Abstract
Objective
The impact of forage feeding strategy on growth performance, ruminal fermentation and nutrient digestibility in post-weaning calves was investigated.
Methods
Forty-five female Holstein calves (body weight [BW] = 79.79±0.38 kg) were enrolled in the 35-d study at one week after weaning and randomly assigned to one of three dietary treatments. All diets were fed as total mixed ration containing 60% (dry matter [DM] basis) of basal starter feed and 40% (DM basis) of forage, but varied in composition of forage source including i) alfalfa (40% DM, AH); ii) alfalfa hay (26.7% DM)+oat hay (13.3% DM; OH); iii) alfalfa hay (26.7% DM)+corn silage (13.3% DM; WS).
Results
Dry matter intake was not different among treatment groups (p>0.05). However, BW (p<0.05) and average daily gain (p<0.05) of calves fed AH and OH were greater than WS-fed calves, whereas heart girth was greater in OH-fed calves than those fed AH and WS (p<0.05). Ruminal fermentation parameters including proportion of butyric acid, acetated-to-propionate ratio, concentration of total volatile fatty acid, protozoal protein, bacterial protein, and microbial protein in rumen were the highest in OH (p<0.05) and the lowest in WS. Compared with the AH and WS, feeding oat hay to postweaning calves increased crude protein digestibility (p<0.05), and decreased duration of diarrhea (p<0.05) and fecal index (p<0.05).
Conclusion
Our results suggested that partially replacing alfalfa hay with oat hay improved ruminal fermentation, nitrogen utilization, and reduced incidence of diarrhea in post-weaning dairy calves.
Keywords: Diarrhea, Forage, Ruminal Development, Serum Metabolism, Weaned Calf
INTRODUCTION
Consumption of solid feed is critical to drive the maturation of functioning rumen in weaning dairy calves. Feeding forage to young dairy calves has been shown to stimulate rumination, promote musculature, and expansion of rumen volume [1,2], and reduce the keratinization of rumen papillae [3]. In addition, high-quality forage supplementation enhanced epithelial development in both rumen and lower gut (e.g. jejunum) [4], increased intestinal villus height [5] and intestinal mucosa area in lambs [6]. Alfalfa hay has been conventionally used in supplementation of grain starters to feed early post-weaning dairy calves in China. However, owing to high protein content of alfalfa hay, this feeding practice is associated with reduced nitrogen utilization, increased nitrogen output to environment, and even trophic diarrhea [7]. There is a lack of literature that investigated the impact of forage feeding strategies on rumen development and growth performance of post-weaning calves. Oat hay has greater contents of neutral detergent fiber (NDF) and pectin than alfalfa hay, whereas corn silage contains higher level of starch which may improve microbial protein yield by providing more readily fermentable carbohydrate. In current study, we evaluated the effect of combined use of different forage sources on nutrient utilization and rumen fermentation of postweaning dairy calves. We hypothesize that partially replacing alfalfa hay with oat hay or corn silage may improve dietary protein efficiency and rumen fermentation and reduce the incidence of diarrhea.
MATERIALS AND METHODS
The study was conducted in National Dairy Industry and Technology System Sanyuan Farm (Beijing, China). The experimental protocol was performed in accordance with the practices outlined in the Guide for the Care and Use of Agriculture Animals in Agriculture Research and Teaching [8].
Animals, treatments and management
Forty-five female Holstein calves (body weight [BW] = 79.79± 0.38 kg) that were weaned at 60 d of age were enrolled in the study at one week after weaning and randomly assigned to one of three dietary treatments with five replicates of three calves for each kind of diet. All diets were fed as total mixed ration that contained 60.0% (dry matter [DM] basis) of texturized starter feed (36.3% pellet starter feed+23.7% flaked corn) and 40% of forage, but differed in forage composition including i) 40.0% alfalfa hay (AH); ii) 26.7% alfalfa hay+13.3% oat hay (OH); iii) 26.7% alfalfa hay+ 13.3% corn silage (WS) (Table 1). Alfalfa hay and oat hay were chopped to 2.5 cm, and the DM content of corn silage was 28%. Total mixed ration was delivered once daily at 0800 for ad libitum intake. Calves were kept in pens bedded with straws and had free access to water. Bedding was replenished and replaced every 1 or 2 d as required. The study lasted 35 days.
Table 1.
Chemical composition of the total mixed ration
| Composition | Treatment1) | ||
|---|---|---|---|
|
| |||
| AH | OH | WS | |
| Ingredient (% DM) | |||
| Alfalfa hay | 40.0 | 26.7 | 26.7 |
| Oat hay | - | 13.3 | - |
| Maize silage | - | - | 13.3 |
| Flaked corn | 23.7 | 23.7 | 23.7 |
| Pellet | 36.3 | 36.3 | 36.3 |
| Pellet (% of DM) | |||
| Corn | 3.2 | 3.2 | 3.2 |
| Wheat middling and reddog | 2.4 | 2.4 | 2.4 |
| Soybean meal | 14.9 | 14.9 | 14.9 |
| Wheat bran | 2.1 | 2.1 | 2.1 |
| DDGS | 5.5 | 5.5 | 5.5 |
| Extruded soybean | 2.1 | 2.1 | 2.1 |
| Extruded corn | 1.8 | 1.8 | 1.8 |
| Cottonseed meal | 1.8 | 1.8 | 1.8 |
| Atox2) | 0.1 | 0.1 | 0.1 |
| 4% premix3) | 2.4 | 2.4 | 2.4 |
| Chemical composition | |||
| DM | 91.6 | 91.8 | 82.8 |
| NDF (% DM) | 34.9 | 35.7 | 34.2 |
| ADF (% DM) | 20.6 | 19.7 | 18.6 |
| CP (% DM) | 17.3 | 15.8 | 16.1 |
| EE (% DM) | 1.61 | 1.63 | 1.86 |
| Ca (% DM) | 0.93 | 0.82 | 0.81 |
| P (% DM) | 0.33 | 0.32 | 0.32 |
DM, dry matter; DDGS, dried distillers grains with solubles; NDF, neutral detergent fiber; ADF, acid detergent fiber; CP, crude protein; EE, ether extract.
AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage.
ITDSA FAR EAST LTD. (Barcelona, Spain).
Premix composition: vitamin A 37,000 IU/kg; vitamin D3, 10,000 IU/kg; vitamin E, 400 IU/kg; Cu, 50 mg/kg; Zn, 300 mg/kg; Mn, 300 mg/kg; Co, 0.5 mg/kg; Se, 1.76 mg/kg; I, 1.7 mg/kg.
Sample collection, measurements and analysis
Growth performance and body measurement
Feed intake of each experimental group was recorded daily. The BW and body configuration including wither height (WH), heart girth (HG), body length (BL) [9], and cannon bone circumference (CC) [10] were measured before and after the experimental period, increment of body frame during the experimental days were calculated.
Digestibility
Nutrient digestibility was performed in five calves of each group during the last five days of the study. Feed offered and refusals from each calf were sampled daily. Total feces was collected, weighed daily and composited for each calf. Daily composite fresh feces were preserved in 1:10 diluted hydrochloric acid (100 mL concentrate hydrochloric acid per liter solution) for analysis of fecal nitrogen. Composite feed, refusal and fecal samples were stored at −20°C until analyses. These samples were dried in forced air oven at 65°C for 48 h, air equilibrated and weighed. Dried samples were then finely ground by mortar and pestle to pass through a 1 mm screen and then retained in sealed containers for determination of DM, NDF, and acid detergent fiber (ADF).
The determination of DM, CP, NDF, and ADF were made according to AOAC methods [11]. Feed, orts and feces were dried at 105°C for 48 h in a forced air oven for determination of DM. Samples were analyzed for nitrogen according to Kjeldahl, and thereafter, CP was determined by total nitrogen (N) ×6.25. Contents of NDF and ADF were residual portion after rinsing according to Van Soest (A2000i, ANKOM, New York, USA) [12].
Diarrhea incidence
Incidences of diarrhea was recorded daily during the experimental period. Fecal score was determined using a 4-point scale system as follows: 1 = normal (firm, well-formed), 2 = mild (soft, pudding-like), 3 = moderate (runny, pancake batter), 4 = severe (liquid, splatters), as described by Diaz et al [13] and Zhang [14]. A fecal of 2 and above was considered as diarrhea. Gentamycin sulfate injection were administered to calves only when feces scored 4. Diarrhea incidence, diarrhea frequency and fecal index were calculated as follows [14]:
Ruminal fermentation parameters
Ruminal samples of five calves randomly selected in each group were collected on d 7, d 14, d 21, d 28, and d 35 of experimental period before feeding. All samples were collected from different locations in the rumen using a rubber tube. Samples were filtered through eight layers of cheesecloth, pH was measured immediately by a digital pH meter (PHB-4, Shanghai Hongyi instrument Limited, Shanghai, China). Ten milliliters of filtrate were placed in a freezing tube and stored at −80°C for analysis of protozoal protein (PCP), bacterial protein (BCP), and microbial protein (MCP). Aliquots of filtrate were centrifuged at 3,000×g for 15 min, and the supernatant was stored at −20°C until analyses for volatile fatty acid (VFA) and ammonia-N (NH3-N) concentrations.
The PCP, BCP, and MCP were analyzed using a trichloroaceticacid protein precipitation kit with the method described by Wang [15]. One milliliter 25% (wt/vol) metaphosphoric acid was added into five milliliters filtrate to determine VFA concentrations using gas chromatography (Model 7980, Agilent Technology Inc., Santa Clata, CA, USA) [16]. Ammonia-N was determined using a phenol-hypochlorite assay [17].
Blood sample collection and analysis
Blood Samples of ten calves in each group were taken by jugular puncture on d 7, d 14, d 21, and d 35 prior to morning feeding. Samples were centrifuged at 3,500×g for 15 min to harvest serum that was stored at −20°C until analysis. Serum concentration of glucose (GLU), triglycerides (TG), blood urea nitrogen (BUN), β-hydroxybutyrate acid (BHBA), and non-esterified fatty acid (NEFA), were determined using corresponding assay kits from Shandong BioBase Biotechnologies Inc. (Shandong, China) in an automated biochemistry analyzer (Model GF-D200, Rainbow, Shandong, China). Aliquots of plasma Serum were used to measure growth hormone (GH) by radioimmunoassay kits from Beijing Boruijie Technology Development Co. Ltd. (Beijing, China) in an absorbance microplate reader (EXL 800, Bio-tek, Winooski, VT, USA). Serum insulin-like growth factor I (IGF-I) was analyzed using a commercial ELISA kit as described by Hammon et al [18].
Calculations and statistical analysis
The apparent digestibility of DM, CP, NDF, and ADF was calculated using the following equation:
Where, E1 = total amount (mg) of the nutrients (DM, CP, NDF, or ADF) ingested by each calf, E2 = total amount (mg) of the corresponding nutrient (DM, CP, NDF, or ADF) in feces collected by each calf.
Data were analyzed using the PROC MIXED procedure of SAS 9.0. The model for BW, body measurement, nutrient digestibility, and diarrhea status was:
Where, μ = overall mean; Ci = the effect of calf (i = 1 to 15); TRj = the effect of treatment (j = 1 to 3); eij = random error.
For variables measured repeatedly (e.g. ruminal fermentation parameters and serum metabolite and hormones, we used the model as following:
Where, μ = overall mean; Ci = the effect of calf (i = 1 to 15); TRj = the main effect of treatment (j = 1 to 3); Tk = the main effect of time (k = 1 to 5 wk for ruminal fermentation parameters, k = 1 to 4 wk for serum metabolites); TRTjk = the interaction of TR and T; eijk = random error.
Mean separation was performed for variable with significant treatment effect using Duncan’s multiple-range test. Significance of difference was declared at p<0.05.
RESULTS
Growth performance
Dry matter intake (DMI) was not different among treatments (p>0.05; Figure 1). Dietary treatment did not affect wither height (p>0.05). Calves in AH and OH had greater average daily gain (ADG) (p<0.05) and cannon bone circumference (p<0.05) than those in WS, whereas calves in OH had greater heart girth (p< 0.05) than that in AH and WS. Body length was the greatest in calves of AH (p<0.05) among three groups.
Figure 1.
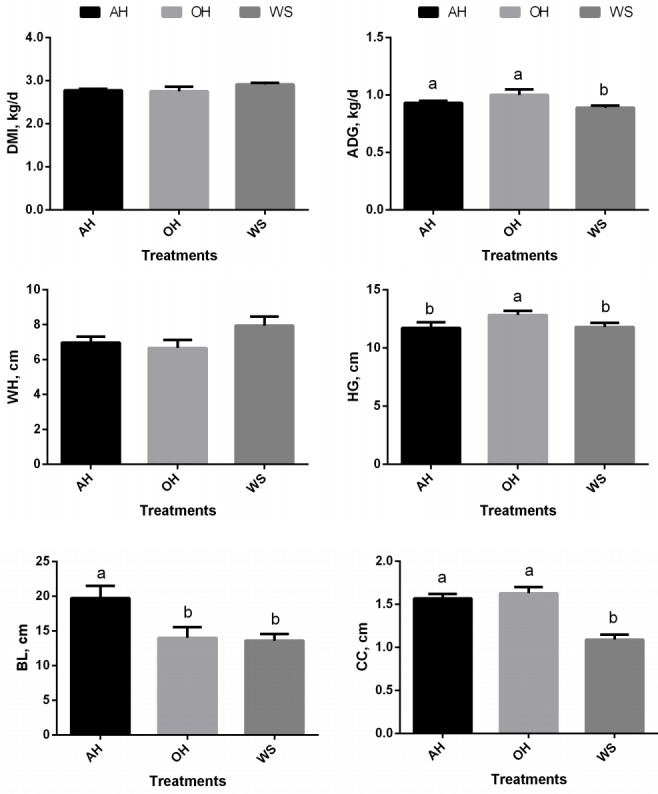
Dry matter intake, average daily gain and increment of body frame of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay. AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage. DMI, dry matter intake; ADG, average daily gain; WH, height at withers; HG, heart girth; BL, body length; CC, cannon bone circumference. a,b Means within a single figure not sharing a common superscript letter are significantly different (p<0.05). ADG and CC increased (p<0.05) in AH and OH. Also, HG in OH was significantly (p<0.05) higher than AH and WS, however, BL in AH was highest (p<0.05).
Total tract apparent digestibility and incidence of diarrhea
Total tract apparent digestibility of DM was not affected (p> 0.05) by forage feeding plane (Figure 2). Compared with AH and OH, NDF and ADF digestibility was lower in WS (p<0.05). Plane of forage feeding significantly affected CP digestibility (p<0.05), which was the highest in OH followed by AH and the lowest in WS.
Figure 2.
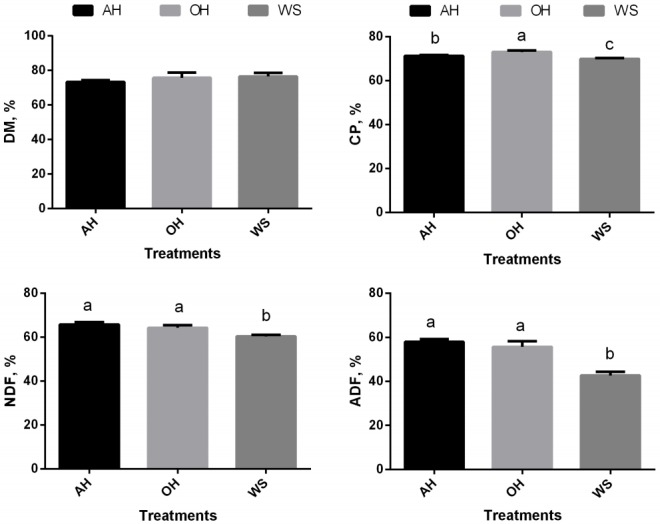
Nutrient digestibility of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay. AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn126.7% alfalfa hay+13.3% maize silage. DM, dry matter; CP, crude protein; NDF, neutral detergent fiber; ADF, acid detergent fiber. a,b Means within a single figure not sharing a common superscript letter are significantly different (p<0.05). NDF and ADF digestibility in WS decreased (p<0.05). CP digestibility were significantly lower (p<0.05) in AH compared to OH, but higher (p<0.05) than in WS.
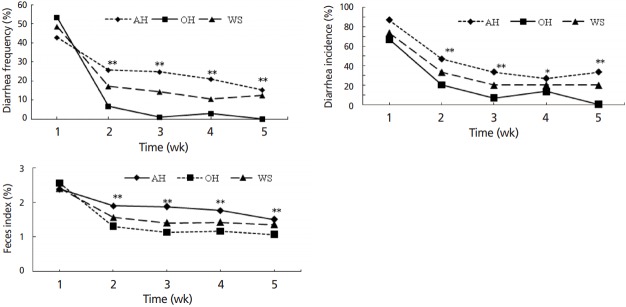
Severe diarrhea was observed during the first week (Figure 3). Feeding OH and WS significantly reduced (p<0.05) incidence and frequency of diarrhea, and fecal index from wk 2 to wk 5.
Figure 3.
Diarrhea status of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay varied with week. AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage. For each time point, * indicates significance at the 0.05 level and ** indicates significance at the 0.01 level among three treatment groups. Calves expressed severely diarrhea in the first week, forage combination feeding of OH and WS reduced (p<0.05) diarrhea incidence, frequency, and fecal index from the second week.
Ruminal fermentation
Ruminal pH and percentage of acetic acid were not affected by dietary treatment (p>0.05; Table 2). Concentration of ammonia-nitrogen in ruminal fluid was the greatest in AH than the other two treatment groups (p<0.05). Percentage of ruminal butyric acid, total volatile fatty acid (TVFA) concentration, acetate to propionate ratio, and BCP concentration were the highest in OH, intermediate in AH and the lowest in WS (p<0.05; Figure 4). There was significant interaction of treatment by week on TVFA, which was increased in OH after wk1 of the study (Figure 4). Partially replacing alfalfa hay with corn silage decreased (p<0.05) ruminal PCP and MCP.
Table 2.
Ruminal fermentation of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay
| Items | Treatment1) | SEM | p-values | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AH | OH | WS | Treatment | Time | Treatment×time | ||
| pH | 6.89 | 6.91 | 6.95 | 0.05 | 0.74 | 0.72 | 0.40 |
| NH3-N (mmol/L) | 5.58a | 5.16b | 5.20b | 0.10 | 0.03 | 0.90 | 0.10 |
| Acetic acid (%) | 64.95 | 64.99 | 65.69 | 0.71 | 0.71 | 0.03 | 0.08 |
| Propionic acid (%) | 20.45b | 19.55c | 21.76a | 0.25 | <0.01 | 0.16 | 0.11 |
| Butyric acid (%) | 6.92b | 7.38a | 4.97c | 0.39 | <0.01 | 0.14 | 0.08 |
| TVFA (mmol/L) | 60.97b | 64.95a | 48.39c | 2.26 | <0.01 | <0.01 | <0.01 |
| Acetate-to-propionate ratio | 3.18b | 3.33a | 3.02c | 0.06 | 0.01 | 0.26 | 0.92 |
| PCP (mg/mL) | 2.35a | 2.54a | 2.03b | 0.08 | <0.01 | 0.85 | 0.38 |
| BCP (mg/mL) | 2.00ab | 2.08a | 1.92b | 0.03 | 0.02 | 0.99 | 0.27 |
| MCP (mg/mL) | 4.35a | 4.62a | 3.96b | 0.09 | <0.01 | 0.95 | 0.89 |
SEM, standard error of the mean; TVFA, total volatile fatty acid; PCP, protozoal protein; BCP, bacterial protein; MCP, microbial protein.
AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage.
Means within a row not sharing a common superscript letter are significantly different (p<0.05).
Figure 4.
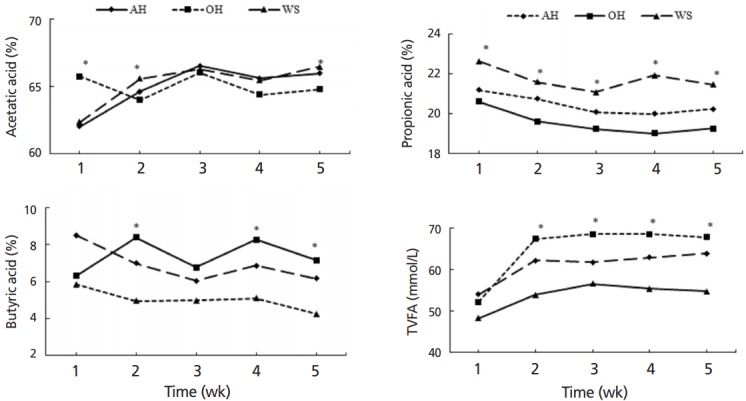
Ruminal volatile fatty acids of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay varied with week. AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage. For each time point, * indicates significance at the 0.05 level among three treatment groups. Proportion of acetic acid and total volatile fatty acid (TVFA) changed following forage supplementation during the experimental procedure (p<0.05).
Serum metabolites and hormones
Plane of forage feeding did not affect serum concentration of GLU, TG, BHBA, NEFA, and IGF-I (Table 3). Serum concentration of BUN was the highest in AH, followed by OH and the lowest in WS (p<0.05). Concentration of GH was higher in OH (p<0.05) than that in AH and WS. There was a significant time effect on serum concentration of GLU, BUN, NEFA, and IGF-I during the study (p<0.01; Figure 5).
Table 3.
Serum metabolism of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay
| Items | Treatment1) | SEM | p-values | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| AH | OH | WS | Treatment | Time | Treatment×time | ||
| GLU (mmol/L) | 5.63 | 5.54 | 5.67 | 0.07 | 0.41 | <0.01 | 0.84 |
| TG (mmol/L) | 0.30 | 0.28 | 0.28 | 0.02 | 0.81 | 0.31 | 0.80 |
| BUN (mmol/L) | 2.40a | 1.94b | 1.65c | 0.14 | 0.01 | <0.01 | 0.93 |
| BHBA (mmol/L) | 0.85 | 0.82 | 0.84 | 0.06 | 0.94 | 0.11 | 0.98 |
| NEFA (μmol/mL) | 99.89 | 93.93 | 92.20 | 6.42 | 0.69 | <0.01 | 0.44 |
| GH (ng/mL) | 1.65b | 1.72a | 1.63b | 0.01 | <0.01 | 0.95 | 0.17 |
| IGF-I (ng/mL) | 246.39 | 250.49 | 243.19 | 5.63 | 0.67 | <0.01 | 0.33 |
SEM, standard error of the mean; GLU, glucose; TG, triglycerides; BUN, blood urea nitrogen; BHBA, β-hydroxybutyrate acid; NEFA, non-esterified fatty acid; GH, growth hormone; IGF-I, insulin-like growth factor I.
AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage.
Means within a row not sharing a common superscript letter are significantly different (p<0.05).
Figure 5.
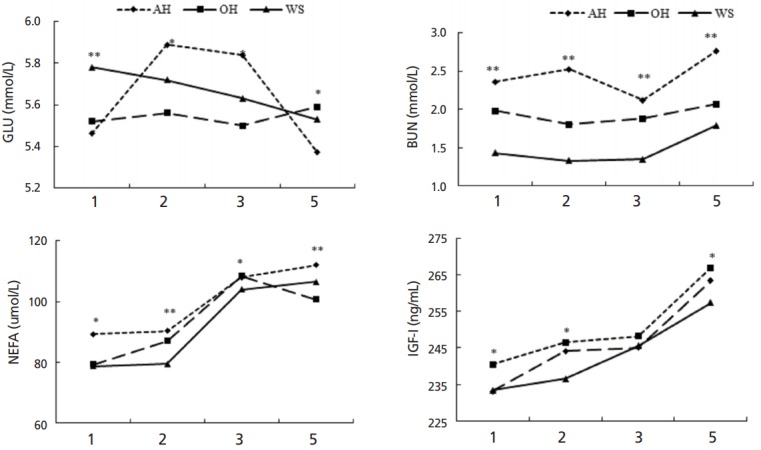
Serum metabolism of weaned calves feeding oat hay or maize silage substituting for portion of alfalfa hay varied with week. AH, 36.3% pellet starter feed+23.7% flaked corn+40.0% alfalfa hay; OH, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% oat hay; WS, 36.3% pellet starter feed+23.7% flaked corn+26.7% alfalfa hay+13.3% maize silage. For each time point, * indicates significance at the 0.05 level and ** indicates significance at the 0.01 level among three treatment groups. During the experimental procedure, concentrations of glucose (GLU), blood urea nitrogen (BUN), non-esterified fatty acid (NEFA), and insulin-like growth factor I (IGF-I) varied significantly following forage supplementation (p<0.05).
DISCUSSION
Growth performance
Greater fiber content may enhance ruminal peristalsis, increase ruminal passage rate and reversely increase feed intake [19]. Castells et al [20] has reported that feeding chopped grass hay together with pelleted starter increased DMI of young dairy calves. Furthermore, supply a certain amount of forage in calf starter could ameliorate the ruminal environment effectively, and improve daily DMI and feed conversion ratio [21]. To our knowledge, there is a paucity of research with regard to forage feeding strategy in early post-weaning calves. However, in present study, partially replacing alfalfa hay with oat hay or corn silage did not affect DMI of weaned calves, which could be explained by the consistent NDF content of all three diets.
The optimal ADG of weaned calves should be maintained at 0.82 to 0.93 kg/d [22], which has been achieved by all three treatments in current study. Stobo et al [23] suggested that forage feeding may improve BW gain through stimulation of gastrointestinal tract development of calves. Live BW was highly related to skeletal configuration, such as heart girth [24]. Cows with larger structural dimensions usually also had larger rumen capacities [25], in our study, larger HG was observed in OH group that had the greatest ADG, suggesting a better rumen development.
Nutrient digestibility and diarrhea
In our study, both DMI and digestibility were not affected by plane of forage feeding. Dietary NDF and ADF could provide substrate and energy for rumen fermentation [26]. Oat hay supplementation balanced the nitrogen content in diet, which could significantly increase nitrogen deposition of calves [27]. However, high organic acid, especially lactic acid, content in corn silage might limit the activity and reproduction of rumen microbes [28], consequently decreased nutrient digestibility. Digestibility of NDF and ADF mirrored ADG of calves. Calves in WS groups apparently displayed a lower HG, BL, and CC values.
Calves experienced severe diarrhea during the first week of the study, which probably because of weaning stress and dietary change (Figure 1). Owing to high protein content of alfalfa hay, the higher protein content of AH diet exceeds the protein requirement of weaned calves [29] and potentially contributed to tropic diarrhea as observed for high incidence of diarrhea in AH group. Replacing alfalfa hay with oat hay diluted dietary protein content and led to increased protein digestion, BW gain, and less environmental nitrogen output.
Ruminal fermentation
The ruminal pH of all treatments groups fell into normal range of weaned calves [30]. VFA is the primary energy substrates for ruminant [31], of which butyric acid is the main energy source for the ruminal epithelial cells growth [32]. Previous research [33,34] suggested that feeding alfalfa and oat hay increased acetic acid and butyric acid production in rumen, and enhanced the development of ruminal papilla. Our study is in agreement with previous studies that proportion of butyric acid was higher in OH and AH group. The higher proportion of propionic acid in WS may result from higher starch content of corn silage [31,35].
Microbial protein contributes 60% to 70% of protein requirement of ruminant [36]. Many dietary factors may affect microbial protein yield, such as synchronized degradation of carbohydrate and protein, diet ruminal retention time, and ruminal pH. Owing to higher NDF content, using oat hay to partially replace alfalfa hay may increase ruminal retention time of the diet, which increased dietary CP digestibility, enhanced recycle of ruminal NH3-N and MCP production and improved dietary N utilization. Our result is in consistent with previous study [37]. It is not unexpected that MCP was also higher in AH than that in WS because of high protein content of alfalfa hay. The higher ruminal NH3-N may due to greater rapidly degraded protein, particularly the higher content of A and B protein fractions in AH than that in OH and WS [29].
Serum metabolites
Although feeding corn silage increased ruminal propionate concentration, the primary precursor of hepatic gluconeogenesis, plane of forage feeding did not affect blood GLU concentration. Most butyric acid is absorbed and metabolized in ruminal epithelial cells with the production of BHBA. Serum BHBA and TG concentration could be altered by ruminal VFA profile [38]. However, in current study, feeding oat hay increased ruminal butyric acid proportion without affecting serum BHBA concentration.
Previous study reported that ADG of heifers positively correlated with GH [39], which supported our results that feeding oat hay increased both serum GH and ADG in post-weaning calves.
The BUN as well as milk urea nitrogen typically mirrors the change of ruminal NH3-N and serves as an indicator of MP balance and dietary protein efficiency in lactating cows [40,41]. The higher BUN level in AH-fed calves agreed with greater ruminal NH3-N content, indicating the lower dietary N efficiency. However, partially replacing alfalfa hay with oat hay improved dietary N utilization and maintained growth performance in comparison with feeding alfalfa alone.
CONCLUSION
In comparison with conventional feeding practice (starter diet with alfalfa hay alone), our data suggest feeding mixed forage of alfalfa and oat hay with starter diet represent a better feeding strategy in postweaning dairy calves by improving dietary N efficiency and growth performance.
ACKNOWLEDGMENTS
The authors acknowledge the financial support of Beijing Nova Program (Z121105002512067), Beijing Young Scientist Program (YETP0305), China Agriculture Research System (CARS-37), and the Special Fund for Agro-scientific Research in the Public Interest (201303061).
Footnotes
CONFLICT OF INTEREST
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
REFERENCES
- 1.Hamada T, Maeda S, Kameoka K. Factors influencing growth of rumen, liver, and other organs in kids weaned from milk replacers to solid foods. J Dairy Sci. 1976;59:1110–8. doi: 10.3168/jds.S0022-0302(76)84330-5. [DOI] [PubMed] [Google Scholar]
- 2.Beiranvand H, Ghorbani GR, Khorvash M, et al. Interactions of alfalfa hay and sodium propionate on dairy calf performance and rumen development. J Dairy Sci. 2014;97:2270–80. doi: 10.3168/jds.2012-6332. [DOI] [PubMed] [Google Scholar]
- 3.Beharka AA, Nagaraja TG, Morrill JL, Kennedy GA, Klemm RD. Effects of form of the diet on anatomical, microbial, and fermentative development of the rumen of neonatal calves. J Dairy Sci. 1998;81:1946–55. doi: 10.3168/jds.S0022-0302(98)75768-6. [DOI] [PubMed] [Google Scholar]
- 4.Rompala RE, Hoagland TA, Meister JA. Modifications in growth and morphology of ovine jejunal and ruminal epithelia as affected by inert dietary substances. J Anim Sci. 1990;68:2530–5. doi: 10.2527/1990.6882530x. [DOI] [PubMed] [Google Scholar]
- 5.Lipkin M. Proliferation and differentiation of gastrointestinal cells. Physiol Rev. 1973;53:891–915. doi: 10.1152/physrev.1973.53.4.891. [DOI] [PubMed] [Google Scholar]
- 6.Sun W, Goetsch AL, Forster LA, Galloway DL, Lewis PK. Forage and splanchnic tissue mass in growing lambs: effects of dietary forage levels and source on splanchnic tissue mass in growing lambs. Br J Nutr. 1994;71:141–51. doi: 10.1079/bjn19940122. [DOI] [PubMed] [Google Scholar]
- 7.Pounden WD, Hibbs JW, Cole CR. Observations on the relation of diet to diarrhea in young dairy calves. J Am Vet Med Assoc. 1951;18(891):400–3. [PubMed] [Google Scholar]
- 8.FASS . Guide for the care and use of agricultural animals in research and teaching. 3rd ed. The Federation of Animal Science Societies; 2010. [Google Scholar]
- 9.Ugur F. Relationships between body measurement of dairy calves at six month of ages and age at first calving and milk production. J Cent Eur Agric. 2005;6:191–4. [Google Scholar]
- 10.Tatum JD, Williams FL, Bowling RA. Effects of feeder-cattle frame size and muscle thickness on subsequent growth and carcass development. I. An objective analysis of frame size and muscle thickness. J Anim Sci. 1986;62:109–20. [Google Scholar]
- 11.AOAC . Association of Official Analytical Chemists. 15th ed. Arlington, VA, USA: AOAC International; 1990. Official methods of analysis. [Google Scholar]
- 12.Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci. 1991;74:3583–97. doi: 10.3168/jds.S0022-0302(91)78551-2. [DOI] [PubMed] [Google Scholar]
- 13.Diaz MC, Van Amburgh ME, Smith JM, Kelsey JM, Hutten EL. Composition of growth of Holstein calves fed milk replacer from birth to 105-kilogram body weight. J Dairy Sci. 2001;84:830–42. doi: 10.3168/jds.S0022-0302(01)74541-9. [DOI] [PubMed] [Google Scholar]
- 14.Zhang NF. PhD Thesis. Beijing, China: Chinese Academy of Agricultural Sciences; 2008. Effects of protein and amino acid nutrition on indexes related to immune response of early weaned dairy calves. [Google Scholar]
- 15.Wang F. Primary research of ruminal bacterial protein and microbial protein determination. Chin J Anim Sci. 1990;2:43–4. [Google Scholar]
- 16.Hamada T, Omori S, Kameoka K, Horii S, Morimoto H. Direct determination of rumen volatile fatty acids by gas chromatography. J Dairy Sci. 1968;51:228–9. doi: 10.3168/jds.S0022-0302(68)86962-0. [DOI] [PubMed] [Google Scholar]
- 17.Broderick GA, Kang JH. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J Dairy Sci. 1980;63:64–75. doi: 10.3168/jds.S0022-0302(80)82888-8. [DOI] [PubMed] [Google Scholar]
- 18.Hammon HM, Zanker IA, Blum JW. Delayed colostrum feeding affects IGF-I and insulin plasma concentrations in neonatal calves. J Dairy Sci. 2000;83:85–92. doi: 10.3168/jds.S0022-0302(00)74859-4. [DOI] [PubMed] [Google Scholar]
- 19.Goetsch AL, Owens PN. Effects of sampling site on passage rate estimates in heifers fed alfalfa hay or a high concentrate diet. J Dairy Sci. 1985;68:914–22. doi: 10.3168/jds.s0022-0302(85)80909-7. [DOI] [PubMed] [Google Scholar]
- 20.Castells L, Bach A, Araujo G, Montoro C, Terré M. Effect of different forage sources on performance and feeding behavior of Holstein calves. J Dairy Sci. 2012;95:286–93. doi: 10.3168/jds.2011-4405. [DOI] [PubMed] [Google Scholar]
- 21.Coverdale JA, Tyler HD, Quigley JD, Brumm JA. Effect of various levesl of forage and fomr of diet on rumen development and growth in calves. J Dairy Sci. 2004;87:2554–62. doi: 10.3168/jds.S0022-0302(04)73380-9. [DOI] [PubMed] [Google Scholar]
- 22.Kertz AF, Barton BA, Reutzel LF. Relative efficiencies of wither height and body weight increase from birth until first calving in Holstein cattle. J Dairy Sci. 1998;81:1479–82. doi: 10.3168/jds.S0022-0302(98)75712-1. [DOI] [PubMed] [Google Scholar]
- 23.Stobo IJ, Roy JHB, Gaston HJ. Rumen development in the calf. Br J Nutr. 1966;20:171–88. doi: 10.1079/bjn19660021. [DOI] [PubMed] [Google Scholar]
- 24.Musa AM, Idam NZ, Elamin KM. Heart girth reflect live body weight in Sudaneseshogur sheep under field conditions. World’s Vet J. 2012;2:54–6. [Google Scholar]
- 25.Nutt BG, Holloway JW, Butts WT., Jr Relationship of rumen capacity of mature angus cows to body measurements, animal performance and forage consumption on pasture. J Anim Sci. 1980;51:1168–76. [Google Scholar]
- 26.Gouet P, Fonty G, Jouany JP, Nebout JM. Cellulolytic bacteria establishment and rumen digestion in lambs isolated after birth. Can J Anim Sci. 1984;64:163–4. [Google Scholar]
- 27.Raven AM, Robinson KL. Studies of the nutrition of the young calf; a comparison of starch, lactose, and hydrogenated palm oil, with butterfat, in milk diets. Br J Nutr. 1958;12:469–82. doi: 10.1079/bjn19580061. [DOI] [PubMed] [Google Scholar]
- 28.Candlish E, Mckirdy J, Candlish E, Mckirdy J. Organic acid determination on treated and untreated corn silage. Can J Plant Sci. 1973;53:105–11. [Google Scholar]
- 29.NRC . Nutrient requirements of dairy cattle. Washington, DC, USA: National Academy Press; 2001. [Google Scholar]
- 30.Feng YL. Ruminant nutrition. Beijing, China: Science Press; 2004. p. 136. [Google Scholar]
- 31.Russell JB, O'Connor JD, Fox DG, Van Soest PJ, Sniffen CJ. A net carbohydrate and protein system for evaluating cattle diets: I. Ruminal fermentation. J Anim Sci. 1992;70:3551–61. doi: 10.2527/1992.70113551x. [DOI] [PubMed] [Google Scholar]
- 32.Tamate H, Mcgilliard AD, Jacobson NL, Getty R. Effect of various dietaries on the anatomical development of the stomach in the calf. J Dairy Sci. 1962;45:408–20. [Google Scholar]
- 33.Lambo-Fodje AM, Oste R, Nyman ME. Short-chain fatty acid formation in the hindgut of rats fed native and fermented oat fibre concentrates. Br J Nutr. 2006;96:47–55. doi: 10.1079/bjn20061797. [DOI] [PubMed] [Google Scholar]
- 34.Sreenath HK, Moldes AB, Koegel RG, Straub RJ. Lactic acid production by simultaneous saccharification and fermentation of alfalfa fiber. J Biosci Bioeng. 2001;92:518–23. doi: 10.1263/jbb.92.518. [DOI] [PubMed] [Google Scholar]
- 35.Allison MJ, Bryant MP, Katz I, Keeney M. Metabolic function of branched-chain volatile fatty acids, growth factors for ruminococci. II. Biosynthesis of higher branched-chain fatty acids and aldehydes. J Bacteriol. 1962;83:1084–93. doi: 10.1128/jb.83.5.1084-1093.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Church DC. Corvallis. III. Van Corvallis, WA, USA: O&B Books City; 1988. Digestive physiology and nutrition of ruminant. [Google Scholar]
- 37.Herrera-Saldana R, Gomez-Alarcon R, Torabi M, Huber JT. Influence of synchronizing protein and starch degradation in the rumen on nutrient utilization and microbial protein synthesis. J Dairy Sci. 1990;73:142–8. doi: 10.3168/jds.S0022-0302(90)78657-2. [DOI] [PubMed] [Google Scholar]
- 38.Quigley JD, Caldwell LA, Sinks GD, Heitmann RN. Changes in blood glucose, nonesterified fatty acids, and ketones in response to weaning and feed intake in young calves. J Dairy Sci. 1991;74:250–7. doi: 10.3168/jds.S0022-0302(91)78167-8. [DOI] [PubMed] [Google Scholar]
- 39.Armstrong DT, Hansel W. The effect of age and plane of nutrition on growth hormone and thyrotropic hormone content of pituitary glands of Holstein heifers. J Anim Sci. 1956;15:640–9. [Google Scholar]
- 40.Stanley CC, Williams CC, Jenny BF, et al. Effects of feeding milk replacer once versus twice daily on glucose metabolism in Holstein and Jersey calves. J Dairy Sci. 2002;85:2335–43. doi: 10.3168/jds.S0022-0302(02)74313-0. [DOI] [PubMed] [Google Scholar]
- 41.Vanhatalo A, Kuoppala K, Ahvenjärvi S, Rinne M. Effects of feeding grass or red clover silage cut at two maturity stages in dairy cows. 1. Nitrogen metabolism and supply of amino acids. J Dairy Sci. 2009;92:5620–33. doi: 10.3168/jds.2009-2249. [DOI] [PubMed] [Google Scholar]