Abstract
Alkaline sphingomyelinase cleaves phosphocholine from sphingomyelin, platelet-activating factor, lysophosphatidylcholine, and less effectively phosphatidylcholine. The enzyme shares no structure similarities with acid or neutral sphingomyelinase but belongs to ecto-nucleotide pyrophosphatase/phosphodiesterase (NPP) family and therefore is also called NPP7 nowadays. The enzyme is expressed in the intestinal mucosa in many species and additionally in human liver. The enzyme in the intestinal tract has been extensively studied but not that in human liver. Studies on intestinal alkaline sphingomyelinase show that it inhibits colonic tumorigenesis and inflammation, hydrolyses dietary sphingomyelin, and stimulates cholesterol absorption. The review aims to summarize the current knowledge on liver alkaline sphingomyelinase in human and strengthen the necessity for close study on this unique human enzyme in hepatobiliary diseases.
Keywords: Sphingomyelin, Alkaline sphingomyelinase, Nucleotide pyrophosphatase/phosphodiesterase 7, Autotaxin, Platelet-activating factor, Cholangiocarcinoma, Liver diseases, Gallstone
Core tip: Alkaline sphingomyelinase is an enzyme expressing in the intestinal tract and additionally human liver. It hydrolyzes sphingomyelin, platelet activating factor and lysophospholipase. In the intestinal tract, it digests dietary sphingomyelin, stimulates cholesterol absorption, and inhibits development of colon cancer. Less is known about the implications of the enzyme in liver diseases. The review summarizes the current knowledge of its roles in hepatobiliary disease and raised special topics for future investigations.
ALK-SMASE IN NPP FAMILY
An enzyme that catalyzes sphingomyelin (SM) hydrolysis to ceramide at optimal alkaline pH was first identified in the intestinal tract by Nilsson in 1969[1]. The enzyme was thereafter named as alkaline sphingomyelinase (alk-SMase)[2] in line with acid and neutral SMases. However, after purification, characterization, and gene cloning[3-6], it was found that alk-SMase actually had no structural similarities with either acid or neutral SMase, but shared about 30% amino acid sequence similarities with enzymes in ecto nucleotide pyrophosphatase/phosphodiesterase (NPP) family. As a novel member in NPP family, alk-SMase is nowadays also called NPP7.
Comparing with other six NPP members, alk-SMase is distinctive in several aspects. Not like most NPP members, alk-SMase has no nucleotidase activity but a phospholipase C activity. It cleaves phosphocholine from phospholipids including SM, platelet activating factor (PAF)[7], lysophosphatidylcholine (lyso-PC), and phosphatidylcholine (PC) less effectively[5]. In NPP family, NPP2 (autotaxin) can also hydrolyze lyso-PC, but with a phospholipase D activity[8]. Another NPP member NPP6 can cleave phosphocholine from lysophospholipids mainly lyso-PC and lyso-PAF but not from SM[9]. Alk-SMase is so far the only one that has decent activities against SM and PAF in this family. In addition, while the activities of other NPP members could be identified in many organs and tissues, expression of alk-SMase is only restricted to intestinal tract in most species[10]. Western blot of rat tissues only shows positive band in intestinal mucosa and content but not in other organs including brain, heart, lung, liver, spleen, kidney and pancreas[4]. Interestingly, additional high activity was found in the bile of human[11], but not in bile of other species including rat, mouse, pig, cow, sheep, dog, guinea pig, and baboon[10] (and unpublished data). Furthermore, most NPP members are functioning as a proliferative and inflammatory factors that are important for cell survival[12], alk-SMase displays inhibitory effects on cell proliferation and inflammation[13,14]. Cell culture studies show that alk-SMase can inhibit cell proliferation by about 50%[15] and its activity in vivo is positively correlated to the activity of caspase 3, the key enzyme that triggers apoptosis[16-18]. Rectal administration of alk-SMase in rats suppresses colitis induced by dextran sulfate sodium (DSS)[19]. Recently the studies with alk-SMase knockout mice clearly showed that both initiation and malignant transformation of colon cancer induced by azoxymethane and DSS was enhanced by about 5 times in the knockout mice comparing with the wild type mice[20]. In agreement with the animal studies, clinical studies also found reduced alk-SMase activity in patients with inflammatory bowel diseases (IBD) and colon cancer, and the reduction is progressive from 25% in IBD to 75% in colonic carcinoma[21-23].
The anticancer effects of alk-SMase are thought to be achieved with a three armed mechanism[13]. First, it hydrolyzes SM to ceramide, which is a well-known antiproliferative and apoptotic molecule[18,24]. Second, it cleaves phosphocholine moiety from PAF and inactivates PAF[7], which is widely expressed in many inflammatory tissues promoting inflammation and tumorigenesis[25]. And finally NPP7 converts lyso-PC to monoacylglycerol[5], thus reducing the production of lysophosphatidic acid (LPA), which otherwise can be formed by NPP2[8]. LPA has emerged as an important messenger with potent inflammatory and carcinogenic effects mediated via several signaling transduction pathways after binding to G protein coupled receptors[26,27]. In supporting this three arm hypothesis, decreased ceramide and increased PAF[20] and LPA (Zhang P et al Abstract presented in AACR symposium, Shanghai, China, 2016) have been found in NPP7 knockout mice.
Similar to other NPP members, alk-SMase is anchored on the surface of the cell membrane with a short hydrophobic domain. The remaining part of the enzyme including the catalytic domain is exposed extracellularly[13]. The enzyme can be released by bile salt[28], and also by pancreatic trypsin, as there is a tryptic site just above the hydrophobic domain embedded inside the membrane[29].
ALK-SMASE IN HUMAN BILE
Alk-SMase in human bile was discovered by Nyberg et al[11] in bile collected from patients in our hospital. The activity in human bile is not derived from bacteria since it is similarly present in the samples with and without bacterial infection. Although gallbladder bile has higher activity than the hepatic bile, no activity was found in the homogenates of gallbladder mucosa, confirming that it is liver not gallbladder that expresses the enzyme. Because PCR experiment identifies alk-SMase mRNA in human HepG2 cells[30], the enzyme is believed to be expressed by hepatocytes, transported to the surface of the microvilli that extend into the bile canaliculi and released by bile salt into the lumen.
Alk-SMase in human bile shares similar characteristics as the one in the intestinal mucosa[31,32]. The enzyme becomes active at the pH around 7 and the maximal activity occurs at pH 9.0. Its activity requires the presence of bile salts[4,6]. The bile salt dependency is type specific, which differs from other lipases such as bile salt stimulated lipase[33,34]. Although different bile salts more or less increase alk-SMase activity with the maximal effects at their critical micelle concentrations, taurocholate (TC) and taurochenodeoxycholate (TCDC) are much more effective than other bile salts. On the other hand, the nonionic detergent Triton X100 and zwitterionic non-denaturing detergent CHAPS with similar structure as TCDC and TC have no stimulatory effect but inhibit alk-SMase activity in the presence of other bile salts[4,31,35]. The finding indicates that the bile salt induced activation is not a simple detergent effect on the physical state of the substrate SM in mixed micelles. Additional interaction between the enzyme protein and the bile salt is likely involved. Supporting this hypothesis, recent studies on crystal structure of human alk-SMase by Gorelik et al[36] showed that the enzyme forms a hydrophobic loop and a positively charged surface which can interact with bile salts. This needs to be proved in further investigations.
Similar also to intestinal alk-SMase, human bile alk-SMase is inhibited by PC, the most abundant phospholipid in the bile[31,37]. This might be related to a competition between PC and SM for the substrate binding site of the enzyme. As shown by both computer homology modelling studies[38] and crystal structural studies[36], alk-SMase forms a specific pocket and a long narrow groove that fits the phosphocholine head group, and the tails of these substrates, respectively. The binding affinity is stronger for SM than for PC[39,40].
Presence of alk-SMase in human bile enhances SM digestion in humans. Intestinal SM is derived from diet, shedding mucosal cells and bile. The enzyme responsible for digesting SM in the gut is alk-SMase and in alk-SMase knockout mice, about 90% of ingested SM cannot be digested but accumulated in the colon[41]. In many species except human, alk-SMase activity is absent in duodenum, increasing in the jejunum and declining in the colon[2]. SM digestion in the gut normally starts in the middle of the jejunum where alk-SMase is high and PC, the major inhibitor of alk-SMase, has been decreased due to the absorption[42]. The process of SM digestion is slow and incomplete in most species, resulting in about 40% ceramide and SM being identified in feces[43,44]. However, due to the presence of additional alk-SMase in the bile of human, human duodenum has considerable alk-SMase activity. Ohlsson et al[45] found that digestion of SM in human is more efficient than other species and about 81% of ingested SM can be digested. The more effective digestion of SM could be important for human health, as dietary SM stimulates the development of the gut of the new born and inhibits colonic tumorigenesis[46-48].
ROLE OF LIVER IN SPHINGOLIPID METABOLISM
It is well known that liver is an important organ for lipid metabolism such as fatty acid beta-oxidation, ketone body generation, cholesterol metabolism, lipoprotein synthesis, and phospholipid metabolism. For the phospholipid metabolism in liver, most previous studies focused on PC not SM, but the interest in SM was increasing in the latest decades. Liver is an organ with relatively high levels of SM. Comparing with subcutaneous and intra-abdominal adipose tissues, SM in human liver is 7-8 fold higher than in these adipose tissues[49].
The high levels of PC and SM in the liver are attributable to the fact that liver efficiently takes up choline containing compounds such as choline, lyso-PC and phosphocholine derived from digestion of phospholipids in the intestinal tract and uses them for synthesis of PC and SM[42,50] (Figure 1). As shown in animal studies, after feeding choline labeled SM, up to 30% of the labeled choline is accumulated in the liver and more than 95% of them is utilized for PC synthesis[42]. This is important for SM synthesis, as at the last step of SM synthesis, phosphocholine is transferred from PC to ceramide, catalyzed by SM synthase, which is highly expressed in the liver[51,52]. For the hydrolysis of SM, liver has high acid SMase activity than most other organs in many species[41]. Acid SMase is an enzyme with two isoforms. One is the lysosome enzyme that breaks down internalized SM in lysosome and the other is a secretory form that can be secreted to the plasma membrane and hydrolyzes membrane bound SM[53]. That is why in Niemann Pick diseases with acid SMase deficiency, liver is one of the most affected organs with SM accumulation[54]. There are many other factors that influence SM levels in the liver, such as high fat diet[55], endotoxin infection[56], hepatitis B virus infection[57] and liver cancer[58].
Figure 1.
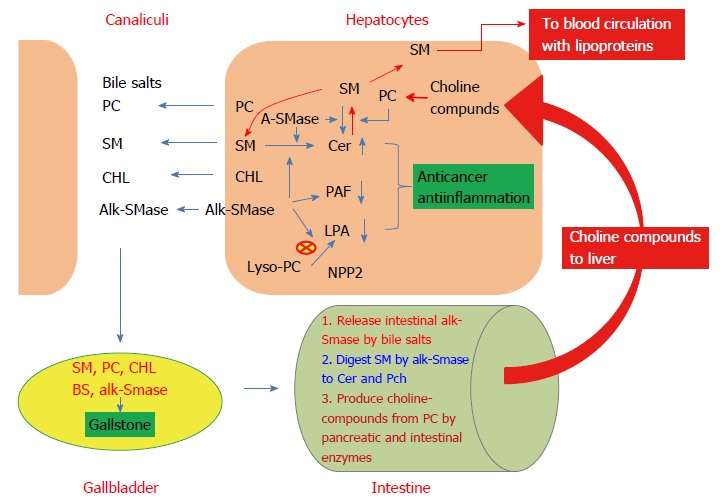
Metabolism of sphingomyelin in the liver and potential implications of alk-SMase in liver diseases. Liver alk-SMase is localized on the hepatocyte canaliculi membrane. It hydrolyzes SM, PAF, and lyso-PC, resulting in increased ceramide (Cer) and decreased PAF and LPA, thus having anticancer and anti-inflammatory effects. Together with PC, cholesterol, and bile salts, SM is released in canaliculi and transported to gallbladder, where the interactions of these compounds affect gallstone formation. When the bile is delivered into the intestinal tract, bile salt will release additional alk-SMase from intestinal mucosa, and digest intestinal SM to ceramide and phosphocholine (Pch). Meanwhile, PC will be hydrolyzed by enzymes from pancreas and intestinal mucosa to choline compounds such as free choline, lyso-PC, and Pch. These choline compounds will be transported to liver where to be used for synthesis of PC. Pch moiety in PC can be transferred to ceramide to form SM by SM synthases. Part of the SM formed will be released into blood together with lipoproteins, part to bile, and part to be degraded by alk-SMase and acid SMase (ASMase) in the liver.
SM synthesized in the liver can be released into plasma and bile (Figure 1). Comparing with other species, human plasma has at least two fold higher levels of SM than other species[59]. The plasma SM from liver is mainly transported with lipoproteins mainly VLDL and less with LDL and HDL[43]. SM in plasma is also derived from intestinal mucosa and other tissue cells, being about 1-1.5 g in total[43]. Most SM secreted from intestine is in chylomicron. SM in chylomicron is mainly not from the dietary products but from the membrane of enterocytes, because most sphingosine, the final digestion product of SM by alk-SMase and neutral ceramidase in the gut is not utilized to resynthesize SM after absorption in mucosal cells, but to convert to fatty acid and then to chyle triglyceride[44,60].
Secretion of phospholipids from hepatocyte canaliculi membrane is by a mechanism related to the interactions of bile salt and ABCB4[61]. Under physiological conditions, more than 95% of the phospholipids in bile is PC, and SM is accounted for about 3%[62]. The levels of SM in human bile is relatively low comparing with other species which don’t have alk-SMase in the bile such as sheep[63]. But under pathological conditions, the level of SM in bile is subject to change. Barnwell et al[64] showed that in vivo perfusion of bile salts in rat significantly reduced PC content and meanwhile induced about 10 time increase of SM in the bile without obvious damage to the liver.
The implications of SM in plasma and bile are getting increasing interest. It has been known that high concentration of plasma SM is a risk factor for atherosclerosis[65]. Recent studies also found that plasma SM could be a biomarker for various liver diseases such as hepatitis, primary sclerosing cholangitis (PSC), and steatosis[57,66,67], indicating SM metabolism in liver significantly contributes to plasma SM levels. SM in the bile may have important implications, as it has stronger van der Waal interactions with cholesterol than PC[40] and its physical properties of SM is affected by bile salt which may affect gallstone formation in gallbladder[68].
ALK-SMASE IN HEPATOBILIARY DISEASES
Comparing the extensive studies on intestinal alk-SMase, which showed its important roles in SM digestion, colon cancer prevention, and cholesterol absorption[13,14,20], the progress of the research on human bile alk-SMase obviously lags behind. The main obstacle is lack of an animal model that expresses alk-SMase in the liver, and lack of a cell line that highly expresses alk-SMase, as the enzyme has already been downregulated in tumorigenesis. In a pilot study, we measured alk-SMase activity in 30 human liver biopsies and the results indicated a reduction of the enzyme activity in steatosis and PSC[30]. Recently we determined alk-SMase activity in 59 bile samples taken under ERCP and found significant reduction of alk-SMase activity in bile of patients with PSC and tumorigenic diseases, with the most remarkable reduction in cholangiocarcinoma[69].
Besides the reduction of activity, an abnormal transcript of alk-SMase was identified in both liver cancer HepG2 cells and colon cancer HT29 cells, which is caused by a shift of RNA splice site at transcriptional level, resulting in exon 4 deletion[30,70]. The enzyme translated from this transcript is totally inactive, as 73 amino acids coded by exon 4 were absent, of which a histidine is critical for formation of the substrate binding site[70]. According to the size of their mRNA, the wild type and the mutant isoform have been called 1.4 kb and 1.2 kb form, respectively. These two forms were found in the bile of many of the 59 patients with different hepatic diseases[69], but the activity of alk-SMase is positively correlated with the ratio of 1.4/1.2 kb form. Decrease in the 1.4 kb product and increase in the 1.2 kb product are likely associated with the development of cholangiocarcinoma. In the bile of one PSC and one cholangiocarcinoma patient, no alk-SMase activity and no 1.4 kb product but only high levels of 1.2 kb form were identified[69].
On the other hand, hepatobiliary diseases may also affect the levels of alk-SMase and SM digestion in the intestine because bile diversion strongly reduced alk-SMase activity in the small intestinal content by 85% and in the feces by 68% in rat[71]. The changes are believed to be related to bile salts, which release the alk-SMase from the intestinal mucosa to gut lumen[28].
HUMAN HEPATIC ALK-SMASE IN PERSPECTIVES
Considering the results from previous studies on alk-SMase, it is predictable that human liver alk-SMase may also have important implications for hepatobiliary diseases. The following questions are worth close investigation: (1) Can the remarkable reduction of alk-SMase activity and the increase in the aberrant isoform in the bile be warning signals for carcinogenesis in the liver, particularly cholangiocarcinoma? It is well known that cholangiocarcinoma is a disease lacking an early biomarker, and most patients are not curable at the time when the disease is diagnosed[72]. Making the situation worsen is the fact that the incidence of cholangiocarcinoma is increasing[73]. To find an early biomarker for cholangiocarcinoma is therefore a challenge for clinical doctors and medical researchers. Alk-SMase activity in bile is significantly decreased in cholangiocarcinoma to an extent greater than in other hepatic diseases associated with increased expression of the 1.2 kb isoform[69]. The reduction of alk-SMase activity seems already occurring in both bile and liver biopsies in PSC patients[31]. PSC is a major risk factor for cholangiocarcinoma and about 15% of PSC patients may finally develop this type of cancer[74]. Future studies in a relatively large scale are necessary to evaluate whether the changed activity and 1.2 kb isoform expression in PSC patients can be biomarker for cholangiocarcinoma. To follow these changes might be helpful for identifying the early carcinogenesis. In addition, it is worthwhile to point out that about 70% of PSC patients may have IBD, particularly ulcerative colitis[75,76]. Reduction of alk-SMase activity in chronic ulcerative colitis has been reported[23]; (2) Are there a cross communication between NPP7 (alk-SMase) and NPP2 (autotaxin) in hepatobiliary diseases? NPP2 hydrolyzes lyso-PC with a phospholipase D activity and generating LPA, a potent inflammatory and proliferative factor[77]. Increased levels of NPP2 are a feature of many important hepatobiliary diseases such as PBC, PSC[78], steatosis[79], liver fibrosis[80], hepatitis C[81] and liver cancer[82]. Alk-SMase shares the same substrate lyso-PC with NPP2 but it cleaves phosphocholine instead of choline and thus generates monoacylglycerol not LPA[5]. Alk-SMase therefore may counteract NPP2 and thus reduce the formation of LPA. Recently we did find that in alk-SMase knockout mice treated with DSS, the levels of LPA in the colonic mucosa is higher in the knockout mice than in the wild type mice (Zhang P et al, Abstract presented in AACR symposium, Shanghai, China, 2016). Interestingly, a recent cohort study showed that primary biliary cirrhosis (PBC) patients who did not respond to ursodeoxycholic acid (UDCA) treatment display higher NPP2 levels than the responders[78]. Changes of alk-SMase may be implicated in the results, as alk-SMase activity can be increased by UDCA in liver cells[17]. No response to UDCA in these PBC patients may indicate a failure to upregulate alk-SMase by UDCA in these patients, leading to increased levels of NPP2; (3) Can alk-SMase protect liver against noxious effects of PAF? PAF is a type of bioactive lipid and can be synthesized rapidly in various inflammatory tissues. After binding to its G protein coupled receptors, PAF triggers several signal transduction pathways leading to activation of various phospholipases including C, D and A2 and to calcium mobilization, MAP kinase activation, and neutrophil mobilization[83,84]. In the liver PAF induces vasoconstriction[85] and may play a key role in several hepatic diseases such as CCl4 induced cirrhosis, ethanol and acetaminophen induced liver injury, viral induced hepatitis, and hepatocellular carcinoma[86-89]. All these diseases are associated with increased formation of PAF levels and PAF receptor expression. To inhibit the effects of PAF, previous studies were focused on PAF acetyl hydrolase and PAF receptor antagonists[90]. PAF is a substrate for alk-SMase which inactivates PAF by degrading it to phosphocholine and alkyl acetyl glycerol[7]. The activity is bile salt dependent with optimal pH at 7.5, which well fits the niche of the hepatobiliary system[7]. In alk-SMase knockout mice, PAF has been found to be significantly increased in the intestinal lumen[20]. It is therefore worthwhile examining the impact of alk-SMase on PAF action in these hepatic diseases and whether upregulation of alk-SMase may counteract the effects of PAF and benefit the patients; (4) What is the role of human bile alk-SMase in regulating SM levels in bile? Alk-SMase affects SM levels in the cell membrane. Overexpression of alk-SMase in COS7 cells[5] and incubation of the cells with purified alk-SMase result in reduced SM in the cell membrane[13]. Alk-SMase in human bile most likely can do the same things and thus affecting SM levels both in the hepatocyte canaliculi and bile. Phospholipids particularly PC and SM affect crystallization of cholesterol which is a key event involved in gallstone formation[68]. SM levels in the bile can be increased in the presence of high concentrations of bile salt[64] , and be decreased in the presence of high levels of alk-SMase released from canaliculi. Considering the influence of the membrane SM on cholesterol translocation and synthesis[40], and the more appreciable interaction of SM than PC with cholesterol[39,40], the impact of bile alk-SMase on gallstone formation through regulating SM levels both in the canalicular membrane and bile might be also worthwhile for close investigation.
CONCLUSION
Additional expression of alk-SMase in liver is unique for humans. As shown in the figure, by hydrolyzing its substrate SM, PAF, and Lyso-PC, alk-SMase generates anticancer and apoptotic molecule ceramide, reduces levels of PAF and LPA, which have been shown to be involved in a series of liver diseases including viral infection, steatosis, fibrosis, sclerosis, and tumorigenesis. Alk-SMase thus may play important roles in protecting the organ from these diseases. In addition, the enzyme is released into bile together with SM, PC, bile salt, and cholesterol and may interfere SM and PC levels and the physical-chemical interactions of these molecules in bile, thus affecting gallstone formation. Liver is an active organ for SM metabolism and for regulating plasma SM levels. Changed alk-SMase activity in bile and SM levels in plasma have been found in several hepatobiliary diseases, and such changes may have diagnostic and prognostic values. The contributions of alk-SMase, a unique human liver enzyme, for these changes need close investigation.
ACKNOWLEDGMENTS
The studies cited from the author’s lab were supported from grants of Swedish Research Council, Swedish Cancerfonden, Albert Påhlsson Foundation, Crafoord Foundation and foundation of Region Skåne University Hospital, Lund, Sweden. Dr. Åke Nilsson is thanked for helpful discussions and suggestions.
Footnotes
Conflict-of-interest statement: No conflict-of-interest to be disclosed.
Manuscript source: Invited manuscript
Peer-review started: November 26, 2017
First decision: December 18, 2017
Article in press: January 23, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Xu RL S- Editor: Wang JL L- Editor: A E- Editor: Li RF
References
- 1.Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969;176:339–347. doi: 10.1016/0005-2760(69)90192-1. [DOI] [PubMed] [Google Scholar]
- 2.Duan RD, Nyberg L, Nilsson A. Alkaline sphingomyelinase activity in rat gastrointestinal tract: distribution and characteristics. Biochim Biophys Acta. 1995;1259:49–55. doi: 10.1016/0005-2760(95)00137-2. [DOI] [PubMed] [Google Scholar]
- 3.Wu J, Cheng Y, Palmberg C, Bergman T, Nilsson A, Duan RD. Cloning of alkaline sphingomyelinase from rat intestinal mucosa and adjusting of the hypothetical protein XP_221184 in GenBank. Biochim Biophys Acta. 2005;1687:94–102. doi: 10.1016/j.bbalip.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Cheng Y, Nilsson A, Tömquist E, Duan RD. Purification, characterization, and expression of rat intestinal alkaline sphingomyelinase. J Lipid Res. 2002;43:316–324. [PubMed] [Google Scholar]
- 5.Duan RD, Bergman T, Xu N, Wu J, Cheng Y, Duan J, Nelander S, Palmberg C, Nilsson A. Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase family. J Biol Chem. 2003;278:38528–38536. doi: 10.1074/jbc.M305437200. [DOI] [PubMed] [Google Scholar]
- 6.Duan RD, Cheng Y, Hansen G, Hertervig E, Liu JJ, Syk I, Sjostrom H, Nilsson A. Purification, localization, and expression of human intestinal alkaline sphingomyelinase. J Lipid Res. 2003;44:1241–1250. doi: 10.1194/jlr.M300037-JLR200. [DOI] [PubMed] [Google Scholar]
- 7.Wu J, Nilsson A, Jönsson BA, Stenstad H, Agace W, Cheng Y, Duan RD. Intestinal alkaline sphingomyelinase hydrolyses and inactivates platelet-activating factor by a phospholipase C activity. Biochem J. 2006;394:299–308. doi: 10.1042/BJ20051121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Umezu-Goto M, Kishi Y, Taira A, Hama K, Dohmae N, Takio K, Yamori T, Mills GB, Inoue K, Aoki J, et al. Autotaxin has lysophospholipase D activity leading to tumor cell growth and motility by lysophosphatidic acid production. J Cell Biol. 2002;158:227–233. doi: 10.1083/jcb.200204026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakagami H, Aoki J, Natori Y, Nishikawa K, Kakehi Y, Natori Y, Arai H. Biochemical and molecular characterization of a novel choline-specific glycerophosphodiester phosphodiesterase belonging to the nucleotide pyrophosphatase/phosphodiesterase family. J Biol Chem. 2005;280:23084–23093. doi: 10.1074/jbc.M413438200. [DOI] [PubMed] [Google Scholar]
- 10.Duan RD, Hertervig E, Nyberg L, Hauge T, Sternby B, Lillienau J, Farooqi A, Nilsson A. Distribution of alkaline sphingomyelinase activity in human beings and animals. Tissue and species differences. Dig Dis Sci. 1996;41:1801–1806. doi: 10.1007/BF02088748. [DOI] [PubMed] [Google Scholar]
- 11.Nyberg L, Duan RD, Axelson J, Nilsson A. Identification of an alkaline sphingomyelinase activity in human bile. Biochim Biophys Acta. 1996;1300:42–48. doi: 10.1016/0005-2760(95)00245-6. [DOI] [PubMed] [Google Scholar]
- 12.Stefan C, Jansen S, Bollen M. NPP-type ectophosphodiesterases: unity in diversity. Trends Biochem Sci. 2005;30:542–550. doi: 10.1016/j.tibs.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Duan RD. Alkaline sphingomyelinase: an old enzyme with novel implications. Biochim Biophys Acta. 2006;1761:281–291. doi: 10.1016/j.bbalip.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Duan RD, Nilsson A. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog Lipid Res. 2009;48:62–72. doi: 10.1016/j.plipres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Hertervig E, Nilsson A, Cheng Y, Duan RD. Purified intestinal alkaline sphingomyelinase inhibits proliferation without inducing apoptosis in HT-29 colon carcinoma cells. J Cancer Res Clin Oncol. 2003;129:577–582. doi: 10.1007/s00432-003-0466-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng Y, Tauschel HD, Nilsson A, Duan RD. Ursodeoxycholic acid increases the activities of alkaline sphingomyelinase and caspase-3 in the rat colon. Scand J Gastroenterol. 1999;34:915–920. doi: 10.1080/003655299750025408. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, Cheng Y, Wu J, Tauschel HD, Duan RD. Ursodeoxycholic acid differentially affects three types of sphingomyelinase in human colon cancer Caco 2 cells. Cancer Lett. 2006;235:141–146. doi: 10.1016/j.canlet.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 18.Hannun YA, Obeid LM. Ceramide: an intracellular signal for apoptosis. Trends Biochem Sci. 1995;20:73–77. doi: 10.1016/s0968-0004(00)88961-6. [DOI] [PubMed] [Google Scholar]
- 19.Andersson D, Kotarsky K, Wu J, Agace W, Duan RD. Expression of alkaline sphingomyelinase in yeast cells and anti-inflammatory effects of the expressed enzyme in a rat colitis model. Dig Dis Sci. 2009;54:1440–1448. doi: 10.1007/s10620-008-0509-2. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, Zhang P, Xu SC, Yang L, Voss U, Ekblad E, Wu Y, Min Y, Hertervig E, Nilsson Å, Duan RD. Enhanced colonic tumorigenesis in alkaline sphingomyelinase (NPP7) knockout mice. Mol Cancer Ther. 2015;14:259–267. doi: 10.1158/1535-7163.MCT-14-0468-T. [DOI] [PubMed] [Google Scholar]
- 21.Hertervig E, Nilsson A, Björk J, Hultkrantz R, Duan RD. Familial adenomatous polyposis is associated with a marked decrease in alkaline sphingomyelinase activity: a key factor to the unrestrained cell proliferation? Br J Cancer. 1999;81:232–236. doi: 10.1038/sj.bjc.6690682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hertervig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79:448–453. [PubMed] [Google Scholar]
- 23.Sjöqvist U, Hertervig E, Nilsson A, Duan RD, Ost A, Tribukait B, Löfberg R. Chronic colitis is associated with a reduction of mucosal alkaline sphingomyelinase activity. Inflamm Bowel Dis. 2002;8:258–263. doi: 10.1097/00054725-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4:604–616. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 25.Yost CC, Weyrich AS, Zimmerman GA. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Erstad DJ, Tager AM, Hoshida Y, Fuchs BC. The autotaxin-lysophosphatidic acid pathway emerges as a therapeutic target to prevent liver cancer. Mol Cell Oncol. 2017;4:e1311827. doi: 10.1080/23723556.2017.1311827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills GB, Moolenaar WH. The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- 28.Duan RD, Cheng Y, Tauschel HD, Nilsson A. Effects of ursodeoxycholate and other bile salts on levels of rat intestinal alkaline sphingomyelinase: a potential implication in tumorigenesis. Dig Dis Sci. 1998;43:26–32. doi: 10.1023/a:1018807600683. [DOI] [PubMed] [Google Scholar]
- 29.Wu J, Liu F, Nilsson A, Duan RD. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am J Physiol Gastrointest Liver Physiol. 2004;287:G967–G973. doi: 10.1152/ajpgi.00190.2004. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y, Wu J, Hertervig E, Lindgren S, Duan D, Nilsson A, Duan RD. Identification of aberrant forms of alkaline sphingomyelinase (NPP7) associated with human liver tumorigenesis. Br J Cancer. 2007;97:1441–1448. doi: 10.1038/sj.bjc.6604013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan RD, Nilsson A. Purification of a newly identified alkaline sphingomyelinase in human bile and effects of bile salts and phosphatidylcholine on enzyme activity. Hepatology. 1997;26:823–830. doi: 10.1002/hep.510260403. [DOI] [PubMed] [Google Scholar]
- 32.Nyberg L, Duan RD, Nilsson A. A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J Nutr Biochem. 2000;11:244–249. doi: 10.1016/s0955-2863(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 33.Bläckberg L, Hernell O. Bile salt-stimulated lipase in human milk. Evidence that bile salt induces lipid binding and activation via binding to different sites. FEBS Lett. 1993;323:207–210. doi: 10.1016/0014-5793(93)81340-6. [DOI] [PubMed] [Google Scholar]
- 34.Hernell O, Bläckberg L. Human milk bile salt-stimulated lipase: functional and molecular aspects. J Pediatr. 1994;125:S56–S61. doi: 10.1016/s0022-3476(06)80737-7. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson A, Duan RD. Alkaline sphingomyelinases and ceramidases of the gastrointestinal tract. Chem Phys Lipids. 1999;102:97–105. doi: 10.1016/s0009-3084(99)00078-x. [DOI] [PubMed] [Google Scholar]
- 36.Gorelik A, Liu F, Illes K, Nagar B. Crystal structure of the human alkaline sphingomyelinase provides insights into substrate recognition. J Biol Chem. 2017;292:7087–7094. doi: 10.1074/jbc.M116.769273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu JJ, Nilsson A, Duan RD. Effects of phospholipids on sphingomyelin hydrolysis induced by intestinal alkaline sphingomyelinase: an in vitro study. J Nutr Biochem. 2000;11:192–197. doi: 10.1016/s0955-2863(00)00064-4. [DOI] [PubMed] [Google Scholar]
- 38.Duan J, Wu J, Cheng Y, Duan RD. Understanding the molecular activity of alkaline sphingomyelinase (NPP7) by computer modeling. Biochemistry. 2010;49:9096–9105. doi: 10.1021/bi101069u. [DOI] [PubMed] [Google Scholar]
- 39.Slotte JP. Sphingomyelin-cholesterol interactions in biological and model membranes. Chem Phys Lipids. 1999;102:13–27. doi: 10.1016/s0009-3084(99)00071-7. [DOI] [PubMed] [Google Scholar]
- 40.Slotte JP. Biological functions of sphingomyelins. Prog Lipid Res. 2013;52:424–437. doi: 10.1016/j.plipres.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Cheng Y, Hansen GH, Niels-Christiansen LL, Koentgen F, Ohlsson L, Nilsson A, Duan RD. Crucial role of alkaline sphingomyelinase in sphingomyelin digestion: a study on enzyme knockout mice. J Lipid Res. 2011;52:771–781. doi: 10.1194/jlr.M012880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyberg L, Nilsson A, Lundgren P, Duan RD. Localization and capacity of sphingomyelin digestion in the rat intestinal tract. J Nutr Biochem. 1997;8:112–118. [Google Scholar]
- 43.Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47:154–171. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Nilsson A. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta. 1968;164:575–584. doi: 10.1016/0005-2760(68)90187-2. [DOI] [PubMed] [Google Scholar]
- 45.Ohlsson L, Hertervig E, Jönsson BA, Duan RD, Nyberg L, Svernlöv R, Nilsson A. Sphingolipids in human ileostomy content after meals containing milk sphingomyelin. Am J Clin Nutr. 2010;91:672–678. doi: 10.3945/ajcn.2009.28311. [DOI] [PubMed] [Google Scholar]
- 46.Nilsson Å. Role of Sphingolipids in Infant Gut Health and Immunity. J Pediatr. 2016;173 Suppl:S53–S59. doi: 10.1016/j.jpeds.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 47.Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J, Merrill AH Jr. Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res. 1996;56:4936–4941. [PubMed] [Google Scholar]
- 48.Zhang P, Li B, Gao S, Duan RD. Dietary sphingomyelin inhibits colonic tumorigenesis with an up-regulation of alkaline sphingomyelinase expression in ICR mice. Anticancer Res. 2008;28:3631–3635. [PubMed] [Google Scholar]
- 49.Kotronen A, Seppänen-Laakso T, Westerbacka J, Kiviluoto T, Arola J, Ruskeepää AL, Yki-Järvinen H, Oresic M. Comparison of lipid and fatty acid composition of the liver, subcutaneous and intra-abdominal adipose tissue, and serum. Obesity (Silver Spring) 2010;18:937–944. doi: 10.1038/oby.2009.326. [DOI] [PubMed] [Google Scholar]
- 50.Le Kim D, Betzing H. Intestinal absorption of polyunsaturated phosphatidylcholine in the rat. Hoppe Seylers Z Physiol Chem. 1976;357:1321–1331. doi: 10.1515/bchm2.1976.357.2.1321. [DOI] [PubMed] [Google Scholar]
- 51.Ullman MD, Radin NS. The enzymatic formation of sphingomyelin from ceramide and lecithin in mouse liver. J Biol Chem. 1974;249:1506–1512. [PubMed] [Google Scholar]
- 52.Huitema K, van den Dikkenberg J, Brouwers JF, Holthuis JC. Identification of a family of animal sphingomyelin synthases. EMBO J. 2004;23:33–44. doi: 10.1038/sj.emboj.7600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jenkins RW, Clarke CJ, Lucas JT Jr, Shabbir M, Wu BX, Simbari F, Mueller J, Hannun YA, Lazarchick J, Shirai K. Evaluation of the role of secretory sphingomyelinase and bioactive sphingolipids as biomarkers in hemophagocytic lymphohistiocytosis. Am J Hematol. 2013;88:E265–E272. doi: 10.1002/ajh.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanier MT. Niemann-Pick diseases. Handb Clin Neurol. 2013;113:1717–1721. doi: 10.1016/B978-0-444-59565-2.00041-1. [DOI] [PubMed] [Google Scholar]
- 55.Choi S, Snider AJ. Sphingolipids in High Fat Diet and Obesity-Related Diseases. Mediators Inflamm. 2015;2015:520618. doi: 10.1155/2015/520618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Memon RA, Holleran WM, Moser AH, Seki T, Uchida Y, Fuller J, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin and cytokines increase hepatic sphingolipid biosynthesis and produce lipoproteins enriched in ceramides and sphingomyelin. Arterioscler Thromb Vasc Biol. 1998;18:1257–1265. doi: 10.1161/01.atv.18.8.1257. [DOI] [PubMed] [Google Scholar]
- 57.Li JF, Qu F, Zheng SJ, Wu HL, Liu M, Liu S, Ren Y, Ren F, Chen Y, Duan ZP, et al. Elevated plasma sphingomyelin (d18:1/22:0) is closely related to hepatic steatosis in patients with chronic hepatitis C virus infection. Eur J Clin Microbiol Infect Dis. 2014;33:1725–1732. doi: 10.1007/s10096-014-2123-x. [DOI] [PubMed] [Google Scholar]
- 58.Koumanov K, Infante R. Phospholipid-transfer proteins in human liver and primary liver carcinoma. Biochim Biophys Acta. 1986;876:526–532. doi: 10.1016/0005-2760(86)90040-8. [DOI] [PubMed] [Google Scholar]
- 59.Dougherty RM, Galli C, Ferro-Luzzi A, Iacono JM. Lipid and phospholipid fatty acid composition of plasma, red blood cells, and platelets and how they are affected by dietary lipids: a study of normal subjects from Italy, Finland, and the USA. Am J Clin Nutr. 1987;45:443–455. doi: 10.1093/ajcn/45.2.443. [DOI] [PubMed] [Google Scholar]
- 60.Nilsson A, Hertervig E, Duan RD. Digestion and absorption of sphingolipids in food. In: Szuhaj BF, van Nieuwenhuyzen W, editors. Nutrition and Biochemistry of phospholipids. Champaign: AOCS Press; 2003. pp. 70–79. [Google Scholar]
- 61.Boyer JL. Bile formation and secretion. Compr Physiol. 2013;3:1035–1078. doi: 10.1002/cphy.c120027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Konikoff FM, Cohen DE, Carey MC. Phospholipid molecular species influence crystal habits and transition sequences of metastable intermediates during cholesterol crystallization from bile salt-rich model bile. J Lipid Res. 1994;35:60–70. [PubMed] [Google Scholar]
- 63.Moschetta A, vanBerge-Henegouwen GP, Portincasa P, Palasciano G, Groen AK, van Erpecum KJ. Sphingomyelin exhibits greatly enhanced protection compared with egg yolk phosphatidylcholine against detergent bile salts. J Lipid Res. 2000;41:916–924. [PubMed] [Google Scholar]
- 64.Barnwell SG, Tuchweber B, Yousef IM. Biliary lipid secretion in the rat during infusion of increasing doses of unconjugated bile acids. Biochim Biophys Acta. 1987;922:221–233. doi: 10.1016/0005-2760(87)90158-5. [DOI] [PubMed] [Google Scholar]
- 65.Jiang XC, Paultre F, Pearson TA, Reed RG, Francis CK, Lin M, Berglund L, Tall AR. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 66.Bladergroen BA, Beynen AC, Geelen MJ. Dietary pectin lowers sphingomyelin concentration in VLDL and raises hepatic sphingomyelinase activity in rats. J Nutr. 1999;129:628–633. doi: 10.1093/jn/129.3.628. [DOI] [PubMed] [Google Scholar]
- 67.Liu J, Zhang H, Li Z, Hailemariam TK, Chakraborty M, Jiang K, Qiu D, Bui HH, Peake DA, Kuo MS, et al. Sphingomyelin synthase 2 is one of the determinants for plasma and liver sphingomyelin levels in mice. Arterioscler Thromb Vasc Biol. 2009;29:850–856. doi: 10.1161/ATVBAHA.109.185223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang DQ, Cohen DE, Carey MC. Biliary lipids and cholesterol gallstone disease. J Lipid Res. 2009;50 Suppl:S406–S411. doi: 10.1194/jlr.R800075-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Duan RD, Hindorf U, Cheng Y, Bergenzaun P, Hall M, Hertervig E, Nilsson Å. Changes of activity and isoforms of alkaline sphingomyelinase (nucleotide pyrophosphatase phosphodiesterase 7) in bile from patients undergoing endoscopic retrograde cholangiopancreatography. BMC Gastroenterol. 2014;14:138. doi: 10.1186/1471-230X-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu J, Cheng Y, Nilsson A, Duan RD. Identification of one exon deletion of intestinal alkaline sphingomyelinase in colon cancer HT-29 cells and a differentiation-related expression of the wild-type enzyme in Caco-2 cells. Carcinogenesis. 2004;25:1327–1333. doi: 10.1093/carcin/bgh140. [DOI] [PubMed] [Google Scholar]
- 71.Duan RD, Verkade HJ, Cheng Y, Havinga R, Nilsson A. Effects of bile diversion in rats on intestinal sphingomyelinases and ceramidase. Biochim Biophys Acta. 2007;1771:196–201. doi: 10.1016/j.bbalip.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Reddy SB, Patel T. Current approaches to the diagnosis and treatment of cholangiocarcinoma. Curr Gastroenterol Rep. 2006;8:30–37. doi: 10.1007/s11894-006-0061-1. [DOI] [PubMed] [Google Scholar]
- 73.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 74.Lazaridis KN, Gores GJ. Primary sclerosing cholangitis and cholangiocarcinoma. Semin Liver Dis. 2006;26:42–51. doi: 10.1055/s-2006-933562. [DOI] [PubMed] [Google Scholar]
- 75.Boberg KM, Lind GE. Primary sclerosing cholangitis and malignancy. Best Pract Res Clin Gastroenterol. 2011;25:753–764. doi: 10.1016/j.bpg.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 76.Kummen M, Schrumpf E, Boberg KM. Liver abnormalities in bowel diseases. Best Pract Res Clin Gastroenterol. 2013;27:531–542. doi: 10.1016/j.bpg.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 77.Moolenaar WH. Lysophospholipids in the limelight: autotaxin takes center stage. J Cell Biol. 2002;158:197–199. doi: 10.1083/jcb.200206094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wunsch E, Krawczyk M, Milkiewicz M, Trottier J, Barbier O, Neurath MF, Lammert F, Kremer AE, Milkiewicz P. Serum Autotaxin is a Marker of the Severity of Liver Injury and Overall Survival in Patients with Cholestatic Liver Diseases. Sci Rep. 2016;6:30847. doi: 10.1038/srep30847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rachakonda VP, Reeves VL, Aljammal J, Wills RC, Trybula JS, DeLany JP, Kienesberger PC, Kershaw EE. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring) 2015;23:965–972. doi: 10.1002/oby.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakagawa H, Ikeda H, Nakamura K, Ohkawa R, Masuzaki R, Tateishi R, Yoshida H, Watanabe N, Tejima K, Kume Y, et al. Autotaxin as a novel serum marker of liver fibrosis. Clin Chim Acta. 2011;412:1201–1206. doi: 10.1016/j.cca.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe N, Ikeda H, Nakamura K, Ohkawa R, Kume Y, Aoki J, Hama K, Okudaira S, Tanaka M, Tomiya T, et al. Both plasma lysophosphatidic acid and serum autotaxin levels are increased in chronic hepatitis C. J Clin Gastroenterol. 2007;41:616–623. doi: 10.1097/01.mcg.0000225642.90898.0e. [DOI] [PubMed] [Google Scholar]
- 82.Zhang G, Zhao Z, Xu S, Ni L, Wang X. Expression of autotaxin mRNA in human hepatocellular carcinoma. Chin Med J (Engl) 1999;112:330–332. [PubMed] [Google Scholar]
- 83.Hanahan DJ. Platelet activating factor: a biologically active phosphoglyceride. Annu Rev Biochem. 1986;55:483–509. doi: 10.1146/annurev.bi.55.070186.002411. [DOI] [PubMed] [Google Scholar]
- 84.Shukla SD. Platelet-activating factor receptor and signal transduction mechanisms. FASEB J. 1992;6:2296–2301. doi: 10.1096/fasebj.6.6.1312046. [DOI] [PubMed] [Google Scholar]
- 85.Buxton DB, Fisher RA, Hanahan DJ, Olson MS. Platelet-activating factor-mediated vasoconstriction and glycogenolysis in the perfused rat liver. J Biol Chem. 1986;261:644–649. [PubMed] [Google Scholar]
- 86.Denizot Y, Descottes B, Truffinet V, Valleix D, Labrousse F, Mathonnet M. Platelet-activating factor and liver metastasis of colorectal cancer. Int J Cancer. 2005;113:503–505. doi: 10.1002/ijc.20585. [DOI] [PubMed] [Google Scholar]
- 87.Murohisa G, Kobayashi Y, Kawasaki T, Nakamura S, Nakamura H. Involvement of platelet-activating factor in hepatic apoptosis and necrosis in chronic ethanol-fed rats given endotoxin. Liver. 2002;22:394–403. doi: 10.1034/j.1600-0676.2002.01552.x. [DOI] [PubMed] [Google Scholar]
- 88.Yang Y, Nemoto EM, Harvey SA, Subbotin VM, Gandhi CR. Increased hepatic platelet activating factor (PAF) and PAF receptors in carbon tetrachloride induced liver cirrhosis. Gut. 2004;53:877–883. doi: 10.1136/gut.2003.024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhou W, Chao W, Levine BA, Olson MS. Role of platelet-activating factor in hepatic responses after bile duct ligation in rats. Am J Physiol. 1992;263:G587–G592. doi: 10.1152/ajpgi.1992.263.5.G587. [DOI] [PubMed] [Google Scholar]
- 90.McIntyre TM, Prescott SM, Stafforini DM. The emerging roles of PAF acetylhydrolase. J Lipid Res. 2009;50 Suppl:S255–S259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]


