ABSTRACT
Aim
This study evaluated poor weight gain as a risk factor for infants who required treatment for retinopathy of prematurity (ROP), by comparing those born before and after the implementation of higher oxygen saturation (SpO2) targets at the Queen Silvia Children's Hospital, Gothenburg, Sweden.
Methods
We compared infants born at less than 31 weeks, who were screened and, or, treated for ROP: 127 in 2011–2012 when SpO2 targets were 88–92% and 142 in 2015–2016 when they were 91–95%. The subjects were reviewed for birth characteristics, weekly weight and ROP treatment. Data were analysed using the weight, insulin‐like growth factor 1, neonatal, ROP (WINROP) prediction tool.
Results
The 2011–2012 infants who needed ROP treatment (12.6%) had significantly poorer postnatal weight gain than those who did not, but this was not seen in the treated (17.6%) and nontreated ROP groups in 2015–2016. WINROP sensitivity decreased from 87.5% in 2011–12 to 48% in 2015–2016.
Conclusion
After the SpO2 target range was increased from 88–92% to 91–95%, postnatal weight gain was no longer a significant risk factor and WINROP lost its ability to predict ROP requiring treatment. Risk factors clearly change as neonatal care develops.
Keywords: Oxygen saturation, Poor weight gain, Preterm birth retinopathy of prematurity, Risk factors
Abbreviations
- GA
Gestational age
- PMA
Postmenstrual age
- ROP
Retinopathy of prematurity
- SDS
Standard deviation sore
- SpO2
Peripheral capillary oxygen saturation
- WINROP
Weight, insulin‐like growth factor 1, neonatal, retinopathy of prematurity
Key notes.
This study evaluated poor weight gain as a risk factor for premature infants treated for retinopathy of prematurity (ROP).
We compared 127 infants in 2011–2012 when oxygen targets were 88–92% and 142 in 2015–2016 when they were 91–95%.
The 2011–2012 infants who needed ROP treatment had significantly poorer postnatal weight gain than those who did not, but this pattern was not repeated in 2015–2016.
Introduction
Retinopathy of prematurity (ROP) causes blindness or severe visual impairment in approximately 20 000 infants each year 1, and excessive oxygen supplementation is the best‐known risk factor. Early studies in the 1960s found restricting oxygen delivery could reduce ROP, but at the cost of increased mortality 2 and the increased incidence of cerebral palsy 3. Randomised controlled trials carried out by the Neonatal Oxygen Prospective Meta‐Analysis Collaboration on more than 4800 infants with a gestational age (GA) of less than 28 weeks compared lower (85–89%) and higher (91–95%) peripheral capillary oxygen saturation (SpO2) targets. They found that the lower range was associated with increased mortality but a lower risk of severe ROP requiring treatment (10.7% needing treatment in lower targets versus 14.5% in higher targets) 4. During 2014, SpO2 targets were gradually increased towards 91–95% in Sweden and that target was fully implemented in 2015.
In settings with advanced neonatal care, other risk factors such as poor prenatal and postnatal weight gain are associated with severe ROP needing treatment 5, 6. A web‐based prediction tool–weight, insulin‐like growth factor 1, neonatal, retinopathy of prematurity (WINROP) – based on weight development, predicts ROP needing treatment with high sensitivity in settings with highly developed neonatal care 7, 8, but with lower sensitivity in settings with poor oxygen control 9.
In the Västra Götaland region of Sweden, approximately 160 infants per year are born with a gestational age (GA) of less than 31 weeks and they all undergo ROP screening. The most immature and sickest infants are cared for at the neonatal intensive care unit at the Queen Silvia Children's Hospital in Gothenburg. WINROP was developed at this clinic to predict ROP needing treatment based on postnatal weight gain 10 and it is now used to alert clinicians about infants with a high risk of severe ROP needing treatment. During 2015, after the implementation of a higher SpO2 target range, we noted that the development of ROP needing treatment was not predicted by WINROP in several cases.
The aim of this retrospective chart review was to evaluate postnatal weight development and WINROP's sensitivity before and after the implementation of higher SpO2 target levels at the Queen Silvia Children's Hospital.
Methods
We analysed the WINROP predicted outcome and frequency of ROP treatment in infants with GAs of less than 31 weeks who had undergone at least one screening examination or treatment for ROP at the Queen Silvia Children's Hospital in Gothenburg. Infants were analysed from two time periods, before and after a change in oxygen saturation targets, from 88–92% in 2011–2012 to 91–95% in 2015–2016. Infants born during the first period had participated in a previous study 11. Data on GA, birthweight, gender and neonatal morbidities, such as bronchopulmonary dysplasia, necrotising enterocolitis and intraventricular haemorrhage were collected from hospital records. Bronchopulmonary dysplasia was defined as the need for continuous supplemental oxygen at 36 weeks of postmenstrual age (PMA), necrotising enterocolitis was defined as stage 2b or more, all grades of intraventricular haemorrhage were included, and sepsis was defined as a positive blood culture. Infants were included if they had survived complete ROP screening and reached approximately 40 weeks of PMA. We retrospectively collected weekly postnatal body weights, from birth to 34 weeks PMA. An online WINROP analysis requires accurate GA, birthweight and weekly weights, and infants were excluded when their weight measurements were incomplete and when their weight gain was considered to have any nonphysiological component, such as hydrocephalus. WINROP was developed for GAs of 23–32 weeks and that is why we excluded infants born at 22 weeks.
Oxygen supplementation
In 2011–2012, our hospital guidelines recommended an SpO2 target range of 88–92%, and by January 2015, the target range of 91−95% had been fully implemented. Oxygen saturation was monitored with individual infant oxygen pulse oximeters.
ROP examination and treatment
Eye examinations were performed according to a routine protocol, which consisted of dilated ocular fundus examinations from once every 2 weeks to twice a week depending on the severity of ROP. The International Classification of Retinopathy of Prematurity was used 12: stages one and two were defined as mild ROP, and stages three to five were defined as severe ROP. Treatments were based on the recommendations of the Early Treatment for Retinopathy of Prematurity Cooperative Group 13.
Postnatal weight development and WINROP analyses
The birthweight standard deviation score (SDS) and weight SDS were calculated 14. WINROP is a web‐based surveillance system that records the infant's GA, birthweight and weekly postnatal weights, measured from birth to the time an alarm. The records are maintained until an alarm is triggered by the data or the infant reaches 32 weeks of PMA. The WINROP algorithm estimates the differences between the expected, safe weekly weight gain and the observed weight gain. An alarm is triggered when the difference exceeds a set limit to warn clinicians that the infant is at high risk of severe ROP needing treatment.
In 2015–2016, WINROP was routinely used to predict ROP needing treatment at the Queen Silvia Children's Hospital and the outcome for this cohort was retrieved from the WINROP database. We performed retrospective WINROP analyses for the 2011–2012 cohort.
Statistical analyses
All statistical analyses were carried out with IBM SPSS Statistics, version 24 (IBM Corp, New York, USA). The negative predictive values (NPVs) and positive predictive values (PPVs) were calculated as well as the sensitivity, specificity and 95% confidence intervals. The Mann–Whitney U‐test, chi‐square test and Fisher's exact test were used to explore differences between variables.
The research ethics committee of the University of Gothenburg approved the study protocol.
Results
Two cohorts of infants born at less than 31 weeks of GA and screened and, or, treated for ROP at the Queen Silvia Children's Hospital were included by year of birth: 2011–2012 (n = 138) or 2015–2016 (n = 154). A number of infants were excluded from WINROP due to hydrocephalus, birth at less than 23 weeks GA or missing weight measurements and this meant that 127 infants were evaluated using WINROP in the 2011–2012 cohort and 142 infants in the 2015–2016 cohort (Fig. 1). The infant's birth characteristics and the number of infants with ROP that were born in the two study periods were similar (Table 1). The proportion of children treated for ROP was 12.6% in the earlier cohort and 17.6% in the later period. There were no significant differences in neonatal morbidities, such as bronchopulmonary dysplasia, necrotising enterocolitis, intraventricular haemorrhage and sepsis between infants receiving ROP treatment in the two study periods or between infants whose data triggered a WINROP alarm or not (Table 1).
Figure 1.
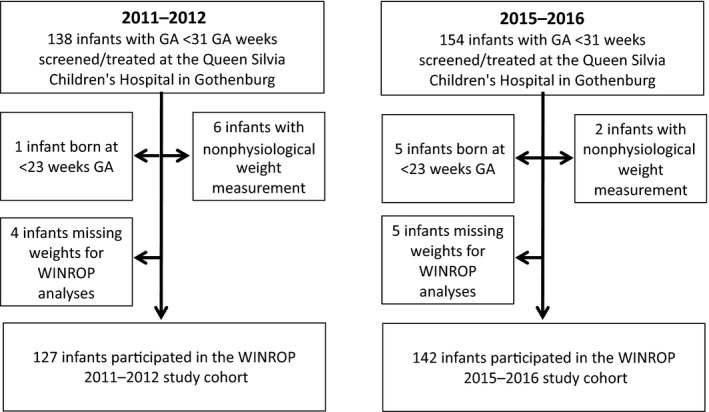
Flow chart of the study population.
Table 1.
Birth characteristics and ROP in infants born at <31 weeks GA and evaluated using WINROP, at the Queen Silvia Children's Hospital, in 2011 to 2012 and in 2015 to 2016
| Birth Characteristics | ||||||
|---|---|---|---|---|---|---|
| All infants | Infants treated for ROP | |||||
| 2011 to 2012 (n = 127) | 2015 to 2016 (n = 142) | p value | 2011 to 2012 (n = 16) | 2015 to 2016 (n = 25) | p value | |
| BW, grams, median (range) | 1065 (440 to 2205) | 933 (420 to 2445) | 0.91† | 615 (440 to 1000) | 705 (420 to 1005) | 0.41† |
| GA, weeks*days, median (range) | 28 + 1 (23 + 0 to 30 + 6) | 27 + 5 (23 + 1 to 30 + 6) | 0.52† | 24 + 2 (23 + 0 to 28 + 0) | 24 + 4 (23 + 1 to 26 + 5) | 0.36† |
| BWSDS*, median (range) | −0.86 (−4.55 to 2.97) | −1.17 (−5.13 to 3.89) | 0.18† | −1.13 (−2.27 to 0.75) | −0.79 (−3.79 to 1.44) | 0.69† |
| Male, % | 49.6% | 50.7% | 0.90‡ | 37.5% | 44.0% | 0.75‡ |
| ROP | |||
|---|---|---|---|
| 2011 to 2012 (n = 127) | 2015 to 2016 (n = 142) | p value | |
| No ROP, % | 59.1% | 50.7% | 0.17‡ |
| Mild ROP (stage 1&2), % | 23.6% | 27.5% | 0.47‡ |
| Severe ROP not tretated (stage 3), % | 4.7% | 4.2% | 0.84‡ |
| ROP treatment, % | 12.6% | 17.6% | 0.25‡ |
| Neonatal morbidities in infants treated for ROP | ||||||
|---|---|---|---|---|---|---|
| 2011 to 2012 (n = 16) | 2015 to 2016 (n = 25) | p value | Infants with no WINROP alarm (n = 15) | Infants with WINROP alarm (n = 26) | p value | |
| BPD, % | 68.8% | 84.0% | 0.77§ | 80.0% | 76.9% | 0.82§ |
| NEC, % | 25.0% | 12.0% | 0.40§ | 20.0% | 15.4% | 0.70§ |
| IVH, % | 43.8% | 52.0% | 0.75‡ | 46.7% | 50.0% | 0.84‡ |
| Sepsis, % | 87.5% | 68.0% | 0.26§ | 60.0% | 84.6% | 0.13§ |
BPD = bronchopulmonary dysplasia; BW = birth weight; BWSDS = birth weight standard deviation score; GA = gestational age; IVH = intraventricular haemorrhage; NEC = necrotizing enterocolitis; PMA = postmenstrual age; ROP = retinopathy of prematurity; WINROP = weight, insulin‐like growth factor‐1, neonatal, retinopathy of prematurity.
*Calculated based on a Scandinavian gender‐specific growth reference 13.
†Mann‐Whitney U Test.
‡Chi‐square test.
§Fisher's Exact Test.
Postnatal weight development and WINROP analyses in the study periods
There were no differences in mean postnatal weight development between the 2011–2012 and 2015–2016 cohorts (Fig. 2A). In the 2011–2012 cohort, very few children with adequate weight increases developed ROP needing treatment. Thus, at a PMA of 26–34 weeks there were significant differences in postnatal weight development between the infants who needed ROP treatment and those who did not need (p < 0.05 to p < 0.001) (Fig. 2B). In the 2015–2016 cohort, there was no difference in weight development between those developing ROP needing treatment and those not (Fig. 2C). In Figure 2D, the weight development in infants not needing treatment for ROP in 2011–2012 and 2015–2016 is presented and in Figure 2E infants needing ROP treatment in 2011–2012 and 2015–2016 are presented. In Figure 2F, infants needing treatment for ROP in 2015–2016, with and without a WINROP alarm, are depicted and this demonstrates a group of infants who developed ROP needing treatment with adequate postnatal weight gain.
Figure 2.
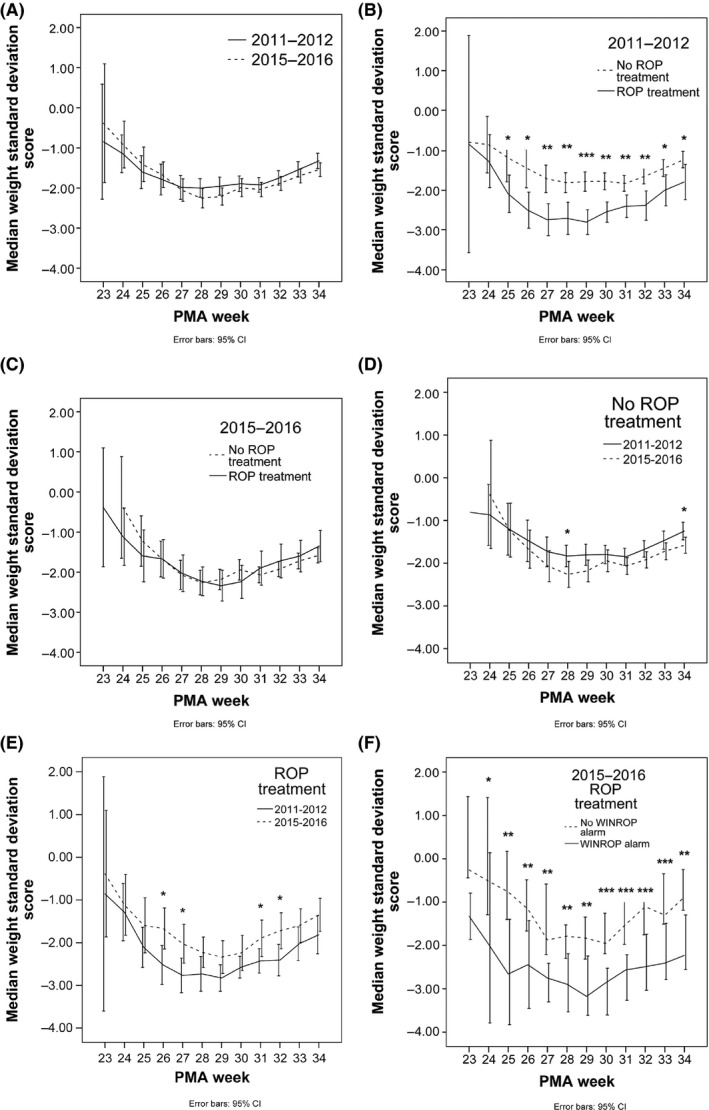
Postnatal weight development, from birth to 34 weeks postmenstrual age (PMA), in infants born <31 weeks GA and screened and/or, treated for ROP at the Queen Silvia Children's Hospital. (A) Infants born in 2011–2012 (solid line) and in 2015–2016 (dashed line). (B, C) Infants that needed ROP treatment (solid line) or no ROP treatment (dashed line), (B) in 2011–2012 and (C) in 2015–2016. (D) Infants that did not need ROP treatment in 2011–2012 (solid line) and in 2015–2016 (dashed line). (E) Infants that needed ROP treatment in 2011–2012 (solid line) and in 2015–2016 (dashed line). (F) Infants in 2015–2016 needing ROP treatment with no WINROP alarm (dashed line) and with WINROP alarm (solid line). Error bars indicate 95% confidence intervals. *p < 0.05, **p < 0.01, ***p < 0.001. [Correction added on 3 October 2017, after online publication: Figure 2e was previously incorrect and has been amended in this current version.]
The sensitivity of WINROP in predicting ROP needing treatment decreased from 87.5% in 2011–2012, to 48.0% in 2015–2016 (Table 2). In the 2011–2012, cohort two infants who were treated for ROP were not identified by WINROP. One of these infants had severe ROP, but did not fulfil the established ROP treatment criteria 12. In the 2015–2016 cohort, all of the 25 infants had fulfilled the established ROP treatment criteria, but just over half of these infants (n‐13) were not identified by WINROP.
Table 2.
WINROP sensitivity, specificity, and positive and negative predictive values for infants screened and/or treated for ROP (GA<31 weeks) at the Queen Silvia Children's Hospital in 2011–2012 and in 2015–2016
| Alarm status | WINROP efficacy | ||||
|---|---|---|---|---|---|
| Alarm | No Alarm | Total | Sensitivity, % (95%CI) | Specificity, % (95%CI) | |
| Study cohort 2011–2012 | |||||
| ROP treatment (n) | 14 | 2 | 16 | 87.5 | 67.5 |
| No ROP treatment (n) | 36 | 75 | 111 | (60.4–97.8) | (57.9–75.9) |
| Total (N) | 50 | 77 | 127 | ||
| PPV, % (95%CI) | 28.0 (16.7–42.7) | ||||
| NPV, % (95%CI) | 97.4 (90.1–99.5) | ||||
| Study cohort 2015–2016 | |||||
| ROP treatment (n) | 12 | 13 | 25 | 48.0 | 60.6 |
| No ROP treatment (n) | 46 | 71 | 117 | (28.3–68.2) | (51.2–69.4) |
| Total (N) | 58 | 84 | 142 | ||
| PPV, % (95%CI) | 20.7 (11.6–33.7) | ||||
| NPV, % (95%CI) | 84.5 (74.6–91.2) | ||||
NPV = negative predictive value; PPV = positive predictive value; ROP = retinopathy of prematurity; WINROP = weight, insulin like growth factor‐1, neonatal, and retinopathy of prematurity.
Discussion
This study shows that postnatal weight gain was no longer a significant risk factor for severe ROP needing treatment after the implementation of a higher SpO2 target of 91–95% in 2015–2016 compared to the 88–92% used in 2011–2012. Detailed analyses of infants born at less than 31 weeks of GA and screened and/or treated at the Queen Silvia Children's Hospital in Gothenburg revealed no significant differences in birth characteristics, morbidities or postnatal weight development between infants born during the two study periods. We found that poor versus adequate weight gain no longer discriminated between severe and less severe ROP, as demonstrated by a low WINROP sensitivity. With the application of higher oxygen targets, many more adequate weight gain infants developed severe ROP needing treatment. The sensitivity of WINROP decreased from 87.5% in 2011–2012 to 48.0% in 2015–2016. Accordingly, we found that WINROP was no longer useful for predicting ROP in the later study period. The development of WINROP was originally based on a cohort of very preterm infants at the Queen Silvia Children's Hospital 10 and previously validated in Sweden and other populations in settings with highly developed neonatal care with a sensitivity of 96–100% 7, 8, 15. WINROP only identified half of the infants that needed treatment in the 2015–2016 cohort and this was comparable to outcomes observed in settings with less developed neonatal care and poor oxygen control 9. The loss of WINROP efficacy indicates that increased SpO2 targets are likely to contribute to severe ROP even in children with adequate postnatal weight gain.
The proportion of children treated for ROP was 12.6% in 2011–2012 and 17.6% in 2015–2016.
Several studies have shown higher incidences of severe ROP in settings with higher oxygen saturation targets, compared to lower targets 16, 17. However, the current study was not designed to compare the frequency of severe ROP needing treatment between the two periods and the increased incidence was not significant. The included cohorts were not strictly population or hospital based since some participating infants were born at other hospitals and spent some time at the Queen Silvia Children's Hospital where some of the eye examinations were performed. Others were just referred for treatment.
One limitation of this study was that the actual SpO2 recordings were not available. Also, we could not ascertain that an SpO2 of 91–95% per se decreased the influence of weight gain on the ROP risk. However, higher targets may increase the risk of hyperoxaemia and oxygenation variability. Compliance with targets is known to be generally low. In one study, SpO2 was reported to exceed the upper target limit 20–73% of the time on infants on supplemental oxygen 18. Alarm limits are often set too high and, in another study in a setting with a target range 88–92%, the alarm limit was 100% for 23.8% of the time 19. In addition, at SpO2 > 93% arterial oxygen tension is often > 80 mm Hg, which has been defined as hyperoxaemia 20 and has been associated with an increased ROP risk 21. The present SpO2 target of 91–95% in our hospital was implemented based on studies that compared two target ranges throughout the period from birth to 36 weeks of PMA and with poor quality of evidence for reduced mortality with the higher target range of 91–94% compared to the lower range of 85–89% 22.
There is growing evidence that initial lower targets followed by higher targets after around 32 weeks of PMA reduce severe ROP 23, 24. Further studies are needed to determine optimal oxygenation at different postmenstrual ages if possible. With our present knowledge, efforts are needed to avoid not only hypoxia but also hyperoxia, which is deleterious for both the retina and other parts of the central nervous system 25.
Conclusion
This study showed that the WINROP prediction tool, which uses postnatal weight development to predict severe ROP that needs treatment, was only minimally effective with higher SpO2 targets. This finding indicates that oxygen is a risk factor that overshadows risk factors associated with growth. Avoiding hyperoxia and saturation variability is mandatory in neonatal intensive care units and in studies of growth‐associated risk factors.
Funding
This study was supported by the Cronqvists Foundation, the Linnéa and Josef Carlssons Foundation, De Blindas Vänner and HKH Kronprinsessan Lovisas förening för barnasjukvård/Stiftelsen Axel Tielmans Minnesfond, Stiftelsen Kronprinsessan Margaretas Arbetsnämnd för synskadade and European Commission (FP7 project 305485 PREVENT–ROP).
Conflict of interest
WINROP is owned by Premacure AB, Uppsala, Sweden. Chatarina Löfqvist, Anna–Lena Hård and Ann Hellström own shares in a company with a financial interest in Premacure AB. No other author has any conflicts of issues to report.
Acknowledegments
We would like to thank the board of the Swedish national register for retinopathy of prematurity and all the doctors that performed the ROP screening in Sweden.
References
- 1. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm‐associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res 2013; 74(Suppl 1): 35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avery ME. Recent increase in mortality from hyaline membrane disease. J Pediatr 1960; 1960: 553–9. [DOI] [PubMed] [Google Scholar]
- 3. McDonald AD. Oxygen Treatment of Premature Babies and Cerebral Palsy. Dev Med Child Neurol 1964; 6: 313–4. [DOI] [PubMed] [Google Scholar]
- 4. Askie LM, Darlow BA, Davis PG, Finer N, Stenson B, Vento M, et al. Effects of targeting lower versus higher arterial oxygen saturations on death or disability in preterm infants. Cochrane Database Syst Rev 2017;4:Cd011190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith LE, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics 2009; 123: e638–45. [DOI] [PubMed] [Google Scholar]
- 6. Klevebro S, Lundgren P, Hammar U, Smith LE, Bottai M, Domellöf M, et al. Cohort study of growth patterns by gestational age in preterm infants developing morbidity. BMJ Open 2016; 6: e012872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu C, Lofqvist C, Smith LE, VanderVeen DK, Hellstrom A, Consortium W . Importance of early postnatal weight gain for normal retinal angiogenesis in very preterm infants: a multicenter study analyzing weight velocity deviations for the prediction of retinopathy of prematurity. Arch Ophthalmol 2012; 130: 992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lofqvist C, Hansen‐Pupp I, Andersson E, Holm K, Smith LE, Ley D, et al. Validation of a new retinopathy of prematurity screening method monitoring longitudinal postnatal weight and insulinlike growth factor I. Arch Ophthalmol 2009; 127: 622–7. [DOI] [PubMed] [Google Scholar]
- 9. Zepeda‐Romero LC, Hard AL, Gomez‐Ruiz LM, Gutierrez‐Padilla JA, Angulo‐Castellanos E, Barrera‐de‐Leon JC, et al. Prediction of retinopathy of prematurity using the screening algorithm WINROP in a Mexican population of preterm infants. Arch Ophthalmol 2012; 130: 720–3. [DOI] [PubMed] [Google Scholar]
- 10. Lofqvist C, Andersson E, Sigurdsson J, Engstrom E, Hard AL, Niklasson A, et al. Longitudinal postnatal weight and insulin‐like growth factor I measurements in the prediction of retinopathy of prematurity. Arch Ophthalmol 2006; 124: 1711–8. [DOI] [PubMed] [Google Scholar]
- 11. Lundgren P, Wilde A, Lofqvist C, Smith LE, Hard AL, Hellstrom A. Weight at first detection of retinopathy of prematurity predicts disease severity. Br J Ophthalmol 2014; 98: 1565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. International Committee for the Classification of Retinopathy of Prematurity . The International Classification of Retinopathy of Prematurity revisited. Arch Ophthalmol 2005; 123: 991–9. [DOI] [PubMed] [Google Scholar]
- 13. Group Etfropc . Revised indications for the treatment of retinopathy of prematurity. Arch Ophthalmol 2003; 121: 1684. [DOI] [PubMed] [Google Scholar]
- 14. Marsal K, Persson PH, Larsen T, Lilja H, Selbing A, Sultan B. Intrauterine growth curves based on ultrasonically estimated foetal weights. Acta Paediatr 1996; 85: 843–8. [DOI] [PubMed] [Google Scholar]
- 15. Lundgren P, Stoltz Sjostrom E, Domellof M, Kallen K, Holmstrom G, Hard AL, et al. WINROP identifies severe retinopathy of prematurity at an early stage in a nation‐based cohort of extremely preterm infants. PLoS One 2013; 8: e73256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Manley BJ, Kuschel CA, Elder JE, Doyle LW, Davis PG. Higher Rates of Retinopathy of Prematurity after Increasing Oxygen Saturation Targets for Very Preterm Infants: experience in a Single Center. J Pediatr 2016; 168: 242–4. [DOI] [PubMed] [Google Scholar]
- 17. Wright KW, Sami D, Thompson L, Ramanathan R, Joseph R, Farzavandi S. A physiologic reduced oxygen protocol decreases the incidence of threshold retinopathy of prematurity. Trans Am Ophthalmol Soc 2006; 104: 78–84. [PMC free article] [PubMed] [Google Scholar]
- 18. Hagadorn JI, Furey AM, Nghiem TH, Schmid CH, Phelps DL, Pillers DA, et al. Achieved versus intended pulse oximeter saturation in infants born less than 28 weeks' gestation: the AVIOx study. Pediatrics 2006; 118: 1574–82. [DOI] [PubMed] [Google Scholar]
- 19. Clucas L, Doyle LW, Dawson J, Donath S, Davis PG. Compliance with alarm limits for pulse oximetry in very preterm infants. Pediatrics 2007; 119: 1056–60. [DOI] [PubMed] [Google Scholar]
- 20. Castillo A, Sola A, Baquero H, Neira F, Alvis R, Deulofeut R, et al. Pulse oxygen saturation levels and arterial oxygen tension values in newborns receiving oxygen therapy in the neonatal intensive care unit: is 85% to 93% an acceptable range? Pediatrics 2008; 121: 882–9. [DOI] [PubMed] [Google Scholar]
- 21. Flynn JT, Bancalari E, Snyder ES, Goldberg RN, Feuer W, Cassady J, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med 1992; 326: 1050–4. [DOI] [PubMed] [Google Scholar]
- 22. Manja V, Lakshminrusimha S, Cook DJ. Oxygen saturation target range for extremely preterm infants: a systematic review and meta‐analysis. JAMA Pediatr 2015; 169: 332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen ML, Guo L, Smith LE, Dammann CE, Dammann O. High or low oxygen saturation and severe retinopathy of prematurity: a meta‐analysis. Pediatrics 2010; 125: e1483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cayabyab R, Arora V, Wertheimer F, Durand M, Ramanathan R. Graded oxygen saturation targets and retinopathy of prematurity in extremely preterm infants. Pediatr Res 2016; 80: 401–6. [DOI] [PubMed] [Google Scholar]
- 25. Reich B, Hoeber D, Bendix I, Felderhoff‐Mueser U. Hyperoxia and the Immature Brain. Dev Neurosci 2016; 38: 311–30. [DOI] [PubMed] [Google Scholar]


