Abstract
Radiation-induced fibrosis (RIF) is a major side effect of radiotherapy in cancer patients with no effective therapeutic options. RIF involves excess deposition and aberrant remodeling of the extracellular matrix (ECM) leading to stiffness in tissues and organ failure. Development of preclinical models of RIF is crucial to elucidate the molecular mechanisms regulating fibrosis and develop therapeutic approaches. In addition to radiation, the main molecular perpetrators of fibrotic reactions are cytokines, including transforming growth factor-β (TGF-β). We hypothesized human oral fibroblasts would develop an in vitro fibrotic reaction in response to radiation and TGF-β. We demonstrate fibroblasts exposed to radiation followed by TGF-β exhibit a fibrotic phenotype with increased collagen deposition, cell proliferation, migration and invasion. This in vitro model of RIF (RIFiv) demonstrates the early biological processes involved in fibrosis. We demonstrate increased levels of several molecules including collagen 1α1, collagen XIα1, integrin-α2 and cyclin D1 mRNA in irradiated cells. A clinically relevant antifibrotic agent, pentoxifylline, and a curcumin analogue both mitigated collagen deposition in irradiated fibroblast cultures. In summary, we established an in vitro model for RIF that facilitates the elucidation of molecular mechanisms in radiation-induced fibrosis and the development of effective therapeutic approaches.
Introduction
Radiation therapy is a common treatment of head and neck cancer, either alone or in combination with surgery and/or chemotherapy. Radiation induces damage in both tumor cells and surrounding tissue. Patients tend to develop significant long-term sequelae, due to both direct changes in cell function, as well as indirect responses to tissue injury. The presence of cytokines and inflammatory mediators contributes to the chronicity of radiation injury and can lead to radiation-induced fibrosis (RIF)1. RIF is a common late complication of radiation therapy and may not manifest for several months after treatment. In the head and neck, fibrosis can lead to trismus, xerostomia, decreased vocal quality, osteoradionecrosis, dysphagia, and aspiration, which all significantly impact quality of life1,2.
Initial inflammation triggered by radiation causes fibroblasts to proliferate, migrate and transdifferentiate into myofibroblasts. These fibroblasts produce excess collagen, which is in abundance in the fibrotic region3. A variety of growth factors have been implicated in fibrosis. Specifically, TGF-β, a key factor secreted by immune cells and fibroblasts, has been observed in both experimental and clinical studies to serve as a potent and primary chemotactic mediator of RIF4–9. Among the pathophysiological roles of TGF-β, induction of ECM expression is one of the critical steps of fibrosis10,11. In vivo studies report increased TGF-β expression in irradiated mouse skin with noted upregulation of a TGF-β receptor12. However, understanding of additional molecular mechanisms and therapeutic targeting thereof has been limited by the lack of an in vitro model.
Based on these observations, we hypothesized in vitro radiation exposure and TGF-β stimulation will recapitulate the fibrotic phenotype. We assessed collagen deposition, cell proliferation, anchorage independent growth, migration and invasion of oral fibroblasts under these circumstances, and determined this to be a useful in vitro model of RIF (RIFiv). We demonstrate RIFiv to be useful in understanding early biological mediators of fibrosis, and as a model to assess therapeutic compounds
Methods and Materials
Cell Culture and Reagents
Multiple lines of primary human oral fibroblasts were isolated from cancer-free patient samples as previously described13. Briefly, cells were isolated from patient tonsillar or uvulopalatoplasty specimens by mincing the samples into less than one-millimeter sections, and adhering to cell culture dish. Patient samples were collected under the auspices of the Biospecimen Repository Core at the University of Kansas Cancer Center with written consent from patients, using protocols approved by the Human Subjects Committee at the University of Kansas Medical Center. Cells were cultured in 4.5 g/L glucose DMEM with 10% heat-inactivated FBS (Invitrogen, Carlsbad, CA) without antibiotics, and maintained for no more than 12 passages. All data presented are confirmed with at least two different patient-derived fibroblast lines.
Direct Red 80 and picric acid were purchased from Thermo Fisher (Waltham, MA). TGF-β was purchased from Sigma Aldrich. TRIzol was purchased from Life Technologies (Grand Island, NY). Pentoxifylline was obtained from Santa Cruz Biotech (Dallas, TX).
In vitro induction of radiation-induced fibrosis
Fibroblasts were plated in 6 well plates (300,000 cells/well), and pre-treated with TGF-β in serum free media for 24 h. Plates were then exposed to gamma radiation (J.L. Shepherd and Associates Mark I Model 68A cesium-137 source irradiator; dose rate = 2.9 Gy/min), and media was replaced with fresh TGF-β in serum free media. Following this, collagen deposition occurred over a 72 h period, at which point the cells were fixed with 75% ethanol. All experiments using RIFiv were confirmed on multiple patient samples.
Collagen Staining
Fixed cells were stained with Direct Red 80 (0.1% in picric acid) for one h. Cells were washed with PBS to remove Direct Red 80 80, air dried at room temperature for five minutes and imaged under light microscope. To quantitate staining intensity, 0.1 M NaOH was used to free bound Direct Red 80 80, and then dye suspension was transferred into clean 96 well plates for optical density at 540 nm emission.
Cell Number
Cell number was measured using the CyQuant proliferation kit (Life Technologies, Grand Island, NY) according to the manufacturer’s protocol. Briefly, cells were plated (2,000 cells/well) in 96 well plates and assayed 72 h later following various experimental treatment conditions in serum free media. Cells were lysed at −80° C, and CyQuant dye used to measure relative cell number by fluorescence assessment.
RNA-Sequencing and RT-PCR
Fibroblasts were washed after 72 h of treatment with PBS, lysed in TRIzol and RNA was collected following manufacturer’s protocol. RNA-sequencing (RNA-Seq) was used to obtain gene expression profile of vehicle control, IR, TGF-β and IR + TGF-β samples. Samples were sequenced in biological duplicates. Sequencing was performed using an Illumina HiSeq 2500 at a 100 bp single read resolution. The sequenced reads were mapped to the human genome (assembly GRCh38.rel77) using STAR software14, transcript abundance estimates were generated using Cufflinks software15 and differential gene expression estimates were calculated using Cuffdiff software16. RNA-Seq generated between 44.0 and 56.2 million reads per sample, of which on average 99.8% mapped to the reference genome. The replicate samples were highly correlated with an average Pearson coefficient of 0.99. mRNA levels of collagen 1α1, collagen XIα1, cyclin D1, HGF, Integrin α2, and β-actin were analyzed using RT-PCR (sequences in Supplemental information). PCR products were visualized on an agarose gel containing Gel Red Nucleic Acid stain. Expression levels assessed using ImageJ.
Migration and Invasion assay
Cell migration was evaluated using in vitro transwell inserts (8 µm pores) (Costar). Fibroblasts were plated in triplicate (50,000 cells/well) in serum-free media in the insert for migration, or on a layer of Matrigel (Corning, Corning, NY) (2 mg/mL) for invasion. Outer wells contained DMEM with 12% FBS cell culture media. Cells were simultaneously plated in 96-well plates to assess cell viability. After 24 h, cells that migrated through the insert were fixed and stained with Hema 3 (Fisher Scientific, Hampton, NH). Parallel proliferation was assessed using CyQuant. The number of migrating cells was normalized to total cell number.
3-D Culture Assay
Fibroblasts seeded in ultra-low attachment plates (50,000 cells/well in 6 well plate (Corning, Ref: 3471, Corning, NY)), and treated with TGF-β (100 ng/mL) and/or radiation (3 Gy) in serum free media. After ten days, cells were imaged at 400× magnification. Celigo imaging cytometer (Nexcelom, Lawrence, MA) used to count number of spheroids and average spheroid diameter.
Immunoblot
Whole-cell lysates were extracted using RIPA lysis buffer and a mixture of protease and phosphatase inhibitors (Minitab, Roche, Basel, Switzerland). Lysates were sonicated on ice, debris removed by centrifugation, and supernatants stored at −80 °C. Sodium dodecyl sulfate-polyacrylamide 12% gels were used to separate proteins, and proteins were transferred to nitrocellulose membranes. Membranes were blocked with Odyssey blocking buffer (Li-Cor, Lincoln, NE) in a 1:1 mixture with PBS 1% Tween-20 (PBST). Primary antibodies (phospho-MAPK, and total MAPK, Cell Signaling, Danvers, MA) were incubated overnight in 1:1 blocking buffer to PBST. Primary antibodies were detected using DyLight conjugated secondary antibodies (anti-rabbit IgG DyLight 488 (#35553), and anti-mouse IgG DyLight 800 (#35521) from Thermo Fisher (Waltham, MA)). Protein bands were detected using Li-Cor odyssey protein imaging system.
Computational Molecular Docking
Protein structure of MAPK-3 (ERK-1) was downloaded from RCSB protein data bank (PDB-ID: 4QTB). Ligand (Difluorocurcumin (CDF)) was downloaded from PubChem (PubChem-CID: 54597187). Autodock tools were used to prepare protein and ligand files for docking. Molecular docking was performed using AutoDock-Vina.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism 6 Version 6.03 (La Jolla, CA). Significance in treatment responses was obtained using two-tailed Mann-Whitney U testing when comparing collagen deposition data, proliferation, migration, invasion and spheroid assays, and two-tailed student’s t-test for RT-PCR data. Normality of RT-PCR data was assessed by Shapiro-Wilk testing using R (ver. 3.3.1). p<0.05 was considered significant.
Results
Radiation and TGF-β induce collagen deposition in oral fibroblasts
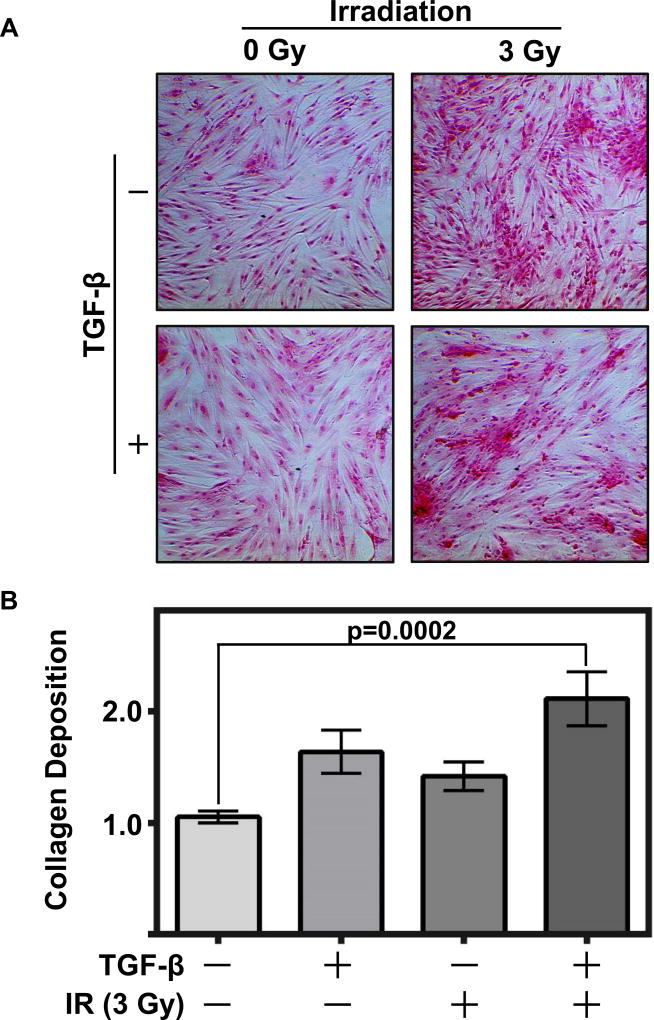
Patients who undergo radiation therapy for head and neck cancer receive 54–70 Gy of fractionated radiation at a rate of 1.9–2.2 Gy per day. In order to optimize conditions for an in vitro model, we tested three different doses of radiation: 3, 12 and 48 Gy. To assess fibrosis, we used Direct Red 80 staining to stain collagen, and found 3 Gy was sufficient to induce collagen deposition in oral fibroblasts. 3 Gy mimics the biology that would occur with each and every fraction of radiation that a patient undergoes, and is an appropriate radiation dose in a cell culture model where there is no shielding from surrounding tissue. Furthermore, marked induction of collagen deposition was observed with a single 3 Gy dose, and this was negligibly different from a 12 Gy dose (Supplemental Figure 1). 3 Gy offered strong induction of the fibrotic phenotype, whereas a high, single dose of 48 Gy was too damaging to the cells (Supplemental Figure 1). Given the central role of TGF-β signaling in RIF1, we treated fibroblasts with increasing concentrations of TGF-β (0, 1, 5, 10, 20, 50, 100 ng/ml) and found collagen deposition increased with the presence of TGF-β (Supplemental Figure 2). Clinically, TGF-β serum levels increase following radiotherapy, with a basal level in non-irradiated patients of 7.2 ng/mL, and a significant increase with an upper range of 147 ng/mL in irradiated patients17. With the caveat that these studies were in lung cancer patients, we selected a dose of 100 ng/mL in our RIFiv model to mimic this significant increase. Given these initial findings, we developed a model, RIFiv, using IR and TGF-β to mimic the post-irradiation environment and induce a significant increase in collagen deposition (Figure1A and 1B).
Figure 1. Radiation and TGF-β induce collagen deposition in oral fibroblasts.
(A) Oral fibroblasts exposed to radiation (3 Gy) and/or treated with TGF-β (100 ng/ml) exhibited collagen deposition. Cells were stained with Direct Red 80 to detect collagen (200× magnification). (B) Cumulative quantitative analyses of collagen deposition by OD assessment of Direct Red 80. Graph depicts three replicated experiments plated in duplicate, with fold change of each experiment indexed to vehicle control treated group, two-tailed Mann-Whitney tests used to calculate p-values, error bars represent +/− SEM.
Radiation and TGF-β enhance cell proliferation and migration
Given that fibrosis is characterized by fibroblast proliferation in addition to ECM deposition1, we next proceeded with testing the effects of radiation and TGF-β on cell growth. We treated oral fibroblasts with TGF-β and/or radiation and determined the effect on cell number over 72 h using the CyQuant proliferation kit. There was a significant increase in relative cell number in the combination radiation + TGF-β group compared to control fibroblasts (p<0.0001) (Figure 2A).
Figure 2. Radiation and TGF-β enhance fibroblast proliferation and migration.
(A) Fibroblasts treated with radiation (3 Gy) and/or TGF-β (100 ng/ml) over 72 h demonstrate an increase in relative cell number (p<0.0001, graph depicts three replicate experiments plated in triplicate), (B) expression of cyclin D1 as determined by RT-PCR and (C) quantified using densitometry analyses of cyclin D1 expression (p=0.05, graph depicts imageJ densitometry analyses of three PCR analyses, and indexed to untreated group). Fibroblasts were assessed for their ability to (D) migrate through a Boyden chamber and (E) invade a Matrigel matrix. Migration and invasion graphs depict three replicate experiments plated in triplicate normalized to parallel proliferation, and indexed to untreated group. Two-tailed Mann-Whitney tests used to calculate p-values, error bars on all graphs represent +/− SEM.
To validate this finding, RT-PCR was performed to quantify the proto-oncogene cyclin D1, which plays an important role in the regulation of G1 to S phase transition in many different cell types. Together with its binding partners, cyclin dependent kinase 4 and 6, cyclin D1 forms active complexes that promote cell cycle progression by phosphorylating and inactivating the retinoblastoma protein18–20. Interestingly, combination treatment increased cyclin D1 mRNA levels compared to untreated fibroblasts (Figure 2B, C). The increased expression of cyclin D1 indicates greater proliferative activity.
During wound healing, fibroblasts migrate and invade into the site of injury in order to repair tissue damage. To understand the role of migration and invasion in wound healing after radiation, we investigated the hypothesis that fibrotic fibroblasts acquire a migratory phenotype. Compared to untreated fibroblasts, we observed an almost 7-fold increase in cell migration in the radiation + TGF-β group (p=0.0006) and an almost 5-fold change in invasion through Matrigel in the combination group (p=0.0006) (Figures 2D, E). The increased propensity of fibrotic cells to proliferate, migrate and invade indicates this RIFiv model simulates the injury-like nature of this pathology.
Radiation and TGF-β enhance spheroid formation of oral fibroblasts under anchorage-independent conditions
Previous in vitro studies of fibroblasts derived from areas of fibrosis such as interstitial lung disease or fibroblasts treated by radiation have exhibited the ability to form spheroids under anchorage-independent conditions21,22. Further exposure of fibroblasts to fibrosis-inducing agents induces an activated phenotype that includes increased α-SMA and collagen type I expression23. In an anchorage independent environment, we observed that the combination of radiation and TGF-β triggered a significant 6-fold increase (p=0.0022) in the number of spheroids compared to untreated fibroblasts (Figure 3A and B). In addition, the average diameter of the spheres was significantly greater than control fibroblasts (p=0.0022) (Figure 3C). Furthermore, spheroids formed from cells treated with combination radiation + TGF-β had significantly higher collagen deposition (p<0.0001) in comparison to untreated fibroblasts (Figure 3D) as assessed by Direct Red 80 80 staining. The increase in spheroids, their diameter, and increase in collagen deposition in the combination group indicate an induction of the fibrotic phenotype.
Figure 3. Radiation and TGF-β enhance anchorage independent growth and collagen deposition.
(A) Fibroblasts formed spheres under non-adherent conditions (400× magnification). (B) The number of spheres was counted and (C) the diameter determined using Image-J software and data presented as fold change indexed to average diameter of controls. (D) Collagen deposition was assessed by measuring the OD of disassociated spheroids stained with Direct Red 80. Graphs depict three replicate experiments, plated in duplicate. Two-tailed Mann-Whitney tests used to calculate pvalues, error bars represent +/− SEM.
Radiation and TGF-β increase collagen and integrin gene expression and decreases hepatocyte growth factor gene expression in oral fibroblasts
RNA-Seq was used to assess the underlying molecular mechanisms of the fibrosis phenotype. Differentially regulated genes in oral fibroblasts treated with the combination of radiation and TGF-β were studied and compared. Figure 4A shows the heat map of differentially expressed genes in the four different groups, grouped into hierarchical clusters. These data were analyzed using QIAGEN’s Ingenuity pathway analysis (IPA) and a network of fibrosis regulator genes was created to visualize upregulation and downregulation of genes in pathways associated with TGF-β (Figure 4B). Notable changes included upregulation of collagen XIα1, collagen 1α1, and integrin-α2β1 and downregulation of hepatocyte growth factor (HGF) in radiation and TGF-β treated cells. To validate these findings, RT-PCR was performed which demonstrated fibroblasts treated with radiation and TGF-β increased levels of collagen XIα1, collagen 1α1, and integrin-α2 expression (Figures 5A–F). RT-PCR also validated a decrease in HGF expression (Figure 5G and H). Furthermore, our RNA-Seq analyses noted an upregulation in mTORC2 gene that may suggest increased cell metabolism in the combination radiation + TGFβ treated group (Figure 4B).
Figure 4. RNA-Seq analysis demonstrates molecular mechanisms of fibrosis.
(A) A heat-map representing the log values of differentially expressed genes relative to the control demonstrates treatment-related changes in gene expression. Differentially expressed genes with an absolute fold change of ≥ 1.5 and q-value (false discovery rate) ≤ 0.05 were mapped. The expression data are hierarchically clustered in rows (distance metric: Euclidean; linkage method: Ward. (B) The network depicts Ingenuity® Knowledge Base pathway analyses of dominant genes with a fold change of 1.5 and p-value ≤ 0.05.
Figure 5. Radiation and TGF-β modulate gene expression in oral fibroblasts.
RT-PCR followed by densitometry quantification demonstrate that radiation (3 Gy) combined with TGF-β (100 ng/mL) increases levels of (A, B) collagen XIα1, (C, D) collagen 1α1 and (E, F) integrin-α2, while significantly decreasing the levels of (G, H) HGF. Gene expression was normalized to β-actin levels, graphs depict three independent experiments and are indexed to control group, p-values determined using t-test with confirmation of normality using Shapiro-Wilk test, error bars represent +/− SEM.
Pentoxifylline and Curcumin reduce radiation-induced fibrosis in oral fibroblasts
Currently, there are no proven treatment options for RIF. The effects of antifibrotic agents, such as pentoxifylline, have been studied in past and ongoing trials. In preliminary studies, pentoxifylline alone or in combination with other antioxidants has demonstrated a modest therapeutic effect in patients affected by RIF24–26. To assess the efficacy of pentoxifylline in mitigating collagen deposition, increasing concentrations of pentoxifylline (0, 2, 20 and 100 µg/ml) were applied to cells after radiation and TGF-β treatment. With concentrations greater than 20 µg/ml of pentoxifylline, we observed a moderate but significant reduction in collagen deposition as assessed by Direct Red 80 80 staining (p=0.0022) when compared to untreated fibroblasts (Figure 6A and B, radiation and TGF-β alone data not shown), suggesting a concentration dependent reduction in collagen deposition.
Figure 6. Pentoxifylline and CDF decrease collagen deposition.
Cells exposed to radiation (3 Gy) and TGF-β (100 ng/ml) were assessed for collagen deposition after treatment with antifibrotic agents. Cells in various treatment groups were stained with Direct Red 80, imaged under light microscopy and the OD quantified and graphed. Increasing concentrations of (A, B) pentoxifylline or (C, D) CDF reduced the level collagen deposition in the cultures. Collagen levels in each treatment arm were normalized to cell number. Graphs depict relative collagen deposition of three independent experiments plated in duplicate, two-tailed Mann-Whitney tests used to calculate p-values, error bars represent +/−SEM.
We also assessed difluorocurcumin (CDF), a derivative of the phytochemical curcumin27. Among many phytochemical agents, curcumin has shown promising antineoplastic properties28,29. Several research reports over the last few decades have established curcumin is a potent anti-inflammatory agent with therapeutic potential against a variety of cancers, and may inhibit the fibrotic process30–36. However, given its low bioavailability and rapid metabolism, analogs such as CDF have been synthesized to improve its therapeutic utility27. As such, fibroblasts were treated with increasing concentrations of CDF (0, 1, 5, 10 and 20 µM) after combination radiation and TGF-β treatment. There was a significant decrease in collagen deposition as assessed by Direct Red 80 80 staining with the use of 5, 10 and 20 µM of CDF (Figures 6C and D, radiation and TGF-β alone data not shown). To elucidate the mechanism of CDF, we assessed binding to MAPK, a common signal transduction component of both TGF-β and integrin signaling. Computational molecular docking analyses demonstrated high binding affinity (−11.6 Kcal/mol) in the catalytic domain of MAPK (Supplemental Fig 3A and B). This was validated by immunoblot, where CDF alleviated MAPK phosphorylation after TGF-β treatment (Supplemental Fig 3C). These findings indicate CDF may be effective in reducing RIF.
Discussion
RIF is a complex and progressive tissue response to injury characterized by constant remodeling and long-term fibroblast activation37,38. After the direct DNA damage resulting from radiation, an acute inflammatory response is triggered. Due to lasting cellular dysfunction from the initial radiotherapy, the inflammatory response persists with an increase in multiple cytokines and growth factors37–40. A long list of inflammatory mediators such as tumor necrosis factor-α (TNF-α), interleukin (IL) 1 (IL-1), IL-6, and a plethora of growth factors play a role in the fibrotic process38,40–44. By far, TGF-β has been established as playing a critically central role in RIF. Its involvement has been addressed in various irradiated tissues mostly through in vivo experiments of skin, intestine, liver, mammary gland, and lung1–3,6,9,12,37,38,44. In the head and neck region, this inflammatory response leads to long-term fibroblast activation leading to progressive stiffening of the connective tissues that can further devastate quality of life45,46. Developing strategies to prevent and treat long-term fibrotic complications would greatly aid in improving patient quality of life.
The previous succession of experiments replicates the fibrosis phenotype in oral fibroblasts leading to a novel in vitro model that can be used for further testing of the processes involved in RIF. In our study, multiple fibroblast lines were cultured from tonsil tissue of cancer free patients using previously established tissue culture models.
In our RIFiv model, stimulated fibroblasts were noted to have increased collagen deposition, a hallmark of fibrosis. IPA revealed an upregulation of genes for collagen 1α1 and collagen XIα1 in these fibroblasts. Alteration in the collagen XIα1 gene has been associated with several connective tissue disorders and is a downstream target of the TGF-β signaling pathway47–50. While clinical fibrosis takes months to years to develop, the pathophysiology of fibrosis starts with the initiation of each and every radiation dose. This RIFiv model represents the early biological processes involved in fibrosis and can be used to elucidate the early mechanisms that cause the continuation of fibrosis.
Additionally, we observed an increase in cyclin D1 expression. Previous in vitro studies suggest that cyclin D1 regulates cell proliferation in fibrotic tissues, such as idiopathic pulmonary fibrosis51,52. Furthermore, using IPA, we observed an upregulation of mTORC2, which regulates cellular metabolism, growth, and proliferation (Figure 4B). A study of fibroblasts implicated in kidney fibrosis demonstrated TGF-β induced mTORC2 signaling activation and reciprocally mTORC2 signaling contributes to TGF-β promoted fibroblast activation53. The previous observations and our current findings together suggest that in RIF not only do cells deposit more collagen but also proliferate with high metabolic activity.
A significant upregulation in the expression of integrin-α2β1 was observed, which likely gives a partial mechanism to the increased migration and spheroid formation observed. Integrin-α2β1 plays an important role in the movement of fibroblasts along collagen fibrils. Fibroblasts interact with surrounding collagen mainly using receptors of the β1 family of integrins54. Previous studies demonstrate fibroblasts exposed to a rich ECM use integrin-α2β1 to form focal contacts on collagens to support migration in response to chemotactic stimuli54–56. More recent evidence suggests a reciprocal connection between integrins and the TGF-β pathway54. While, just radiation alone and TGF-β alone were noted to cause minimal increases in collagen deposition, cell proliferation, migration, invasion, and ability to form spheroids, the combination together resulted in a significant change in fibroblast activity.
Interestingly, although HGF has been established as upregulated in the HNSCC tumor microenvironment57, HGF was downregulated in our system following IR. Along with its role in embryonic development, evidence is now emerging that HGF is an antifibrotic factor that plays a critical role in preventing tissue fibrosis. In vitro studies have observed that HGF counteracts many pro-fibrotic actions of TGF-β suggesting the balance between the two may play a role in fibrosis development58–62. After initial acute injury, both HGF and TGF-β are induced, but if the injury is chronic in nature, TGF-β expression progressively increases, with a gradual decrease in HGF levels. Thus in chronic injury states, such created via radiation damage, the balance shifts to favor the pro-fibrotic TGF-β61,63. Noting a decrease in the expression of HGF in our study further lends credence to this RIFiv model.
Given our RIFiv model replicates the early fibrosis phenotype induced by radiation damage and is concurrent with previously performed experiments, it can thus be used to test anti-fibrotic agents. Although we focus on head and neck fibroblasts, this model is potentially useful in studying fibrosis with fibroblasts from other anatomical locations, such as lung, breast, or liver. Clinically, it is important to mitigate fibrosis early on in the process of development. We tested both pentoxifylline, currently FDA approved for chronic occlusive arterial disease, as well as curcumin analogue CDF, and noted a decrease in collagen deposition after treatment with each agent. Further studies on the mechanism of CDF function are warranted and are a subject of current investigation.
Supplementary Material
Acknowledgments
This work was supported by the Department of Otolaryngology, University of Kansas Medical Center, and the University of Kansas Cancer Center CCSG 1-P30-CA168524-02. The Genomics Core is supported by the University of Kansas Medical Center, School of Medicine, the Kansas Intellectual and Developmental Disability Research Center (NIH U54 HD090216) and the Molecular Regulation of Cell Development and Differentiation - COBRE (5P20GM104936-10). The Flow Cytometry Core Laboratory is sponsored, in part, by NIH/NIGMS COBRE P30GM103326.
Kumar D., Yalamanchali, S., New, J., Parsel, S., New, N., Holcomb, A., Gunewardena, S., Tawfik, O., Lominska, C., Kimler, B.F., Anant, S., Kakarala, K., Tsue, T., Shnayder, Y., Sykes, K., Padhye, S., Thomas, S.M. Development and characterization of an in vitro model for radiation-induced fibrosis. Radiat. Res.
Footnotes
Conflict of interest: The authors declare no conflicts of interest
References
- 1.Straub JM, New J, Hamilton CD, et al. Radiation-induced fibrosis: mechanisms and implications for therapy. Journal of cancer research and clinical oncology. 2015;141(11):1985–94. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stubblefield MD. Radiation Fibrosis Syndrome: Neuromuscular and Musculoskeletal Complications in Cancer Survivors. Pm&R. 2011;3(11):1041–54. doi: 10.1016/j.pmrj.2011.08.535. [DOI] [PubMed] [Google Scholar]
- 3.Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. Journal of Clinical Investigation. 2007;117(3):524–29. doi: 10.1172/JCI31487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200(4):500–03. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 5.Tomasek JJ, Gabbiani G, Hinz B, et al. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Bio. 2002;3(5):349–63. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 6.Yan C, Wang L, Li B, et al. The expression dynamics of transforming growth factor-beta/Smad signaling in the liver fibrosis experimentally caused by Clonorchis sinensis. Parasites & vectors. 2015;8:70. doi: 10.1186/s13071-015-0675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez EM, Knackstedt RJ, Jenrette JM. Significant fibrosis after radiation therapy in a patient with Marfan syndrome. Radiation oncology journal. 2014;32(3):208–12. doi: 10.3857/roj.2014.32.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller K, Meineke V. Radiation-induced alterations in cytokine production by skin cells. Experimental hematology. 2007;35(4 Suppl 1):96–104. doi: 10.1016/j.exphem.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113(6):685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 10.Cabello-Verrugio C, Santander C, Cofre C, et al. The internal region leucine-rich repeat 6 of decorin interacts with low density lipoprotein receptor-related protein-1, modulates transforming growth factor (TGF)-beta-dependent signaling, and inhibits TGF-beta-dependent fibrotic response in skeletal muscles. The Journal of biological chemistry. 2012;287(9):6773–87. doi: 10.1074/jbc.M111.312488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2004;18(7):816–27. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 12.Schultze-Mosgau S, Wehrhan F, Rodel F, et al. Transforming growth factor-beta receptor-II up-regulation during wound healing in previously irradiated graft beds in vivo. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2003;11(4):297–305. doi: 10.1046/j.1524-475x.2003.11410.x. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler SE, Shi H, Lin F, et al. Enhancement of head and neck squamous cell carcinoma proliferation, invasion, and metastasis by tumor-associated fibroblasts in preclinical models. Head & neck. 2013;36(3):358–92. doi: 10.1002/hed.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobin A, Davis CA, Schlesinger F, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trapnell C, Williams BA, Pertea G, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nature biotechnology. 2010;28(5):511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trapnell C, Hendrickson DG, Sauvageau M, et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nature biotechnology. 2013;31(1):46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novakova-Jiresova A, van Gameren MM, Coppes RP, et al. Transforming growth factor-β plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiotherapy and Oncology. 2004;71(2):183–89. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 18.Kato J, Matsushime H, Hiebert SW, et al. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev. 1993;7(3):331–42. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 19.Lundberg AS, Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18(2):753–61. doi: 10.1128/mcb.18.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 21.Torry DJ, Richards CD, Podor TJ, et al. Anchorage-independent colony growth of pulmonary fibroblasts derived from fibrotic human lung tissue. The Journal of clinical investigation. 1994;93(4):1525–32. doi: 10.1172/JCI117131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Reilly S, Walicka M, Kohler SK, et al. Dose-dependent transformation of cells of human fibroblast cell strain MSU-1.1 by cobalt-60 gamma radiation and characterization of the transformed cells. Radiation research. 1998;150(5):577–84. [PubMed] [Google Scholar]
- 23.Hinz B, Phan SH, Thannickal VJ, et al. The myofibroblast - One function, multiple origins. Am J Pathol. 2007;170(6):1807–16. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okunieff P, Augustine E, Hicks JE, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(11):2207–13. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson G, Bhatia S, Smith BJ, et al. Randomized trial of pentoxifylline and vitamin E vs standard follow-up after breast irradiation to prevent breast fibrosis, evaluated by tissue compliance meter. Int J Radiat Oncol Biol Phys. 2013;85(3):604–8. doi: 10.1016/j.ijrobp.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 26.Chua DT, Lo C, Yuen J, et al. A pilot study of pentoxifylline in the treatment of radiation-induced trismus. American journal of clinical oncology. 2001;24(4):366–9. doi: 10.1097/00000421-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Vyas A, Dandawate P, Padhye S, et al. Perspectives on new synthetic curcumin analogs and their potential anticancer properties. Current pharmaceutical design. 2013;19(11):2047–69. [PMC free article] [PubMed] [Google Scholar]
- 28.Craig WJ. Health-promoting properties of common herbs. The American journal of clinical nutrition. 1999;70(3 Suppl):491S–99S. doi: 10.1093/ajcn/70.3.491s. [DOI] [PubMed] [Google Scholar]
- 29.Orlando RA, Gonzales AM, Royer RE, et al. A Chemical Analog of Curcumin as an Improved Inhibitor of Amyloid Abeta Oligomerization. Plos One. 2012;7(3) doi: 10.1371/journal.pone.0031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ammon HP, Safayhi H, Mack T, et al. Mechanism of antiinflammatory actions of curcumine and boswellic acids. Journal of ethnopharmacology. 1993;38(2–3):113–9. doi: 10.1016/0378-8741(93)90005-p. [DOI] [PubMed] [Google Scholar]
- 31.Lantz RC, Chen GJ, Solyom AM, et al. The effect of turmeric extracts on inflammatory mediator production. Phytomedicine : international journal of phytotherapy and phytopharmacology. 2005;12(6–7):445–52. doi: 10.1016/j.phymed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Wilken R, Veena MS, Wang MB, et al. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Molecular cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao W, Wang Y, Wang Y, et al. Potential anti-cancer effect of curcumin in human lung squamous cell carcinoma. Thoracic cancer. 2015;6(4):508–16. doi: 10.1111/1759-7714.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avasarala S, Zhang F, Liu G, et al. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS One. 2013;8(2):e57285. doi: 10.1371/journal.pone.0057285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang SS, Gong ZJ, Li WH, et al. Antifibrotic effect of curcumin in TGF-beta 1-induced myofibroblasts from human oral mucosa. Asian Pacific journal of cancer prevention : APJCP. 2012;13(1):289–94. doi: 10.7314/apjcp.2012.13.1.289. [DOI] [PubMed] [Google Scholar]
- 36.Wu SJ, Tam KW, Tsai YH, et al. Curcumin and saikosaponin a inhibit chemical-induced liver inflammation and fibrosis in rats. The American journal of Chinese medicine. 2010;38(1):99–111. doi: 10.1142/S0192415X10007695. [DOI] [PubMed] [Google Scholar]
- 37.Denham JW, Hauer-Jensen M. The radiotherapeutic injury--a complex 'wound'. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2002;63(2):129–45. doi: 10.1016/s0167-8140(02)00060-9. [DOI] [PubMed] [Google Scholar]
- 38.Martin M, Lefaix J, Delanian S. TGF-beta1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiat Oncol Biol Phys. 2000;47(2):277–90. doi: 10.1016/s0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 39.Delanian S, Porcher R, Rudant J, et al. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. Journal of Clinical Oncology. 2005;23(34):8570–79. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 40.Haase O, Rodemann HP. Fibrosis and cytokine mechanisms: relevant in hadron therapy? Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2004;73(Suppl 2):S144–7. doi: 10.1016/s0167-8140(04)80037-9. [DOI] [PubMed] [Google Scholar]
- 41.Bentzen SM. Preventing or reducing late side effects of radiation therapy: radiobiology meets molecular pathology. Nature reviews Cancer. 2006;6(9):702–13. doi: 10.1038/nrc1950. [DOI] [PubMed] [Google Scholar]
- 42.Abdollahi A, Li M, Ping G, et al. Inhibition of platelet-derived growth factor signaling attenuates pulmonary fibrosis. J Exp Med. 2005;201(6):925–35. doi: 10.1084/jem.20041393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vozenin-Brotons MC, Milliat F, Sabourin JC, et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int J Radiat Oncol Biol Phys. 2003;56(2):561–72. doi: 10.1016/s0360-3016(02)04601-1. [DOI] [PubMed] [Google Scholar]
- 44.Yarnold J, Brotons MC. Pathogenetic mechanisms in radiation fibrosis. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology. 2010;97(1):149–61. doi: 10.1016/j.radonc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Murphy BA, Gilbert J. Dysphagia in head and neck cancer patients treated with radiation: assessment, sequelae, and rehabilitation. Semin Radiat Oncol. 2009;19(1):35–42. doi: 10.1016/j.semradonc.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(21):3582–9. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myllyharju J, Kivirikko KI. Collagens and collagen-related diseases. Annals of medicine. 2001;33(1):7–21. doi: 10.3109/07853890109002055. [DOI] [PubMed] [Google Scholar]
- 48.Raglow Z, Thomas SM. Tumor matrix protein collagen XIalpha1 in cancer. Cancer letters. 2015;357(2):448–53. doi: 10.1016/j.canlet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu YH, Chang TH, Huang YF, et al. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33(26):3432–40. doi: 10.1038/onc.2013.307. [DOI] [PubMed] [Google Scholar]
- 50.Cheon DJ, Tong Y, Sim MS, et al. A collagen-remodeling gene signature regulated by TGF-beta signaling is associated with metastasis and poor survival in serous ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(3):711–23. doi: 10.1158/1078-0432.CCR-13-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagood JS, Prabhakaran P, Kumbla P, et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol. 2005;167(2):365–79. doi: 10.1016/S0002-9440(10)62982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Conte E, Gili E, Fruciano M, et al. PI3K p110gamma overexpression in idiopathic pulmonary fibrosis lung tissue and fibroblast cells: in vitro effects of its inhibition. Laboratory investigation; a journal of technical methods and pathology. 2013;93(5):566–76. doi: 10.1038/labinvest.2013.6. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Ren J, Liu X, et al. Rictor/mTORC2 signaling mediates TGFbeta1-induced fibroblast activation and kidney fibrosis. Kidney international. 2015;88(3):515–27. doi: 10.1038/ki.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margadant C, Sonnenberg A. Integrin-TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO reports. 2010;11(2):97–105. doi: 10.1038/embor.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tiger CF, Fougerousse F, Grundstrom G, et al. alpha11beta1 integrin is a receptor for interstitial collagens involved in cell migration and collagen reorganization on mesenchymal nonmuscle cells. Developmental biology. 2001;237(1):116–29. doi: 10.1006/dbio.2001.0363. [DOI] [PubMed] [Google Scholar]
- 56.Eckes BMCZ, Ralf Hallinger ZGZ, et al. Mechanical Tension and Integrin a2b1 Regulate Fibroblast Functions. Journal of Investigative Dermatology Symposium Proceedings. 2006;11:66–72. doi: 10.1038/sj.jidsymp.5650003. [DOI] [PubMed] [Google Scholar]
- 57.Knowles LM, Stabile LP, Egloff AM, et al. HGF and c-Met participate in paracrine tumorigenic pathways in head and neck squamous cell cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(11):3740–50. doi: 10.1158/1078-0432.CCR-08-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rubin JS, Bottaro DP, Aaronson SA. Hepatocyte growth factor/scatter factor and its receptor, the c-met proto-oncogene product. Biochimica et biophysica acta. 1993;1155(3):357–71. doi: 10.1016/0304-419x(93)90015-5. [DOI] [PubMed] [Google Scholar]
- 59.Florquin S, Rouschop KM. Reciprocal functions of hepatocyte growth factor and transforming growth factor-beta1 in the progression of renal diseases: a role for CD44? Kidney international Supplement. 2003;(86):S15–20. doi: 10.1046/j.1523-1755.64.s86.4.x. [DOI] [PubMed] [Google Scholar]
- 60.Mizuno S, Matsumoto K, Kurosawa T, et al. Reciprocal balance of hepatocyte growth factor and transforming growth factor-beta 1 in renal fibrosis in mice. Kidney international. 2000;57(3):937–48. doi: 10.1038/sj.ki.4491416. [DOI] [PubMed] [Google Scholar]
- 61.Liu Y. Hepatocyte growth factor in kidney fibrosis: therapeutic potential and mechanisms of action. American journal of physiology Renal physiology. 2004;287(1):F7–16. doi: 10.1152/ajprenal.00451.2003. [DOI] [PubMed] [Google Scholar]
- 62.Yu Y, Lu L, Qian X, et al. Antifibrotic effect of hepatocyte growth factor-expressing mesenchymal stem cells in small-for-size liver transplant rats. Stem cells and development. 2010;19(6):903–14. doi: 10.1089/scd.2009.0254. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159(4):1465–75. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.