Abstract
We reported previously that fibroblast growth factor 9 (FGF9) acts as an anti-differentiation factor, stimulating proliferation of granulosa (GC) and theca (TC) cells while suppressing hormone-induced steroidogenesis of these cells. How FGF9 acts to simultaneously suppress steroidogenesis and stimulate proliferation remains to be fully elucidated. Thus, this study was undertaken to clarify the effects of FGF9 on the TC transcriptome. Ovaries were obtained from beef heifers at a local abattoir, TC were isolated from large antral follicles, and cultured with or without 30 ng/mL of FGF9 for 24 h in the presence of LH and IGF-1. Following treatment, total RNA was extracted from TC and processed for microarray using Affymetrix GeneChip Bovine Genome Arrays (n = 4/group). Transcriptome analysis comparing FGF9-treated TC with control TC using 1.3-fold cut-off and a P < 0.05 significance level identified 355 differentially expressed transcripts, with 164 elements up-regulated and 191 elements down-regulated by FGF9. The Ingenuity Pathway Analysis (IPA) was utilized to investigate how FGF9 treatment affects molecular pathways, biological functions, and the connection between molecules in bovine TC. The IPA software identified 346 pathways in response to FGF9 in TC involved in several biological functions, and unveiled interesting relationships among genes related to cell proliferation (e.g., CCND1, FZD5 and MYB), antioxidation/cytoprotection (e.g., HMOX1 and NQO1) and steroidogenesis (e.g., CYP11A1 and STAR). Overall, genes, pathways, and networks identified in this study painted a picture of how FGF9 may regulate folliculogenesis, providing novel candidate genes for further investigation of FGF9 functions in ovarian follicular development.
Keywords: cattle, cell proliferation, Fibroblast growth factor-9 (FGF9), microarray, steroidogenesis, theca cell
1. Introduction
Fibroblast growth factors (FIGFs) constitute a large family of single chain polypeptide factors present in both vertebrates and invertebrates [1, 2]. Currently, 22 different members have been reported in mammals (FGF1 to 23), binding to high affinity receptors and several cofactors to regulate a variety of biological processes in various tissues [3–5]. One of these, fibroblast growth factor 9 (FGF9), was originally isolated from human glioma cells and characterized as a mitogenic factor [6]. In the last two decades, research has shown that FGF9 plays diverse roles in many different tissues, including heart [7, 8], cartilage [9], liver [10], and the reproductive system [11, 12]. In these tissues, FGF9 binds to FGF receptors (FGFR1c, FGFR2c, FGFR3c, FGFR3b, and FGFR4) to activate specific tyrosine residues and downstream intracellular signaling pathways, including the RAS-MAPK, PI3K-AKT, PLCγ, and STAT pathways, which regulate cell proliferation, survival, metabolism, and differentiation [13]. In fact, FGF9 has been shown to regulate sex determination [14], steroidogenesis [15–17], tissue development [18], and even mood disorders [19]. Moreover, expression of FGF9 has been related to brain [20] and gastric [21] cancers.
In the ovaries, FGF9 was first described to be present in murine corpora lutea, stromal, and theca cells (TC) and was observed to stimulate progesterone (P4) production by granulosa cells (GC) in a paracrine way [12]. In porcine GC, FGF9 in the presence of insulin-like growth factor 1 (IGF-1) stimulated cell proliferation and steroidogenesis [22]. In cattle, FGF9 has been suggested to be an anti-differentiation factor by stimulating in vitro proliferation of TC and GC while suppressing P4 production by TC and GC and estradiol (E2) production by GC in the presence of IGF-1 [15, 16]. Abundance of FGF9 mRNA in GC and TC is hormonally regulated [15, 16] and changes according to the size and estrogenic status of follicles and days post-ovulation in cattle [23]. Hence, it seems clear that FGF9 is an important regulator of ovarian function in mammals, but its role may differ between monotocous and polytocous animals. Nevertheless, detailed information about signaling mechanisms activated by FGF9 in the ovary is lacking.
The technology of microarray is a powerful tool for one to investigate how a specific cell type reacts to certain stimuli, enabling the simultaneous measurement of thousands of gene transcripts [24]. Therefore, we utilized this resource to investigate how bovine TC from large ovarian follicles respond to exogenous FGF9 in vitro. The present study unveils novel signaling pathways activated by FGF9 in TC and may provide valuable information to understand molecular aspects of cell proliferation, steroidogenesis, and apoptosis in a mono-ovulatory species such as cattle and humans.
2. Material and methods
2.1. Reagents and Hormones
The reagents used in cell culture were Ham’s F-12 (F12), DMEM, gentamicin, glutamine, sodium bicarbonate, trypan blue, deoxyribonuclease (DNase), and collagenase from Sigma-Aldrich Chemical Co. (St. Louis, MO), and fetal calf serum (FCS) from Equitech-Bio, Inc. (Kerrville, TX). The hormones used in cell culture were recombinant human FGF9 and IGF-1 (R&D Systems, Minneapolis, MN; all carrier-free), and ovine LH (NIDDK-oLH-26; activity: 1.0× NIH-LH-S1 U/mg) from the National Hormone & Pituitary Program (Torrance, CA).
2.2. Cell Collection and In Vitro Culture
Ovaries were obtained from beef heifers (n = 76) at a local abattoir and transported to the laboratory in 0.9% saline with 1% streptomycin/penicillin on ice. TC were isolated from large antral follicles (8.1–22 mm in surface diameter) with adequate vascularity and moderately transparent follicular fluid as previously described [16, 25]. Briefly, follicles were bisected, GC were scraped free from the theca interna and the theca interna tissue was removed via microdissection and enzymatically digested for 1 h at 37°C on a rocking platform. Non-digested thecal tissue was removed via filtration through a 149 μm mesh screen (Gelman Sciences, Ann Arbor, MI, USA). TC were then centrifuged at 50 × g for 7 min, washed twice in medium (1:1 DMEM and F12 containing 2.0 mM glutamine, 0.12 mM gentamicin, and 38.5 mM sodium bicarbonate), and resuspended in serum-free medium containing collagenase and DNase at 1.25 and 0.5 mg/mL, respectively, to prevent clumping of cells before plating [16, 26].
Viability of TC was determined by trypan blue exclusion test and 3 × 105 viable cells (per well) were transferred to Falcon 24-well multiwell plates (Becton Dickinson, Lincoln Park, NJ, USA) with medium containing 10% FCS. Cells were cultured at 38.5°C in a humidified 95% air and 5% CO2 environment for the first 48 h with a medium change at 24 h. Then, TC were washed twice in serum-free medium and cultured in 1 mL serum-free medium containing 15 ng/mL of LH and 15 ng/mL of IGF-1 with or without 30 ng/mL of FGF9 for 24 h. Dose of IGF1, LH and FGF9 were based on the previous studies [16, 25].
2.3. RNA Extraction, Microarray and Statistical Analyses
Following treatment, TC were lysed with 0.5 mL of TRIzol® Reagent (Life Technologies Inc., Gaithersburg, MD) and total RNA was extracted as previously described [26, 27]. Affymetrix GeneChip Bovine Genome Arrays (Affymetrix, Santa Clara, CA) were used for the microarray as previously described [28]. This particular array is designed to monitor expression of approximately 23,000 bovine transcripts through 24,072 probe sets. A total of eight chips were hybridized with RNA extracted from four biological replicates of the same number of TC pools in a paired design for the two treatments (FGF9 or control). Each pool of TC was generated from 5 to 7 large follicles collected from 4 to 5 animals. The processing of RNA, including RNA purification and hybridization of microarray slides, was performed by the University of Tulsa Microarray Core Facility. Affymetrix GeneChip Operating Software (GCOS ver. 1.1.1, Affymetrix, Santa Clara, CA) was used to quantitate each GeneChip®. A false discovery rate (FDR) threshold was set at p = 0.10. Summary intensities for each probe were loaded into DNA-Chip Analyzer (dChip), version 1.3, for normalization, standardization, and analysis. Paired t-tests were calculated using dChip to evaluate significant differences between treatments as previously described [27, 28].
2.4. Microarray Functional Data Analysis
To explore the biological knowledge associated with the statistically significant probe sets from the microarray chips in addition to the annotation produced along with statistical comparisons in dChip, the QIAGEN’s Ingenuity® Pathway Analysis (IPA®; QIAGEN Redwood City, http://www.qiagen.com/ingenuity) was utilized. The analyses were performed to investigate how FGF9 treatment affects molecular pathways, biological functions, and the connection between molecules in bovine TC.
2.5. Quantitative One-Step Real-Time RT-PCR
Abundance of mRNA for the selected target genes detected as up- and downregulated in the microarray experiment was validated through one-step real-time PCR (Table 1) as described previously [28–30]. Then, a simple comparison of the fold changes estimated from the microarray analysis and RT-PCR was performed. Fold change was calculated relative to the level of expression in samples from cultured TC treated with FGF9 vs. controls. Selection of genes was based on microarray result and previous studies indicating their regulation by FGF9 [16, 22, 26, 31, 32].
Table 1.
Sequences and characteristics for primers (Forward and Reverse) and probes for real-time RT-PCR amplification of target genes.
| Affymetrix ID1 & Accesion ID | Target gene2 | Sequences (forward/reverse/probe)3 | Melting temperature (°C) |
|---|---|---|---|
| Bt.24862.1.A1_at NM_001046273 | CCND1 | CGACTTCATCGAGCACTTCCT TCTGTGCCACAGACGTGAAGTT ATGCCGGTGGCCGAGGAGAAC |
56.9 58.8 65.3 |
| Bt.28178.1.A1_at XM_005213881.3 | SPRY2 | GGGCTGCACGCCTACAAGT CGCAGGTCCCGTAGTCGAT TGCCGTCAGACTGGATCTGCGACA |
60.7 59.2 63.9 |
| Bt.29166.1.S1_at NM_001082045.2 | CASP3 | CTTCCACGAAAATACTGGCATG TGAATGTTTCCCTGAGGTTTGC TCGATCTGGTACAGACGTG |
54.3 55.9 55.3 |
| Bt.7190.1.S1_at NM_176644 | CYP11A1 | ACAGGGAGAAGCTTGGCAATT GTAGGATCCCTCGAACTTGAAGA AGTTTATATCATTCACCCTGAAGACGTGGCCC |
57.2 55.8 63.3 |
| Bt.11656.1.S1_at NM_001192786.1 | CGN | GCAGAGAACAAAAAACGCTCTCA ACCTGATCTCGGCCACGAT AGAGCCGGCAACTCAAGAGCCTTGAG |
56.1 58.6 65.3 |
| Bt.21540.1.S1_at XM_010820096.2 | FGFR2c | GTTCCAATGCGGAAGTGCTG GTTTTGGCAGGACAGTGAGC AGGCGGATGCTGGCGAGTATATTTGTAAGG |
57.1 56.5 63.9 |
Probe set ID from Affymetrix.
Target genes: CCND1 = cyclin D1; SPRY2 = sprouty 2; CASP3 = caspase 3; CYP11A1 = side-chain cleavage enzyme; CGN = cingulin.
Forward and reverse primers, and fluorescent probe for each target gene.
3. Results
Analysis of hybridized GeneChip Bovine Genome arrays comparing FGF9-treated TC with control TC identified 2277 of 24,128 genes significantly up- and down-regulated at a level of P < 0.05 and with a FDR of P = 0.10, and of those genes, 355 were identifiable and differentially expressed using a cut-off of P < 0.05 and fold change of >1.3, with 164 elements up-regulated and 191 elements down-regulated. A 1.3-fold cut-off was based on our previous porcine cultured granulosa cell microarray data set [27] and other published ovarian microarrays using 1.2–1.3-fold cut-offs [33, 34]. The IPA software utilized a total of 345 of the 355 differentially expressed transcripts in its analysis. The 20 genes found to be the most up- and down-regulated among the differentially expressed genes are summarized in Table 2 and Table 3, respectively, in order of fold-change.
Table 2.
20 most up-regulated genes by FGF9 treatment in bovine TC.
| Probe set | Gene symbol | Gene Name | Fold change | P-value | |
|---|---|---|---|---|---|
| 1 | Bt.16538.2.A1 at | CCND1 | Cyclin D1 | 4.96 | 0.0000569 |
|
|
|||||
| 2 | Bt.12927.1.S1_at | HAS2 | hyaluronan synthase 2 | 3.90 | 0.000803649 |
|
|
|||||
| 3 | Bt. 1817.1.S1_at | ETV1 | Ets variant 1 | 3.49 | 0.00142088 |
|
|
|||||
| 4 | Bt.996.1.S1_at | — | transcribed locus | 3.25 | 0.00080151 |
|
|
|||||
| 5 | Bt.19826.1.A1_at | — | transcribed locus | 3.23 | 0.0210203 |
|
|
|||||
| 6 | Bt.6983.1.S1_at | SOX9 | SRY (Sex determining region Y)-box 9 | 3.16 | 0.0377339 |
|
|
|||||
| 7 | Bt.29956.1.A1_at | BMP2 | bone morphogenetic protein 2 | 2.98 | 0.00193277 |
|
|
|||||
| 8 | Bt.10212.1.S1_at | PHLDA1 | Pleckstrin homology-like domain, family A, member 1 | 2.97 | 0.000000737 |
|
|
|||||
| 9 | Bt.3051.1.S1 at | — | Transcribed locus | 2.92 | 0.0000611 |
|
|
|||||
| 10 | Bt.72.1.S1_at | MMP1 | matrix metallopeptidase 1 (interstitial collagenase) | 2.90 | 0.00506371 |
|
|
|||||
| 11 | Bt.16100.1.S1_at | FZD5 | Frizzled family receptor 5 | 2.81 | 0.000317748 |
|
|
|||||
| 12 | Bt.303.1.S1_at | P2RY1 | purinergic receptor P2Y, G-protein coupled, 1 | 2.74 | 0.0194217 |
|
|
|||||
| 13 | Bt.13073.1.S1_at | SERPINB2 | serpin peptidase inhibitor, clade B (ovalbumin), member 2 | 2.63 | 0.0182733 |
|
|
|||||
| 14 | Bt.568.1.S1_at | IBSP | integrin-binding sialoprotein | 2.52 | 0.0337404 |
|
|
|||||
| 15 | Bt.10648.1.S1_at | RRM2 | ribonucleotide reductase M2 | 2.43 | 0.0240124 |
|
|
|||||
| 16 | Bt.11109.1.S1_at | MIR221 | microRNA mir-221 | 2.43 | 0.0000000273 |
|
|
|||||
| 17 | Bt.12781.1.S1_at | MYB | v-myb myeloblastosis viral oncogene homolog (avian) | 2.40 | 0.0397893 |
|
|
|||||
| 18 | Bt.17951.1.A1_at | — | Transcribed locus | 2.36 | 0.0257273 |
|
|
|||||
| 19 | Bt.5618.1.A1_at | CNTNAP2 | contactin associated protein-like 2 | 2.33 | 0.000372504 |
|
|
|||||
| 20 | Bt.20507.1.S1_at | CYP26B1 | cytochrome P450, family 26, subfamily B, polypeptide 1 | 2.31 | 0.00301863 |
Table 3.
20 most down-regulated genes by FGF9 treatment in bovine TC.
| Probe set | Gene symbol | Gene Name | Fold change | P-value | |
|---|---|---|---|---|---|
| 1 | Bt.4939.1.S1_at | SFRP2 | secreted frizzled-related protein 2 | −4.09 | 0.00392234 |
|
|
|||||
| 2 | Bt.7190.1.S1_at | CYP11A1 | cytochrome P450, family 11, subfamily A, polypeptide 1 | −4.09 | 0.0236564 |
|
|
|||||
| 3 | Bt.13482.1.S1_at | NOV | nephroblastoma overexpressed | −3.25 | 0.0116386 |
|
|
|||||
| 4 | Bt.13855.2.S1_at | ADAMDEC1 | ADAM-like, decysin 1 | −3.25 | 0.00490709 |
|
|
|||||
| 5 | Bt.11656.1.S1_at | CGN | cingulin | −3.06 | 0.000463423 |
|
|
|||||
| 6 | Bt.19661.1.S1_at | PPP2R2B | protein phosphatase 2, regulatory subunit B, beta | −3.01 | 0.000310913 |
|
|
|||||
| 7 | Bt.20574.1.S1_at | AQP11 | aquaporin 11 | −2.99 | 0.00102593 |
|
|
|||||
| 8 | Bt.10021.1.S1_at | MYL7 | myosin, light chain 7, regulatory | −2.72 | 0.0261566 |
|
|
|||||
| 9 | Bt.19322.1.A1_at | COL4A6 | collagen, type IV, alpha 6 | −2.69 | 0.0213395 |
|
|
|||||
| 10 | Bt.5341.1.S1_at | OGN | osteoglycin | −2.68 | 0.00729922 |
|
|
|||||
| 11 | Bt.23113.1.A1_at | — | transcribed locus | −2.66 | 0.0000184 |
|
|
|||||
| 12 | Bt.22768.1.A1_at | SVEP1 | sushi, von Willebrand factor type A, EGF and pentraxin domain containing 1 | −2.65 | 0.0126702 |
|
|
|||||
| 13 | Bt.24211.1.A1_at | ASPN | asporin | −2.61 | 0.00459269 |
|
|
|||||
| 14 | Bt.1745.1.S1_at | KRT18 | keratin 18 | −2.59 | 0.00608511 |
|
|
|||||
| 15 | Bt.7042.1.S1_at | NPVF | neuropeptide VF precursor | −2.58 | 0.00210952 |
|
|
|||||
| 16 | Bt.16495.1.A1_at | COL4A5 | collagen, type IV, alpha 5 | −2.56 | 0.0140642 |
|
|
|||||
| 17 | Bt.5530.1.S1_at | DHRS3 | dehydrogenase/reductase (SDR family) member 3 | −2.49 | 0.00124302 |
|
|
|||||
| 18 | Bt.27645.1.A1_at | ISM1 | isthmin 1 homolog (zebrafish) | −2.46 | 0.00446444 |
|
|
|||||
| 19 | Bt.3885.4.S1_X_at | CLCA3P | chloride channel accessory 3 (pseudogene) | −2.36 | 0.00487499 |
|
|
|||||
| 20 | Bt.12685.1.S1_at | MYH11 | myosin, heavy chain 11, smooth muscle | −2.30 | 0.0294988 |
3.1. Identification of molecular canonical pathways altered by FGF9 treatment
The IPA software identified differentially expressed transcripts in 346 pathways in response to FGF9 in TC. The top ten canonical pathways affected by FGF9 (based on p-value on negative log scale) are shown in Fig. 1. The main functions associated with the top altered pathways include cellular assembly and organization, and cell cycle, growth, development, proliferation, death and survival. In addition to these, it is noteworthy to mention that FGF9 treatment affected transcripts for genes in pathways related to ovarian steroidogenesis, including: WNT/β-catenin signaling, IGF-1 signaling, PI3K/AKT signaling, TGF-β signaling, EGF signaling, and pregnenolone biosynthesis.
Fig. 1.
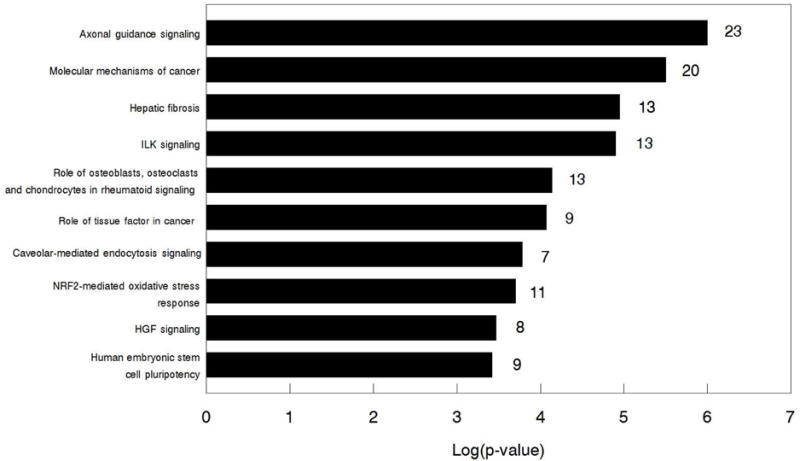
Top 10 canonical pathways altered by FGF9 in bovine TC identified by IPA. Numbers to the right on the bars indicate the number of differentially expressed transcripts in each pathway.
3.2. Identification of biological functions altered by FGF9 treatment
The IPA identified 500 annotated biological functions for the differentially expressed transcripts altered by FGF9 treatment in bovine TC. The top ten identified biological functions predicted to be increased by FGF9 in TC are shown in Fig. 2 in order of activation z-scores (z-scores measure the correlation between relationship direction and gene expression, but only z-scores greater than 2 or smaller than-2 are considered significant for predicted activation state).
Fig. 2.
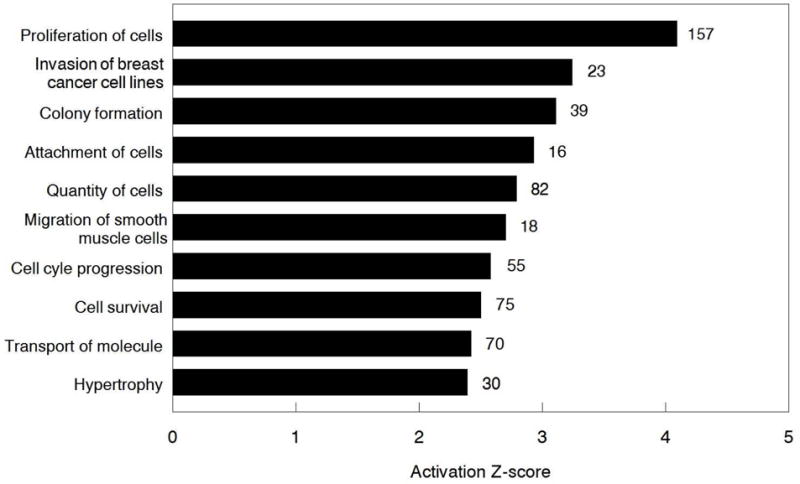
Top 10 biological functions for the differentially expressed transcripts altered by FGF9 treatment in bovine TC identified by IPA. Numbers to the right on the bars indicate the number of differentially expressed transcripts in each pathway.
3.3. Identification of molecular networks affected by FGF9 treatment
Seeking to understand how molecules affected by FGF9 connect to each other and relate to specific functions, we analyzed the networks generated by IPA. The differentially expressed transcripts affected by FGF9 were categorized in 21 different networks. The top 10 networks according to p-score are shown in Fig. 3.
Fig. 3.
Top 10 networks generated by IPA according to score. The greater the area in the graph, the higher the score of the network.
In order to assess how FGF9 treatment may affect ovarian function, special attention was given to networks involving cell proliferation and steroidogenesis, two actions already reported to be affected by FGF9 in bovine TC [16]. These networks are shown in Figs. 5 and 6, respectively. For cell proliferation, up-regulated (P < 0.05) transcripts were CCND1, FZD5, MYB, CASP3, FRMD4A, ACKR3, JADE2, RBPJ, HAUS8, ARRDC2, DENND2A, H2AFZ, while down-regulated (P < 0.05) transcripts were HLA-DRA, AARSD1, MLF1, JADE3, UBE2D4, PPP2R2B, NQO1, HMOX1, AGTR1, RNF150, SOD, KCNAB1. For steroidogenesis, up-regulated genes (P < 0.05) by FGF9 were: TFPI, VLDLR, SLC38A2, ACSL4, ARG2, PER2 while down-regulated genes (P < 0.05) by FGF9 were: COL14A1, OGN, IGF2, SORT1, AGTR1, GPIIB-IIIA, CYP2C9, STAR, CYP11A1, SIRT3, RIMS1, ARID5B; frizzled molecules were either down-regulated (SRFP2, FZD2, FZD4) or up-regulated (FZD5).
Fig. 5.
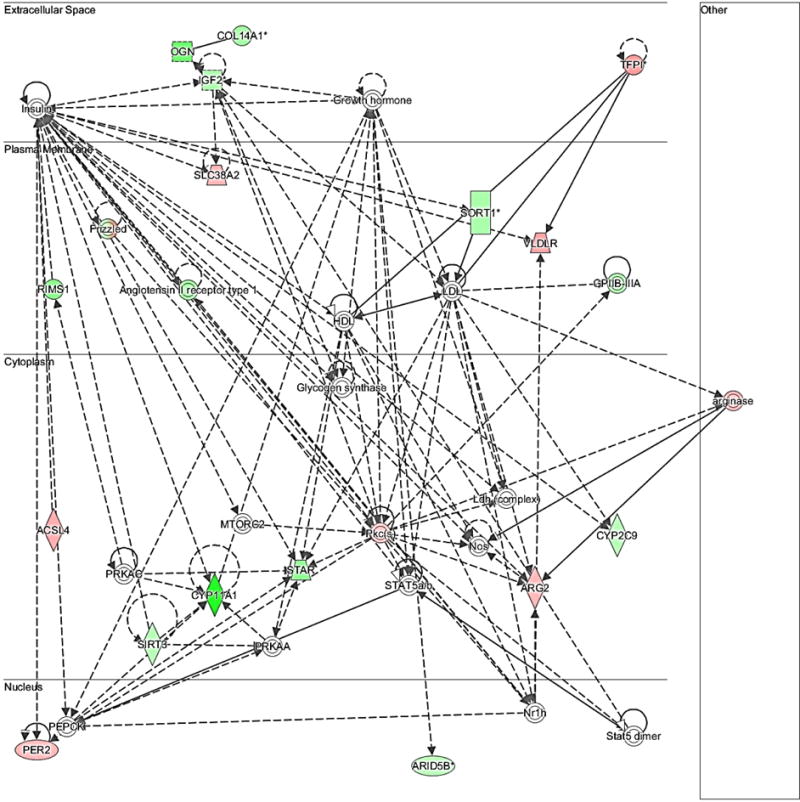
Network of differentially expressed transcripts related to steroidogenesis (Network 15: Lipid metabolism, molecular transport, small molecule biochemistry). The bold lines show a direct association among molecules while dashed lines show an indirect association among the molecules. Different shapes of molecules indicate different functions. Significantly up-regulated genes (P < 0.05) by FGF9 in this network were: TFPI, VLDLR, SLC38A2, ACSL4, ARG2, PER2; significantly down-regulated genes (P < 0.05) by FGF9 in this network were: COL14A1, OGN, IGF2, SORT1, AGTR1, GPIIB-IIIA, CYP2C9, STAR, CYP11A1, SIRT3, RIMS1, ARID5B. Frizzled molecules were either down-regulated (SRFP2, FZD2, FZD4) or up-regulated (FZD5).
Fig. 6.
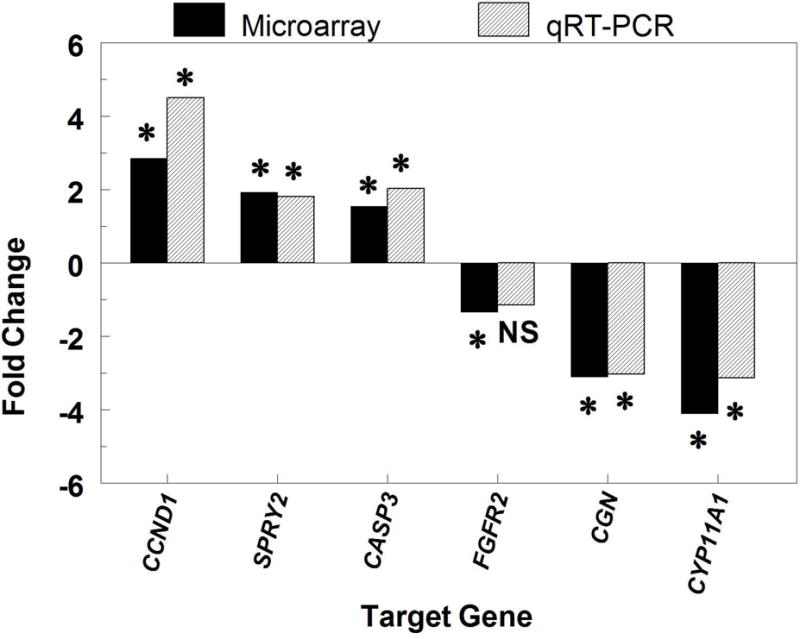
Comparison of fold changes in mRNA expression detected through Affymetrix microarray analysis (Affymetrix, Santa Clara, CA; solid bar) and quantitative reverse-transcription PCR (qRT-PCR) analysis (hatched bar) of RNA obtained from theca cells treated with or without FGF9. All fold changes from the microarray analysis are statistically significant (P < 0.05). CCND1 = cyclin D1; SPRY2 = sprouty 2; CASP3 = caspase 3; CYP11A1 = side-chain cleavage enzyme; CGN = cingulin; FGFR2c = FGF receptor 2c. Asterisks indicate values are significantly (P < 0.05) altered by FGF9 as determined by qRT-PCR and/or microarray analysis. NS = not significant.
3.4. qRT-PCR of up and down regulated genes
Abundance of mRNA for the target genes selected from the pool of DNA sequences detected as differentially expressed in TC treated with or without FGF9 was validated through qRT-PCR. In addition, a comparison of the fold changes in expression of the target genes on Control and FGF9-treated TC samples estimated by microarray analysis and through qRT-PCR was performed (Fig. 6).
Microarray Upregulated Genes
Expression patterns for genes detected as upregulated in the microarray analysis were consistent with the quantitative analysis (Fig. 6). The fold changes detected in the microarrays for CCND1, SPRY2, and CASP3 were 2.86, 1.93, and 1.54, respectively, whereas in the quantitative analysis using real-time RT-PCR the estimated fold changes were 4.50, 1.81, and 2.03, respectively.
Microarray Downregulated Genes
For genes detected as downregulated in the microarray analysis, the same pattern of expression was detected only for CYP11A1 (−4.09 and −3.13 fold change for microarray and quantitative analysis, respectively), CGN (−3.06 and –3.03 fold change in the microarray and quantitative analysis, respectively) and FGFR2 (−1.34 and −1.15 fold change in the microarray and quantitative analysis, respectively) (Fig. 6).
4. Discussion
For the first time in any species, the effect of FGF9 on an ovarian cell transcriptome has been evaluated. FGF9 is a powerful mitogen that stimulates cell proliferation in several tissues and has been implicated in many types of cancer [20, 21, 35]. In the ovary, FGF9 has been reported to stimulate proliferation of both GC and TC [15, 16]. Hence, it is no surprise that, based on Z-activation scores, cell proliferation was the biological function most affected by FGF9 in the present analysis (Fig. 2), with 157 out of 346 differentially expressed transcripts related with this function.
The fact that the gene encoding for cyclin D1 (CCND1), a protein required for progression of the G1 phase of the cell cycle [36] that has been previously detected in proliferating TC (but not GC) in mice [37] and in proliferating TC and GC in cattle [32], was the most up-regulated transcript by FGF9 treatment in the present microarray further supports the importance of FGF9 in stimulating TC proliferation. In addition, two other genes among the top 20 up-regulated transcripts by FGF9 (FZD5 and MYB) are related to cell proliferation (as shown in Table 2 and Fig. 4). In fact, WNT proteins that bind to these cell-surface receptors (i.e., FZD5) stimulate cell proliferation of human endometrial adenocarcinoma cells [38] and bovine TC [39], while MYB (v-myb myeloblastosis viral oncogene homolog) encodes the protein c-Myb, a transcription factor that stimulates cell proliferation while suppressing apoptosis and differentiation of a variety of cell types, including human myeloid leukemia cells [40, 41] and human breast cancer cells [42]. The protein c-Myb is a positive regulator of CCND1 in human liver cancer [43] and breast carcinoma [44] cells. Furthermore, Bartunek and colleagues [45] reported that MYB and FGF2 cooperate to sustain avian hematopoietic cell proliferation while preventing them to differentiate into red blood cells. Hence, FGF9 appears to be stimulating the progression of the G1 phase of the cell cycle for bovine TC proliferation through the WNT signaling pathway and the regulation of CCND1. Interestingly, WNT3A, a member of the WNT family of ligands has been reported to increase FGF9 mRNA abundance in bovine TC [16], reinforcing the association between FGF9 and the WNT signaling pathway.
Fig. 4.
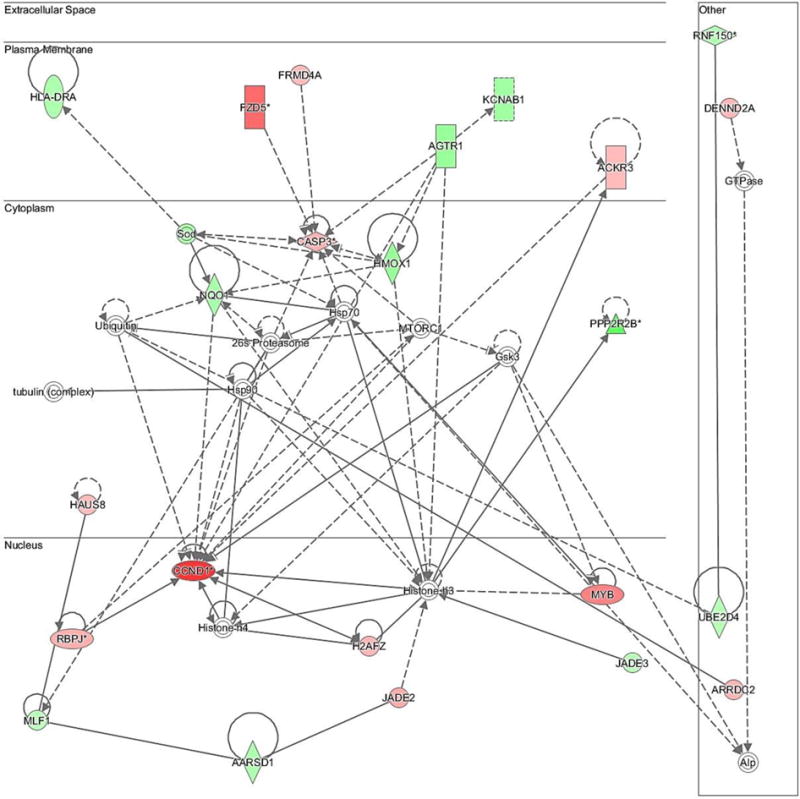
Network of differentially expressed transcripts related to cellular growth and proliferation (Network 3). The bold lines show a direct association among molecules while dashed lines show an indirect association among the molecules. Different shapes of molecules indicate different functions. Significantly up-regulated genes (P < 0.05) by FGF9 in this network were: CCND1, FZD5, MYB, CASP3, FRMD4A, ACKR3, JADE2, RBPJ, HAUS8, ARRDC2, DENND2A, H2AFZ; significantly down-regulated genes (P < 0.05) by FGF9 in this network were: HLA-DRA, AARSD1, MLF1, JADE3, UBE2D4, PPP2R2B, NQO1, HMOX1, AGTR1, RNF150, SOD, KCNAB1.
Another intriguing observation from the cell proliferation Network generated by IPA (Fig. 4) is the fact that transcripts for caspase-3 (CASP3) were increased in TC in response to FGF9. Indeed, although CASP3 is a major downstream effector of apoptosis in GC of atretic follicles, CASP3 immunostaining has also been reported in TC of healthy follicles [46]. Reports imply that CASP3 may be involved in selective destruction of organelles and may be enhancing cell survival and proliferation under certain situations [47, 48]. Interestingly, CASP3 cleaves the cyclin-dependent kinase inhibitor 1B (CDKN1B, p27Kip1), a mammalian regulator of the cell cycle that induces G1 arrest, thus stimulating lymphoid cell proliferation [49]. Because p27Kip1 decreases during TC proliferation and increases during TC terminal differentiation in mice [37], the present data indicate that, like with lymphoid cells, CASP3 may be stimulating bovine TC proliferation by preventing p27Kip1 to arrest cells at G1, but further research will be required to verify this suggestion. A lack of change in p27Kip1 mRNA following FGF9 treatment supports a post-transcriptional regulation of p27Kip1. Furthermore, MIR221, one of the top up-regulated genes in the present study, has been reported to down-regulate p27Kip1 via prevention of translation in human hepatocellular carcinoma [50].
It is also noteworthy that the genes HMOX1 and NQO1 were down-regulated by FGF9, as shown in the cell proliferation Network (Fig. 4). These genes are antioxidant and cytoprotective, being activated by the transcription activator NRF2 in response to oxidative stress in order to protect the cells [51]. Not by coincidence, NRF2-mediated oxidative stress response is among the top canonical pathways affected by FGF9 in the present study (Fig. 1). Reactive oxygen species (ROS) are constantly produced during metabolic processes and it is now well accepted that ROS at high concentrations are cytotoxic, but, at moderate concentrations, ROS regulates signal transduction processes, including cell proliferation [43, 52]. In the ovary, high levels of ROS have been shown to stimulate the initiation of apoptosis in GC of antral follicles in rats [53] and mice [54]. Nevertheless, in rat theca-interstitial cells, generation of ROS is required to maintain cell proliferation, and a reduction in the level of ROS inhibits cell proliferation [55]. Hence, the FGF9-induced reduction in transcripts for HMOX1 and NQO1, factors that would suppress oxidative stress and likely reduce ROS concentrations, observed in the present study suggests that ROS may be important for bovine TC proliferation. This idea is further supported by the fact that: 1) ROS mediate FGF2 biological effects in chondrocytes (as reviewed in [56]); 2) FGF2 activates antioxidant pathways in cultures of rat hippocampal neurons [57]; and 3) FGF10 attenuates H2O2-induced human and rat alveolar epithelia cell DNA damage [58]. In addition, Cui and co-authors [59] recently reported that NQO1 is frequently up-regulated in human ovarian carcinomas, supporting the idea that controlled regulation of NQO1 may play an important role in ovarian function. Nonetheless, further research should be conducted to verify the effects of FGF9 on oxidative stress measures in theca cells, such as RSO concentrations.
FGF9 has also been reported as a regulator of steroidogenesis by murine, bovine, and porcine follicular somatic cells [12, 15, 16, 22]. In bovine GC, FGF9 decreases IGF-1 plus FSH-induced E2, P4, and pregnenolone production while decreasing mRNA abundance for the steroidogenic enzyme CYP11A1 and for the FSH receptor (FSHR) of both small (1–5 mm) and large (8–22 mm) antral follicles [15]. In bovine TC, FGF9 decreases IGF-1 plus LH-induced P4 and androstenedione (A4) production while decreasing mRNA abundance for the steroidogenic enzymes CYP11A1 and CYP17A1 and for the LH receptor (LHCGR) of large antral follicles [16]. Therefore, the fact that CYP11A1 is among the top down-regulated genes is not surprising (Table 3). In addition, other genes related to steroidogenesis of TC were down-regulated by FGF9 in this study (Fig. 5), including the genes encoding for steroidogenic acute regulatory protein (STAR), insulin-like growth factor 2 (IGF2), angiotensin II receptor, type I (AGTR1), frizzled class receptor 4 (FZD4), and cytochrome P450, family 2, subfamily C, polypeptide 9 (CYP2C9). STAR is responsible for mediating the transport of cholesterol to mitochondria [60]; IGF2, AGTR1 and FZD4 appear to be important to P4 production, since both IGF2 [61] and angiotensin II [62] have been reported to stimulate P4 release while FZD4 knockout mice have lower P4 production [63]; CYP2C9 is involved in catabolism of P4 [64]. Hence, these observations suggest that FGF9 affects several genes that may act in concert to suppress P4 and A4 production by bovine TC. The fact that STAR mRNA was down-regulated in this study is in contrast to a previous studies showing that FGF9 has no effect on abundance of IGF1-induced STAR mRNA in bovine GC [15] and TC [16], and thus will require further elucidation. Finally, the observation that the frizzled proteins encoding genes FZD2, FZD4, and SRFP2 were down-regulated in the steroidogenesis Network suggests that these genes can be also linking the WNT signaling pathway with steroidogenesis in TC, but this hypothesis also requires further investigation. Recent studies support this idea by showing that WNT5A increases androstenedione production by bovine TC [39].
Steroidogenesis by TC and GC of large antral follicles is crucial for follicular differentiation [65–68]. Because FGF9 decreases synthesis of P4 and A4 and reduces mRNA abundance for both LHCGR and CYP17A1 [16), critical factors for TC differentiation in preovulatory follicles [67,69], it seems likely to suppose that FGF9 acts as an anti-differentiation factor of TC of large antral follicles, at least in cattle. This idea is further reinforced by the observation that abundance of bovine FGF9 mRNA is greater in TC of large E2-inactive follicles (i.e., future atretic follicles) than in large E2-active follicles (i.e., dominant follicles) at an early growing phase of the first dominant follicle [23]. Although TC were initally grown in 10% FCS in the present study, it is unlikely that TC from bovine follicles luteinized with time in culture because: 1) progesterone production does not increase with time using this culture paradigm [25]; 2) the morphology of TC in this culture system retains a fibroblastic appearance [70]; and 3) TC remain responsive to LH and IGF1 in terms of CYP17A1 mRNA and androstenedione production [16, 25, 26 71].Thus, the fact that FGF9 up-regulated CCND1 and MYB, factors that suppress cell differentiation while stimulating cell proliferation [37, 41], together with the observation that FGF9 was not predicted to upregulate transcripts related to TC differentiation in the current study corroborates the idea that FGF9 is stimulating bovine TC proliferation rather than differentiation. In a previous study [23], the IC50 for FGF9 (i.e., 9–14 ng/mL) reducing TC steroidogenesis suggested that the FGF9 effects are mediated by high affinity receptors because these values are in line with the Kd values obtained for FGF9 and bFGF binding to their respective receptors in other tissues [72, 73], but what the concentrations of FGF9 are in follicular fluid are unknown. Previously, we reported that attempts to use FGF9 ELISA and Western blotting to detect FGF9 in follicular fluid were unsuccessful, most likely because of the small quantities of FGF9 present [23]. Thus, additional developmental work will be required to ascertain intraovarian levels of FGF9.
In summary, the current microarray study allowed the identification of 345 differentially expressed transcripts in bovine TC following in vitro FGF9 treatment, several of them related to cell proliferation, survival, and steroidogenesis. Furthermore, the present study provides a genome-wide view of how FGF9 may promote TC proliferation while suppressing TC differentiation. Future investigations of the candidate genes and pathways unraveled here by IPA will not only provide an insight of how FGF9 plays a role in folliculogenesis in cattle, but will also help to unveil how this growth factor affects pathological conditions such as cystic ovarian follicles and ovarian cancer.
Highlights.
We examine changes in theca cell transcriptome after FGF9 treatment.
A total of 164 transcripts were up-regulated and 191 were down-regulated by FGF9.
IPA software identified 346 biological pathways in theca cells in response to FGF9.
Genes regulated were involved cell proliferation, antioxidation and steroidogenesis.
Acknowledgments
The authors thank the assistance of Dr. E.-S. Han and Jacob Crowley at the University of Tulsa Microarray Core Facility for microarray assistance; Creekstone Farms (Arkansas City, KS) for donation of bovine ovaries; the Oklahoma State University Recombinant DNA/Protein Core Facility for use of their equipment. This work supported in part by: the NICHD, National Institutes of Health, through Agreement R15-HD-066302, and the Oklahoma State University Agricultural Experiment Station (Project OKL02970).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The authors have nothing to discolose.
References
- 1.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2 doi: 10.1186/gb-2001-2-3-reviews3005. REVIEWS3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Itoh N, Ornitz DM. Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem. 2011;149:121–130. doi: 10.1093/jb/mvq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Givol D, Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. FASEB J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- 4.Itoh N, Ornitz DM. Evolution of the Fgf and Fgfr gene families. Trends Genet. 2004;20:563–569. doi: 10.1016/j.tig.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Miyamoto M, Naruo K, Seko C, Matsumoto S, Kondo T, Kurokawa T. Molecular-cloning of a novel cytokine cDNA-encoding the 9th member of the fibroblast growth-factor family, which has a unique secretion property. Mol Cell Biol. 1993;13:4251–4259. doi: 10.1128/mcb.13.7.4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavine KJ, Yu K, White AC, Zhang X, Smith C, Partanen J, Ornitz DM. Endocardial and epicardial derived FGF signals regulate myocardial proliferation and differentiation in vivo. Dev Cell. 2005;8:85–95. doi: 10.1016/j.devcel.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Singla DK, Singla RD, Abdelli LS, Glass C. Fibroblast growth factor-9 enhances M2 macrophage differentiation and attenuates adverse cardiac remodeling in the infarcted diabetic heart. PLoS One. 2015;10:e0120739. doi: 10.1371/journal.pone.0120739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weksler NB, Lunstrum GP, Reid ES, Horton WA. Differential effects of fibroblast growth factor (FGF) 9 and FGF2 on proliferation, differentiation and terminal differentiation of chondrocytic cells in vitro. Biochem J. 1999;342(Pt 3):677–682. [PMC free article] [PubMed] [Google Scholar]
- 10.Antoine M, Wirz W, Tag CG, Gressner AM, Marvituna M, Wycislo M, Hellerbrand C, Kiefer P. Expression and function of fibroblast growth factor (FGF) 9 in hepatic stellate cells and its role in toxic liver injury. Biochem Biophys Res Commun. 2007;361:335–341. doi: 10.1016/j.bbrc.2007.06.189. [DOI] [PubMed] [Google Scholar]
- 11.Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- 12.Drummond AE, Tellbach M, Dyson M, Findlay JK. Fibroblast growth factor-9, a local regulator of ovarian function. Endocrinology. 2007;148:3711–3721. doi: 10.1210/en.2006-1668. [DOI] [PubMed] [Google Scholar]
- 13.Ornitz DM, Itoh N. The Fibroblast Growth Factor signaling pathway. Wiley Interdiscip Rev Dev Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Kobayashi A, Sekido R, DiNapoli L, Brennan J, Chaboissier MC, Poulat F, Behringer RR, Lovell-Badge R, Capel B. Fgf9 and Wnt4 act as antagonistic signals to regulate mammalian sex determination. PLoS Biol. 2006;4:e187. doi: 10.1371/journal.pbio.0040187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber NB, Spicer LJ. Effects of fibroblast growth factor 9 (FGF9) on steroidogenesis and gene expression and control of FGF9 mRNA in bovine granulosa cells. Endocrinology. 2012;153:4491–4501. doi: 10.1210/en.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schreiber NB, Totty ML, Spicer LJ. Expression and effect of fibroblast growth factor 9 in bovine theca cells. J Endocrinol. 2012;215:167–175. doi: 10.1530/JOE-12-0293. [DOI] [PubMed] [Google Scholar]
- 17.Lai MS, Cheng YS, Chen PR, Tsai SJ, Huang BM. Fibroblast growth factor 9 activates akt and MAPK pathways to stimulate steroidogenesis in mouse Leydig cells. PLoS One. 2014;9:e90243. doi: 10.1371/journal.pone.0090243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harada M, Murakami H, Okawa A, Okimoto N, Hiraoka S, Nakahara T, Akasaka R, Shiraishi Y, Futatsugi N, Mizutani-Koseki Y, Kuroiwa A, Shirouzu M, Yokoyama S, Taiji M, Iseki S, Ornitz DM, Koseki H. FGF9 monomer-dimer equilibrium regulates extracellular matrix affinity and tissue diffusion. Nat Genet. 2009;41:289–298. doi: 10.1038/ng.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aurbach EL, Inui EG, Turner CA, Hagenauer MH, Prater KE, Li JZ, Absher D, Shah N, Blandino P, Jr, Bunney WE, Myers RM, Barchas JD, Schatzberg AF, Watson SJ, Jr, Akil H. Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci USA. 2015;112:11953–11958. doi: 10.1073/pnas.1510456112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todo T, Kondo T, Kirino T, Asai A, Adams EF, Nakamura S, Ikeda K, Kurokawa T. Expression and growth stimulatory effect of fibroblast growth factor 9 in human brain tumors. Neurosurgery. 1998;43:337–346. doi: 10.1097/00006123-199808000-00098. [DOI] [PubMed] [Google Scholar]
- 21.Sun C, Fukui H, Hara K, Zhang X, Kitayama Y, Eda H, Tomita T, Oshima T, Kikuchi S, Watari J, Sasako M, Miwa H. FGF9 from cancer-associated fibroblasts is a possible mediator of invasion and anti-apoptosis of gastric cancer cells. BMC Cancer. 2015;15:333. doi: 10.1186/s12885-015-1353-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans JR, Schreiber NB, Williams JA, Spicer LJ. Effects of fibroblast growth factor 9 on steroidogenesis and control of FGFR2IIIc mRNA in porcine granulosa cells. J Anim Sci. 2014;92:511–519. doi: 10.2527/jas.2013-6989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schütz LF, Schreiber NB, Gilliam JN, Cortinovis C, Totty ML, Caloni F, Evans JR, Spicer LJ. Changes in fibroblast growth factor 9 mRNA in granulosa and theca cells during ovarian follicular growth in dairy cattle. J Dairy Sci. 2016;99:9143–9151. doi: 10.3168/jds.2015-10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schena M, Shalon D, Davis RW, Brown PO. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science. 1995;270:467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 25.Stewart RE, Spicer LJ, Hamilton TD, Keefer BE. Effects of insulin-like growth factor I and insulin on proliferation and on basal and luteinizing hormone-induced steroidogenesis of bovine thecal cells: involvement of glucose and receptors for insulin-like growth factor I and luteinizing hormone. J Anim Sci. 1995;73:3719–3731. doi: 10.2527/1995.73123719x. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Schütz LF, Robinson CL, Totty ML, Spicer LJ. Evidence that gene expression of ovarian follicular tight junction proteins is regulated in vivo and in vitro in cattle. J Anim Sci. 2017;95:1313–1324. doi: 10.2527/jas.2016.0892. [DOI] [PubMed] [Google Scholar]
- 27.Grado-Ahuir JA, Aad PY, Ranzenigo G, Caloni F, Cremonesi F, Spicer LJ. Microarray analysis of insulin-like growth factor-I-induced changes in messenger ribonucleic acid expression in cultured porcine granulosa cells: possible role of insulin-like growth factor-I in angiogenesis. J Anim Sci. 2009;87:1921–1933. doi: 10.2527/jas.2008-1222. [DOI] [PubMed] [Google Scholar]
- 28.Grado-Ahuir JA, Aad PY, Spicer LJ. New insights into the pathogenesis of cystic follicles in cattle: Microarray analysis of gene expression in granulosa cells. J Anim Sci. 2011;89:1769–1786. doi: 10.2527/jas.2010-3463. [DOI] [PubMed] [Google Scholar]
- 29.Voge JL, Aad PY, Santiago CA, Goad DW, Malayer JR, Allen D, Spicer LJ. Effect of insulin-like growth factors (IGF), FSH, and leptin on IGF-binding-protein mRNA expression in bovine granulosa and theca cells: quantitative detection by real-time PCR. Peptides. 2004;25:2195–2203. doi: 10.1016/j.peptides.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Dentis JL, Schreiber NB, Gilliam JN, Schutz LF, Spicer LJ. Changes in brain ribonuclease (BRB) messenger RNA in granulosa cells (GCs) of dominant vs subordinate ovarian follicles of cattle and the regulation of BRB gene expression in bovine GCs. Domest Anim Endocrinol. 2016;55:32–40. doi: 10.1016/j.domaniend.2015.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Z, Price CA. Differential actions of fibroblast growth factors on intracellular pathways and target gene expression in bovine ovarian granulosa cells. Reproduction. 2012;144:625–632. doi: 10.1530/REP-12-0199. [DOI] [PubMed] [Google Scholar]
- 32.Totty ML, Morrell BC, Spicer LJ. Fibroblast growth factor 9 (FGF9) regulation of cyclin D1 and cyclin-dependent kinase-4 in ovarian granulosa and theca cells of cattle. Mol Cell Endocrinol. 2017;440:25–33. doi: 10.1016/j.mce.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruebel M, Shankar K, Gaddy D, Lindsey F, Badger T, Andres A. Maternal obesity is associated with ovarian inflammation and upregulation of early growth response factor 1. Am J Physiol Endocrinol Metab. 2016;311:E269–77. doi: 10.1152/ajpendo.00524.2015. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Zhuang X, Xu T, Mao M, Wang C, Chen Y, Han X, Wu J. Expression analysis of microRNAs and mRNAs in ovarian granulosa cells after microcystin-LR exposure. Toxicon. 2017;129:11–19. doi: 10.1016/j.toxicon.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Hendrix ND, Wu R, Kuick R, Schwartz DR, Fearon ER, Cho KR. Fibroblast growth factor 9 has oncogenic activity and is a downstream target of Wnt signaling in ovarian endometrioid adenocarcinomas. Cancer Res. 2006;66:1354–1362. doi: 10.1158/0008-5472.CAN-05-3694. [DOI] [PubMed] [Google Scholar]
- 36.Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- 37.Robker RL, Richards JS. Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol. 1998;12:924–940. doi: 10.1210/mend.12.7.0138. [DOI] [PubMed] [Google Scholar]
- 38.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 39.Spicer LJ. Wingless-type mouse mammary tumor virus integration site (WNT) regulation of ovarian theca cells of cattle. J Anim Sci. 2016;94(E-Suppl. 5):539. doi: 10.1093/jas/skab197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anfossi G, Gewirtz AM, Calabretta B. An oligomer complementary to c-myb-encoded mRNA inhibits proliferation of human myeloid leukemia cell lines. Proc Natl Acad Sci USA. 1989;86:3379–3383. doi: 10.1073/pnas.86.9.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lyon J, Robinson C, Watson R. The role of Myb proteins in normal and neoplastic cell proliferation. Crit Rev Oncog. 1994;5:373–388. doi: 10.1615/critrevoncog.v5.i4.30. [DOI] [PubMed] [Google Scholar]
- 42.Drabsch Y, Robert RG, Gonda TJ. MYB suppresses differentiation and apoptosis of human breast cancer cells. Breast Cancer Res. 2010;12:R55. doi: 10.1186/bcr2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, Luo N, Luo Y, Peng Z, Zhang T, Li S. microRNA-150 inhibits human CD133-positive liver cancer stem cells through negative regulation of the transcription factor c-Myb. Int J Oncol. 2012;40:747–756. doi: 10.3892/ijo.2011.1242. [DOI] [PubMed] [Google Scholar]
- 44.Mitra P, Yang RM, Sutton J, Ramsay RG, Gonda TJ. CDK9 inhibitors selectively target estrogen receptor-positive breast cancer cells through combined inhibition of MYB and MCL-1 expression. Oncotarget. 2016;7:9069–9083. doi: 10.18632/oncotarget.6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bartunek P, Pajer P, Karafiat V, Blendinger G, Dvorak M, Zenke M. bFGF signaling and v-Myb cooperate in sustained growth of primitive erythroid progenitors. Oncogene. 2002;21:400–410. doi: 10.1038/sj.onc.1205103. [DOI] [PubMed] [Google Scholar]
- 46.Boone DL, Tsang BK. Caspase-3 in the rat ovary: localization and possible role in follicular atresia and luteal regression. Biol Reprod. 1998;58:1533–1539. doi: 10.1095/biolreprod58.6.1533. [DOI] [PubMed] [Google Scholar]
- 47.Perfettini JL, Kroemer G. Caspase activation is not death. Nat Immunol. 2003;4:308–310. doi: 10.1038/ni0403-308. [DOI] [PubMed] [Google Scholar]
- 48.Kuranaga E. Caspase signaling in animal development. Dev Growth Differ. 2011;53:137–148. doi: 10.1111/j.1440-169X.2010.01237.x. [DOI] [PubMed] [Google Scholar]
- 49.Frost V, Al-Mehairi S, Sinclair AJ. Exploitation of a non-apoptotic caspase to regulate the abundance of the cdkI p27(KIP1) in transformed lymphoid cells. Oncogene. 2001;20:2737–2748. doi: 10.1038/sj.onc.1204367. [DOI] [PubMed] [Google Scholar]
- 50.Fornari F, Gramantieri L, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Giovannini C, Croce CM, Bolondi L, Negrini M. MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene. 2008;27:5651–5661. doi: 10.1038/onc.2008.178. [DOI] [PubMed] [Google Scholar]
- 51.Attucks OC, Jasmer KJ, Hannink M, Kassis J, Zhong Z, Gupta S, Victory SF, Guzel M, Polisetti DR, Andrews R, Mjalli AM, Kostura MJ. Induction of heme oxygenase I (HMOX1) by HPP-4382: a novel modulator of Bach1 activity. PLoS One. 2014;9:e101044. doi: 10.1371/journal.pone.0101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu Y, Rosen DG, Zhou Y, Feng L, Yang G, Liu J, Huang P. Mitochondrial manganese-superoxide dismutase expression in ovarian cancer: role in cell proliferation and response to oxidative stress. J Biol Chem. 2005;280:39485–39492. doi: 10.1074/jbc.M503296200. [DOI] [PubMed] [Google Scholar]
- 53.Devine PJ, Perreault SD, Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol Reprod. 2012;86:1–10. doi: 10.1095/biolreprod.111.095224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang JQ, Gao BW, Wang J, Ren QL, Chen JF, Ma Q, Zhang ZJ, Xing BS. Critical role of FoxO1 in granulosa cell apoptosis caused by oxidative stress and protective effects of grape seed procyanidin B2. Oxid Med Cell Longev. 2016;2016:6147345. doi: 10.1155/2016/6147345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19:1519–1524. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- 56.Sainz RM, Lombo F, Mayo JC. Radical decisions in cancer: redox control of cell growth and death. Cancers (Basel) 2012;4:442–474. doi: 10.3390/cancers4020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mark RJ, Keller JN, Kruman I, Mattson MP. Basic FGF attenuates amyloid beta-peptide-induced oxidative stress, mitochondrial dysfunction, and impairment of Na+/K+-ATPase activity in hippocampal neurons. Brain Res. 1997;756:205–214. doi: 10.1016/s0006-8993(97)00196-0. [DOI] [PubMed] [Google Scholar]
- 58.Upadhyay D, Bundesmann M, Panduri V, Correa-Meyer E, Kamp DW. Fibroblast growth factor-10 attenuates H2O2-induced alveolar epithelial cell DNA damage: role of MAPK activation and DNA repair. Am J Respir Cell Mol Biol. 2004;31:107–113. doi: 10.1165/rcmb.2003-0064OC. [DOI] [PubMed] [Google Scholar]
- 59.Cui X, Li L, Yan G, Meng K, Lin Z, Nan Y, Jin G, Li C. High expression of NQO1 is associated with poor prognosis in serous ovarian carcinoma. BMC Cancer. 2015;15:244. doi: 10.1186/s12885-015-1271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin D, Sugawara T, Strauss JF, 3rd, Clark BJ, Stocco DM, Saenger P, Rogol A, Miller WL. Role of steroidogenic acute regulatory protein in adrenal and gonadal steroidogenesis. 1995; Science. 267:1828–1831. doi: 10.1126/science.7892608. [DOI] [PubMed] [Google Scholar]
- 61.Spicer LJ, Voge JL, Allen DT. Insulin-like growth factor-II stimulates steroidogenesis in cultured bovine thecal cells. Mol Cell Endocrinol. 2004;227:1–7. doi: 10.1016/j.mce.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Acosta TJ, Berisha B, Ozawa T, Sato K, Schams D, Miyamoto A. Evidence for a local endothelin-angiotensin-atrial natriuretic peptide systemin bovine mature follicles in vitro: effects on steroid hormones and prostaglandin secretion. Biol Reprod. 1999;61:1419–1425. doi: 10.1095/biolreprod61.6.1419. [DOI] [PubMed] [Google Scholar]
- 63.Hsieh M, Boerboom D, Shimada M, Lo Y, Parlow AF, Luhmann UF, Berger W, Richards JS. Mice null for Frizzled4 (Fzd4−/−) are infertile and exhibit impaired corpora lutea formation and function. Biol Reprod. 2005;73:1135–1146. doi: 10.1095/biolreprod.105.042739. [DOI] [PubMed] [Google Scholar]
- 64.Yamazaki H, Shimada T. Progesterone and testosterone hydroxylation by cytochromes P450 2C19, 2C9, and 3A4 in human liver microsomes. Arch Biochem Biophys. 1997;346:161–169. doi: 10.1006/abbi.1997.0302. [DOI] [PubMed] [Google Scholar]
- 65.Knecht M, Darbon JM, Ranta T, Baukal AJ, Catt KJ. Estrogens enhance the adenosine 3',5'-monophosphate-mediated induction of follicle-stimulating hormone and luteinizing hormone receptors in rat granulosa cells. Endocrinology. 1984;115:41–49. doi: 10.1210/endo-115-1-41. [DOI] [PubMed] [Google Scholar]
- 66.Evans AC, Fortune JE. Selection of the dominant follicle in cattle occurs in the absence of differences in the expression of messenger ribonucleic acid for gonadotropin receptors. Endocrinology. 1997;138:2963–2971. doi: 10.1210/endo.138.7.5245. [DOI] [PubMed] [Google Scholar]
- 67.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–214. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 68.Young JM, McNeilly AS. Theca: the forgotten cell of the ovarian follicle. Reproduction. 2010;140:489–504. doi: 10.1530/REP-10-0094. [DOI] [PubMed] [Google Scholar]
- 69.Richards JS. Hormonal control of gene expression in the ovary. Endocr Rev. 1994;15:725–751. doi: 10.1210/edrv-15-6-725. [DOI] [PubMed] [Google Scholar]
- 70.Chamberlain CS, Spicer LJ. Hormonal control of ovarian cell production of insulin-like growth factor binding proteins. Mol Cell Endocrinol. 2001;182:69–81. doi: 10.1016/s0303-7207(01)00541-x. [DOI] [PubMed] [Google Scholar]
- 71.Spicer LJ, Aad PY, Allen DT, Mazerbourg S, Payne AH, Hsueh AJ. Growth differentiation factor 9 (GDF9) stimulates proliferation and inhibits steroidogenesis by bovine theca cells: influence of follicle size on responses to GDF9. Biol Reprod. 2008;78:243–53. doi: 10.1095/biolreprod.107.063446. [DOI] [PubMed] [Google Scholar]
- 72.Neufeld G, Gospodarowicz D. The identification and partial characterization of the fibroblast growth factor receptor of baby hamster kidney cells. J Biol Chem. 1985;260:13860–13868. 1985. [PubMed] [Google Scholar]
- 73.Hecht D, Zimmerman N, Bedford M, Avivi A, Yayon A. Identification of fibroblast growth factor 9 (FGF9) as a high affinity, heparin dependent ligand for FGF receptors 3 and 2 but not for FGF receptors 1 and 4. Growth Factors. 1995;12:223–233. doi: 10.3109/08977199509036882. [DOI] [PubMed] [Google Scholar]


