Abstract
Layered oxide NaxMO2 (M: transition metal) is a promising cathode material for sodium-ion secondary battery. Crystal structure of O3- and P2-type NaxMO2 with various M against temperature (T) was systematically investigated by synchrotron x-ray diffraction mainly focusing on the T-dependences of a- and c-axis lattice constants (a and c) and z coordinate (z) of oxygen. Using a hard-sphere model with minimum Madelung energy, we confirmed that c/a and z values in O3-type NaxMO2 were reproduced. We further evaluated the thermal expansion coefficients (αa and αc) along a- and c-axis at 300 K. The anisotropy of the thermal expansion was quantitatively reproduced without adjustable parameters for O3-type NaxMO2. Deviations of z from the model for P2-type NaxMO2 are ascribed to Na vacancies characteristic to the structure.
Introduction
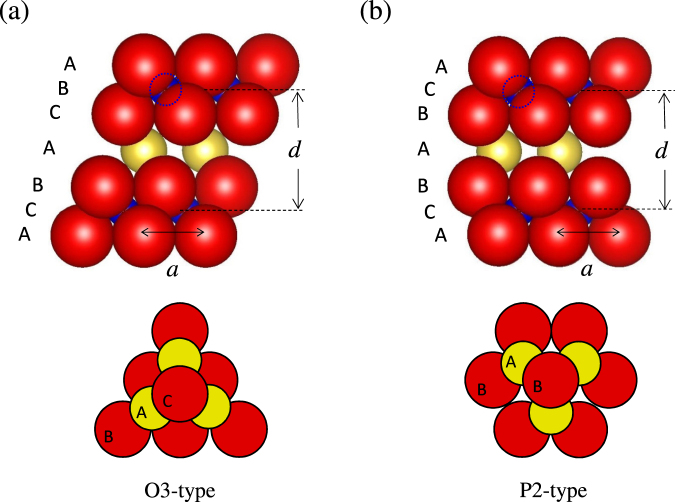
Sodium-ion-secondary battery (SIB) stores electrochemical energy through Na+ intercalation/deintercalation process. Due to large Clark number (=2.63) of Na compared with that (=0.006) of Li, SIBs can be a promising next-generation battery for storage of natural energy at a power plant and for a large-scale device such as electrical vehicle. Layered oxide NaxMO2 (M: transition metal) is a typical cathode material for SIBs1–3. Crystal structure of this material is categorized into two typical structures: O3 and P2 types4. Figure 1 shows schematic structures of (a) O3-type and (b) P2-type NaMO2. Red, yellow, and blue spheres represent O, Na, and M, respectively. M is surrounded by six oxygens, and a MO6 octahedron is formed. The edge-sharing MO6 octahedra form a MO2 layer. Both O3- and P2-type NaMO2 exhibit alternately stacked MO2 layers and Na sheets. The sodium sheet, upper and lower oxygen sheets stack as BAC resulting in the octahedral Na site. In the P2-type NaMO2, the sodium and oxygen sheets stack as BAB resulting in the prismatic Na site. O3-type NaMO2 (M = Ti, Cr, Mn, Co, Ni) and P2-type NaxMO2 (M = Mn and Co) were found to exhibit Na+ intercalation/deintercalation in early 1980s5–9. Concerning the discovery of hard carbon (≥200 mAh/g) as anode material of SIB10, electrochemical properties of NaxMO2 are extensively reported11–20. Very recently, substitution effects on the battery properties in O3-type structure21–27 and P2-type structure28–45 were extensively studied to reduce expensive element and improve the cyclability and capacity.
Figure 1.
Schematic figure of (a) O3- and (b) P2-NaMO2 structure. Red, yellow, and blue spheres represent O, Na, and M (transition metal element), respectively. M is sandwiched by upper and lower oxygen layers. In the P2-type structure, only the Na1 site [the atomic coordinates of Na1 are ] is shown. The bottom panels show top views of O-Na-O stackings in the O3- and P2-type structures, respectively. The BAC stacking in O3 forms NaO6 octahedron, and the BAB stacking in P2 forms NaO6 triangular prism.
Not only electrochemical properties but also superconductivity46, crystal structure47–57, magnetism58–61, thermoelectric effect62,63, and first-principle calculation64–67 of the end family are also studied. Fujita et al. found that NaxCoO2−δ single crystal shows a large dimensionless figure-of-merit of ZT = 1 at 800 K63, which has motivated practical use for waste heat recovery at high temperatures (T). An isostructural O3-type LiMO2 is widely used as a cathode material in lithium-ion-secondary battery (LIB)68,69. This family is also studied as a thermoelectric material70 and a cathode material of solid oxide fuel cell (SOFC)71. In particular, Lan and Tao found that LixAl0.5Co0.5O2 shows good proton conductivity of 0.1 Scm−1 at 773 K71, which is the highest among those of known polycrystalline proton-conducting materials. During operation of energy devices such as LIB(SIB), thermoelectric device, and SOFC, these materials are exposed to a variation and/or a gradient of temperature. A mismatch in thermal expansion coefficients in between the components can result in high stresses around the interface leading to deterioration of the device. Thus, evaluation and systematical comprehension of thermal expansion behaviors in this class of materials are important.
In this paper, we report systematic structural analysis of four O3- and five P2-type NaxMO2 samples against T (300 K ≤ T ≤ 800 K) performed by synchrotron x-ray diffraction focusing on thermal expansion. To understand the thermal expansion behavior, we constructed a hard-sphere model with constraint that M, upper and lower oxygens are connected each other. We confirmed that the calculated d/a [d: interlayer distance, a: a-axis lattice constant] and z well reproduced the experimental values for O3-type NaxMO2. By introducing T-linear expansion of the hard sphere, the anisotropy of the thermal expansion was quantitatively reproduced without adjustable parameter for O3-type NaxMO2.
Results
Temperature dependence of a(c)-axis lattice constants and z coordinate of oxygen
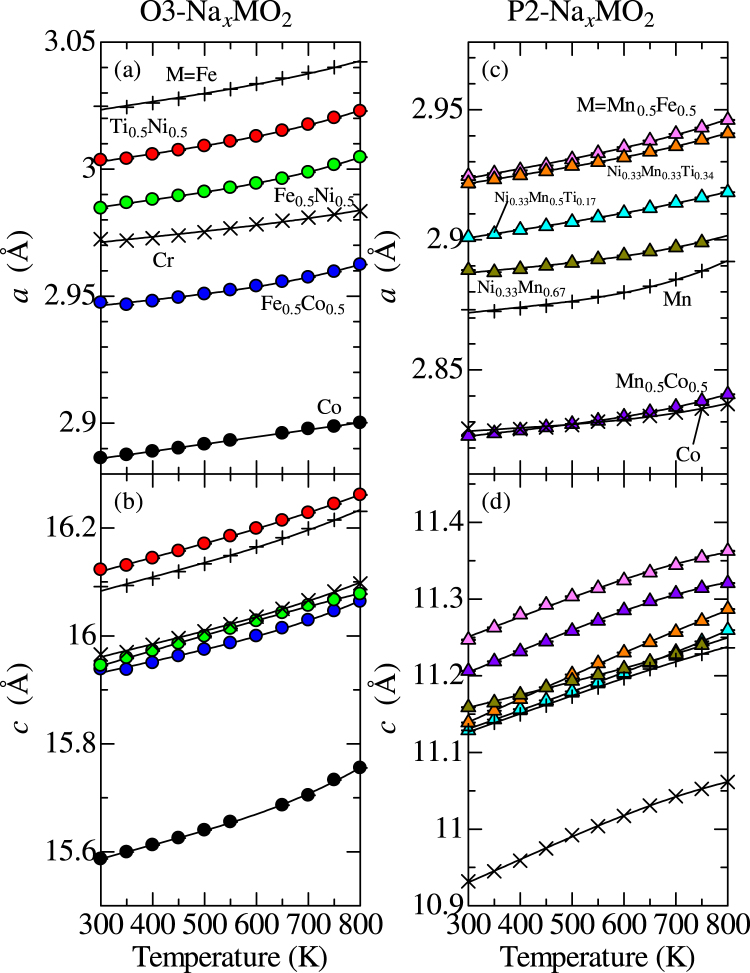
Figure 2(a) and (b) show a-axis lattice constant (a), and (b) c-axis lattice constant (c) of O3-type Na0.99CrO2, Na0.99FeO2, Na1.00CoO2, Na0.98Fe0.5Co0.5O2, Na0.99Fe0.5Ni0.5O2, and Na0.94Ti0.5Ni0.5O2 against T. With T, a and c monotonically increase. Raw x-ray diffraction data and results of Rietveld refinements at 300 K are shown in Figs S1–S3. The solid line represents a least-square fitting with use of a degree 3 polynomial function. By using , a linear thermal expansion coefficient along a- and c-axis was evaluated. Figure 2(c) and (d) show a and c of P2-type Na0.52MnO2, Na0.59CoO2, Na0.50Mn0.5Co0.5O2, Na0.70Ni0.33Mn0.67O2, Na0.69Ni0.33Mn0.5Ti0.17O2, Na0.70Ni0.33Mn0.33Ti0.34O2, and Na0.48Mn0.5Fe0.5O2. The P2-type compounds also show monotonical T-dependences of a and c. In Table 1, the values of a, c at 300 K, αa, and αc of O3- and P2-type NaxMO2 at 300 K were listed.
Figure 2.
Temperature (T) dependence of (a) a-axis, (b) c-axis lattice constants (a and c) of O3-type NaxMO2. T dependence of (c) a, and (d) c of P2-type NaxMO2. The solid line represents a least-square fitting with use of a degree 3 polynomial function for a and c. a and c values of O3-Na0.99CrO2, O3-Na0.99FeO2, P2-Na0.52MnO2, and P2-Na0.59CoO2 were referred from our previous reports56,57.
Table 1.
a-axis, c-axis lattice constants (a and c), z coordinate of oxygen (z) at 300 K, the linear thermal expansion coefficient [αa (αc)] along a(c)-axis, and αc/αa of O3- and P2-type NaxMO2 at 300 K.
| Compound | a (Å) | c (Å) | z | αa (10−5 K−1) | αc (10−5 K−1) | αc/αa |
|---|---|---|---|---|---|---|
| 56O3-Na0.99CrO2 | † 2.97247(3) | †15.96540(18) | 0.23213(13) | *0.73 | *1.52 | *2.07 |
| 56O3-Na 0.99 FeO 2 | †3.02477(2) | †16.09135(10) | 0.23389(12) | *1.07 | *1.63 | *1.52 |
| O3-Na1.00CoO2 | 2.88627(2) | 15.58680(12) | 0.23046(9) | 0.98 | 1.71 | 1.74 |
| O3-Na0.98Fe0.5Co0.5O2 | 2.94748(3) | 15.93844(23) | 0.23238(12) | 0.79 | 1.37 | 1.73 |
| O3-Na0.99Fe0.5Ni0.5O2 | 2.98463(9) | 15.94545(63) | 0.23334(17) | 1.01 | 1.76 | 1.74 |
| O3-Na0.94Ti0.5Ni0.5O2 | 3.00358(4) | 16.12273(24) | 0.23382(13) | 1.06 | 1.61 | 1.51 |
| 57P2-Na0.52MnO2 | †2.87311(9) | †11.1287(5) | 0.0858(3) | *0.81 | *2.11 | *2.59 |
| 57P2-Na0.59CoO2 | †2.82748(4) | †10.9319(2) | 0.0881(3) | *0.48 | *2.70 | *5.61 |
| P2-Na0.50Mn0.5Co0.5O2 | 2.82459(5) | 11.20617(33) | 0.08704(24) | 0.84 | 2.42 | 2.87 |
| P2-Na0.70Ni0.33Mn0.67O2 | 2.88835(5) | 11.15881(29) | 0.09120(28) | 0.67 | 1.51 | 2.26 |
| P2-Na0.69Ni0.33Mn0.5Ti0.17O2 | 2.90101(3) | 11.12868(16) | 0.09263(17) | 1.07 | 2.20 | 2.05 |
| P2-Na0.70Ni0.33Mn0.33Ti0.34O2 | 2.92173(4) | 11.13896(22) | 0.09381(21) | 1.12 | 2.72 | 2.42 |
| P2-Na0.48Mn0.5Fe0.5O2 | 2.92452(8) | 11.24690(48) | 0.08592(32) | 1.30 | 2.26 | 1.74 |
Figure 3(a) shows z coordinate of oxygen for O3- and P2-type structure (zO3 and zP2) against a at 300 and 700 K. Blue and red circles represent zO3 at 300 and 700 K, respectively. Light green and pink triangles represent zP2 at 300 and 700 K, respectively. The values were almost independent of T. Figure 3(b) shows a ratio of interlayer distance (d) to a against a at 300 K, where d is c/3 for O3-type and c/2 for P2-type structure. Light blue and purple circles represent d/a of O3-type compounds at 300 and 700 K, respectively. Green and yellow triangles represent d/a of P2-type compounds at 300 and 700 K, respectively. d/a slightly decreases with an increase in a.
Figure 3.
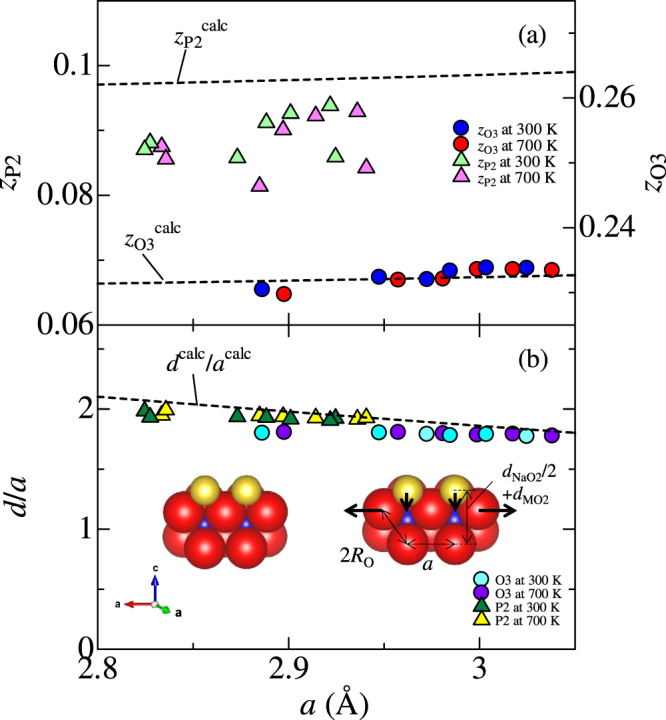
(a) z coordinate of oxygen for O3- (zO3) and P2-type (zP2) structure against a-axis lattice constant (a). Blue and red circles represent zO3 at 300 and 700 K, respectively. Light green and pink triangles represent zP2 at 300 and 700 K, respectively. The broken lines represent z calculated by the hard sphere model with minimum Madelung energy for O3 and P2-type compounds ( and ) against a, respectively. (b) The ratio of interlayer distance (d) to a against a. Light blue and purple circles represent d/a of O3-type compounds at 300 and 700 K, respectively. Green and yellow triangles represent d/a of P2-type compounds at 300 and 700 K, respectively. The broken line represents d/a calculated by the hard sphere model with minimum Madelung energy (dcalc/acalc) against a. The inset of Fig. 3(b) shows a schematic view of the local atomic configuration around M.
Thermal expansion coefficients
Figure 4 shows (a) αa, (b) αc, and (c) αc/αa against a. αa and αc of O3-type Na1.00CoO2 were 0.98 × 10−5 K−1 and 1.71 × 10−5 K−1, respectively. These values are comparable to those of inorganic compounds; αa = 1.44 × 10−5 K−1 for LiMn2O472, and αa(αc) = 0.85(2.5) × 10−5 K−1 for layered BaFe1.84Co0.16As273. αa(αc) in Fig. 4 is rather scattered against a around the averaged value of 0.92(1.96) × 10−5 K−1. The ratio αc/αa is also scattered around the averaged value (=2.30). However, the data point for P2-Na0.59CoO2 are seriously deviated from the average value. This is probably due to the Na ordering67.
Figure 4.
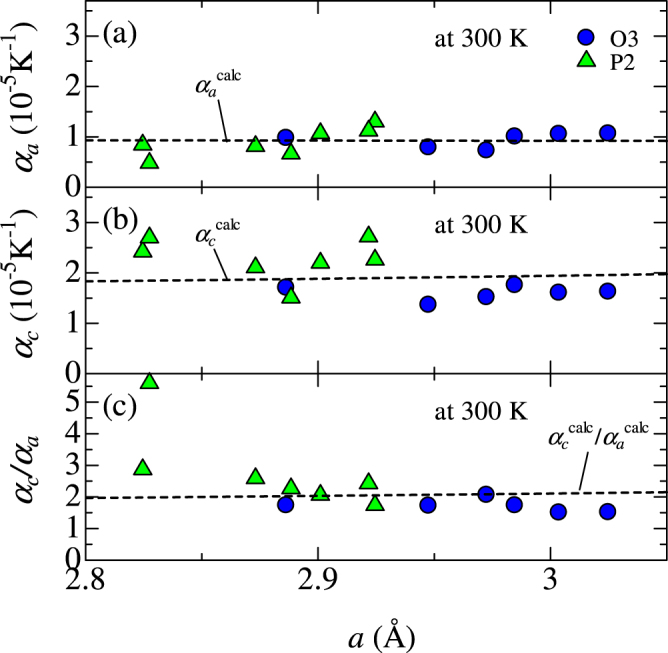
Linear thermal expansion coefficient (a) along a-axis (αa), (b) along c-axis (αc), and (c) αc/αa against a-axis lattice constant (a). The broken lines in Fig. 4(a,b and c) represent αa, αc, and αc/αa against a calculated by the hard-sphere model with minimum Madelung energy (, , and /).
Discussion
A hard-sphere model with minimum Madelung energy
We have constructed a hard-sphere structural model for O3- and P2-type NaMO2 to reproduce the experimental results (d/a, z, αc/αa). Firstly, imagine a sheet consists of hard spheres that were arrayed on triangular lattice, and then the sheet is alternately stacked as shown in Fig. 1. In the model, hard spheres of Na, M, and O were assumed to have Shannon’s ionic radius74; RNa = 1.02 Å, RO = 1.40 Å, and RM is a variable parameter that takes 0.58–0.7 Å, respectively. Since the ionic radius of the hard sphere is different from one another, the structure can not be the hexagonal close-packed structure, and several structures with different a are possible for the unique value of RM. Here, we adopted a constraint that M, upper, and lower oxygens coroneted each other, because the constraint minimizes the Madelung energy against a (vide infra). For a calculation of thermal expansion coefficient, the hard sphere is assumed to expand in proportion to the temperature difference (ΔT). ΔT dependence of RNa is expressed as , where mNa(=22.99) is atomic weight of Na atom. Similarly, ΔT dependences of RO and RM are expressed as , and , where mO(=16.00) and mM(=55.85) (We used 55.85 of the atomic weight of Fe as mM although M is not only Fe but also mixture of Ti, Mn, Fe, and Co. When we used 47.88 of the atomic weight of Ti as mM, the calculated αc/αa worse reproduces the experiments.) are atomic weights of O and M, respectively.
Now, let us derive the expression (acalc) of a-axis lattice constant as a function of ΔT. Note that the in-plane nearest-neighbor oxygen distance is a for both the P2- and O3-structure. Considering the above-mentioned constraint, acalc(ΔT) for both O3- and P2-structures is expressed as
| 1 |
This equation is easily derived using Pythagorean theorem. As shown in Eq. 1, acalc strongly depends on RM value. Due to the finite ionic radius of oxygen (RO = 1.40 Å), minimum value of acalc is 2.80 Å. At acalc = 2.80 Å, RM is evaluated to be ≈0.5799 Å using Eq. 1. With an increase in RM, the oxygen triangular lattice expands in order to keep the connection between M, upper and lower oxygens.
Na sheet is sandwiched by the MO2 layers as BAC (BAB) in the O3-type (P2-type) structure. We noted that the expression (dcalc) of interlayer distance is independent of the stacking manner, and the ΔT-dependence of dcalc for O3- and P2-type structure is expressed as,
| 2 |
The first and the second terms correspond to and , where and are the thicknesses of MO2 and NaO2 layers, respectively. A relationship between dcalc and the expression of c (ccalc) is expressed as and for O3- and P2-type structures. By using Pythagorean theorem, Eq. 2 is easily derived. Expressions of z (zcalc) for O3- and P2-type structures are derived as
| 3 |
and
| 4 |
respectively.
Now, let us consider the stability of the hard-sphere model with the constraint that M, upper and lower oxygens coroneted to each other. For this purpose, we calculated the Madelung energy at a specific RM (=0.65 Å) against a. We show that this model exhibits minimum Madelung energy (EME). Figure 5 shows EME of O3- and P2-type NaMO2 against a. Our constraint gives acalc ≈ 2.995 Å [Eq. 1] at RM = 0.65 Å. With the O3 structure, the acalc value corresponds to the minimum position of EME (−8.67 eV). With an increase in a from 2.995 Å, M becomes isolated from the surrounding oxygens (the right-side inset of Fig. 5), and EME increases (Note that the oxygen positions were controlled by Eqs 3 and 4). With an decrease in a from 2.995 Å, the upper and lower oxygens are separated (the left-side inset of Fig. 5), and EME increases as well (We used , , and for the calculation below a = 2.995 Å). Similar results are obtained for the P2 structure. Thus, our model is energetically stable against the variation of a. Our constraint that M, upper and lower oxygens connected to each other causes the compact layered structure and minimized the long-range Coulomb energy between the layers. We call our model “hard-sphere model with minimum Madelung energy”.
Figure 5.
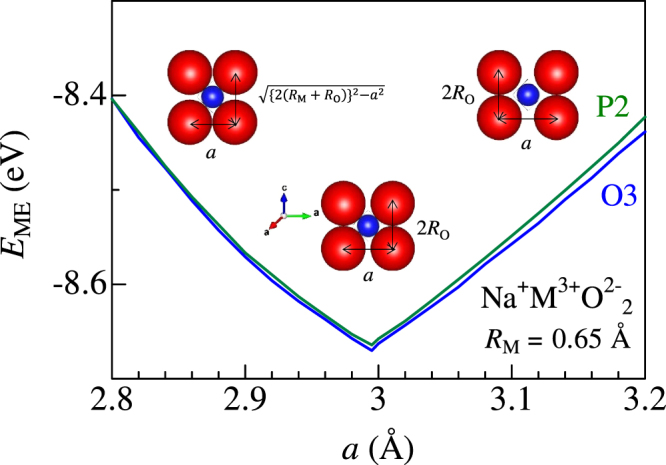
Madelung energy (EME) of O3- and P2-type NaMO2 against a for the hard-sphere model. RM was fixed at 0.65 Å.
Companion of the structural parameters and the thermal expansion coefficients with the model
The broken line in Fig. 3(b) is the calculated d/a based on the hard-sphere model with minimum Madelung energy (see Eqs 1 and 2). dcalc/acalc decreases with an increase in acalc, which reproduces experimental results. The decrease in dcalc/acalc is schematically depicted in the inset of Fig. 3(b). When O-O distance along in-plane direction elongates due to increase in RM, Na atoms relatively sink down along out-of-plane direction. (Decrease in against a is displayed in Fig. S5). We further calculated z and plotted them in Fig. 3(a). In the O3-type compounds, zcalc well reproduces the experimental data. In the P2-type compounds, however, zcalc is slightly smaller than . We ascribed the smaller zP2 to the Na vacancies characteristic to the P2 structure. With the vacancies, the nominal valence of M became higher, and hence RM becomes smaller. Our model tells us that becomes smaller if RM becomes smaller.
The broken lines in Fig. 4 represent the calculated αa, αc, and αc/αa (, , and /), respectively. () was evaluated by using the equation, . The only fitting parameter A (=2.56 × 10−4 K−1) was chosen to fit the average value of αa (=0.92 × 10−5 K−1). Using the same value of A, was found to reproduce the magnitude of experimental value for the O3 materials. On the other hand, larger αc for the P2 materials is possibly due to Na vacancies except for the data point of Na0.59CoO2. We note that is expressed without the adjustable parameter A,
| 5 |
where . The hard-sphere model examined in this paper gives intuitive and easy comprehension of the thermal expansion behavior of the layered oxides. The density-functional-thery (DFT) calculation successfully reproduces the linear thermal expansion coefficients of several materials such as Al75, S76, 4d transition metals77,78, Os78, MgO79, CaO79, and ZnO80, which is beyond the scope of this paper.
Conclusion
We systematically determined the temperature dependent lattice constant and z-coordinates of P2- and O3-type NaMO2. We proposed a simple hard-sphere model with constraint that M, upper and lower oxygens are coroneted to each others. The model quantitatively reproduced a, c, z, αa and αc for O3-type NaxMO2. On the other hand, z coordinate of P2-type NaxMO2 deviates from the hard-sphere model possibly due to Na vacancies. This simple model can be easily applied for the other layered compounds to intuitively understand and design the thermal expansion behaviors.
Methods
Sample preparation
Powders of O3- and P2-NaxMO2 (M: transition metal) were synthesized by using conventional solid state reaction. For O3-Na1.00CoO2, Na2O2 and Co3O4 were mixed under the molar ratio of Na:Co = 1.1:1, and calcined at 550°C in O2 for 16 h. Then, the product was finely ground, and again calcined in the same condition (this process was repeated once again.). For O3-Na0.98Fe0.5Co0.5O2, Na2CO3, Fe3O4 and Co3O4 were mixed under the molar ratio of Na:Fe:Co = 1.05:0.5:0.5, and calcined at 900 °C in air for 15 h. For O3-Na0.99Fe0.5Ni0.5O2, Na2O2, Fe2O3 and NiO were mixed under the molar ratio of Na:Fe:Ni = 1.2:0.5:0.5, and calcined at 650°C in O2 for 15 h. Then the product was finely ground and again calcined in the same condition. For O3-Na0.94Ti0.5Ni0.5O2, Na2CO3, TiO2 and NiO were mixed under the molar ratio of Na:Ti:Ni = 1.05:0.5:0.5, and calcined at 900°C in air for 15 h. Then the product was finely ground and again calcined in the same condition.
For P2-Na0.50Mn0.5Co0.5O2, Na2CO3, MnCO3 and Co3O4 were mixed under the molar ratio of Na:Mn:Co = 0.7:0.5:0.5, and calcined at 900°C in air for 12 h, Then, the product was finely ground and again calcined in the same condition. For P2-Na0.70Ni0.33Mn0.67O2, Na2CO3, NiO and Mn2O3 were mixed in ethanol under the molar ratio of Na:Ni:Mn = 0.7:0.33:0.67, and calcined at 900°C in air for 24 h. Then, the product was finely ground and again calcined in the same condition. For P2-Na0.69Ni0.33Mn0.5Ti0.17O2, Na2CO3, NiO, Mn2O3 and TiO2 were mixed under the molar ratio of Na:Ni:Mn:Ti = 0.7:0.33:0.5: 0.17, and calcined at 900°C in air for 18 h. For P2-Na0.70Ni0.33Mn0.33Ti0.34O2, Na2CO3, NiO, Mn2O3 and TiO2 were mixed under the molar ratio of Na:Ni:Mn:Ti = 0.7:0.33:0.33:0.34, and calcined at 900°C in air for 12 h. For P2-Na0.48Mn0.5Fe0.5O2, Na2O2, Mn2O3 and Fe2O3 were mixed under the molar ratio of Na:Mn:Fe = 0.7:0.5:0.5, and calcined at 900°C in air for 12 h. Then, the product was finely ground and again calcined in the same condition. All the samples were taken out from the hot furnace (>200°C), and then immediately transferred into a vacuum desiccator to avoid moisture in air.
X-ray diffraction
The synchrotron radiation x-ray diffraction (XRD) patterns were measured at BL02B2 beamline81 at SPring-8. The capillary was placed on the Debye-Scherrer camera at the beamline. The sample temperature was controlled by blowing a hot N2 in the temperature range of 300 K ≤ T ≤ 800 K. The XRD patterns were detected with an imaging plate (IP). The exposure time was 5 min. The wavelength of the x-ray was 0.499420 Å for P2-Na0.48Mn0.5Fe0.5O2 and P2-Na0.50Mn0.5Co0.5O2, and 0.499892 Å for O3-Na0.98Fe0.5Co0.5O2 and Na0.94Ti0.5Ni0.5O2, and 0.499838 Å for the others. The wavelengths are calibrated by the cell parameter of standard CeO2 powders. Crystal structure was analyzed by RIETAN-FP program82. Schematic figure of the crystal structure were drawn by VESTA program83. All the reflections can be indexed with the O3-type () or P2-type (P63/mmc) structures except for a tiny amount of impurity of O3-type Fe-rich phase for Na0.99Fe0.5Ni0.5O2 and NiO for Na0.94Ti0.5Ni0.5O2. All the structural parameters against T (300 K ≤ T ≤ 800 K) were listed in Tables S1–S9. During heating process, any extra impurity peaks were not appeared. We observed no tendency of Na deintercalation due to heating (Fig. S4).
The actual Na concentrations in the compound were determined by the Rietveld refinement based on the synchrotron XRD patterns at 300 K. We note that ref.45 reported a consistency of Na contents determined by ICP-AES (Inductively Coupled Plasma Atomic Emission Spectroscopy) and Rietveld refinement using synchrotron x-ray diffraction for P2-NaxMn1/2Fe1/2O2 phase.
Madelung energy calculation
Madelung energy (EME) was computed by the MADEL program in the VESTA software using the Fourier method83. The site potential ϕi is calculated by the formula , where gj is the occupancy of the j th ion, Zi is the valence of the j th ion, ε0 is the vacuum permittivity, and lij is the distance between ions i and j. EME is calculated by using the formula , where Wi is a factor depending on gi and the number of equivalent atomic positions at the site i in the unit cell. For O3-type structure (space group: ), we put +1, +3, and −2 charges on 3a Na (0,0,0), 3b M (0,0, ), and 6c O (0,0, z) sites in stoichiometric NaMO2. In the calculation of the P2-type structure (P63/mmc), we assume a stoichiometric NaMO2 with fully occupied 2d Na site. We put +e, +3e, and −2e charges on 2d Na , 2a M (0,0,0), and 4f O . A radius (s) of the hard sphere was set to 0.3 Å, and Fourier coefficients are summed up to 10 Å−1 in the reciprocal space.
Electronic supplementary material
Acknowledgements
This work was supported by Grant-in-Aids for Scientific Research (No. 23684022, No. 15K13513) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. The synchrotron-radiation X-ray powder diffraction experiments were performed at the SPring-8 BL02B2 beamline with the approval (2012A1094, 2013A1649, 2014A1056, 2015B1077) of the Japan Synchrotron Radiation Research Institute (JASRI).
Author Contributions
W.K. measured synchrotron XRD patterns, analyzed the XRD data, calculated linear thermal expansion coefficient based on a hard-sphere model, and wrote the manuscript. A.Y. synthesized O3-type layered oxides, and measured synchrotron XRD patterns. T.A., T.S., and D.T. synthesized P2-type layered oxides, and measured synchrotron XRD patterns. Y.M. contributed discussion and critically examined the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22279-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim SW, Seo DH, Ma X, Ceder G, Kang K. Electrode materials for rechargeable sodium-ion batteries: Potential alternatives to current lithium-ion batteries. Advanced Energy Materials. 2012;2:710–721. doi: 10.1002/aenm.201200026. [DOI] [Google Scholar]
- 2.Pan H, Hu Y-S, Chen L. Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ. Sci. 2013;6:2338–2360. doi: 10.1039/c3ee40847g. [DOI] [Google Scholar]
- 3.Yabuuchi N, Kubota K, Dahbi M, Komaba S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014;114:11636–11682. doi: 10.1021/cr500192f. [DOI] [PubMed] [Google Scholar]
- 4.Delmas C, Fouassier C, Hagenmuller P. Physica. 1980. Structural classification and properties of the layered oxides; pp. 81–85. [Google Scholar]
- 5.Parant JP, Olazcuaga R, Devalette M, Fouassier C, Hagenmuller P. Sur quelques nouvelles phases de formule NaxMnO2 (x ≤ 1) J. Solid State Chem. 1971;3:1–11. doi: 10.1016/0022-4596(71)90001-6. [DOI] [Google Scholar]
- 6.Delmas C, Braconnier JJ, Fouassier C, Hagenmuller P. Electrochemical intercalation of sodium in NaxCoO2 bronzes. Solid State Ionics. 1981;3/4:165–169. doi: 10.1016/0167-2738(81)90076-X. [DOI] [Google Scholar]
- 7.Braconnier JJ, Delmas C, Hagenmuller P. Etude par Desintercalation Electrochimique des Systemes NaxCrO2 et NaxNiO2. Mat. Res. Bull. 1982;17:993–1000. doi: 10.1016/0025-5408(82)90124-6. [DOI] [Google Scholar]
- 8.Maazaz A, Delmas C, Hagenmuller P. A study of the NaxTiO2 system by electrochemical deintercalation. J. Incl. Phenom. 1983;1:45–51. doi: 10.1007/BF00658014. [DOI] [Google Scholar]
- 9.Mendiboure A, Delmas C, Hagenmuller P. Electrochemical Intercalation and Deintercalation of NaxMnO2 Bronzes. J. Solid State Chem. 1985;57:323–331. doi: 10.1016/0022-4596(85)90194-X. [DOI] [Google Scholar]
- 10.Komaba S, et al. Electrochemical Na Insertion and Solid Electrolyte Interphase for Hard-Carbon Electrodes and Application to Na-Ion Batteries. Adv. Energy Mater. 2011;21:3859–3867. [Google Scholar]
- 11.Komaba S, Takei C, Nakayama T, Ogata A, Yabuuchi N. Electrochemical intercalation activity of layered NaCrO2 vs. LiCrO2. Electrochem. commun. 2010;12:355–358. doi: 10.1016/j.elecom.2009.12.033. [DOI] [Google Scholar]
- 12.Didier C, et al. Electrochemical Na-Deintercalation from NaVO2. Electrochem. Solid-State Lett. 2011;14:A75–A78. doi: 10.1149/1.3555102. [DOI] [Google Scholar]
- 13.Ma X, Chen H, Ceder G. Electrochemical Properties of Monoclinic NaMnO2. J. Electrochem. Soc. 2011;158:1307–1312. doi: 10.1149/2.035112jes. [DOI] [Google Scholar]
- 14.Nohira T, Ishibashi T, Hagiwara R. Properties of an intermediate temperature ionic liquid NaTFSA-CsTFSA and charge-discharge properties of NaCrO2 positive electrode at 423 K for a sodium secondary battery. J. Power Sources. 2012;205:506–509. doi: 10.1016/j.jpowsour.2011.11.086. [DOI] [Google Scholar]
- 15.Lee E, et al. New Insights into the Performance Degradation of Fe-Based Layered Oxides in Sodium-Ion Batteries: Instability of Fe3+/Fe4+ Redox in α-NaFeO2. Chem. Mater. 2015;27:6755–6764. doi: 10.1021/acs.chemmater.5b02918. [DOI] [Google Scholar]
- 16.Wu D, et al. NaTiO2: a layered anode material for sodium-ion batteries. Energy Environ. Sci. 2015;8:195–202. doi: 10.1039/C4EE03045A. [DOI] [Google Scholar]
- 17.Caballero A, et al. Synthesis and characterization of high-temperature hexagonal P2-Na0.6MnO2 and its electrochemical behaviour as cathode in sodium cells. J. Mater. Chem. 2002;12:1142–1147. doi: 10.1039/b108830k. [DOI] [Google Scholar]
- 18.Hamani D, Ati M, Tarascon JM. & Rozier, P2-NaxVO2 as possible electrode for Na-ion batteries. Electrochem. commun. 2011;13:938–941. doi: 10.1016/j.elecom.2011.06.005. [DOI] [Google Scholar]
- 19.D’Arienzo M, et al. Layered Na0.71CoO2: a powerful candidate for viable and high performance Na-batteries. Phys. Chem. Chem. Phys. 2012;14:5945–5952. doi: 10.1039/c2cp40699c. [DOI] [PubMed] [Google Scholar]
- 20.Zhu K, et al. A New Layered Sodium Molybdenum Oxide Anode for Full Intercalation-Type Sodium-Ion Batteries. J. Mater. Chem. A. 2015;3:22012–22016. doi: 10.1039/C5TA05444C. [DOI] [Google Scholar]
- 21.Saadoune I, Maazaz A, Mé Né Trier M, Delmas C. On the NaxNi0.6Co0.4O2 System: Physical and Electrochemical Studies. J. Solid State Chem. 1996;122:111–117. doi: 10.1006/jssc.1996.0090. [DOI] [Google Scholar]
- 22.Yoshida H, Yabuuchi N, Komaba S. NaFe0.5Co0.5O2 as high energy and power positive electrode for Na-ion batteries. Electrochem. commun. 2013;34:60–63. doi: 10.1016/j.elecom.2013.05.012. [DOI] [Google Scholar]
- 23.Mu L, et al. Prototype Sodium-Ion Batteries Using an Air-Stable and Co/Ni-Free O3-Layered Metal Oxide Cathode. Adv. Mater. 2015;27:6928–6933. doi: 10.1002/adma.201502449. [DOI] [PubMed] [Google Scholar]
- 24.Singh G, et al. Electrochemical performance of NaFex(Ni0.5Ti0.5)1 − xO2 (x = 0.2 and x = 0.4) cathode for sodium-ion battery. J. Power Sources. 2015;273:333–339. doi: 10.1016/j.jpowsour.2014.09.050. [DOI] [Google Scholar]
- 25.Vassilaras P, Toumar AJ, Ceder G. Electrochemical properties of NaNi1/3Co1/3Fe1/3O2 as a cathode material for Na-ion batteries. Electrochem. commun. 2014;38:79–81. doi: 10.1016/j.elecom.2013.11.015. [DOI] [Google Scholar]
- 26.Wang X, Liu G, Iwao T, Okubo M, Yamada A. Role of Ligand-to-Metal Charge Transfer in O3-Type NaFeO2-NaNiO2 Solid Solution for Enhanced Electrochemical Properties. J. Phys. Chem. C. 2014;118:2970–2976. doi: 10.1021/jp411382r. [DOI] [Google Scholar]
- 27.Yu H, Guo S, Zhu Y, Ishida M, Zhou H. Novel titanium-based O3-type NaTi0.5Ni0.5O2 as a cathode material for sodium ion batteries. Chem. Commun. 2014;50:457–459. doi: 10.1039/C3CC47351A. [DOI] [PubMed] [Google Scholar]
- 28.Yabuuchi N, et al. P2-type Nax[Fe1/2Mn1/2]O2 made from earth-abundant elements for rechargeable Na batteries. Nat. Mater. 2012;11:512–517. doi: 10.1038/nmat3309. [DOI] [PubMed] [Google Scholar]
- 29.Buchholz D, Chagas LG, Winter M, Passerini S. P2-type layered Na0.45Ni0.22Co0.11Mn0.66O2 as intercalation host material for lithium and sodium batteries. Electrochimica Acta. 2013;110:208–213. doi: 10.1016/j.electacta.2013.02.109. [DOI] [Google Scholar]
- 30.Doubaji S, Valvo M, Saadoune I, Dahbi M, Edström K. Synthesis and characterization of a new layered cathode material for sodium ion batteries. J. Power Sources. 2014;266:275–281. doi: 10.1016/j.jpowsour.2014.05.042. [DOI] [Google Scholar]
- 31.Yoshida H, et al. P2-type Na2/3Ni1/3Mn2/3−xTixO2 as a new positive electrode for higher energy Na-ion batteries. Chem. Commun. 2014;50:3677–3680. doi: 10.1039/C3CC49856E. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, et al. Stable layered P3/P2 Na0.66Co0.5Mn0.5O2 cathode materials for sodium-ion batteries. J. Mater. Chem. A. 2015;3:20708–20714. doi: 10.1039/C5TA05205J. [DOI] [Google Scholar]
- 33.Kang W, et al. Copper substituted P2-type Na0.67CuxMn1 − xO2: a stable high-power sodium-ion battery cathode. J. Mater. Chem. A. 2015;3:22846–22852. doi: 10.1039/C5TA06371J. [DOI] [Google Scholar]
- 34.Han MH, et al. High-Performance P2-Phase Na2/3Mn0.8Fe0.1Ti0.1O2 Cathode Material for Ambient-Temperature Sodium-Ion Batteries. Chem. Mater. 2016;28:106–116. doi: 10.1021/acs.chemmater.5b03276. [DOI] [Google Scholar]
- 35.Komaba S, et al. Study on the reversible electrode reaction of Na1 − xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery. Inorg. Chem. 2012;51:6211–6220. doi: 10.1021/ic300357d. [DOI] [PubMed] [Google Scholar]
- 36.Shanmugam R, Lai W. Study of Transport Properties and Interfacial Kinetics of Na2/3[Ni1/3MnxTi2/3− x]O2 (x = 0,1/3) as Electrodes for Na-Ion Batteries. J. Electrochem. Soc. 2015;162:A8–A14. doi: 10.1149/2.0201501jes. [DOI] [Google Scholar]
- 37.Shanmugam R, Lai W. Na2/3Ni1/3Ti2/3O2: ebi-functional f electrode materials for Na-ion batteries. ECS Electrochem. Lett. 2014;3:A23–A25. doi: 10.1149/2.007404eel. [DOI] [Google Scholar]
- 38.Sharma N, et al. Rate Dependent Performance Related to Crystal Structure Evolution of Na0.67Mn0.8Mg0.2O2 in a Sodium-Ion Battery. Chem. Mater. 2015;27:6976–6986. doi: 10.1021/acs.chemmater.5b02142. [DOI] [Google Scholar]
- 39.Wang, X., Tamaru, M., Okubo, M. & Yamada, A. Electrode Properties of P2-Na2/3MnyCo1−yO2 as Cathode Materials for Sodium-Ion Batteries. J. Phys. Chem. C117, 15545–15551 (2013).
- 40.Wang Y, Xiao R, Hu Y-S, Avdeev M, Chen L. P2-Na0.6[Cr0.6Ti0.4]O2 cation-disordered electrode for high-rate symmetric rechargeable sodium-ion batteries. Nat. Commun. 2015;6:6954(9). doi: 10.1038/ncomms7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan D, et al. P2-type Na0.67Mn0.65Fe0.2Ni0.15O2 Cathode Material with High-capacity for Sodium-ion Battery. Electrochim. Acta. 2014;116:300–305. doi: 10.1016/j.electacta.2013.10.211. [DOI] [Google Scholar]
- 42.Zhao J, et al. Electrochemical and thermal properties of P2-type Na2/3Fe1/3Mn2/3O2 for Na-ion batteries. J. Power Sources. 2014;264:235–239. doi: 10.1016/j.jpowsour.2014.04.048. [DOI] [Google Scholar]
- 43.Zhao W, et al. Synthesis of metal ion substituted P2-Na2/3Ni1/3Mn2/3O2 cathode material with enhanced performance for Na ion batteries. Mater. Lett. 2014;135:131–134. doi: 10.1016/j.matlet.2014.07.153. [DOI] [Google Scholar]
- 44.Zhao W, et al. Enhanced electrochemical performance of Ti substituted P2-Na2/3Ni1/4Mn3/4O2 cathode material for sodium ion batteries. Electrochim. Acta. 2015;170:171–181. doi: 10.1016/j.electacta.2015.04.125. [DOI] [Google Scholar]
- 45.Mortemard de Boisse B, Carlier D, Guignard M, Bourgeois L, Delmas C. P2-NaxMn1/2Fe1/2O2 Phase Used as Positive Electrode in Na Batteries: Structural Charges Induced by the Electrochemical (De)intercalation Process. Inorg. Chem. 2014;53:11197–11205. doi: 10.1021/ic5017802. [DOI] [PubMed] [Google Scholar]
- 46.Takada K, et al. Superconductivity in two-dimensional CoO2 layers. Nature. 2003;422:53–55. doi: 10.1038/nature01450. [DOI] [PubMed] [Google Scholar]
- 47.Berthelot R, Carlier D, Delmas C. Electrochemical investigation of the P2-NaxCoO2 phase diagram. Nat. Mater. 2011;10:74–80. doi: 10.1038/nmat2920. [DOI] [PubMed] [Google Scholar]
- 48.Huang Q, et al. Coupling between electronic and structural degrees of freedom in the triangular lattice conductor NaxCoO2. Phys. Rev. B. 2004;70:184110. doi: 10.1103/PhysRevB.70.184110. [DOI] [Google Scholar]
- 49.Viciu L, et al. Crystal structure and elementary properties of NaxCoO2 (x = 0.32, 0.51, 0.6, 0.75, and 0.92) in the three-layer NaCoO2 family. Phys. Rev. B. 2006;73(1):74104(10). [Google Scholar]
- 50.Guignard M, et al. Vanadium Clustering/Declustering in P2-Na1/2VO2 Layered Oxide. Chem. Mater. 2014;26:1538–1548. doi: 10.1021/cm403114k. [DOI] [Google Scholar]
- 51.Kubota K, et al. New insight into structural evolution in layered NaCrO2 during electrochemical sodium extraction. J. Phys. Chem. C. 2015;119:166–175. doi: 10.1021/jp5105888. [DOI] [Google Scholar]
- 52.Jung YH, Christiansen AS, Johnsen RE, Norby P, Kim DK. In Situ X-Ray Diffraction Studies on Structural Changes of a P2 Layered Material during Electrochemical Desodiation/Sodiation. Adv. Funct. Mater. 2015;25:3227–3237. doi: 10.1002/adfm.201500469. [DOI] [Google Scholar]
- 53.Lu X, et al. Direct imaging of layered O3- and P2-NaxFe1/2Mn1/2O2 structures at the atomic scale. Phys. Chem. Chem. Phys. 2014;16:21946–21952. doi: 10.1039/C4CP02957G. [DOI] [PubMed] [Google Scholar]
- 54.Shimono T, Tanabe D, Kobayashi W, Moritomo Y. Structural Response of P2-Type NaxMnO2 against Na+ Intercalation. J. Phys. Soc. Jpn. 2013;82:083601. doi: 10.7566/JPSJ.82.083601. [DOI] [Google Scholar]
- 55.Shimono T, Tanabe D, Kobayashi W, Nitani H, Moritomo Y. Electronic State of P2-Type NaxMO2 (M= Mn and Co) as Investigated by In situ X-ray Absorption Spectroscopy. J. Phys. Soc. Jpn. 2013;82:124717(5). [Google Scholar]
- 56.Tanabe D, Shimono T, Kobayashi W, Moritomo Y. Temperature dependence of anisotropic displacement parameters in O3-type NaMO2 (M = Cr and Fe): Comparison with isostructural LiCoO2. Phys. Status Solidi - Rapid Res. Lett. 2014;8:287–290. doi: 10.1002/pssr.201308295. [DOI] [Google Scholar]
- 57.Tanabe D, Shimono T, Kobayashi W, Moritomo Y. Na-site energy of P2-type NaxMO2 (M=Mn and Co) Phys. Status Solidi RRL. 2013;7:1097–1101. doi: 10.1002/pssr.201308101. [DOI] [Google Scholar]
- 58.Clarke SJ, Fowkes AJ, Harrison A, Ibberson RM, Rosseinsky MJ. Synthesis, Structure, and Magnetic Properties of NaTiO2. Chem. Mater. 1998;10:372–384. doi: 10.1021/cm970538c. [DOI] [Google Scholar]
- 59.McQueen TM, et al. Successive orbital ordering transitions in NaVO2. Phys. Rev. Lett. 2008;101:166402. doi: 10.1103/PhysRevLett.101.166402. [DOI] [PubMed] [Google Scholar]
- 60.McQueen TM, et al. Magnetic Structure and Properties of the S = 5/2 Triangular Antiferromagnet α-NaFeO2. Phys. Rev. B. 2007;76:024420. doi: 10.1103/PhysRevB.76.024420. [DOI] [Google Scholar]
- 61.Onoda M. Geometrically frustrated triangular lattice system NaxVO2: superparamagnetism in x = 1 and trimerization in x≈0.7. J. Phys. Condens. Matter. 2008;20:145205. doi: 10.1088/0953-8984/20/14/145205. [DOI] [Google Scholar]
- 62.Lee M, et al. Large enhancement of the thermopower in NaxCoO2 at high Na doping. Nat. Mater. 2006;5:537. doi: 10.1038/nmat1669. [DOI] [PubMed] [Google Scholar]
- 63.Fujita K, Mochida T, Nakamura K. High-Temperature Thermoelectric Properties of NaxCoO2 − δ Single Crystals. Jpn. J. Appl. Phys. 2001;40:4644–4647. doi: 10.1143/JJAP.40.4644. [DOI] [Google Scholar]
- 64.Kim S, Ma X, Ong SP, Ceder G. A comparison of destabilization mechanisms of the layered NaxMO2 and LixMO2 compounds upon alkali de-intercalation. Phys. Chem. Chem. Phys. 2012;14:15571–15578. doi: 10.1039/c2cp43377j. [DOI] [PubMed] [Google Scholar]
- 65.Mo Y, Ong SP, Ceder G. Insights into Diffusion Mechanisms in P2 Layered Oxide Materials by First-Principles Calculations. Chem. Mater. 2014;26:5208–5214. doi: 10.1021/cm501563f. [DOI] [Google Scholar]
- 66.Toumar AJ, Ong SP, Richards WD, Dacek S, Ceder G. Vacancy Ordering in O3-Type Layered Metal Oxide Sodium-Ion Battery Cathodes. Phys. Rev. Appl. 2015;4:064002(9). doi: 10.1103/PhysRevApplied.4.064002. [DOI] [Google Scholar]
- 67.Hinuma Y, Meng YS, Ceder G. Temperature-concentration phase diagram of P2-NaxCoO2 from first-principles calculations. Phys. Rev. B. 2008;77:224111. doi: 10.1103/PhysRevB.77.224111. [DOI] [Google Scholar]
- 68.Goodenough JB, Kim Y. Challenges for Rechargeable Li Batteries. Chem. Mater. 2010;22:587–603. doi: 10.1021/cm901452z. [DOI] [Google Scholar]
- 69.Park, J.-K.(ed) Principles and Applications of Lithium Secondary Batteries. Wiley-VCH Verlag GmbH & Co. KGaA (2012).
- 70.Motohashi T, et al. Impact of lithium composition on the thermoelectric properties of the layered cobalt oxide system LixCoO2. Phys. Rev. B. 2011;83:195128. doi: 10.1103/PhysRevB.83.195128. [DOI] [Google Scholar]
- 71.Lan R, Tao S. Novel Proton Conductors in the Layered Oxide Material LixAl0.5Co0.5O2. Adv. Energy Mater. 2014;4:1301683. doi: 10.1002/aenm.201301683. [DOI] [Google Scholar]
- 72.Sparks TD, Gurlo A, Clarke DR. Enhanced n-type thermopower in distortion-free LiMn2O4. J. Mater. Chem. 2012;22:4631. doi: 10.1039/c2jm16297k. [DOI] [Google Scholar]
- 73.da Luz MS, et al. High-resolution measurements of the thermal expansion of superconducting Co-doped BaFe2As2. Phys. Rev. B. 2009;79:214505. doi: 10.1103/PhysRevB.79.214505. [DOI] [Google Scholar]
- 74.Shannon RD. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta. Cryst. A. 1976;32:751. doi: 10.1107/S0567739476001551. [DOI] [Google Scholar]
- 75.Togo A, Tanaka I. First principles phonon calculations in materials science. Scripta Mater. 2015;108:1–5. doi: 10.1016/j.scriptamat.2015.07.021. [DOI] [Google Scholar]
- 76.George J, et al. Lattice thermal expansion and anisotropic displacements in α-sulfur from diffraction experiments and first-principles theory. J. Chem. Phys. 2016;145:234512. doi: 10.1063/1.4972068. [DOI] [PubMed] [Google Scholar]
- 77.Souvatzis P, Eriksson O. Ab initio calculations of the phonon spectra and the thermal expansion coefficients of the 4d metals. Phys. Rev. B. 2008;77:024110. doi: 10.1103/PhysRevB.77.024110. [DOI] [Google Scholar]
- 78.Palumbo M, Dal Corso A. Lattice dynamics and thermophysical properties of h.c.p. Os and Ru from the quasi-harmonic approximation. J. Phys.: Condens. Matter. 2017;29:395401. doi: 10.1088/1361-648X/aa7dca. [DOI] [PubMed] [Google Scholar]
- 79.Erba A, Shahrokhi M, Moradian R, Dovesi R. On how differently the quasi-harmonic approximation works for two isostructural crystals: Thermal properties of periclase and lime. J. Chem. Phys. 2015;142:044114. doi: 10.1063/1.4906422. [DOI] [PubMed] [Google Scholar]
- 80.Wang Z, Wang F, Wang L, Jia Y, Sun Q. First-principles study of negative thermal expansion in zinc oxide. J. Appl. Phys. 2013;114:063508. doi: 10.1063/1.4817902. [DOI] [Google Scholar]
- 81.Nishibori E, et al. The large Debye-Scherrer camera installed at SPring-8 BL02B2 for charge density studies. Nucl. Instrum. Methods A. 2001;467-468:1045. doi: 10.1016/S0168-9002(01)00639-8. [DOI] [Google Scholar]
- 82.Izumi F, Momma K. Three-Dimensional Visualization in Powder Diffraction. Solid State Phenom. 2007;130:15. doi: 10.4028/www.scientific.net/SSP.130.15. [DOI] [Google Scholar]
- 83.Momma K, Izumi F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Cryst. 2011;44:1272–1276. doi: 10.1107/S0021889811038970. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.