Bronchiectasis is a disease defined by a permanent and usually progressive bronchial dilation associated with multiple exacerbations and decreased health-related quality of life [1–3]. Improvement in the current knowledge of this condition's pathophysiology has clearly highlighted its complex and heterogeneous profile, whose severity or prognosis cannot be defined using a single variable [4]. Accordingly, multidimensional scores including demographical, clinical, microbiological and radiological data have recently been developed and validated as useful tools to better evaluate the disease's severity and prognosis: FACED (forced expiratory volume in 1 s (FEV1), age, chronic colonisation by Pseudomonas aeruginosa, radiological extension and dyspnoea), E-FACED (FACED plus exacerbations) and the bronchiectasis severity index (BSI) [5–8].
Short abstract
Both FACED and E-FACED scores have shown good short-term prognostic value for predicting mortality in bronchiectasis http://ow.ly/albl30i11bv
To the Editor:
Bronchiectasis is a disease defined by a permanent and usually progressive bronchial dilation associated with multiple exacerbations and decreased health-related quality of life [1–3]. Improvement in the current knowledge of this condition's pathophysiology has clearly highlighted its complex and heterogeneous profile, whose severity or prognosis cannot be defined using a single variable [4]. Accordingly, multidimensional scores including demographical, clinical, microbiological and radiological data have recently been developed and validated as useful tools to better evaluate the disease's severity and prognosis: FACED (forced expiratory volume in 1 s (FEV1), age, chronic colonisation by Pseudomonas aeruginosa, radiological extension and dyspnoea), E-FACED (FACED plus exacerbations) and the bronchiectasis severity index (BSI) [5–8].
The FACED and E-FACED scores have been shown to predict all-cause and respiratory mortality in large cohorts of patients with bronchiectasis from different countries, both at 5 years [5–7] and after long-term follow-up [9]. However, while the BSI score has demonstrated good predictive value for mortality risk from the first to the fourth year from the radiological diagnosis of bronchiectasis [8], this parameter has not been yet analysed in FACED and E-FACED scores. Since new therapies are emerging to treat this disease, it is important to identify possible surrogate markers or clinical tools that could act as relevant end-points and predict short-term outcomes [10]. Therefore, the aim of this study was to investigate the ability of the FACED and E-FACED scores to predict annual mortality in patients with bronchiectasis from the first to the fifth year of follow-up, beginning at the radiological diagnosis.
Data were analysed from an international, observational and multicentre study of historical cohorts in adult patients with bronchiectasis from Spain and Latin America. These data were originally used to create and validate the FACED and E-FACED scores published elsewhere [5–7]. The diagnosis of bronchiectasis was made using high-resolution computed tomography in all patients. Patients with cystic fibrosis were excluded from the study. All centres used the same protocol to collect patients' information as previously published [5–7]. The variables studied were: age, sex, body mass index, dyspnoea (modified Medical Research Council score), presence of chronic respiratory failure, exacerbations and hospitalisations in the previous year, radiological findings, bronchiectasis aetiology, microbiological profile, respiratory function test and usual treatments. All variables were collected as close as possible to the bronchiectasis diagnosis date to avoid interference from the various treatments during its clinical evolution. The vital status of each patient was dated yearly from the first to the fifth year, from the date of the radiological diagnosis of bronchiectasis. The area under the receiver operating characteristic curve (AUC ROC) and confidence interval at 95% were used to calculate the annual predictive power of FACED and E-FACED scores for all-cause mortality.
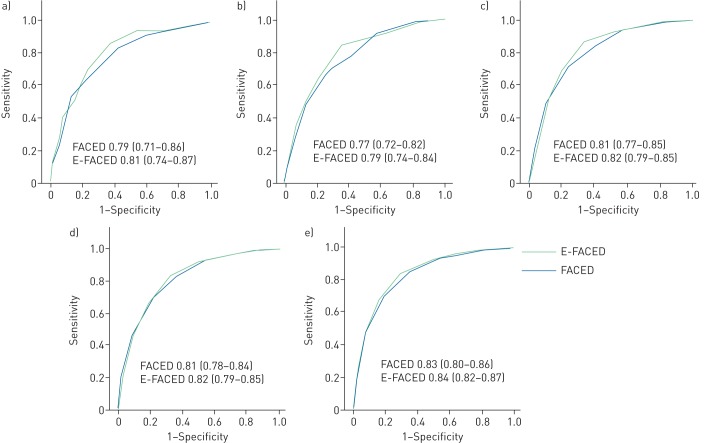
This study included 1470 consecutive patients: 819 from Spain (seven centres) and 651 from three different countries in Latin America (one centre from Argentina, four from Brazil and one from Chile). The mean±sd age of the included patients was 54.1±17.7 years, and 61% of them were female. The mean post-bronchodilator FEV1 was 65.7±25.2%. The number of exacerbations in the previous year was 1.62±1.8, and the number of hospitalisations was 0.56±1.1. Chronic bronchial infection by P. aeruginosa was identified in 27.5% of the patients. Mean FACED and E-FACED scores were 2.36±1.8 and 3.02±2.23, respectively. According to the FACED and E-FACED scores, 56.6% and 61.5% of patients presented mild bronchiectasis; 29.5% and 30% moderate bronchiectasis and 13.8% and 8.6% severe bronchiectasis, respectively. The cumulative mortality was as follows. First year: 37 (2.5%) patients; second year: 72 (4.9%) patients; third year: 129 (8.8%) patients; fourth year 184 (12.5%) patients; and fifth year 249 (16.9%) patients. Figure 1 presents annual AUC ROC for FACED (range 0.77–0.83) and E-FACED scores (range 0.79–0.84) during the 5-year follow-up period. There were no statistical differences between the yearly predictive power of BSI compared with FACED/E-FACED scores. The range value of AUC ROC for BSI (0.79–0.82) was very similar to the range value for FACED (0.77–0.83) and E-FACED (0.79–0.84). Moreover, bronchiectasis classified as mild by FACED or E-FACED presented a decreased yearly mortality risk compared with those categorised as moderate and severe, as assessed by the log rank (Mantel–Cox) test. The results were similar for both scores and for every year. The differences between groups were more evident from the third year, due to the increasing number of accumulated deaths.
FIGURE 1.
Receiver operating characteristic (ROC) curves (area under the curve (AUC) (95% CI)) for FACED (forced expiratory volume in 1 s, age, chronic colonisation by Pseudomonas aeruginosa, radiological extension and dyspnoea) and E-FACED (FACED plus exacerbations) for each year of follow-up. a) year 1; b) year 2; c) year 3; d) year 4; e) year 5.
Our study shows that both FACED and E-FACED scores maintained an excellent predictive power for all-cause mortality, even in shorter periods of time than originally described [5–7]. We analysed data from a large cohort of patients, who were well-characterised using standardised protocols in specialised outpatient clinics. Mortality results were similar in all the participating centres, even though the patients presented very different clinical characteristics and underlying aetiologies of bronchiectasis.
FACED and E-FACED scores are easy to remember and to apply, and have proved useful for predicting mid- and long-term mortality in different cohorts from several countries [5–7, 9]. The results of our study show that they may also be useful, in the same way as the BSI score, for predictions of short-term mortality. This prognostic ability could improve bronchiectasis management in both daily practice and clinical trial scenarios. In daily practice, this short-term prognosis may help to identify patients who need more intensive or preventive treatments, such as chronic antibiotics or anti-inflammatory agents, and to develop personalised management strategies that allow an individualised approach. New guidelines that standardise bronchiectasis management based on the best available evidence may help decision making of when to apply treatments of higher or lower intensity, depending on the multidimensional severity of the disease and its expected mortality [11, 12]. As regards the clinical trials scenario, we believe that these scores may play an important role in identifying a more precise population that could benefit from specific interventions based on a shorter or longer expected mechanism of action [13].
One strength of this study is the large number of patients included from very different settings with a well-characterised baseline profile. Although all the clinical variables used to establish both scores were recorded as near as possible to the diagnosis of bronchiectasis, one potential limitation of this study is that the treatments administered over the 5 years of follow-up could have influenced the mortality results. However, no medical treatment for bronchiectasis has yet been proven to reduce short- or long-term mortality. Another limitation is that we could not assess the possible role of comorbidities in mortality risk, as we did not have enough data to permit the calculation of the Charlson index or the bronchiectasis aetiology comorbidity index [14].
In conclusion, FACED and E-FACED scores have been shown to be useful to predict all-cause mortality, not only in the long term, but also for short-term follow-up (yearly from the first to the fifth year after diagnosis). Further studies should validate our results in different populations of patients with bronchiectasis, evaluate whether the two scores are able to predict exacerbations and other key outcomes in short-term periods and assess which variables could influence the prognostic capacity of these scores over time, as well as to evaluate both scores' sensitivity to change after therapeutic interventions.
Footnotes
Conflict of interest: None declared.
Authors' contributions to the study: D. de la Rosa, R. Athanazio and M.A. Martínez-García designed the study, contributed to data acquisition and interpretation, supervised the study, and wrote the manuscript. The other authors contributed to data acquisition and interpretation, critically revised the manuscript, and approved the final version to be published.
References
- 1.O'Donnell AE. Bronchiectasis. Chest 2008; 134: 815–823. [DOI] [PubMed] [Google Scholar]
- 2.Barker AF. Bronchiectasis. N Engl J Med 2002; 346: 1383–1393. [DOI] [PubMed] [Google Scholar]
- 3.McShane PJ, Naureckas ET, Tino G, et al. . Non-cystic fibrosis bronchiectasis. Am J Respir Crit Care Med 2013; 188: 647–656. [DOI] [PubMed] [Google Scholar]
- 4.Loebinger MR, Wells AU, Hansell DM, et al. . Mortality in bronchiectasis: a long-term study assessing the factors influencing survival. Eur Respir J 2009; 34: 843–849. [DOI] [PubMed] [Google Scholar]
- 5.Martínez-García MÁ, de Gracia J, Vendrell Relat M, et al. . Multidimensional approach to non-cystic fibrosis bronchiectasis: the FACED score. Eur Respir J 2014; 43: 1357–1367. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Garcia MA, Athanazio RA, Girón R, et al. . Predicting high risk of exacerbations in bronchiectasis: the E-FACED score. Int J Chron Obstruct Pulmon Dis 2017; 12: 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Athanazio R, Pereira MC, Gramblicka G, et al. . Latin America validation of FACED score in patients with bronchiectasis: an analysis of six cohorts. BMC Pulm Med 2017; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chalmers JD, Goeminne P, Aliberti S, et al. . The Bronchiectasis Severity Index. An international derivation and validation study. Am J Respir Crit Care Med 2014; 189: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis HC, Cowman S, Fernandes M, et al. . Predicting mortality in bronchiectasis using bronchiectasis severity index and FACED scores: a 19-year cohort study. Eur Respir J 2016; 47: 482–489. [DOI] [PubMed] [Google Scholar]
- 10.Aliberti S, Masefield S, Polverino E, et al. . Research priorities in bronchiectasis: a consensus statement from the EMBARC Clinical Research Collaboration. Eur Respir J 2016; 48: 632–647. [DOI] [PubMed] [Google Scholar]
- 11.Polverino E, Goeminne PC, McDonnell MJ, et al. . European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50: 1700629. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-García MA, Máiz L, Olveira C, et al. . Spanish guidelines for the treatment of bronchiectasis in adults. Arch Bronconeumol 2018; 54: 88–98. [DOI] [PubMed] [Google Scholar]
- 13.Chalmers JD, McDonnell MJ, Rutheford R, et al. . The generalizability of bronchiectasis randomized controlled trials: a multicenter cohort study. Respir Med 2016; 112: 51–58. [DOI] [PubMed] [Google Scholar]
- 14.McDonnell MJ, Aliberti S, Goeminne PC, et al. . Comorbidities and the risk of mortality in patients with bronchiectasis: an international multicentre cohort study. Lancet Respir Med 2016; 4: 969–979. [DOI] [PMC free article] [PubMed] [Google Scholar]