratio testing. Furthermore, miR-495 reduced responding during the first 3 days of extinction training and attenuated cocaine-primed reinstatement (Bastle et al. 2017).
Expression patterns of several other miRNA are also regulated by cocaine exposure. Two weeks of daily experimenter-administered cocaine, miR-124a and let-7d are both reduced in the DS, whereas miR-181a is increased in the accumbens, prefrontal cortex and hippocampus. Interestingly, these miRNA are predicted to target a large number of genes that have been previously implicated in cocaine addiction, such as CREB, BDNF, the GluA2 subunit of AMPA receptors, mGluR5 receptors and the β1 subunit of integrin cell-adhesion receptors (Fig. 1) (Boudreau & Wolf 2005; Chandrasekar & Dreyer 2009; Conrad et al. 2008; Hollander et al. 2010; Moussawi et al. 2009; Wiggins et al. 2011). Manipulation of the expression of these miRNA within the NAc is able to bidirectionally control cocaine CPP after extinction training. That is, overexpression of miR-124 or let-7 attenuated CPP, whereas their inhibition augmented this preference. Conversely, overexpression of miR-181a augmented cocaine CPP, whereas its inhibition attenuated CPP (Chandrasekar & Dreyer 2011).
As mentioned previously, the striatum is comprised of two major cell types that act in opposition of each other: striatonigral MSNs that express the D1 receptor, and striatopallidal neurons that express the D2 receptor. A major question left unanswered is which cell type these microRNAs act within to exert their effects over cocaine reinforcement. Schaefer et al. began to broadly answer that question by generating a mouse line in which Ago2 is selectively knocked out in D2-expressing neurons, hypothetically abolishing miRNA signaling within these cells. Under these conditions, knockout mice self-administer significantly less cocaine than wild-type littermates. Furthermore, the mutant mice have impaired cocaine CPP, consistent with the hypothesis that the drug is less rewarding without the presence of Ago2. Interestingly, in contrast with Dicer knockout mice that are not viable, Ago2 knockout mice had no impairments in normal operant learning, sucrose preference or cocaine-induced hyperlocomotion (Schaefer et al. 2010).
Alcohol
While the vast majority of attention regarding miRNAs in addiction has been paid to cocaine, there is also some literature regarding miRNA contributions to alcoholism. While the effects of alcohol on miR-212 have not been examined, a peripheral effect linking these two has been determined. In patients with alcoholic liver disease (ALD), there is an upregulation of miR-212 in the colon that underlies decreased expression of Zona Occludens-1 (ZO-1). This causes disruptions of tight junctions within the gut, allowing intestinal permeability, endotoxin leakage into circulation and the progression of ALD (Tang et al. 2008).
miR-9 is a brain-enriched miRNA that is upregulated in the striatum following exposure to alcohol that regulates neuronal responses to alcohol through alternative splicing of BK channels. These Ca2+ and voltage-gated K+ channels contribute to neuronal excitability, and their expression is downregulated by alcohol. miR-9 controls tolerance to alcohol by targeting the 3′UTR of a specific splice variant with increased sensitivity to alcohol. By decreasing the expression of this splice variant, the channels sensitivity to alcohol is decreased (Pietrzykowski et al. 2008).
Following 2 weeks of voluntary ethanol drinking in rats, miR-124 expression is downregulated, and protein expression of its target BDNF is upregulated in the dorsolateral, but not DMS. Overexpression of miR-124 enhances ethanol-induced CPP, whereas siRNA knockdown of miR-124 attenuates this CPP. Consistently, overexpression of BDNF also attenuates ethanol CPP. Furthermore, overexpression of miR-124 increases ethanol consumption during a two-bottle choice paradigm, whereas BDNF overexpression or miR-124 knockdown decreases alcohol consumption (Bahi & Dreyer 2013). A second microRNA that regulates BDNF expression, miR-206, is also altered by chronic alcohol exposure in rats. Tapocik et al. showed upregulation of miR-206 specifically in the medial prefrontal cortex (mPFC), but not the ventral tegmental area (VTA), amygdala or NAc, following 3 weeks of withdrawal from chronic ethanol exposure (Tapocik et al. 2014). Furthermore, viral overexpression of miR-206 in the mPFC recapitulated escalation of alcohol self-administration in nonchronically exposed rats, and also caused a decrease in BDNF expression. Together, these data form a causal link between miR-206 expression and function and the development of alcohol dependence-like behaviors (Tapocik et al. 2014).
Opiates
The miRNA let-7 downregulates expression of the mu opioid receptor (MOR) through binding to its 3′UTR, and morphine upregulation of let-7 expression contributes to opioid tolerance via downregulation of the receptor (He et al. 2010; He & Wang 2012). Chronic morphine treatment decreased Ago2 expression in the VTA, whereas naloxone-precipitated withdrawal induced an upregulation of Ago2. However, the expression of miR-133b, which has previously been shown to be enriched in TH-expressing dopaminergic midbrain neurons, was not altered (Garcia-Perez et al. 2015). Morphine downregulates the expression of miR-27a, which directly targets the 3′UTR of Serpini1 (also called Neuroserpin), a protein previously implicated in dendritic spine development. Surprisingly, miR-27a targeting of Serpini1 serves as a translational activator (Borges et al. 2010; Tapocik et al. 2016). Translational activation by miRNA binding occurs when AU-rich motifs near miRNA binding sites enhance translation, although the mechanism by which this happens is not understood (Vasudevan et al. 2007). Mu opioid receptors also regulate miR-190 in a manner that is agonist-dependent. That is, fentanyl, but not morphine decreases miR-190 in the rodent hippocampus. This is because of fentanyl’s ability to stimulate beta-Arrestin signaling through the MOR, which stimulates ERK phosphorylation of Yin Yang 1 (YY1) and transcription of talin2 (Zheng et al. 2010).
Only a single study has examined the effects of self-administered morphine on miRNA expression. Tapocik et al. examined two strains of mice, C57BL/6J and DBA/2J, comparing mice that actively self-administered morphine with mice that had passive morphine infusions yoked to self-administering counterparts. They found that in C57BL6/J mice there were over 200 genes regulated specifically by morphine self-administration in the ventral striatum, and over 100-altered genes in the ventral mesencephalon. Interestingly, <3% of these genes were also altered in DBA/2J mice (Tapocik et al. 2013). Standout miRNA that were changed in both strains included miR-675 and miR-154. miR-675 has been implicated in dopaminergic neuronal differentiation, while miR-154 targets the 3′UTR of the MOR (Tapocik et al. 2013).
Most recently, Yan et al. (2017) showed that chronically administered heroin reduces miR-218 expression in the accumbens (but not the hippocampus or PFC), and virally rescuing this expression decreases heroin self-administration. Many plasticity-related genes that were predicted targets of miR-218 were upregulated, including Gabrb3, GluR2, Ube3a, Nrxn1, Gng3 and Mecp2. The authors went on to perform a luciferase reporter assay and quantitative polymerase chain reaction (qPCR) to verify that MeCP2 is a bona fide target of miR-218 (Yan et al. 2017).
Nicotine
Nicotine, the primary psychoactive component of tobacco, induces coordinated changes in gene expression for many genes related to synaptic plasticity that underlie the development of nicotine addiction. However, contributions of miRNA to nicotine-induced plasticity have only been recently studied (Dreyer 2010). In 2009, Huang and Li published the first two papers on the topic. First, they showed that miR-140-5P targets the 3′UTR and decreases expression of the gene encoding Dynamin-1, a protein involved in protein endocytosis that binds to the β2 subunit of the α4β2 nicotinic acetylcholine receptors (nAChR), a crucial receptor in the brain for nicotine reward (Huang & Li 2009b). In the second paper, they show that miR-504 directly targets the 3′ UTR of the dopamine D1 receptor and that this targeting upregulates expression of the receptor (Huang & Li 2009a).
Circulating miRNA as biomarkers for addiction
The vast majority of miRNA are found intracellularly, however, a significant number of miRNAs have been observed in the extracellular environment, including the bloodstream. Given the presence of ribonucleases in serum and other bodily fluids, it is quite surprising to find stably expressed RNAs of any type here, indicating that they must be protected somehow, possibly by a lipid vesicle (Etheridge et al. 2011). Indeed, 40–100 nm diameter lipid exosomes have been described as vesicles that are released into the extracellular space to deliver their content to neighboring cells, sometimes being released from neurons in response to depolarization, and targeted to specific cell types depending on the expression profile of surface proteins (Goldie et al. 2014; Zhang et al. 2015). Although the mechanisms of miRNA packaging into exosomes and intercellular delivery is not well understood, this discovery has led to significant attention paid to circulating miRNAs as biomarkers for disease. At the time of this publication, a PubMed search for ‘circulating miRNA cancer’, returns 1367 results. A search for ‘circulating miRNA addiction’ produces only a single result, although more diligent searching does uncover some interesting information, primarily regarding nicotine and alcohol. It is important to note the difficulty of interpretation of results from studies measuring circulating miRNAs. Many pathological states can result in migration of cell populations (such as leukocytes) from endothelium-attached to freely circulating states, or between intrastitial and intravascular compartments (Muller 2014). Thus, changes observed in circulating miRNA are potentially because of more broad immune activation or cellular migration. This is an important caveat that should be considered when designing experiments or considering therapeutic value of these results. Techniques to isolate exosomes and specifically measure noncellular circulating miRNAs, or examining correlation of circulating miRNAs with leukocyte counts, would help to address this caveat.
In a relatively small study of Japanese smokers vs. non-smokers, 44 of 66 miRNA detected in plasma were significantly different between the two groups. Forty-three of those miRNA were significantly increased in smokers, while only miR-188-5P was decreased compared with nonsmokers. This was a reversible change, as subjects who had quit smoking had similar plasma miRNA profiles as nonsmokers. Interestingly, the effect of smoking may not be directly attributed to nicotine, because one patient who had quit smoking but used a nicotine patch had a similar plasma miRNA profile to nonsmokers (Takahashi et al. 2013). A second larger study conducted in the UK detected a significant increase in circulating miR-124 in smokers compared with nonsmokers and ex-smokers. They also detected a significant difference in let-7a, but only in smokers compared with ex-smokers, and not compared with nonsmokers. The discovery of miR-124 as a circulating miRNA is quite interesting because it is a neuron-specific miRNA, indicating that it is being actively secreted from neurons into the bloodstream (Banerjee et al. 2015).
Acute ethanol consumption (blood concentration 113 mg/dl) results in a modest (approximately twofold) increase in miR-122 concentration in serum. miR-122 is of particular interest because it is considered an early biomarker for liver injury, for example, acetaminophen toxicity produces 100–10 000-fold increase in serum miR-122 (McCrae et al. 2016). A second study looking at the effects of alcohol use during pregnancy showed that at the time of birth, women who reported 1 or more binge drinking episode, or ≥3 drinks per week during pregnancy had an altered miRNA circulation profile compared with women who did not report drinking during pregnancy. Fifty-five miRNAs were significantly altered between the two groups, 46 of which were elevated, and 9 were downregulated. Pathway analysis highlighted miRNA known to bind to genes related to alcohol use and plasticity such as BDNF, ERK1/2, PI3K, Akt, etc. (Gardiner et al. 2016).
A single paper has examined the expression profiles of circulating miRNA in patients with methamphetamine use disorder (MUD). They found that in 124 patients with MUD, miR-181a, miR-15b, let-7e and let-7d were each decreased compared with in normal controls. Further study is needed to determine whether circulating miRNA expression is also detected in patients diagnosed with substance use disorder for other psychostimulants, but these may be a promising new biomarker for drug addiction (Zhao et al. 2016).
Therapeutic potential of miRNA manipulation
Although the first mammalian miRNA was only discovered less than two decades ago (Reinhart et al. 2000), their pleiotropic nature of regulating protein expression was rapidly recognized as a potentially powerful pharmacotherapeutic target in a huge number of disorders. While none of these compounds have been tested in clinical trials for neuropsychiatric disorders, we may be able to look to other fields for a glimpse into the future of potential therapeutics. miRNA-based therapeutics can be separated into mimics and inhibitors (anti-miRs) of the endogenous miRNA (Rupaimoole & Slack 2017). Very talented chemists have already created a huge number of variations of these compounds, considering how recently miRNA were discovered. Currently registered on clinicaltrails.gov are dozens of trials administering either microRNA mimics or anti-miRs. Most notably, there are three microRNA mimic delivery strategies being tested in phase 1 for safety for a scleroderma (miR-29), mesothelioma (miR-16), and multiple solid tumor syndrome (miR-34). Additionally, there are four notable anti-miRs currently being investigated in the clinic. Current antisense oligonucleotides against microRNA have been modified into ‘LNA’ structures, protecting them from RNAse degradation and allowing insertion into membranes and delivery to intracellular compartments. Most notably, Miraversen, by Santaris Pharma, is an LNA-anti-miR-122 and is showing promising results against hepatitis virus C. The drug has completed phase IIa trials and is currently being investigated in multicenter phase IIb trials. Regulus Therapeutics is also developing an anti-miR-122, although their compound relies on N-Acetylgalactocosamine (GalNAc) conjugation to anti-miR-122, rather than a LNA conformation. This compound is also currently in phase 2. A second GalNAc-conjucated antimer from Regulus Therapeutics, against miR-103/107, is currently in phase 2 trials for type 2 diabetes and nonalcoholic fatty liver disease.
Conclusions
MicroRNAs are highly pleiotropic epigenetic regulators of gene expression that are able to coordinate the expression of hundreds of genes in response to internal and external stimuli. Many miRNAs have been implicated in synaptic plasticity and drug addiction, and neuron-specific miRNAs have been shown to be secreted into the bloodstream in response to at least one drug of abuse (tobacco), indicating that these may have potential as biomarkers for addiction in the future. Furthermore, a great deal of attention has been paid to miRNA for development of novel pharmacotherapeutics, and these may represent a novel target for treatment of drug addiction and other neuropsychiatric diseases.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Bahi A, Dreyer JL. Striatal modulation of BDNF expression using microRNA124a-expressing lentiviral vectors impairs ethanol-induced conditioned-place preference and voluntary alcohol consumption. Eur J Neurosci. 2013;38:2328–2337. doi: 10.1111/ejn.12228. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Waters D, Camacho OM, Minet E. Quantification of plasma microRNAs in a group of healthy smokers, ex-smokers and non-smokers and correlation to biomarkers of tobacco exposure. Biomarkers. 2015;20:123–131. doi: 10.3109/1354750X.2014.1000970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastle RM, Oliver RJ, Gardiner AS, Pentkowski NS, Bolognani F, Allan AM, Chaudhury T, St Peter M, Galles N, Smith C, Neisewander JL, Perrone-Bizzozero NI. In silico identification and in vivo validation of miR-495 as a novel regulator of motivation for cocaine that targets multiple addiction-related networks in the nucleus accumbens. Mol Psychiatry. 2017 doi: 10.1038/mp.2016.238. https://doi.org/10.1038/mp.2016.238. [DOI] [PMC free article] [PubMed]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Carrie A, de Roux N, Vinet MC, Jonveaux P, Couvert P, Villard L, Arzimanoglou A, Beldjord C, Fontes M, Tardieu M, Chelly J. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9:1377–1384. doi: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Borges VM, Lee TW, Christie DL, Birch NP. Neuroser-pin regulates the density of dendritic protrusions and dendritic spine shape in cultured hippocampal neurons. J Neurosci Res. 2010;88:2610–2617. doi: 10.1002/jnr.22428. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Involvement of the ventral tegmental dopamine system in opioid and psychomotor stimulant reinforcement. NIDA Res Monogr. 1986;67:190–196. [PubMed] [Google Scholar]
- Caine SB, Heinrichs SC, Coffin VL, Koob GF. Effects of the dopamine D-1 antagonist SCH 23390 microinjected into the accumbens, amygdala or striatum on cocaine self-administration in the rat. Brain Res. 1995;692:47–56. doi: 10.1016/0006-8993(95)00598-k. [DOI] [PubMed] [Google Scholar]
- Cassel S, Carouge D, Gensburger C, Anglard P, Burgun C, Dietrich JB, Aunis D, Zwiller J. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–492. doi: 10.1124/mol.106.022301. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, Qin J, Zoghbi HY. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. microRNAs miR-124, let-7d and miR-181a regulate cocaine-induced plasticity. Mol Cell Neurosci. 2009;42:350–362. doi: 10.1016/j.mcn.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Chandrasekar V, Dreyer JL. Regulation of miR-124, Let-7d, and miR-181a in the accumbens affects the expression, extinction, and reinstatement of cocaine-induced conditioned place preference. Neuropsychopharmacology. 2011;36:1149–1164. doi: 10.1038/npp.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen DY, Bambah-Mukku D, Pollonini G, Alberini CM. Glucocorticoid receptors recruit the CaMKIIalpha-BDNF-CREB pathways to mediate memory consolidation. Nat Neurosci. 2012;15:1707–1714. doi: 10.1038/nn.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010;2:1–7. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR, Kaczmarek L. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 2012;32:14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Pretto DI, Janusz A, Kaczmarek L, Leigh MJ, Gabriel N, Durbin-Johnson B, Hagerman RJ, Tassone F. High MMP-9 activity levels in fragile X syndrome are lowered by minocycline. Am J Med Genet, Part A. 2013;161:1897–1903. doi: 10.1002/ajmg.a.36023. [DOI] [PubMed] [Google Scholar]
- Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M. Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron. 2010;65:373–384. doi: 10.1016/j.neuron.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etheridge A, Lee I, Hood L, Galas D, Wang K. Extracellular microRNA: a new source of biomarkers. Mutat Res. 2011;717:85–90. doi: 10.1016/j.mrfmmm.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- Gao J, Wang WY, Mao YW, Graff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010;466:1105–1109. doi: 10.1038/nature09271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Frausto SF, Guedea AL, Tronson NC, Jovasevic V, Leaderbrand K, Corcoran KA, Guzman YF, Swanson GT, Radulovic J. IQGAP1 regulates NR2A signaling, spine density, and cognitive processes. J Neurosci. 2011;31:8533–8542. doi: 10.1523/JNEUROSCI.1300-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Perez D, Lopez-Bellido R, Hidalgo JM, Rodriguez RE, Laorden ML, Nunez C, Milanes MV. Morphine regulates Argonaute 2 and TH expression and activity but not miR-133b in midbrain dopaminergic neurons. Addict Biol. 2015;20:104–119. doi: 10.1111/adb.12083. [DOI] [PubMed] [Google Scholar]
- Gardiner AS, Gutierrez HL, Luo L, Davies S, Savage DD, Bakhireva LN, Perrone-Bizzozero NI. Alcohol use during pregnancy is associated with specific alterations in MicroRNA levels in maternal serum. Alcohol Clin Exp Res. 2016;40:826–837. doi: 10.1111/acer.13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013a;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW. Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A. 2013b;110:9124–9129. doi: 10.1073/pnas.1220591110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- Goldie BJ, Dun MD, Lin M, Smith ND, Verrills NM, Dayas CV, Cairns MJ. Activity-associated miRNA are packaged in Map1b-enriched exosomes released from depolarized neurons. Nucleic Acids Res. 2014;42:9195–9208. doi: 10.1093/nar/gku594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. Basal ganglia – input, neural activity, and relation to the cortex. Curr Opin Neurobiol. 1991;1:644–651. doi: 10.1016/s0959-4388(05)80043-1. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang H, Wang Q, Chen Y, Chen S. Expression of p-CREB and activity-dependent miR-132 in temporal lobe epilepsy. Int J Clin Exp Med. 2014;7:1297–1306. [PMC free article] [PubMed] [Google Scholar]
- He Y, Wang ZJ. Let-7 microRNAs and opioid tolerance. Front Genet. 2012;3:110. doi: 10.3389/fgene.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Yang C, Kirkmire CM, Wang ZJ. Regulation of opioid tolerance by let-7 family microRNA targeting the mu opioid receptor. J Neurosci. 2010;30:10251–10258. doi: 10.1523/JNEUROSCI.2419-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Schwendt M, McGinty JF. Suppression of activity-regulated cytoskeleton-associated gene expression in the dorsal striatum attenuates extinction of cocaine-seeking. Int J Neuropsychopharmacol. 2011;14:784–795. doi: 10.1017/S1461145710001173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller EA, Hamilton PJ, Burek DD, Lombroso SI, Pena CJ, Neve RL, Nestler EJ. Targeted epigenetic remodeling of the Cdk5 gene in nucleus accumbens regulates cocaine- and stress-evoked behavior. J Neurosci. 2016;36:4690–4697. doi: 10.1523/JNEUROSCI.0013-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch SM, Ciliax BJ, Gutekunst CA, Rees HD, Heilman CJ, Yung KK, Bolam JP, Ince E, Yi H, Levey AI. Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J Neurosci. 1995;15:5222–5237. doi: 10.1523/JNEUROSCI.15-07-05222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho VM, Dallalzadeh LO, Karathanasis N, Keles MF, Vangala S, Grogan T, Poirazi P, Martin KC. GluA2 mRNA distribution and regulation by miR-124 in hippocampal neurons. Mol Cell Neurosci. 2014;61:1–12. doi: 10.1016/j.mcn.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Im HI, Amelio AL, Kocerha J, Bali P, Lu Q, Willoughby D, Wahlestedt C, Conkright MD, Kenny PJ. Striatal microRNA controls cocaine intake through CREB signalling. Nature. 2010;466:197–202. doi: 10.1038/nature09202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horman SR, Janas MM, Litterst C, Wang B, MacRae IJ, Sever MJ, Morrissey DV, Graves P, Luo B, Umesalma S, Qi HH, Miraglia LJ, Novina CD, Orth AP. Akt-mediated phosphorylation of argonaute 2 downregulates cleavage and upregulates translational repression of microRNA targets. Mol Cell. 2013;50:356–367. doi: 10.1016/j.molcel.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li MD. Differential allelic expression of dopamine D1 receptor gene (DRD1) is modulated by microRNA miR-504. Biol Psychiatry. 2009a;65:702–705. doi: 10.1016/j.biopsych.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Li MD. Nicotine modulates expression of miR-140*, which targets the 3′-untranslated region of dynamin 1 gene (Dnm1) Int J Neuropsychopharmacol. 2009b;12:537–546. doi: 10.1017/S1461145708009528. [DOI] [PubMed] [Google Scholar]
- Im HI, Hollander JA, Bali P, Kenny PJ. MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212. Nat Neurosci. 2010;13:1120–1127. doi: 10.1038/nn.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusz A, Milek J, Perycz M, Pacini L, Bagni C, Kaczmarek L, Dziembowska M. The fragile X mental retardation protein regulates matrix metalloproteinase 9 mRNA at synapses. J Neurosci. 2013;33:18234–18241. doi: 10.1523/JNEUROSCI.2207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska M, Milek J, Cymerman IA, Leski S, Kaczmarek L, Dziembowska M. miR-132 regulates dendritic spine structure by direct targeting of matrix metalloproteinase 9 mRNA. Mol Neurobiol. 2015;53(7):4701–4712. doi: 10.1007/s12035-015-9383-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas P, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry. 2011;16:974–986. doi: 10.1038/mp.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano T, Suzuki Y, Shibuya M, Kiuchi K, Hagiwara M. Cocaine-induced CREB phosphorylation and c-Fos expression are suppressed in Parkinsonism model mice. NeuroReport. 1995;6:2197–2200. doi: 10.1097/00001756-199511000-00023. [DOI] [PubMed] [Google Scholar]
- Kawashima H, Numakawa T, Kumamaru E, Adachi N, Mizuno H, Ninomiya M, Kunugi H, Hashido K. Glucocorticoid attenuates brain-derived neurotrophic factor-dependent upregulation of glutamate receptors via the suppression of microRNA-132 expression. Neuroscience. 2010;165:1301–1311. doi: 10.1016/j.neuroscience.2009.11.057. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Brain reward systems and compulsive drug use. Trends Pharmacol Sci. 2007;28:135–141. doi: 10.1016/j.tips.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Kenny PJ. Epigenetics, microRNA, and addiction. Dialogues Clin Neurosci. 2014;16:335–344. doi: 10.31887/DCNS.2014.16.3/pkenny. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Lendeckel W, Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- Lai EC. Micro RNAs are complementary to 3′ UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–364. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- Leung AK. The whereabouts of microRNA actions: cytoplasm and beyond. Trends Cell Biol. 2015;25:601–610. doi: 10.1016/j.tcb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li M, Dai FR, Du XP, Yang QD, Zhang X, Chen Y. Infusion of BDNF into the nucleus accumbens of aged rats improves cognition and structural synaptic plasticity through PI3K-ILK-Akt signaling. Behav Brain Res. 2012;231:146–153. doi: 10.1016/j.bbr.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute 2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Magill ST, Cambronne XA, Luikart BW, Lioy DT, Leighton BH, Westbrook GL, Mandel G, Goodman RH. microRNA-132 regulates dendritic growth and arborization of newborn neurons in the adult hippocampus. Proc Natl Acad Sci U S A. 2010;107:20382–20387. doi: 10.1073/pnas.1015691107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeyev EV, Zhang J, Carrasco MA, Maniatis T. The microRNA miR-124 promotes neuronal differentiation by triggering brain-specific alternative pre-mRNA splicing. Mol Cell. 2007;27:435–448. doi: 10.1016/j.molcel.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson ME, Lynch S. The CBHSQ Report. SAMHSA; Rockville, MD: 2013. Medication prescribing and behavioral treatment for substance use disorders in physician office settings. [PubMed] [Google Scholar]
- McCrae JC, Sharkey N, Webb DJ, Vliegenthart AD, Dear JW. Ethanol consumption produces a small increase in circulating miR-122 in healthy individuals. Clin Toxicol. 2016;54:53–55. doi: 10.3109/15563650.2015.1112015. [DOI] [PubMed] [Google Scholar]
- Mellios N, Sugihara H, Castro J, Banerjee A, Le C, Kumar A, Crawford B, Strathmann J, Tropea D, Levine SS, Edbauer D, Sur M. miR-132, an experience-dependent microRNA, is essential for visual cortex plasticity. Nat Neurosci. 2011;14:1240–1242. doi: 10.1038/nn.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–327. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller WA. How endothelial cells regulate transmigration of leukocytes in the inflammatory response. Am J Pathol. 2014;184:886–896. doi: 10.1016/j.ajpath.2013.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy V, Bozdagi O, Matynia A, Balcerzyk M, Okulski P, Dzwonek J, Costa RM, Silva AJ, Kaczmarek L, Huntley GW. Matrix metalloproteinase-9 is required for hippocampal late-phase long-term potentiation and memory. J Neurosci. 2006;26:1923–1934. doi: 10.1523/JNEUROSCI.4359-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudelman AS, DiRocco DP, Lambert TJ, Garelick MG, Le J, Nathanson NM, Storm DR. Neuronal activity rapidly induces transcription of the CREB-regulated microRNA-132, in vivo. Hippocampus. 2010;20:492–498. doi: 10.1002/hipo.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci U S A. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet DL, Perron MP, Gobeil LA, Plante P, Provost P. MicroRNAs in gene regulation: when the smallest governs it all. J Biomed Biotechnol. 2006;2006:69616. doi: 10.1155/JBB/2006/69616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panayotis N, Pratte M, Borges-Correia A, Ghata A, Villard L, Roux JC. Morphological and functional alterations in the substantia nigra pars compacta of the Mecp2-null mouse. Neurobiol Dis. 2011;41:385–397. doi: 10.1016/j.nbd.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Post-transcriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn RK, Brown AL, Goldie BJ, Levi EM, Dickson PW, Smith DW, Cairns MJ, Dayas CV. Distinct miRNA expression in dorsal striatal subregions is associated with risk for addiction in rats. Transl Psychiatry. 2015;5:e503. doi: 10.1038/tp.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn RK, James MH, Hawkins GE, Brown AL, Heathcote A, Smith DW, Cairns MJ, Dayas CV. Temporally specific miRNA expression patterns in the dorsal and ventral striatum of addiction-prone rats. Addict Biol. 2017 doi: 10.1111/adb.12520. https://doi.org/10.1111/adb.12520. [DOI] [PubMed]
- Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E. Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron. 2009;63:803–817. doi: 10.1016/j.neuron.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhart BJ, Slack FJ, Basson M, Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR, Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- Remenyi J, van den Bosch MW, Palygin O, Mistry RB, McKenzie C, Macdonald A, Hutvagner G, Arthur JS, Frenguelli BG, Pankratov Y. miR-132/212 knockout mice reveal roles for these miRNAs in regulating cortical synaptic transmission and plasticity. PLoS One. 2013;8:e62509. doi: 10.1371/journal.pone.0062509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Structural plasticity associated with drugs of abuse. Neuropharmacology. 2004;47:561–572. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci. 2011;12:623–637. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Ortiz CJ, Baglietto-Vargas D, Martinez-Coria H, LaFerla FM, Kitazawa M. Upregulation of miR-181 decreases c-Fos and SIRT-1 in the hippocampus of 3xTg-AD mice. J Alzheimers Dis. 2014;42:1229–1238. doi: 10.3233/JAD-140204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- Sadakierska-Chudy A, Frankowska M, Miszkiel J, Wydra K, Jastrzebska J, Filip M. Prolonged induction of miR-212/132 and REST expression in rat striatum following cocaine self-administration. Mol Neurobiol. 2017;54:2241–2254. doi: 10.1007/s12035-016-9817-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Im HI, Veno MT, Fowler CD, Min A, Intrator A, Kjems J, Kenny PJ, O’Carroll D, Greengard P. Argonaute 2 in dopamine 2 receptor-expressing neurons regulates cocaine addiction. J Exp Med. 2010;207:1843–1851. doi: 10.1084/jem.20100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Wolf ME, Tseng KY. A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J Neurosci. 2014;34:3095–3100. doi: 10.1523/JNEUROSCI.4940-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt WJ. Balance of transmitter activities in the basal ganglia loops. J Neural Transm Suppl. 1995;46:67–76. [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci U S A. 2011;108:19407–19412. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW. Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci. 2014;17:1655–1657. doi: 10.1038/nn.3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Kalivas PW. The tetrapartite synapse: extracellular matrix remodeling contributes to corticoaccumbens plasticity underlying drug addiction. Brain Res. 2015;1628(Pt A):29–39. doi: 10.1016/j.brainres.2015.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ACW, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC, 3rd, Boger HA, Kalivas PW. Accumbens nNOS Interneurons Regulate Cocaine Relapse. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2017;37:742–756. doi: 10.1523/JNEUROSCI.2673-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Neural plasticity & behavior – sixty years of conceptual advances. J Neurochem. 2016;139(Suppl 2):179–199. doi: 10.1111/jnc.13580. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yokota S, Tatsumi N, Fukami T, Yokoi T, Nakajima M. Cigarette smoking substantially alters plasma microRNA profiles in healthy subjects. Toxicol Appl Pharmacol. 2013;272:154–160. doi: 10.1016/j.taap.2013.05.018. [DOI] [PubMed] [Google Scholar]
- Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- Tapocik JD, Luu TV, Mayo CL, Wang BD, Doyle E, Lee AD, Lee NH, Elmer GI. Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict Biol. 2013;18:480–495. doi: 10.1111/j.1369-1600.2012.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Barbier E, Flanigan M, Solomon M, Pincus A, Pilling A, Sun H, Schank JR, King C, Heilig M. microRNA-206 in rat medial prefrontal cortex regulates BDNF expression and alcohol drinking. J Neurosci. 2014;34:4581–4588. doi: 10.1523/JNEUROSCI.0445-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapocik JD, Ceniccola K, Mayo CL, Schwandt ML, Solomon M, Wang BD, Luu TV, Olender J, Harrigan T, Maynard TM, Elmer GI, Lee NH. MicroRNAs are involved in the development of morphine-induced analgesic tolerance and regulate functionally relevant changes in Serpini1. Front Mol Neurosci. 2016;9:20. doi: 10.3389/fnmol.2016.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognini P, Pizzorusso T. MicroRNA212/132 family: molecular transducer of neuronal function and plasticity. Int J Biochem Cell Biol. 2012;44:6–10. doi: 10.1016/j.biocel.2011.10.015. [DOI] [PubMed] [Google Scholar]
- Vaquero L, Camara E, Sampedro F, Perez de Los Cobos J, Batlle F, Fabregas JM, Sales JA, Cervantes M, Ferrer X, Lazcano G, Rodriguez-Fornells A, Riba J. Cocaine addiction is associated with abnormal prefrontal function, increased striatal connectivity and sensitivity to monetary incentives, and decreased connectivity outside the human reward circuit. Addict Biol. 2017;22(3):844–856. doi: 10.1111/adb.12356. [DOI] [PubMed] [Google Scholar]
- Vasudevan S, Tong Y, Steitz JA. Switching from repression to activation: microRNAs can up-regulate translation. Science. 2007;318:1931–1934. doi: 10.1126/science.1149460. [DOI] [PubMed] [Google Scholar]
- Viola TW, Wearick-Silva LE, De Azeredo LA, Centeno-Silva A, Murphy C, Marshall P, Li X, Singewald N, Garcia F, Bredy TW, Grassi-Oliveira R. Increased cocaine-induced conditioned place preference during periadolescence in maternally separated male BALB/c mice: the role of cortical BDNF, microRNA-212, and MeCP2. Psychopharmacology. 2016;233:3279–3288. doi: 10.1007/s00213-016-4373-z. [DOI] [PubMed] [Google Scholar]
- Vo N, Klein ME, Varlamova O, Keller DM, Yamamoto T, Goodman RH, Impey S. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc Natl Acad Sci U S A. 2005;102:16426–16431. doi: 10.1073/pnas.0508448102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Skolnick P. New medications for substance use disorders: challenges and opportunities. Neuropsychopharmacology. 2012;37:290–292. doi: 10.1038/npp.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins A, Smith RJ, Shen HW, Kalivas PW. Integrins modulate relapse to cocaine-seeking. J Neurosci. 2011;31:16177–16184. doi: 10.1523/JNEUROSCI.3816-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JW, Masino AJ, Reichert JR, Turner GD, Meighan SE, Meighan PC, Harding JW. Ethanol-induced impairment of spatial memory and brain matrix metalloproteinases. Brain Res. 2003;963:252–261. doi: 10.1016/s0006-8993(02)04036-2. [DOI] [PubMed] [Google Scholar]
- Wright KN, Hollis F, Duclot F, Dossat AM, Strong CE, Francis TC, Mercer R, Feng J, Dietz DM, Lobo MK, Nestler EJ, Kabbaj M. Methyl supplementation attenuates cocaine-seeking behaviors and cocaine-induced c-Fos activation in a DNA methylation-dependent manner. J Neurosci. 2015;35:8948–8958. doi: 10.1523/JNEUROSCI.5227-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Lu J, Kulbokas EJ, Golub TR, Mootha V, Lindblad-Toh K, Lander ES, Kellis M. Systematic discovery of regulatory motifs in human promoters and 3′ UTRs by comparison of several mammals. Nature. 2005;434:338–345. doi: 10.1038/nature03441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan B, Hu Z, Yao W, Le Q, Xu B, Liu X, Ma L. MiR-218 targets MeCP2 and inhibits heroin seeking behavior. Sci Rep. 2017;7(40):413. doi: 10.1038/srep40413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Zhang R, Li M, Wu X, Wang J, Huang L, Shi X, Li Q, Su B. A functional MiR-124 binding-site polymorphism in IQGAP1 affects human cognitive performance. PLoS One. 2014;9:e107065. doi: 10.1371/journal.pone.0107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Xu H, Su X, He X. Role of microRNA in governing synaptic plasticity. Neural Plast. 2016;2016(2016):4959523. doi: 10.1155/2016/4959523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Smith AD, Levey AI, Bolam JP. Synaptic connections between spiny neurons of the direct and indirect pathways in the neostriatum of the rat: evidence from dopamine receptor and neuropeptide immunostaining. Eur J Neurosci. 1996;8:861–869. doi: 10.1111/j.1460-9568.1996.tb01573.x. [DOI] [PubMed] [Google Scholar]
- Zapata A, Minney VL, Shippenberg TS. Shift from goal-directed to habitual cocaine seeking after prolonged experience in rats. J Neurosci. 2010;30:15457–15463. doi: 10.1523/JNEUROSCI.4072-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Sankala H, Zhang X, Graves PR. Phosphorylation of argonaute 2 at serine-387 facilitates its localization to processing bodies. Biochem J. 2008;413:429–436. doi: 10.1042/BJ20080599. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang D, Wang Q, Shen D, Wang Y, Chen B, Zhang J, Gai L. MiR-132 inhibits expression of SIRT1 and induces pro-inflammatory processes of vascular endothelial inflammation through blockade of the SREBP-1c metabolic pathway. Cardiovasc Drugs Therapy. 2014;28:303–311. doi: 10.1007/s10557-014-6533-x. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Zhang K, Jiang H, Du J, Na Z, Hao W, Yu S, Zhao M. Decreased expression of plasma microRNA in patients with methamphetamine (MA) use disorder. J Neuroimmune Pharmacol. 2016;11(3):542–548. doi: 10.1007/s11481-016-9671-z. [DOI] [PubMed] [Google Scholar]
- Zheng H, Chu J, Zeng Y, Loh HH, Law PY. Yin Yang 1 phosphorylation contributes to the differential effects of mu-opioid receptor agonists on microRNA-190 expression. J Biol Chem. 2010;285:21994–22002. doi: 10.1074/jbc.M110.112607. [DOI] [PMC free article] [PubMed] [Google Scholar]