Abstract
It has been previously reported that HSP27 is released from platelets in type 2 diabetes mellitus (DM) patients according to phosphorylation. In the present study, we investigated the effect of ristocetin, a glycoprotein (GP)Ib/IX/V activator, on the release of HSP27 and the effect of anti-platelet agents, such as acetylsalicylic acid (ASA), on this release. Forty-six patients with type 2 DM were recruited, and classified into two groups depending on administration of anti-platelet agents, resulting in 31 patients without these agents (control group) and 15 patients with them (anti-platelet group). Ristocetin potently induced the aggregation of platelets in the two groups. Ristocetin-induced changes of the area under the curve of light transmittance and the ratio of the size of platelet aggregates in the anti-platelet group were slightly different from those in the control group. On the other hand, the levels of phosphorylated-HSP27 induced by ristocetin in the platelets from a patient of the anti-platelet group taking ASA were significantly lower than those from a patient of the control group. The levels of HSP27 release from the ristocetin-stimulated platelets were significantly correlated with the levels of phosphorylated-HSP27 in the platelets from patients in the two groups. The levels of HSP27 release and those of platelet-derived growth factor-AB (PDGF-AB) secretion stimulated by ristocetin in the anti-platelet group were lower than those in the control group. In addition, the levels of HSP27 release and those of PDGF-AB secretion stimulated by ADP in the anti-platelet group were lower than those in the control group. These results strongly suggest that anti-platelet agents inhibit the HSP27 release from platelets stimulated by ristocetin but not the aggregation in type 2 DM patients.
Keywords: ristocetin, platelet, aggregation, HSP27, diabetes
Introduction
Atherothrombosis is contributed to the sudden onset of arterial occlusion induced by platelet activation and aggregation, which initiates cerebrovascular and cardiovascular diseases causing high risk for mortality and disability (1,2). Once the platelets in blood flow are tethered by the injury sites of vessel, the formation of glycoprotein (GP)Ib/IX/V and von Willebrand factor complex followed by GPIIb/IIIa activation induces platelet aggregation (1,2). By contrast, the secretion of autocrine/paracrine mediators such as ADP, and the release of thromboxane A2 (TXA2) from platelets are triggered by activation, which accerelates thrombus formation (1). In addition, the secretion of platelet granule contents such as platelet-derived growth factor-AB (PDGF-AB) is elicited by activation, which modifies vascular endothelial/smooth muscle cell function resulting in the development of atherosclerosis (1). In a variety of pathophysiological conditions, shear stress is recognized to induce platelet activation (3), which depends on the interaction of GPIb/IX/V and von Willebrand factor (3,4). Ristocetin is a potent inducer of the interaction as an activator of GPIb/IX/V (5). Ristocetin-inducible GPIb activation reportedly elicits the generation of TXA2 via the activation of phospholipase A2 in human platelets (4). We previously indicated that ristocetin stimulates the release of soluble CD40 (sCD40) ligand from human platelets mediated through TXA2 production (6).
Type 2 diabetes mellitus (DM) is a worldwide concern of public health due to the elevating incidence of cardiovascular diseases (7). In addition to the progression of atherosclerotic change, the spontaneous platelet aggregation develops from early stage of the disease, resulting in the increased risk of cardiovascular complications (8). Regarding the functional changes of platelets in type 2 DM patients, we previously showed that irreversible platelet microaggregation is inducible by a low-dose (1 µM) of ADP, and that the notable sensitivity of platelets is mediated through not P2Y1 but P2Y12 receptors (9). We have also demonstrated that the upregulation of platelet aggregation is closely correlated with the activation of p44/p42 mitogen-activated protein (MAP) kinase and p38 MAP kinase stimulated by collagen in the platelets from type 2 DM patients (10). Thus, the adequate regulation of platelet dysfunctions underlying the pathogenesis may be a useful strategy for the improvement of prognosis of DM patients. Anti-platelet agents, such as acetylsalicylic acid (ASA), are widely accepted therapeutic tools for the prevention of ischemic cardiovascular diseases in DM patients (11), whereas non-responders known as ‘aspirin resistance’, reduce the effectiveness of ASA (12).
On the other hand, it is recognized that heat shock proteins (HSPs) induced by various environmental stresses such as heat and chemicals, intracellularly act as molecular chaperones protecting the unfolded proteins against aggregation (13). Among HSPs, low-molecular weight HSPs (HSPB) such as HSP27 (HSPB1) and αB-crystallin (HSPB5), characterized by the homological sequences of amino acid, termed ‘α-crystallin domain’, possess a variety of pleiotropic functions including an anti-apoptotic effect (14,15), and play roles as stabilizer of actin and microtubules of cytoskeleton and molecular chaperones (13,16). Regarding the functional modulation of HSPBs, it is known that post-translational modification, such as phosphorylation, changes their functions (13). In human HSP27, the serine residues Ser-15, Ser-78 and Ser-82, are recognized as potential sites of phosphoryation (13,17). HSP27 at rest exists as an unphosphorylated aggregated form, which is rapidly dissociated into dimer or monomer due to phosphorylation, considered as a hinge of its substrate binding and presenting its characteristic functions (13,18). It is well known that the members of MAP kinase superfamily such as p38 MAP kinase are involved in the phosphorylation of HSP27 in the process of human platelet activation (13,19). We previously reported that Rac, a low-molecular weight GTP-binding protein, regulates HSP27 phosphorylation stimulated by collagen via p44/p42 MAP kinase in human platelets (20). Furthermore, we have recently shown that phosphorylated-HSP27 stimulated by collagen or thrombin receptor-activating protein (TRAP) in the platelets of type 2 DM patients is released together with the secretion of PDGF-AB (21,22). Accumulating evidence indicates that HSP27, not only intracellularly, but also extracellularly plays a role in and modulates inflammation (23). However, the clinical relevance of HSP27 phosphorylation and the release in human platelets especially in the diabetic conditions is not precisely understood.
In the present study, we investigated the effect of ristocetin on the release of HSP27 in platelets in type 2 DM patients, and the effect of anti-platelet agents on this relase. Our present findings strongly suggest that anti-platelet agents inhibit the HSP27 release from platelets stimulated by ristocetin but not the aggregation in type 2 DM patients.
Materials and methods
Materials
Ristocetin and ADP were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). Phospho-specific HSP27 (Ser-78) rabbit anti-human polyclonal antibodies (cat. no. SPA-523) were purchased from Stressgen Biotechnologies (Victoria, BC, Canada). GAPDH rabbit polyclonal antibodies (cat. no. SC-25778) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The PDGF-AB enzyme-linked immunosorbent assay (ELISA) kit was purchased from R&D Systems, Inc. (Minneapolis, MN, USA). The phosphorylated-HSP27 ELISA kit was purchased from Enzo Life Sciences, Inc. (Farmingdale, NY, USA). Other materials and chemicals were obtained from commercial sources. Ristocetin was dissolved in dimethyl sulfoxide. The maximum concentration of dimethyl sulfoxide was 0.1%, which did not affect platelet aggregation, western blot analysis or ELISA.
Subjects
The inclusion criteria for the study were the presence of type 2 DM according to the criteria of the World Health Organization. We excluded the patients who were complicated with a malignancy, infectious diseases, including hepatitis B and hepatitis C, or autoimmune disorders. All the participants were advised to avoid sleep deprivation or blood donation. The study was approved by the committee of the conduct of human research at the National Center for Geriatrics and Gerontology (Obu, Japan) and at Gifu University Graduate School of Medicine (Gifu, Japan). Written informed consent was obtained from all the patients.
Blood sampling
Blood (10 ml) was drawn from the vein between 8:00 a.m. and 9:00 a.m. after at least 15 min of bed rest to preserve steady state conditions. Sodium citrate (14 µM) was added to the blood immediately as an anticoagulant, and platelet-rich plasma (PRP) was obtained by centrifugation at 155 × g for 12 min at room temperature. Platelet-poor plasma (PPP) was prepared from the residual blood by centrifugation at 1,400 × g for 5 min.
Platelet aggregation
Platelet aggregation was measured using an aggregometer (PA-200 apparatus; Kowa Co. Ltd., Tokyo, Japan) with a laser-scattering system, as previously described (9,10). The system can determine the size of platelet aggregates based on particle counting (small size, 9–25 µm; medium size, 25–50 µm; large size, 50–70 µm). Briefly, PRP was preincubated at 37°C for 1 min with a stirring speed of 800 rpm, and then stimulated by 1.5 mg/ml of ristocetin, 1 µM of ADP or vehicle. The percentage of transmittance of isolated platelets was recorded as 0%, and that of corresponding PPP (blank) was recorded as 100%. Platelet aggregation was then terminated by adding ice-cold EDTA (10 mM). The conditioned mixture was collected and centrifuged at 10,000 × g at 4°C for 2 min. The supernatant was immediately stored at −80°C until the determination of PDGF-AB and phosphorylated-HSP27 levels. The pellet was washed twice with PBS, and then lysed immediately by boiling in a lysis buffer containing 62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate (SDS), 50 mM dithiothreitol and 10% glycerol for a western blot analysis.
Western blot analysis
A western blot analysis was performed as previously described (24). Briefly, SDS-polyacrylamide gel electrophoresis (PAGE) was performed by the method described by Laemmli (25) in a 12.5% polyacrylamide gel. The proteins fractioned in the gels were transferred onto polyvinylidene fluoride (PVDF) membranes, and the membranes were blocked with 5% fat-free dry milk in phosphate-buffered saline (PBS) with 0.1% Tween-20 (PBS-T; 10 mM Na2HPO4, 1.8 mM KH2PO4, pH 7.4, 137 mM NaCl, 0.1% Tween-20) for 2 h before incubation with the indicated primary antibodies. Peroxidase-labeled antibodies raised in goat against rabbit IgG (KPL, Gaithersburg, MD, USA, cat. no. 5220-0336) were used as the secondary antibodies. The primary and secondary antibodies were diluted to 1:10,000 with 5% fat-free dry milk in PBS-T. The peroxidase activity on the PVDF membrane was visualized with X-ray film by means of an ECL Western blotting detection system (GE Healthcare, Buckinghamshire, UK) according to the manufacturer's protocol. The bands were analyzed by densitometry using the ImageJ software program (National Institutes of Health, Bethesda, MD, USA). The quantitative data of each sample were obtained as the counts of pixels.
ELISA for PDGF-AB and phosphorylated-HSP27
The levels of PDGF-AB and phosphorylated-HSP27 in the supernatant of the conditioned mixture after platelet aggregation were determined by corresponding ELISA kits, according to their manufacturer's protocol.
Statistical analysis
The data except representatives were expressed as the mean ± standard deviation. The statistical significance of the correlation between two variables, linear regression analysis was adopted. The comparison of means between the two groups were performed by one-way ANOVA. Statistical analyses were performed with SPSS version 19.0 (IBM Japan Ltd., Tokyo, Japan) as a software. P<0.05 was considered to indicate a statistically significant difference.
Results
Characterization of the subjects for western blot analysis and ELISA
The clinical and biochemical characteristics of the recruited patients with type 2 DM (n=46) are shown in Table I. Of the 46 patients, 15 patients administered anti-platelet agents such as ASA (n=12), ticlopidine (n=1) or clopidgrel (n=3), were classified as the anti-platelet group (one patient was administered both ASA and clopidgrel). The HbA1c levels of the control and anti-platelet groups, which were significantly higher than the upper limit of normal range (5.9%), were 8.4±1.4% and 8.7±1.2%, respectively. No significant difference was found between the two groups (P<0.05). The anthropometric indexes were within the normal limits in Japanese patients. Significant changes of metabolic variables were not observed.
Table I.
Characteristics of the study subjects.
| Group | ||
|---|---|---|
| Parameters | Control | Anti-platelet |
| Total number | 31 | 15 |
| Sex (F/M) | 12/19 | 9/6 |
| Age (years) | 70.9±6.9 | 74.3±5.6 |
| DM duration (years) | 14.0±8.5 | 14.1±5.7 |
| Height (cm) | 159.1±9.0 | 153.7±10.3 |
| Weight (kg) | 62.9±12.6 | 54.1±9.8a |
| BMI | 24.7±3.6 | 22.7±2.4 |
| sBP (mmHg) | 120.3±20.6 | 117.4±15.1 |
| dBP (mmHg) | 68.1±10.2 | 63.8±12.5 |
| HbA1c (%) | 8.4±1.4 | 8.7±1.2 |
| Glu (mg/dl) | 169.9±60.3 | 159.0±48.4 |
| TC (mg/dl) | 185.2±34.3 | 181.6±46.7 |
| TG (mg/dl) | 128.1±57.6 | 140.6±109.8 |
| HDL (mg/dl) | 49.1±14.0 | 48.1±10.6 |
| Plt (×104/µl) | 21.3±5.3 | 20.0±4.2 |
F, female; M, male; DM, diabetes mellitus; BMI, body mass index; sBP, systolic blood pressure; dBP, diastolic blood pressure; HbA1c, hemoglobin A1c; Glu, plasma glucose; TC, total cholesterol; TG, triglyceride; HDL, high-density lipoprotein; Plt, platelet counts. The data are presented as the mean ± standard deviation.
P<0.05 vs. control.
Comparison of the ristocetin-induced platelet aggregation between the control and anti-platelet groups of type 2 DM patients
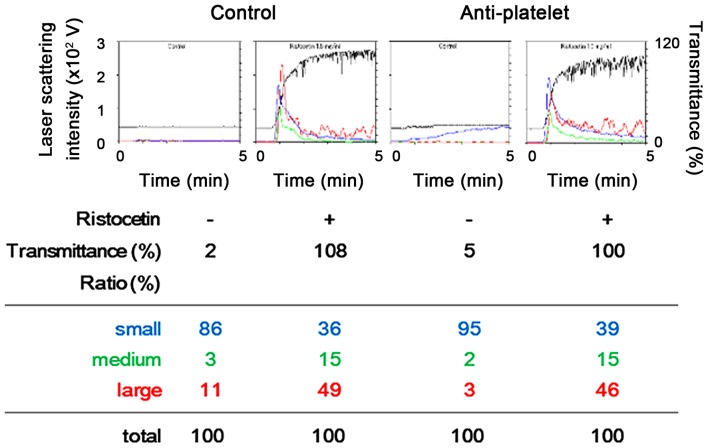
A representative pattern of ristocetin-induced platelet aggregation in the study groups analyzed by an aggregometer with a laser scattering system are shown in Fig. 1. Ristocetin (1.5 mg/ml) potently induced platelet aggregation in the two study groups, and it seems that there was no difference between the effect observed in the anti-platelet group and that in the control group. Thus, we compared the changes induced by restocetin of the area under the curve (AUC) of light transmittance and the ratio of aggregated particle sizes of platelets between the two groups. The ristocetin-induced change of AUC of light transmittance in the anti-platelet group was not significantly different from that in the control group (Fig. 2). Ristocetin significantly induced the changes of the aggregated particle size ratio, namely, the ratio of large aggregates (50–70 µm) and medium aggregates (25–50 µm) was increased, but that of small aggregates (9–25 µm) was decreased (Table II). There was little difference between the two groups in the change induced by ristocetin of the aggregated particle size ratio (Table II).
Figure 1.
Representative patterns of platelet aggregation induced by 1.5 mg/ml of ristocetin as detected by an aggregometer with laser-scattering system in platelets from type 2 DM patients. PRP from a patient of type 2 DM in the anti-platelet group taking aspirin and PRP from a patient of control group was stimulated by 1.5 mg/ml of ristocetin or vehicle in an aggregometer at 37°C for 4 min with a stirring speed of 800 rpm. Time-dependent changes in the platelet aggregation after the stimulation are shown. The black line indicates the percentage of transmittance of each sample (the isolated platelets were recorded as 0%, and platelet-free plasma was recorded as 100%). The blue line indicates small aggregates (9–25 µm); green line, medium aggregates (25–50 µm); red line, large aggregates (50–70 µm). The ratio is presented for each value. The upper panel indicates the results of platelet aggregation, and the lower panel indicates each ratio detected at the end of stimulation. PRP, platelet-rich plasma; DM, diabetes mellitus.
Figure 2.
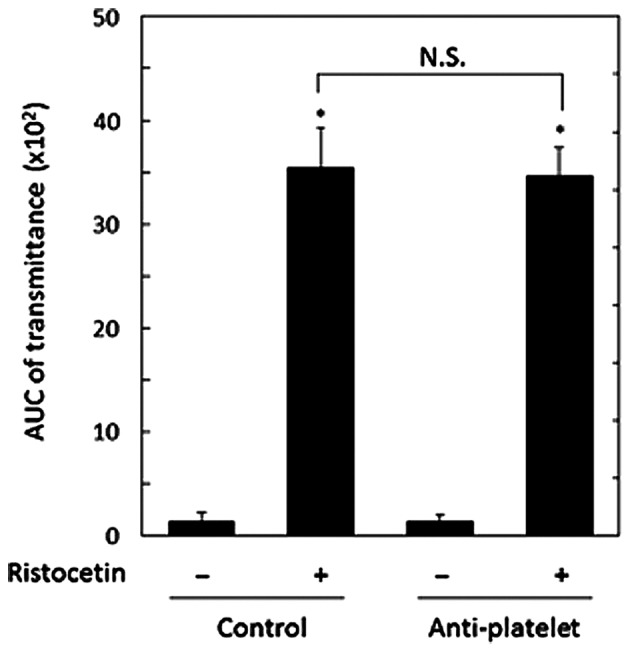
Comparison of the ristocetin-induced platelet aggregation between the anti-platelet and control groups of type 2 DM patients. PRP from patients of type 2 DM in the anti-platelet group (n=15) and PRP from patients of the control group (n=31) were stimulated by 1.5 mg/ml of ristocetin or vehicle in an aggregometer at 37°C for 4 min with a stirring speed of 800 rpm. The value of AUC of transmittance in the two groups are presented as mean ± standard deviation. *P<0.05, compared to the value without ristocetin. N.S. designates statistically not significant between the pair. PRP, platelet-rich plasma; DM, diabetes mellitus; AUC, area-under curve.
Table II.
Comparison of ristocetin effect on the ratio of aggregated particle size between the control and anti-platelet groups in type 2 DM patients.
| Ratio of aggregated particle size | ||||
|---|---|---|---|---|
| Group | Ristocetin | Large (%) | Medium (%) | Small (%) |
| Control | − | 5.4±6.3 | 2.8±2.9 | 91.5±9.0 |
| + | 45.5±4.6a | 14.7±1.1a | 39.8±3.9a | |
| Anti-platelet | − | 3.7±3.7 | 4.2±5.6 | 91.9±8.4 |
| + | 46.6±3.5a | 15.3±2.1a | 37.9±3.3a | |
PRP from patients of type 2 DM in the anti-platelet group (n=15) and PRP from patients of the control group (n=31) were stimulated by 1.5 mg/ml of ristocetin or vehicle in an aggregometer at 37°C for 4 min with a stirring speed of 800 rpm. The results obtained from the aggregometer for the ratio of large aggregates, medium aggregates and small aggregates are summarized. Each value are presented as mean ± standard deviation.
P<0.05 compared to the value without ristocetin. PRP, platelet-rich plasma; DM, diabetes mellitus.
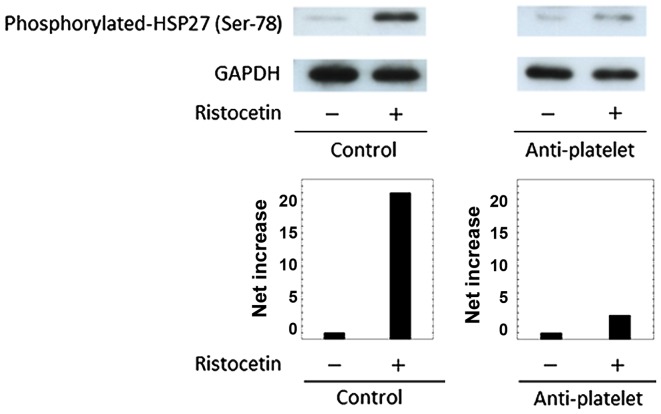
Comparison of the ristocetin-induced HSP27 phosphorylation between the control and anti-platelet groups of type 2 DM patients
The phosphorylation of HSP27 in human is detected at three serine residues (Ser-15, Ser-78 and Ser-82) (13,17). In previous studies (21,22), we demonstrated that the levels of HSP27 phosphorylation at Ser-78 are significantly correlated with the levels of released phosphorylated-HSP27 in human platelets stimulated by collagen or TRAP. Thus, we examined the effect of ristocetin on the phosphorylation of HSP27 (Ser-78) in the platelet in the study groups, and compared the phosphorylation levels. Ristocetin (1.5 mg/ml) elicited the phosphorylation of HSP27 (Ser-78) in the two groups, but the levels observed in a patient of the anti-platelet group taking ASA were remarkably weaker than those in a patient of the control group (Fig. 3). Similar results were obtained from patients taking other types of anti-platelet agents such as ticlopidine and clopidgrel (data not shown). It is probable that the administration of anti-platelet agents suppresses the ristocetin-induced phosphorylation of HSP27 in the platelets of type 2 DM patients.
Figure 3.
Representative data showing the ristocetin-induced HSP27 phosphorylation in platelets from a patient of the anti-platelet group and that of the control group of type 2 DM, which are identical to the subjects in Fig. 1. PRP was stimulated by 1.5 mg/ml of ristocetin or vehicle for 4 min, and then the reaction was terminated by the addition of an ice-cold EDTA (10 mM) solution. The extracts of platelets were subjected to a western blot analysis using antibodies against phospho-specific HSP27 (Ser-78) and GAPDH. The bands were quantified using the ImageJ software program as the counts of pixels. The bands of phospho-HSP27 were normalized to the GAPDH bands, and the levels of vehicle were subtracted from the ristocetin-stimulated levels, presented as the net increase. Upper panel indicates the results of a western blot analysis, and lower panel indicate the net increase. PRP, platelet-rich plasma; DM, diabetes mellitus.
Association between the release of phosphorylated-HSP27 and the levels of HSP27 phosphorylation (Ser-78) induced by ristocetin in the platelets from the two groups-combined type 2 DM patients
Since we found that ristocetin stimulated the release of phosphorylated-HSP27 from platelets, we next investigated the relationship between the levels of released phosphorylated-HSP27 and the levels of HSP27 (Ser-78) phosphorylation induced by ristocetin (1.5 mg/ml) in the platelets from two groups-combined type 2 DM patients (n=34; 25 from control group and 9 from anti-platelet group). The levels were significantly correlated with the levels of HSP27 (Ser-78) phosphorylation in the platelets (R2=0.284, P=0.001; Fig. 4). It is likely that the ristocetin-induced phosphorylation of HSP27 results in the release of phosphorylated-HSP27 from the platelets of type 2 DM patients.
Figure 4.
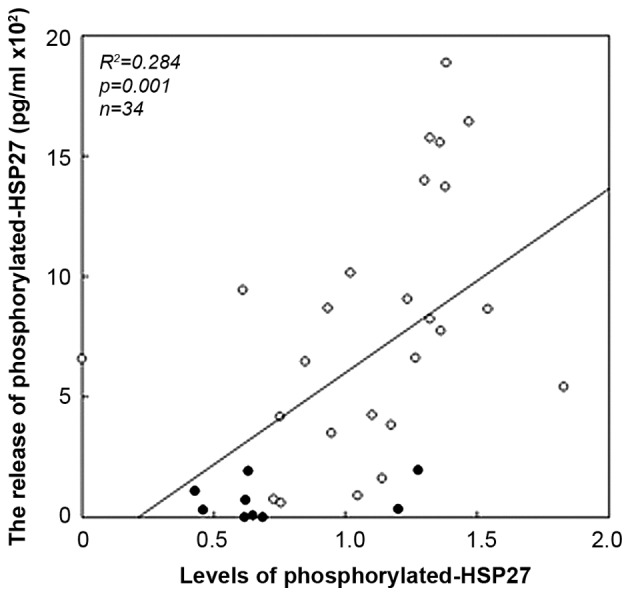
Association between individual levels of HSP27 phosphorylation (Ser-78) in the platelets and the release of phosphorylated HSP27 levels induced by ristocetin in type 2 DM patients. The extracts of the stimulated-platelets derived from the patients of anti-platelet group (n=9) and those of control group (n=25) were subjected to a western blot analysis using antibodies against phospho-specific HSP27 (Ser-78) and GAPDH, and the bands were quantified using ImageJ software program as the counts of pixels. The baseline levels in unstimulated samples were subtracted from each of the individual HSP27 phosphorylation levels (phosphorylated-HSP27/GAPDH). The levels of phosphorylated HSP27 in the conditioned mixture were determined using an ELISA. These data were plotted individually and analyzed by linear regression analysis. Closed circles and open circles indicate the data of anti-platelet group and those of control group, respectively. DM, diabetes mellitus.
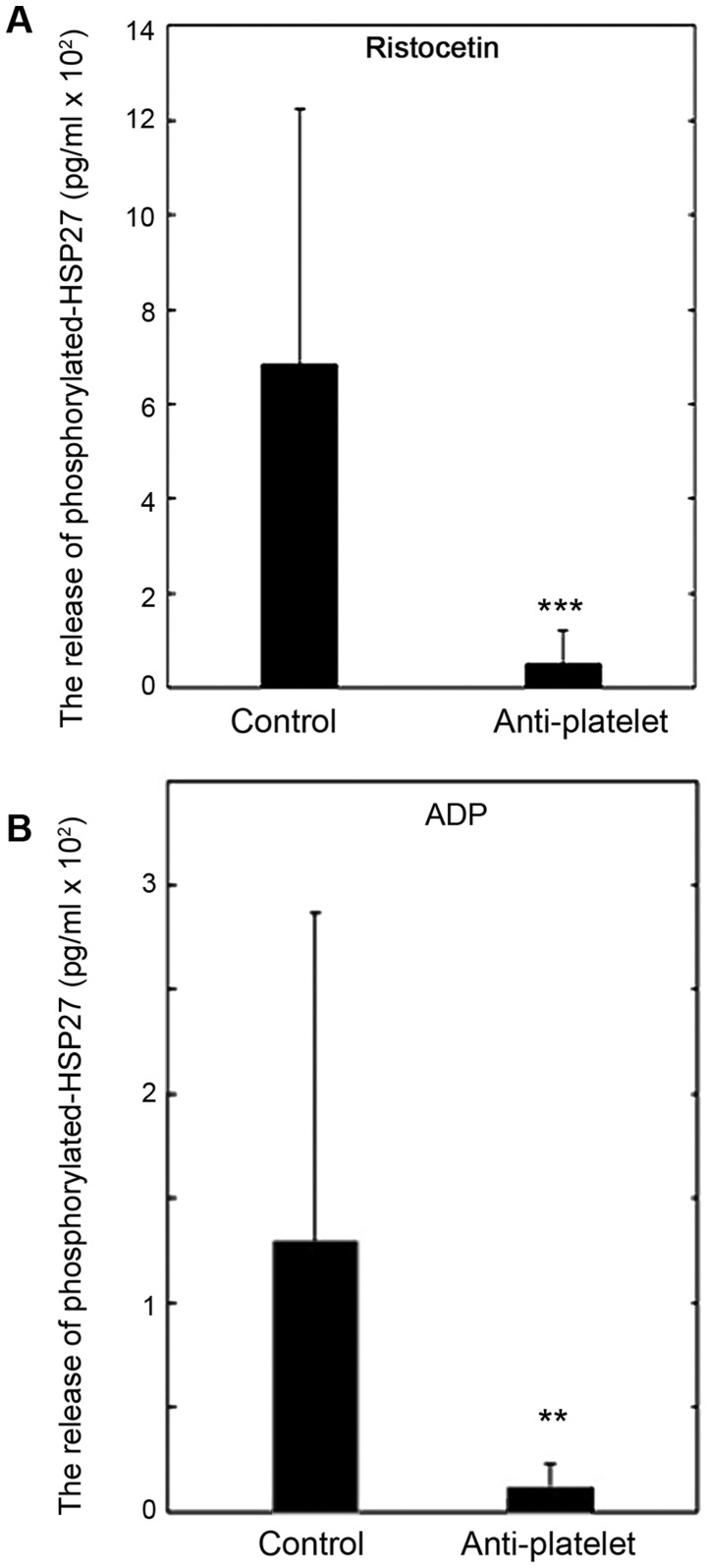
Comparison of the release of phosphorylated-HSP27 between the control and anti-platelet groups of type 2 DM patients
We compared the released levels of phosphorylated-HSP27 induced by ristocetin or ADP between the control and anti-platelet groups of type 2 DM patients. The levels of the phosphorylated-HSP27 release induced by 1.5 mg/ml of ristocetin in the anti-platelet group were significantly lower than those in the control group (Fig. 5A). The levels of phosphorylated-HSP27 release induced by 1 µM of ADP in the anti-platelet group, as well as ristocetin, were significantly lower than those in the control group (Fig. 5B).
Figure 5.
Comparison of the release of phosphoryrated HSP27 between the anti-platelet and control groups of type 2 DM patients. PRP from patients of type 2 DM in the anti-platelet group (n=9) and PRP from patients of the control group (n=25) were stimulated by 1.5 mg/ml of (A) ristocetin, 1 µM of (B) ADP or vehicle for 4 min, and the levels of phosphorylated HSP27 in the conditioned mixture were determined using ELISA. The value of the levels of phosphorylated-HSP27 are expressed as mean ± standard deviation. **P<0.01 or***P<0.001, compared to the value of control. PRP, platelet-rich plasma; DM, diabetes mellitus.
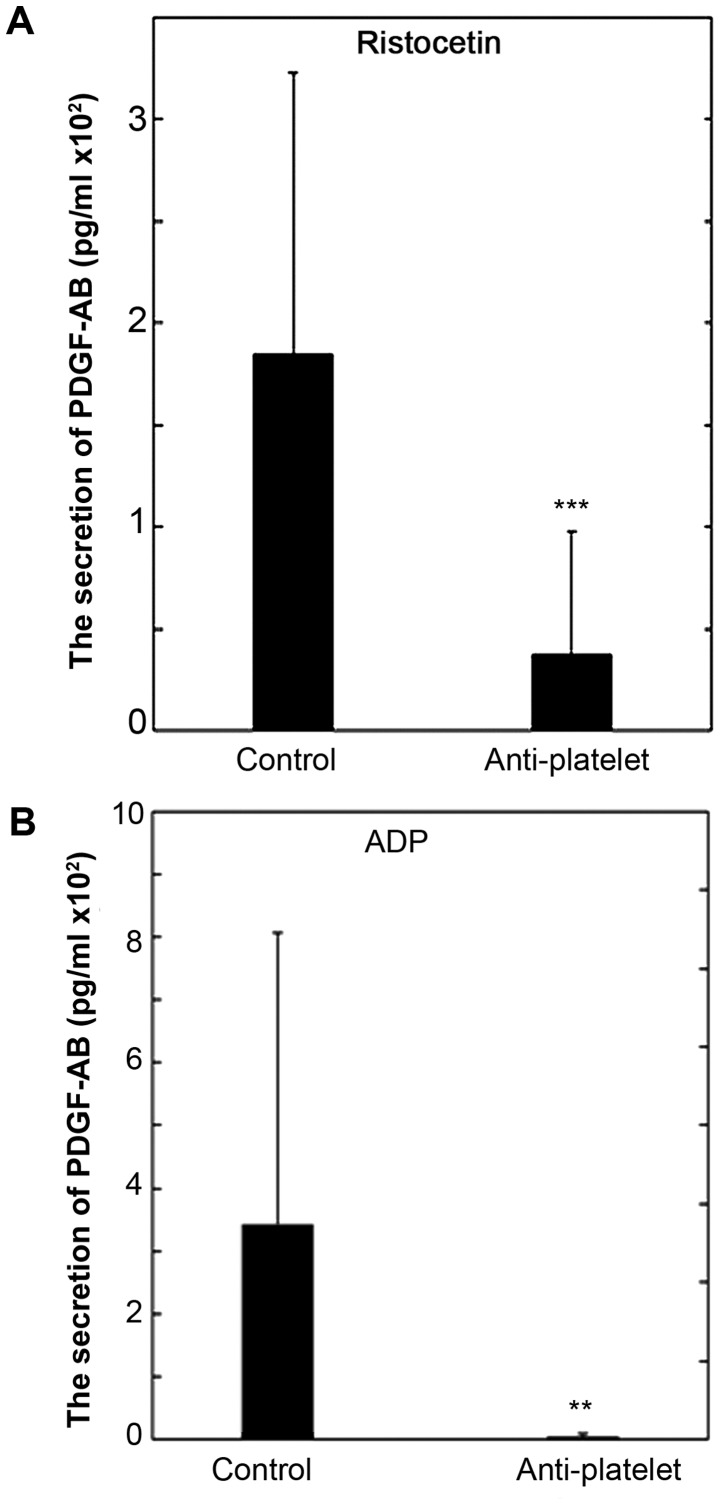
Comparison of the secretion of PDGF-AB between the control group and the anti-platelet group of type 2 DM patients
We recently reported that the release of phosphorylated HSP27 induced by collagen or TRAP is accompanied with the secretion of PDGF-AB from the platelets of type 2 DM subjects (21,22). We also compared the secretion of PDGF-AB induced by ristocetin or ADP between the control and anti-platelet groups of type 2 DM patients. The levels of the PDGF-AB secretion induced by 1.5 mg/ml of ristocetin in the anti-platelet group were significantly lower than those in the control group (Fig. 6A). In addition, the levels of PDGF-AB secretion induced by 1 µM of ADP in the anti-platelet group were significantly lower than those in the control group (Fig. 6B).
Figure 6.
The comparison of the secretion of PDGF-AB between the anti-platelet and control groups of type 2 DM patients. PRP from patients of type 2 DM in the anti-platelet group (n=9) and PRP from patients of the control group (n=25) were stimulated by 1.5 mg/ml of (A) ristocetin, 1 µM of (B) ADP or vehicle for 4 min, and the levels of PDGF-AB in the conditioned mixture were determined using ELISA. The value of the levels of PDGF-AB are presented as mean ± standard deviation. **P<0.01 or***P<0.001, compared to the value of control. PRP, platelet-rich plasma; DM, diabetes mellitus.
Discussion
In the present study, we investigated the effect of ristocetin on the release of HSP27 from platelets of type 2 DM patients, and the effect of anti-platelet agents on the release. At first, we compared the platelet aggregation induced by ristocetin between the control and anti-platelet groups of type 2 DM, and found that there were few differences in the AUC of light transmittance or the ratio of aggregated particle sizes of platelets between the two groups. These findings indicate that anti-platelet agents hardly affect the ristocetin-induced platelet aggregation in the study subjects. Ristocetin has been established as a potent inducer of the interaction between GPIb/IX/V and von Willebrand factor (5), which is a model of shear stress-activated platelets in vitro (3,4). Therefore, the present findings strongly suggest that anti-platelet agents have no effect on the shear stress-induced platelets aggregation in type 2 DM subjects.
We have recently reported that collagen or TRAP induces HSP27 phosphorylation in platelets of type 2 DM patients, and that the phosphorylated HSP27 is released from the platelets, accompanying the secretion of PDGF-AB (21,22). Thus, we next examined whether ristocetin induced the phosphorylation and the release of HSP27, and found that the phosphorylation of HSP27 was truly induced by ristocetin, and that the released levels of phosphorylated HSP27 was significantly correlated with the phosphorylated levels of HSP27 stimulated by ristocetin in the study subjects. Thus, it seems likely that the ristocetin-stimulated release of phosphorylated HSP27 results from the phosphorylation of HSP27. This is probably the first report showing that the ristocetin-induced phosphorylation of HSP27, resulting in the release of phosphorylated HSP27 from human platelets, as far as we know. Of note, the levels of phosphorylated HSP27 release stimulated by ristocetin in the anti-platelet group were markedly lower than those in the control group, whereas little difference between the two groups was observed in the ristocetin-stimulated aggregation. Therefore, it is probable that anti-platelet agents may successfully reduce the phosphorylation and release of HSP27 induced by ristocetin without affecting the aggregation in platelets of type 2 DM patients. In addition, we investigated the secretion of PDGF-AB induced by ristocetin between the control and anti-platelet groups. As well as the release of phosphorylated HSP27, we found that the secretion of PDGF-AB stimulated by ristocetin in the anti-platelet group was significantly lower than that in the control group. Therefore, it is likely that ristocetin-induced PDGF-AB secretion from platelets, in addition to the release of phosphorylated HSP27, could be suppressed by anti-platelet agents in type 2 DM subjects.
It has been reported that GPIb/IX/V activation promotes the generation of TXA2, leading to ADP secretion in rodent platelets (26). We previously demonstrated that ristocetin elicits TXA2 synthesis through cyclooxygenase-1, resulting in the release of soluble CD40 ligand via the activation of MAP kinases including p38 MAP kinase and p44/p42 MAP kinase through the binding of TXA2 to TP in human platelets (6). We have also reported that ADP induces HSP27 phosphorylation through p38 MAP kinase and p44/p42 MAP kinase in human platelets (27). Based on these findings, we compared the effect of ADP on the release of HSP27 between the study groups, and found that the levels of HSP27 release in the anti-platelet group were significantly lower than those in the control group. It seems likely that ADP in fact induces the phosphorylation of HSP27 and its subsequent release from platelets of type 2 DM patients, which is suppressible by anti-platelet agents. Therefore, these results suggest that the ristocetin-induced phosphorylation and the release of HSP27 reducible by anti-platelet agents are dependent on the downstream events of GPIb/XI/V activation such as TXA2-involved release of ADP in the platelets of type 2 DM patients. Additionally, we confirmed that the levels of PDGF-AB secretion induced by ADP in the anti-platelet group were lower than those in the control group Taking our findings into account as a whole, it is most likely that the ristocetin-induced phosphorylation and release of HSP27 accompanying the secretion of PDGF-AB, are mediated through the downstream events of GPIb/IX/V activation including ADP release, which could be suppressed by anti-platelet agents in type 2 DM patients.
In addition to intracellular functions of HSP27 such as molecular chaperone, HSP27 released into extracellular space could affect the functions of adjacent cells (13,23). It has been reported that HSP27 released from macrophages protectively acts against the development of atherosclerosis (28). In vascular endothelial cells, extracellular HSP27 has been shown to increase transcription of VEGF promoting angiogenesis (29). It has also been reported that HSP27 diversely upregulates pro-inflammatory factors including interleukin (IL)-1β in addition to anti-inflammatory factors including IL-10 in macrophages (30). Recently, global myocardial ischemia reportedly causes HSP27 release, which could elicit pro-inflammatory effect through toll-like receptors in vascular endothelial cells of coronary arteries (31). It is well recognized that platelets in blood flow are tethered at the injured sites of vessel through the formation of GPIb/IX/V and von Willebrand factor complex, initiating adhesion, first step of the platelet activation (1,2). Ristocetin is known to mimic the interaction of GPIb/IX/V and von Willbrand factor (5). We have recently reported that collagen and TRAP stimulate the phosphorylation of HSP27 and the release from the platelets of type 2 DM patients, which is associated with PDGF-AB secretion (21,22). It is well established that PDGF-AB is involved in the pathological development of atherosclerosis (1). Taking account of these findings, it is likely that the pathways of platelet activation cooperatively upregulates the phosphorylation of HSP27 leading to the release accompanying PDGF-AB secretion, which may be involved in the pathogenesis of atherosclerosis, and inflammation. Therefore, the regulation of HSP27 phosphorylation in human platelets suggests a novel therapeutic possibility for the diseases caused by atherosclerosis, major concerns in type 2 DM patients. Further investigations are required to elucidate the details underlying the release of phosphorylated-HSP27 from human platelets.
In conclusion, our present findings strongly suggest that anti-platelet agents inhibit the HSP27 release from platelets stimulated by ristocetin but not the aggregation in type 2 DM patients.
Acknowledgements
Not applicable.
Funding
This study was supported by the Research Funding for Longevity Sciences (28–9) from National Center for Geriatrics and Gerontology, Japan.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HT and OK conceived and designed the experiments. HT, GK and TO performed the experiments. HT, TD, KK and OK analyzed the data. HT, YE, HI, TO, SO, TI and OK wrote the paper. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by the committee of the conduct of human research at National Center for Geriatrics and Gerontology, and at Gifu University Graduate School of Medicine. Written informed consent was obtained from all the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Davì G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 2.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 3.Berndt MC, Shen Y, Dopheide SM, Gardiner EE, Andrews RK. The vascular biology of the glycoprotein Ib-IX–V complex. Thromb Haemost. 2001;86:178–188. [PubMed] [Google Scholar]
- 4.Garcia A, Quinton TM, Dorsam RT, Kunapuli SP. Src family kinase-mediated and Erk-mediated thromboxane A2 generation are essential for VWF/GPIb-induced fibrinogen receptor activation in human platelets. Blood. 2005;106:3410–3414. doi: 10.1182/blood-2005-05-1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong JF, Berndt MC, Schade A, McIntire LV, Andrews RK, López JA. Ristocetin-dependent, but not botrocetin-dependent, binding of von Willebrand factor to the platelet glycoprotein Ib-IX–V complex correlates with shear-dependent interactions. Blood. 2001;97:162–168. doi: 10.1182/blood.V97.1.162. [DOI] [PubMed] [Google Scholar]
- 6.Enomoto Y, Adachi S, Matsushima-Nishiwaki R, Doi T, Niwa M, Akamatsu S, Tokuda H, Yoshimura S, Iwama T, Kozawa O. Thromboxane A2 promotes soluble CD40 ligand release from platelets in atherosclerotic patients. Athelosclerosis. 2010;209:415–421. doi: 10.1016/j.atherosclerosis.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 8.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Jr, Sowers JR. Diabetes and cardiovascular disease: A statement for healthcare professionals from the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.CIR.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 9.Matsuno H, Tokuda H, Ishisaki A, Zhou Y, Kitajima Y, Kozawa O. P2Y12 receptors play a significant role in the development of platelet microaggregation in patients with diabetes. J Clin Endocrinol Metab. 2005;90:920–927. doi: 10.1210/jc.2004-0137. [DOI] [PubMed] [Google Scholar]
- 10.Hanai Y, Adachi S, Yasuda I, Takai S, Matsushima-Nishiwaki R, Kato H, Enomoto Y, Akamatsu S, Sakakibara S, Ogura S, et al. Collagen-induced p38 MAP kinase activation is a biomarker of platelet hyper-aggregation in patients with diabetes mellitus. Life Sci. 2009;85:386–394. doi: 10.1016/j.lfs.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 11.Colwell JA, American Diabetes Association Aspirin therapy in diabetes. Diabetes Care. 2003;26(Suppl 1):S87–S88. doi: 10.2337/diacare.26.2007.S87. [DOI] [PubMed] [Google Scholar]
- 12.Hankey GJ, Eikelboom JW. Aspirin resistance. Lancet. 2006;367:606–617. doi: 10.1016/S0140-6736(06)68040-9. [DOI] [PubMed] [Google Scholar]
- 13.Mymrikov EV, Seit-Nebi AS, Gusev NB. Large potentials of small heat shock proteins. Physiol Rev. 2011;91:1123–1159. doi: 10.1152/physrev.00023.2010. [DOI] [PubMed] [Google Scholar]
- 14.Bruey JM, Ducasse C, Bonniaud P, Ravagnan L, Susin SA, Diaz-Latoud C, Gurbuxani S, Arrigo AP, Kroemer G, Solary E, et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat Cell Biol. 2000;2:645–652. doi: 10.1038/35023595. [DOI] [PubMed] [Google Scholar]
- 15.Arrigo AP. The cellular ‘networking’ of mammalian Hsp27 and its functions in the control of protein folding, redox state and apoptosis. Adv Exp Med Biol. 2007;594:14–26. doi: 10.1007/978-0-387-39975-1_2. [DOI] [PubMed] [Google Scholar]
- 16.Pockley AG. Heat shock proteins, inflammation, and cardiovascular disease. Circulation. 2002;105:1012–1017. doi: 10.1161/hc0802.103729. [DOI] [PubMed] [Google Scholar]
- 17.Landry J, Lambert H, Zhou M, Lavoie JN, Hickey E, Weber LA, Anderson CW. Human HSP27 is phosphorylated at serines 78 and 82 by heat shock and mitogen-activated kinases that recognize the same amino acid motif as S6 kinase II. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 18.Acunzo J, Katsogiannou M, Rocchi P. Small heat shock proteins HSP27 (HspB1), αB-crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol. 2012;44:1622–1631. doi: 10.1016/j.biocel.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 19.Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, Barnes MJ, Farndale RW. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- 20.Kageyama Y, Doi T, Akamatsu S, Kuroyanagi G, Kondo A, Mizutani J, Otsuka T, Tokuda H, Kozawa O, Ogura S. Rac regulates collagen-induced HSP27 phosphorylation via p44/p42 MAP kinase in human platelets. Int J Mol Med. 2013;32:813–818. doi: 10.3892/ijmm.2013.1455. [DOI] [PubMed] [Google Scholar]
- 21.Tokuda H, Kuroyanagi G, Tsujimoto M, Enomoto Y, Matsushima-Nishiwaki R, Onuma T, Kojima A, Doi T, Tanabe K, Akamatsu S, et al. Release of phosphorylated HSP27 (HSPB1) from platelets is accompanied with the accerelation of aggregation in diabetic patients. PLoS One. 2015;10:e0128977. doi: 10.1371/journal.pone.0128977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tokuda H, Kuroyanagi G, Tsujimoto M, Matsushima-Nishiwaki R, Akamatsu S, Enomoto Y, Iida H, Otsuka T, Ogura S, Iwama T, et al. Thrombin receptor-activating protein (TRAP)-activated Akt is involved in the release of phosphorylated-HSP27 (HSPB1) from platelets in DM patients. Int J Mol Sci. 2016;17(pii):E737. doi: 10.3390/ijms17050737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones. 2017 Oct 30; doi: 10.1007/s12192-017-0856-z. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato K, Ito H, Hasegawa K, Inaguma Y, Kozawa O, Asano T. Modulation of the stress-induced synthesis of hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells. J Neurochem. 1996;66:946–950. doi: 10.1046/j.1471-4159.1996.66030946.x. [DOI] [PubMed] [Google Scholar]
- 25.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Pestina TI, Berndt MC, Steward SA, Jackson CW, Gartner TK. The roles of ADP and TXA in botrocetin/VWF-induced aggregation of washed platelets. J Thromb Haemost. 2004;2:2213–2222. doi: 10.1111/j.1538-7836.2004.01023.x. [DOI] [PubMed] [Google Scholar]
- 27.Kato H, Takai S, Matsushima-Nishiwaki R, Adachi S, Minamitani C, Otsuka T, Tokuda H, Akamatsu S, Doi T, Ogura S, et al. HSP27 phosphorylation is correlated with ADP-induced platelet granule secretion. Arch Biochem Biophys. 2008;475:80–86. doi: 10.1016/j.abb.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Rayner K, Chen YX, McNulty M, Simard T, Zhao X, Wells DJ, de Belleroche J, O'Brien ER. Extracellular release of the atheroprotective heat shock protein 27 is mediated by estrogen and competitively inhibits acLDL binding to scavenger receptor-A. Circ Res. 2008;103:133–141. doi: 10.1161/CIRCRESAHA.108.172155. [DOI] [PubMed] [Google Scholar]
- 29.Thuringer D, Jego G, Wettstein G, Terrier O, Cronier L, Yousfi N, Hébrard S, Bouchot A, Hazoumé A, Joly AL, et al. Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 2013;27:4169–4183. doi: 10.1096/fj.12-226977. [DOI] [PubMed] [Google Scholar]
- 30.Salari S, Seibert T, Chen YX, Hu T, Shi C, Zhao X, Cuerrier CM, Raizman JE, O'Brien ER. Extracellular HSP27 acts as a signaling molecule to activate NF-κB in macrophages. Cell Stress Chaperones. 2013;18:53–63. doi: 10.1007/s12192-012-0356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin C, Cleveland JC, Ao L, Li J, Zeng Q, Fullerton DA, Meng X. Human myocardium releases heat shock protein 27 (HSP27) after global ischemia: The proinflammatory effect of extracellular HSP27 through toll-like receptor (TLR)-2 and TLR4. Mol Med. 2014;20:280–289. doi: 10.2119/molmed.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.