Abstract
Sarcomas account for 3% of all uterine malignancies and many of them are characterized by acquired, specific fusion genes whose detection has increased pathogenetic knowledge and diagnostic precision. We describe a novel fusion gene, GREB1‐NCOA2, detected by transcriptome sequencing and validated by reverse transcriptase polymerase chain reaction and Sanger sequencing in an undifferentiated uterine sarcoma. The chimeric transcript was an in‐frame fusion between exon 3 of GREB1 and exon 15 of NCOA2. The fusion is reported here for the first time, but it involves the GREB1 gene, an important promoter of tumor growth and progression, and NCOA2 which is known to be involved in transcriptional regulation. The alteration and recombination of these genes played a role in the tumorigenesis and/or progression of this sarcoma.
Keywords: fusion gene, GREB1, NCOA2, RNA sequencing, uterine sarcoma
1. INTRODUCTION
Uterine sarcomas are rare malignant mesenchymal tumors that account for 3% of uterine malignancies.1 The most recent World Health Organization (WHO) classification recognizes low‐grade and high‐grade endometrial stromal sarcomas (ESS), leiomyosarcomas, and undifferentiated sarcomas as the most common uterine sarcomas.2 Though most sarcomas can be classified unambiguously and meaningfully based on a distinctive histology and/or immunophenotype, some show overlapping features.3 It is now known that cytogenetic and molecular analyses may help diagnose and classify such tumors.4, 5 This is because many uterine sarcomas are associated with recurrent chromosomal rearrangements leading to highly specific, tumorigenic gene fusions. The use in recent years of next generation sequencing (NGS) methodology has played an important role in identifying such molecular rearrangements. By way of example, NGS identified a YWHAE‐NUTM2 fusion brought about by a 10;17‐translocation and a ZC3H7B‐BCOR caused by an X;22‐translocation in ESS, thus adding two new pathogenetic subgroups to this spectrum of tumors.6, 7
The identification of specific fusion transcripts is especially important in tumors that are phenotypically difficult to classify. In the present study, we report the molecular consequences of a translocation t(2;8)(p25;q13) identified in an undifferentiated uterine sarcoma. High‐Throughput Paired‐End RNA‐Sequencing revealed that it led to a novel fusion transcript between the Growth Regulation by Estrogen in Breast cancer 1 (GREB1) gene and the Nuclear Receptor Coactivator 2 (NCOA2) gene.
2. MATERIALS AND METHODS
2.1. Case History
A 51‐year‐old female presented with a uterine tumor in 1997. The tumor was diagnosed as high‐grade malignant, probably a leiomyosarcoma, based on a pre‐operative biopsy. The patient subsequently underwent total hysterectomy with bilateral salpingo‐oophorectomy and lymphadenectomy. Gross evaluation showed a 6.5 cm tumor of the uterine corpus with a homogeneous, gray‐white, fleshy cut surface. Morphological assessment showed a high‐grade tumor consisting of spindle and polygonal cells displaying pronounced atypia, multiple mitotic figures, and necrosis. Invasion of the cervix, parametrium bilaterally, and vessels was seen. No dissemination was seen to adnexal lymph nodes. Immunostaining of the uterine tumor showed weak positivity for desmin but absence of actin (Figure 1). A diagnosis of high‐grade endometrial sarcoma was made at the time, but subsequently changed (see below).
Figure 1.
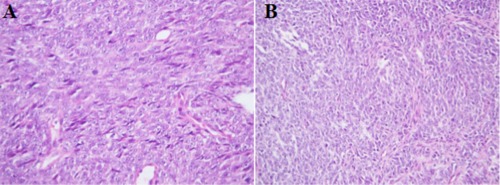
Histological examination (H‐E) of the malignant tumor. A, Uterine primary, 1997: high‐grade tumor consisting of spindle and polygonal cells with pronounced atypia and mitoses. B, Lung metastasis, 1999: high‐grade undifferentiated tumor with solid growth pattern, consisting of epithelioid cells with marked atypia [Color figure can be viewed at wileyonlinelibrary.com]
The patient developed lung metastases in 1999 and 2008, and both were resected. These metastases resembled to a certain extent the uterine tumor, but the tumor cells were now predominantly epithelioid rather than having spindle cell morphology. The 1999 lesion was extensively investigated using immunohistochemistry and was shown to be positive for vimentin and pan‐cytokeratin (AE1/AE3) as well as estrogen and progesterone receptors. The tumor was negative for CK5/6, CK7, CK14, CD10, CD45, CD117, chromogranin A, synaptophysin, actin, SMA, Myf‐4, HMB‐45, and TTF1 (Figure 1). This immunohistochemical profile was not diagnostic for any specific uterine sarcoma entity, nor did it allow certain differentiation between sarcoma and undifferentiated carcinoma. Given the unclear nature of the metastasis, additional/repeat stains were retrospectively performed on the uterine tumor. This analysis showed diffuse, strong expression of pan‐cytokeratin (AE1/AE3) but absence of CD10, Ber‐EP4, and PAX8.
The morphology and immunohistochemical profile of this tumor do not allow for any certain classification in the authors’ opinion, although the case history as well as the histological features of in particular the primary tumor lead us to conclude that it was probably of mesenchymal origin. Our final diagnosis is therefore sarcoma, not otherwise classifiable.
2.2. Cell Culturing and Karyotyping
Short‐term cultured cells from the lung metastasis surgically removed in 1999 were analyzed cytogenetically as part of our diagnostic routine.8 The karyotyping followed the recommendations of the International System for Human Cytogenomic Nomenclature (ISCN).9 The tumor's cytogenetic features have previously been published.10
The study was approved by the Regional Committee for Medical and Health Research Ethics, South‐East Norway (REK Sør‐Øst; http://helseforskning.etikkom.no). Written informed consent was obtained from the patient. The consent included acceptance that the clinical details be published. The ethics committee's approval included a review of the consent procedure. All patient information has been de‐identified.
2.3. RNA Extraction and High‐Throughput Paired‐End RNA‐Sequencing
Total RNA was extracted from formalin‐fixed, paraffin embedded (FFPE) tissue from the primary tumor (1997) and lung metastasis (1999) using RNeasy FFPE (Qiagen, Hilden, Germany). The RNA quality was evaluated using 2100 Bioanalyzer (Agilent, Santa Clara, CA) according to the manufacturer's instructions. One µg of total RNA was sent for High‐Throughput Paired‐End RNA‐sequencing at the Genomics Core Facility, Oslo University Hospital and University of Oslo (http://oslo.genomics.no/). The sequencing was performed using an Illumina HiSeq 2000 instrument and the Illumina software pipeline. The FusionCatcher program (version 0.99.3a beta‐April 15, 2014) with the associated ENSEMBL, UCSC, and RefSeq databases automatically downloaded by FusionCatcher (https://code.google.com/p/fusioncatcher/) were used for the discovery of fusion transcripts.
2.4. Reverse Transcriptase Polymerase Chain Reaction (RT‐PCR) and Sanger Sequencing
The primers used for PCR reactions and Sanger sequencing are listed in Table 1. For RT‐PCR, one µg of total RNA was reverse‐transcribed in a 20 µl reaction volume using iScript Advanced cDNA synthesis Kit according to the manufacturer's instructions (Bio‐Rad Laboratories, Oslo, Norway). The 25 μl PCR volume contained 12.5 μl Premix Ex Taq DNA Polymerase Hot Start Version (Takara Bio Europe/SAS, Saint‐Germain‐en‐Laye, France), 1 μl of cDNA, and 1 µl of each of the forward and reverse primers. The primer combination GREB1–518F1/NCOA2–3377R1 was used to detect the presence of GREB1‐NCOA2 chimeric transcript. For amplification of the possible reciprocal NCOA2‐GREB1 fusion transcripts, the primer set NCOA2–3244F1/GREB1–648R1 was used. The PCR amplifications were run on a C‐1000 Thermal cycler (Bio‐Rad Laboratories) with an initial denaturation at 94°C for 30 sec followed by 35 cycles at 98°C for 7 sec, 55°C for 30 sec, 1 min at 72°C, and a final extension at 72°C for 5 min. Three µl of the PCR product were stained with GelRed (Biotium, Hayward, CA), analyzed by electrophoresis through 1.0% agarose gel, and photographed. The remaining 22 µl PCR product were purified using the QIAquick PCR Purification Kit (Qiagen) and sequenced using 3500 Genetic Analyzer (Applied Biosystems). The BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi) and BLAT (http://genome.ucsc.edu/cgi-bin/hgBlat) softwares were used for computer analysis of sequence data.
Table 1.
Primers used for PCR and Sanger sequencing analyses
| Name | Sequence | Position | Gene | Accession number |
|---|---|---|---|---|
| GREB1–518F1 | 5′‐agaaggagggctggaaacaaa‐3’ | 498–518 | GREB1 | NM_014668.3 |
| NCOA2–3377R1 | 5′‐catggggcagtctgatttgg‐3′ | 3377–3396 | NCOA2 | NM_001321703.1 |
| NCOA2–3244F1 | 5′‐gtatgattcggaacccagca‐3′ | 3225–3244 | NCOA2 | NM_001321703.1 |
| GREB1–648R1 | 5′‐gacccccacgaggagaaag‐3′ | 648–666 | GREB1 | NM_014668.3 |
3. RESULTS
The cytogenetic investigation of the uterine sarcoma cells showed an abnormal karyotype described as 45,XX,t(2;8)(p25;q13),‐8,inv(11)(p15q23),del(14)(q22q32),der(17)t(8;17)(q11;p13) [20] (Figure 2A).
Figure 2.
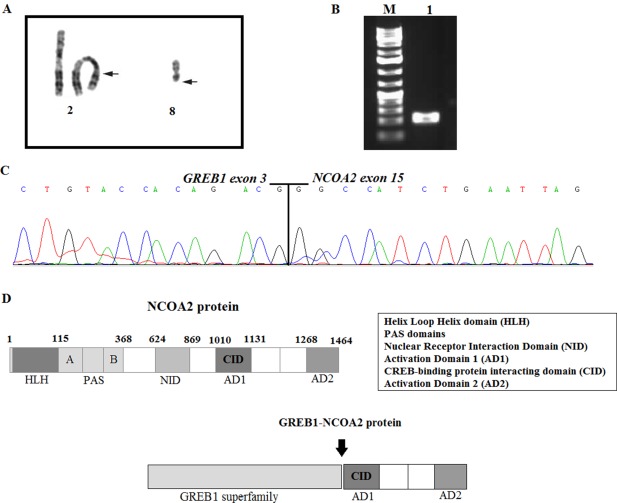
A, Partial karyotype showing the translocation t(2;8)(p25;q13). Breakpoint positions are indicated by arrows. B, Gel electrophoresis showing the amplified cDNA fragments. M, 1 Kb DNA ladder (GeneRuler, ThermoFisher); lane 1, amplification of cDNA fragment using the primers GREB1–518F/NCOA2–3377R1. C, Partial sequence chromatogram of the amplified cDNA fragment showing the junction point of the GREB1‐NCOA2 fusion. D, Illustration of the protein NCOA2 and chimeric GREB1‐NCOA2. All domains are shown, the arrow is pointing at the breakpoint position [Color figure can be viewed at wileyonlinelibrary.com]
The FusionCatcher software, with the FASTQ files obtained from the Genomics Core Facility, Oslo University Hospital and University of Oslo (http://oslo.genomics.no/), was used to detect fusion transcripts in the lung metastasis (1999) of the uterine sarcoma. A total of six chimeric transcripts were obtained by the algoritm (Table 2). All fusions were tested using the BLAT command (https://genome-euro.ucsc.edu/cgi-bin/hgBlat?command=start the program) to identify those with 100% identity in the genome according to the UCSC Genome Browser (update Dec. 2013, GRCh38/hg38). Only two of six transcripts showed such identity: GREB1‐NCOA2 and its reciprocal NCOA2‐GREB1.
Table 2.
Fusion transcripts detected using FusionCatcher
| 5'‐Chr | 3'‐Chr | 5'‐Partner gene | 3'‐Partner gene | Fusion sequence |
|---|---|---|---|---|
| 2 | 8 | GREB1 | NCOA2 | TTCCAGCTGCACCCTCTGCCTGAAGGATGCTGTACCACAGACG*GGCCATCTGAATTAGAGATGAACATGGGGGGACCTCAGTATAG |
| 8 | 2 | NCOA2 | GREB1 | CCTGGCCAAAGACAGACGCTTCAGTCTCAGGTCATGAATATAG*GGTTTTGCCAGGCCGGGAAGGACCTGCGCCTTGTCTCCATTTC |
| 2 | 11 | GREB1 | MALAT1 | CCAGTTGGAAGGGCTACTGCTGAATTTTTTTTTTTTTTTTTTTTTTGGTT*TTTTTTTTTTTTACACGAATTTGAGGAAAACCAAATGAATTTGATAGCCA |
| 3 | 19 | AC099535.4 | UBA52 | AGAGTCCACCCTGCACCTGGTGCTGCGCCTGTAAGGTGGCATTATTGAGC*CTCTCCGCCAGCTTGCCCAGAAATACAACTGCGACAAGATGATCTGCCGC |
| 6 | 6 | TRAF3IP2 | FYN | AGAGCCGACTACCCTCCGGGCCCAGTCTGTCTGTCCGTGGTGGATCTAAG*GTGCAAAGTTCCCCATCAAGTGGACGGCCCCCGAGGCAGCCCTGTACGGG |
| 19 | 3 | UBA52 | AC099535.4 | CTCACTGGCAAAACCATCACCCTTGAGGTCGAGCCCAGTGACACCATTGA*AATGTCAAAGCCAAAATTCAAGACAAGGAGGTATCCCACCTGACCAGCAG |
To validate the presence of the GREB1‐NCOA2 and NCOA2‐GREB1 fusions, RT‐PCR with specific primers was performed, followed by Sanger sequencing. The presence of an in‐frame GREB1‐NCOA2 was confirmed, whereas the reciprocal NCOA2‐GREB1 fusion was not identified by RT‐PCR. Sanger sequencing analysis of the amplified fragment GREB1‐NCOA2 showed presence of a fusion between exon 3 of GREB1 (accession number NM_014668.3) and exon 15 of NCOA2 (accession number NM_001321703.1; Figure 2B,C). The GREB1 and NCOA2 genes map to chromosomal bands 2p25 and 8q13, respectively, making it overwhelmingly likely that the fusion was brought about by the t(2;8)(p25;q13). Since the fusion transcript was identified in the lung metastasis, we tested also the primary tumor and found the same fusion (data not shown).
4. DISCUSSION
The identification of specific fusion transcripts coming from tumor‐specific chromosomal aberrations has proven essential in the differential diagnosis of many sarcomas, for example, synovial sarcoma and myxoid liposarcoma,11, 12 but still others exist with unclear phenotypic as well as genotypic features leading to an uncertain diagnosis. To increase existing knowledge about fusion transcript(s) characteristic of uterine sarcomas in general and this undifferentiated uterine sarcoma in particular, we performed transcriptome sequencing on tumor RNA finding a novel GREB1‐NCOA2 fusion gene corresponding to the t(2;8) detected by karyotyping. The first 3 exons of GREB1 were fused to exon 15 of the NCOA2 transcript.
GREB1, which maps to chromosome band 2p25, is an estrogen‐responsive gene that is an early responder in the estrogen receptor‐regulated pathway. It is a critical mediator of both estrogen‐stimulated proliferation of breast cancer cells and androgen‐stimulated proliferation of prostate cancer cells.13, 14 According to the Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer (http://cgap.nci.nih.gov/Chromosomes/Mitelman), GREB1 was previously found involved in various types of neoplasia and with different partners: a GREB1‐E2F6 fusion was seen in T‐cell acute lymphoblastic leukemia15 and a GREB1‐PDE1A in adenocarcinoma of the prostate.16 Although its exact function in the cascade of hormone action remains unclear, GREB1 may regulate proliferation in hormone responsive ovarian and endometrial cancer cells and thus be a candidate for further consideration as a potential therapeutic target.17
NCOA2, which maps to chromosomal band 8q13, is a member of the p160 steroid receptor coactivator gene family.18 It interacts with ligand‐bound nuclear receptor through its nuclear receptor interaction domain (NID) and C‐terminal transcriptional activation domains, AD1/CID (activation domain 1/CREB‐binding protein interacting domain) and AD2, to recruit histone acetyltransferases and methyltransferases to specific enhancer/promoter regions, thereby facilitating chromatin remodeling and transcription of nuclear receptor target genes.19 NCOA2 is well known for its involvement in chimeric transcripts in hematologic malignancies and soft tissue tumors. It has been reported to be fused with different partners: KAT6A‐NCOA2 (also known as MOZ‐TIF2) was found in acute myeloid leukemia (AML),20, 21 ETV6 (TEL)‐NCOA2 was reported in childhood acute leukemia, PAX3‐NCOA2 in alveolar rhabdomyosarcoma,22 HEY1‐NCOA2 in mesenchymal chondrosarcomas,23, 24 SRF‐NCOA2, TEAD1‐NCOA2, and VGLL2‐NCOA2 in rhabdomyosarcomas,25, 26 and AHRR‐NCOA2 27 and GTF2‐NCOA2 28 in angiofibromas. In all the mentioned fusions, NCOA2 is the 3’‐partner gene, as it was in the present case. The breakpoint position varies, however; in SRF‐NCOA2 it involves exon 12, in HEY1‐NCOA2 and TEAD1‐NCOA2 exon 13, in VGLL2‐NCOA2 and GTF2‐NCOA2 exon 14, and in ETV6‐NCOA2, PAX3‐NCOA2, and AHRR‐NCOA it is in exon 15. The fusion transcript GREB1‐NCOA2 is in‐frame coding for a chimeric protein that retains the interval 116–207 amino acids of GREB1 (NP_055483) and the nuclear receptor coactivator domain (amino acids 1071–1399) from the C‐terminal part of NCOA2 (NP_006531). The nuclear receptor coactivator from NCOA2 contains the AD1/CID and AD2 domains that seem to be essential for the transformation capacity of various cancer gene fusions (Figure 2 D).29, 30
The fusion GREB1‐NCOA2 has not been previously reported, but one of the genes involved, NCOA2, is known to play a role in transcriptional regulation19 which probably holds a key to its contribution to the tumorigenic and/or progression process. The importance of such chimeric transcripts is well documented in endometrial stromal sarcomas via the fusions JAZF1‐SUZ12, JAZF1‐PHF1, EPC1‐PHF1, MEAF6‐PHF1, ZC3H7B‐BCOR, and possibly also MBTD‐Cxorf67, whose presumed oncogenic effects are mediated through altered transcriptional control.31
The karyotype contained also other structural rearrangements of chromosomes, but no fusion transcript(s) were found involving gene(s) mapping to the other breakpoints. This is strong, albeit indirect, evidence that the translocation t(2;8)(p25;q13) generated the essential pathogenetic change in this sarcoma. To what extent this corresponds to a particular set of phenotypic (both morphological and immunophenotypic) features can only be resolved when more tumors with the same gene fusion are described. Experience tells us that whenever one tumor with a seemingly unique tumor‐associated translocation and corresponding fusion gene is reported, other examples of the same alteration eventually turn up. Also for this reason it is important that even single rare tumors with credible gene fusion candidates for a primary pathogenetic role are brought to the attention of the scientific community dealing with sarcoma classification and tumorigenesis.
CONFLICTS OF INTEREST
The author(s) declare that they have no competing interests.
ACKNOWLEDGMENTS
This study was supported by grants from the Radium Hospital Foundation.
Brunetti M, Panagopoulos I, Gorunova L, Davidson B, Heim S, Micci F. RNA‐sequencing identifies novel GREB1‐NCOA2 fusion gene in a uterine sarcoma with the chromosomal translocation t(2;8)(p25;q13). Genes Chromosomes Cancer. 2018;57:176–181. https://doi.org/10.1002/gcc.22518
Funding information Radium Hospital Foundation.
REFERENCE LIST
- 1. D'Angelo E, Prat J. 2010. Uterine sarcomas: A review. Gynecol Oncol. 116:131–139. [DOI] [PubMed] [Google Scholar]
- 2. Kurman RJ, Carcangiu ML, Herrington CS, Young RH. WHO Classification of Tumors of Female Reproductive Organs. Volume 6 ed. Lyon: IARC; 2014. [Google Scholar]
- 3. Nucci MR. 2016. Practical issues related to uterine pathology: Endometrial stromal tumors. Modern Pathol. 29:S92–103. [DOI] [PubMed] [Google Scholar]
- 4. Micci F, Heim S. 2015. Tumors of the female genital organs. Cancer Cytogenet. 4:447–480. [Google Scholar]
- 5. Davidson B, Micci F. 2017. Molecular characteristics of uterine sarcomas. Exp Rev Mol Diagnos. 17:515–522. [DOI] [PubMed] [Google Scholar]
- 6. Panagopoulos I, Thorsen J, Gorunova L, et al. 2013. Fusion of the ZC3H7B and BCOR genes in endometrial stromal sarcomas carrying an X;22‐translocation. Genes Chromosom Cancer. 52:610–618. [DOI] [PubMed] [Google Scholar]
- 7. Lee C‐H, Ou W‐B, Marino‐Enriquez A, et al. 2012. 14‐3‐3 fusion oncogenes in high‐grade endometrial stromal sarcoma. Proc Natl Acad Sci USA. 109:929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandahl N. Methods in solid tumor cytogenetics In: Rooney DE, ed. Human Cytogenetics: Malignancy and Acquired Abnormalities. 3rd ed. New York: Oxford University Press; 2001. [Google Scholar]
- 9. McGowan‐Jordan J, Simons A, Schmid M. ISCN An International System for Human Cytogenomic Nomenclature. Basel: Karger; 2016. [Google Scholar]
- 10. Teixeira MR, Micci F, Dietrich CU, Heim S. 2000. Detailed genome‐wide screening for inter‐ and intrachromosomal abnormalities by sequential G‐banding and RxFISH color banding of the same metaphase cells. Cancer Genet Cytogenet. 119:94–101. [DOI] [PubMed] [Google Scholar]
- 11. Fukuoka K. 2006. Molecular detection of SYT‐SSX fusion gene transcripts currently represents the most specific and sensitive tool for diagnosing intrathoracic synovial sarcoma. Internal Med. 45:881–882. [DOI] [PubMed] [Google Scholar]
- 12. Fletcher CD, Bridge J, Hogendoorn PC, Mertens F. WHO Classification of Tumors of Soft Tissue and Bone. Lyon: IARC; 2013. [Google Scholar]
- 13. Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. 2005. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treatment. 92:141–149. [DOI] [PubMed] [Google Scholar]
- 14. Rae JM, Johnson MD, Cordero KE, et al. 2006. GREB1 is a novel androgen‐regulated gene required for prostate cancer growth. Prostate. 66:886–894. [DOI] [PubMed] [Google Scholar]
- 15. Atak ZK, Gianfelici V, Hulselmans G, et al. 2013. Comprehensive analysis of transcriptome variation uncovers known and novel driver events in T‐cell acute lymphoblastic leukemia. PLoS Genet. 9:e1003997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshihara K, Wang Q, Torres‐Garcia W, Zheng S, Vegesna R, Kim H, Verhaak RGW. 2015. The landscape and therapeutic relevance of cancer‐associated transcript fusions. Oncogene. 34:4845–4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodgkinson KM, Vanderhyden BC. 2014. Consideration of GREB1 as a potential therapeutic target for hormone‐responsive or endocrine‐resistant cancers. Expert Opin Ther Targets. 18:1065–1076. [DOI] [PubMed] [Google Scholar]
- 18. Xu J, Li Q. 2003. Review of the in vivo functions of the p160 steroid receptor coactivator family. Mol Endocrinol. 17:1681–1692. [DOI] [PubMed] [Google Scholar]
- 19. Voegel JJ, Heine MJ, Tini M, Vivat V, Chambon P, Gronemeyer H. 1998. The coactivator TIF2 contains three nuclear receptor‐binding motifs and mediates transactivation through CBP binding‐dependent and ‐independent pathways. EMBO J. 17:507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carapeti M, Aguiar RC, Goldman JM, Cross NC. 1998. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood. 91:3127–3133. [PubMed] [Google Scholar]
- 21. Carapeti M, Aguiar RC, Watmore AE, Goldman JM, Cross NC. 1999. Consistent fusion of MOZ and TIF2 in AML with inv(8)(p11q13). Cancer Genet Cytogenet. 113:70–72. [DOI] [PubMed] [Google Scholar]
- 22. Sumegi J, Streblow R, Frayer RW, et al. 2010. Recurrent t(2;2) and t(2;8) translocations in rhabdomyosarcoma without the canonical PAX‐FOXO1 fuse PAX3 to members of the nuclear receptor transcriptional coactivator family. Genes Chromosom Cancer. 49:224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Panagopoulos I, Thorsen J, Gorunova L, Micci F, Heim S. 2014. Sequential combination of karyotyping and RNA‐sequencing in the search for cancer‐specific fusion genes. Int J Biochem Cell Biol. 53:462–465. [DOI] [PubMed] [Google Scholar]
- 24. Wang L, Motoi T, Khanin R, Olshen A, Mertens F, Bridge J, Cin PD, Antonescu CR, Singer S, Hameed M, Bovee JVMG, Hogendoorn PCW, Socci N, Ladanyi M. 2012. Identification of a novel, recurrent HEY1‐NCOA2 fusion in mesenchymal chondrosarcoma based on a genome‐wide screen of exon‐level expression data. Genes Chromosom Cancer. 51:127–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alaggio R, Zhang L, Sung YS, et al. 2016. A Molecular Study of Pediatric Spindle and Sclerosing Rhabdomyosarcoma: Identification of Novel and Recurrent VGLL2‐related Fusions in Infantile Cases. Am J Surg Pathol. 40:224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mosquera JM, Sboner A, Zhang L, Kitabayashi N, Chen C‐L, Sung YS, Wexler LH, LaQuaglia MP, Edelman M, Sreekantaiah C, Rubin MA, Antonescu CR. 2013. Recurrent NCOA2 gene rearrangements in congenital/infantile spindle cell rhabdomyosarcoma. Genes Chromosom Cancer. 52:538–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jin Y, Möller E, Nord KH, Mandahl N, Von Steyern FV, Domanski HA, Mariño‐Enríquez A, Magnusson L, Nilsson J, Sciot R, Fletcher CDM, Debiec‐Rychter M, Mertens F. 2012. Fusion of the AHRR and NCOA2 genes through a recurrent translocation t(5;8)(p15;q13) in soft tissue angiofibroma results in upregulation of aryl hydrocarbon receptor target genes. Genes Chromosom Cancer. 51:510–520. [DOI] [PubMed] [Google Scholar]
- 28. Arbajian E, Magnusson L, Mertens F, Domanski HA, Vult von Steyern F, Nord KH. 2013. A novel GTF2I/NCOA2 fusion gene emphasizes the role of NCOA2 in soft tissue angiofibroma development. Genes Chromosom Cancer. 52:330–331. [DOI] [PubMed] [Google Scholar]
- 29. Panagopoulos I, Gorunova L, Bjerkehagen B, Boye K, Heim S. 2014. Chromosome aberrations and HEY1‐NCOA2 fusion gene in a mesenchymal chondrosarcoma. Oncol Rep. 32:40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Deguchi K, Ayton PM, Carapeti M, Kutok JL, Snyder CS, Williams IR, Cross NCP, Glass CK, Cleary ML, Gilliland DG. 2003. MOZ‐TIF2‐induced acute myeloid leukemia requires the MOZ nucleosome binding motif and TIF2‐mediated recruitment of CBP. Cancer Cell. 3:259–271. [DOI] [PubMed] [Google Scholar]
- 31. Micci F, Gorunova L, Agostini A, Johannessen LE, Brunetti M, Davidson B, Heim S, Panagopoulos I. 2016. Cytogenetic and molecular profile of endometrial stromal sarcoma. Genes Chromosom Cancer. 55:834–846. [DOI] [PMC free article] [PubMed] [Google Scholar]


