Abstract
AIM
To investigate the impact of alpha-fetoprotein (AFP) on long-term recurrence rate and overall survival and we also aimed to define the level of AFP leading to a higher risk of disease recurrence and affecting patient survival.
METHODS
Data of adult patients who received liver transplant (LT) for hepatocellular carcinoma (HCC) at our hospital from January 2000 to December 2013 were reviewed. Reviewed data included demographic characteristics, preoperative AFP level, operative details, follow-up details, and survival outcomes. Patients were mostly listed for LT based on Milan or UCSF criteria. For the purpose of this study, normal AFP level was defined as AFP value < 10 ng/mL, high AFP level was defined as AFP value ≥ 10 to < 400 ng/mL, and very high AFP level was defined as AFP ≥ 400 ng/mL. The patients were divided into these 3 groups accordingly. Survival rates were plotted as Kaplan-Meier curves and compared by log-rank analysis. Continuous variables were expressed as median (interquartile range). Categorical variables were compared by Spearman’s test. Discriminative analysis was used to define the lowest value of AFP that could affect the overall survival in study population. Statistical significance was defined by a P value of < 0.05.
RESULTS
Totally 250 adult patients underwent LT for HCC in the study period. Eight-four of them received deceased-donor LT and 166 had living-donor LT. The patients were divided into 3 groups: Group A, AFP < 10 ng/mL (n = 83); Group B, AFP ≥ 10 to < 400 ng/mL (n = 131); Group C, AFP ≥ 400 ng/mL (n = 36). The commonest etiology was hepatitis-B-related cirrhosis. The Model for End-stage Liver Disease scores in these groups were similar (median, 13 vs 13 vs 12; P = 0.745). The time to operation in Group A was longer (median, 94 vs 31 vs 35 d; P = 0.001). The groups were similar in hospital mortality (P = 0.626) and postoperative complication (P = 0.702). Pathology of explants showed that the 3 groups had similar numbers of tumor nodules, but the tumors in Group C were larger (A: 2.5 cm, B: 3.0 cm, C: 4.0 cm; P = 0.003). Group C had a bigger proportion of patients who were beyond Milan criteria (P = 0.010). Poor differentiation and vascular permeation were also more common in this group (P = 0.017 and P = 0.003 respectively). It also had poorer 5-year survival (A: 85.5%, B: 82.4%, C: 66%; P = 0.029). The 5-year disease-free survival was 84.3% in Group A, 80.1% in Group B, and 61.1% in Group C. Receiver operating characteristic area under the curve for AFP in predicting tumor recurrence was 0.685. The selected cut-off value was 54 ng/mL for AFP (C-index 0.685; 95%CI: 0.592-0.779; sensitivity 0.595; specificity 0.687). On discriminative analysis, AFP value of 105 ng/mL was shown to affect the overall survival of the patients.
CONCLUSION
HCC patients with a high preoperative AFP level had inferior survival after LT. AFP level of 54 ng/mL was associated with disease recurrence, and AFP level of 105 ng/mL was found to be the cut-off value for overall survival difference.
Keywords: Alpha-fetoprotein, Liver transplantation, Recurrence, Survival
Core tip: Various established criteria have been used to identify patients with hepatocellular carcinoma who would benefit from liver transplant with reasonable survival. Alpha-fetoprotein (AFP) level has been identified as an important factor associated with suboptimal survival with high recurrence rate. This study demonstrated that AFP level correlated well with the pathological findings of tumor differentiation and microvascular invasion, which are usually confirmed in explant pathology. In this set of data, AFP level of 54 ng/mL was associated with disease recurrence, and AFP level of 105 ng/mL was found to be the cut-off value for overall survival difference.
INTRODUCTION
Liver transplant (LT) is the best treatment option for hepatocellular carcinoma (HCC) as it removes both the tumor and the cirrhotic liver. The Milan criteria have been well adopted worldwide as a set of guidelines for listing patients for LT. Patients within the Milan criteria have a 5-year post-LT survival of 65%-80%, with a recurrence risk of 8%-15%[1]. However, the Milan criteria are criticized for being too stringent, since many patients beyond the criteria could still have reasonable post-LT survival[2-8]. Therefore, in additional to morphological consideration of tumor, the adoption of biological markers such as alpha-fetoprotein (AFP), response to therapy and evolution after therapy[9] is advocated.
AFP has been used as a tumor marker for HCC, and a high AFP level has been shown to be associated with poorer outcomes[10,11]. In previous studies, suboptimal results with high recurrence rates were seen in patients who had received LT with an AFP level of > 1000 ng/mL[12-14]. Such a level is considered a contraindication to LT. This level is applied not only to extended criteria but also to patients within the Milan criteria. Unfortunately, the exact consensual cut-off value remains undefined.
In this study, we investigated the impact of AFP on long-term recurrence rate and overall survival. We also aimed to define the level of AFP leading to a higher risk of disease recurrence and affecting patient survival.
MATERIALS AND METHODS
Prospectively collected data of adult patients who received deceased-donor LT (DDLT) or living-donor LT (LDLT) for HCC at our hospital in the period from January 2000 to December 2013 were reviewed and analyzed. These data included demographic characteristics, preoperative AFP level, operative details, follow-up details, and survival outcomes. Institutional review board approval was not required for this study because it was a retrospective analysis of anonymous data. Patient treatments were not affected by this study.
Patient selection for LT
The strategies adopted for selection of patients with known HCC for LT have been described elsewhere[15,16]. In brief, tumor evaluation was done with computed tomography of the abdomen and thorax, in addition to radionuclide bone scan at initial diagnosis. In recent years, dual-tracer (11C-acetate and 18 F-fluorodeoxyglucose) positron emission tomography (PET) was performed to exclude extrahepatic metastasis. Patients who were 65 years old or younger and not eligible for partial hepatectomy or local ablation were considered for LT. The age limit as a selection criterion was getting relatively loose as long as the patient was physically fit. The Milan criteria[1] and the UCSF criteria[14] were used for selection of patients for listing. Patients who had recurrent HCC after hepatectomy would still be considered for LT if their disease was still within selection criteria. There was no mandatory waiting period prior to LT, and bridging therapy with transarterial chemoembolization was offered to LT candidates with reasonable liver function. From October 2009 onwards, an arbitrary Model for end-stage liver disease (MELD) score of 18 points were given to DDLT candidates with HCC remaining at stage 2 six months after radiological confirmation of their stage-2 disease. Two MELD points were added every three months as long as their disease remained at stage 2 or below[17].
Patients who were beyond the Milan and UCSF criteria because they had slightly larger tumors or slightly more tumors were not eligible for DDLT but could be considered for LDLT if they had no portal or hepatic vein invasion.
Treatments
Surgery was performed using standard techniques. Cell-saver device was not used. Explants were examined by pathologists for tumor size and number, differentiation, and presence of microscopic vascular invasion. Tumors found on explant examination were regarded as incidental tumors. Neither medical nor radiation adjuvant treatment was given to any patient after LT. The patients were monitored regularly by measurement of serum AFP level, chest radiography and abdominal and chest computed tomography every 3 mo. Recurrences suspected on clinical grounds were confirmed by histological examination as far as possible.
Donor and recipient operations were performed as described elsewhere[18]. The decision to use left-lobe graft vs right-lobe graft was based on a number of donor and recipient factors, the most important of which were the ratio of graft weight to standard liver volume, the ratio of graft weight to recipient weight, MELD score, and donor liver anatomy. The Urata formula [Liver volume (mL) = Body surface area (m2) × 706.2 + 2.4] was used to calculate standard liver volume[19]. Implantation process and techniques were similar for left and right lobe grafts[18,20]. The immunosuppression and prophylaxis regimens prescribed have been described earlier[21].
Statistical analysis
For the purpose of this study, normal AFP level was defined as AFP value < 10 ng/mL, high AFP level was defined as AFP value ≥ 10 to < 400 ng/mL, and very high AFP level was defined as AFP ≥ 400 ng/mL. The patients were divided into these 3 groups accordingly.
Receiver operating characteristic (ROC) analysis was used to evaluate the ability of AFP to predict postoperative recurrence and to choose the optimal cut-off value for subsequent analysis. For indication for LDLT, high specificity was essential for avoiding excluding a large number of patients who would not develop recurrence.
Clinical profiles and outcomes of patients were compared on the basis of AFP level. Comparisons were made on short- and long-term outcomes, including graft function, graft survival, patient survival, and incidence of biliary complication. Continuous variables were expressed as median (range), and the Mann-Whitney U test was used for subgroup comparison. Categorical variables were compared by χ2 test or Fisher’s exact test. The cumulative probability of recurrence and survival was estimated by the life-table method and compared by the log-rank test. Deaths from all causes were included in the calculation of survival. Patients without recurrence were regarded as censored observations in the calculation of cumulative recurrence rates. Variables related to graft, tumor and tumor treatment before LT were analyzed for prognostic significance. The Kaplan-Meier method was used for survival analysis and the log-rank test was used for survival comparison. Discriminative analysis was used to define the lowest value of AFP that could affect the overall survival in the study population. Statistical significance was defined by a P value of < 0.05. The computer software SPSS, version 20.0 (SPSS Inc., Chicago, IL, United States), was used for all statistical calculations.
RESULTS
From January 2000 to December 2013, 250 adult patients underwent LT for HCC. Eight-four of them received DDLT and 166 had LDLT. The patients were divided into 3 main groups according to their preoperative AFP level: Group A, AFP < 10 ng/mL (normal); Group B, AFP ≥ 10 to < 400 ng/mL (high); Group C, AFP ≥ 400 ng/mL (very high) (Table 1). Patients in Group C were significantly younger (P = 0.037). The 3 groups has similar distribution of sex (P = 0.492). The commonest etiology was hepatitis-B-related cirrhosis. The median MELD scores in the 3 groups were similar (P = 0.745). The median time to operation in Group A was significantly longer (P = 0.001).
Table 1.
Comparison of Group A, Group B and Group C
| Group A (n = 83) | Group B (n = 131) | Group C (n = 36) | P value | |
| Age (yr) | 56 (38-65) | 55 (3-72) | 51.5 (11-66) | 0.037 |
| Male/Female | 67/16 | 113/18 | 29/7 | 0.492 |
| Diagnosis: | ||||
| Cirrhosis | ||||
| Cryptogenic | 2 | 2 | 1 | |
| Hepatitis B | 67 | 89 | 25 | |
| Hepatitis C | 4 | 22 | 3 | |
| Alcoholic | 0 | 1 | 1 | |
| Hepatitis B + C | 1 | 2 | 0 | |
| Alcoholic + hepatitis C | 1 | 0 | 1 | |
| Alcoholic + hepatitis B | 0 | 1 | 0 | |
| Autoimmune | 1 | 0 | 0 | |
| Wilson's disease | 0 | 0 | 0 | |
| Preoperative MELD score | 13 (6-35) | 12 (6-35) | 12 (8-43) | 0.745 |
| Waiting time (d) | 94 (1-2735) | 31 ( 1-1874) | 35 (1-1473) | 0.001 |
| Blood transfusion (units) | 4.2 (0-32) | 2 (0-56) | 4 (0-31) | 0.128 |
| Fresh frozen plasma transfusion (units) | 8 (0-24) | 6 (0-30) | 6 (0-22) | 0.609 |
| Platelet transfusion (units) | 8 (0-26) | 6 (0-32) | 8 (0-22) | 0.978 |
| Operation time (min) | 650 (370-1105) | 678 (333-1110) | 707 (300-1273) | 0.598 |
| Cold ischemic time (min) | 182 (62-652) | 125 (60-633) | 133 (70-500) | 0.206 |
| Warm ischemic time (min) | 49.5 (25-102) | 52 (26-108) | 55.5 (30-93) | 0.209 |
| Hospital stay (d) | 1.7 (8-132) | 15 (0-83) | 15 (7-47) | 0.251 |
| Intensive care unit stay (d) | 3 (1-42) | 3 (0-30) | 3 (2-16) | 0.283 |
| Follow-up (mo) | 82.4 (0.59-204.9) | 89.1 (0-210.82) | 68.2 (5.95-204.24) | 0.242 |
| Hospital mortality | 2 (2.4%) | 2 (1.5%) | 0 | 0.626 |
| LDLT:DDLT | 45:38 | 93:38 | 28:8 | 0.012 |
| Explant Milan Within:Beyond | 56:23 | 84:46 | 15:21 | 0.010 |
| Explant UCSF Within:Beyond | 63:16 | 98:32 | 23:13 | 0.188 |
| No. of tumor in explant | 1 (1-multiple) | 2 (1-multiple) | 1 (1-20) | 0.272 |
| Largest size of tumor in explant (cm) | 2.5 (0.90-7.00) | 3.0 (0.25-9.00) | 4.0 (1.5-19.5) | 0.003 |
| Differentiation: | 0.017 | |||
| Well | 26 | 41 | 3 | |
| Moderate | 41 | 66 | 25 | |
| Poor | 2 | 8 | 6 | |
| Undifferentiated | 0 | 2 | 0 | |
| Unknown | 10 | 13 | 2 | |
| Vascular permeation: | 0.003 | |||
| No | 60 | 85 | 14 | |
| Yes | 18 | 40 | 21 | |
| Unknown | 1 | 5 | 1 | |
| Graft loss | 18 (21.7%) | 28 (21.4%) | 15 (41.7%) | 0.033 |
| Patient status Alive:Dead | 65:18 | 104:27 | 21:15 | 0.027 |
| Graft survival, yr | 0.038 | |||
| 1 | 96.40% | 93.10% | 97.20% | |
| 3 | 89.20% | 84.70% | 80.60% | |
| 5 | 85.50% | 81.60% | 66.00% | |
| Patient survival, yr | 0.029 | |||
| 1 | 96.40% | 94.70% | 97.20% | |
| 3 | 89.20% | 85.50% | 80.60% | |
| 5 | 85.50% | 82.40% | 66.00% | |
| Disease-free survival, yr | 0.007 | |||
| 1 | 92.80% | 89.30% | 80.60% | |
| 3 | 88.00% | 81.70% | 72.20% | |
| 5 | 84.30% | 80.10% | 61.10% | |
| Postoperative early complication by Clavien grading: | 0.702 | |||
| No | 40 | 68 | 20 | |
| I | 19 | 24 | 9 | |
| II | 5 | 13 | 2 | |
| IIIA | 11 | 11 | 3 | |
| IIIB | 6 | 6 | 2 | |
| IVA | 1 | 7 | 0 | |
| IVB | 0 | 0 | 0 | |
| V | 1 | 2 | 0 |
Group A-AFP < 10 ng/mL; Group B-AFP ≥ 10 to < 400 ng/mL; Group C, AFP ≥ 400 ng/mL. MELD: Model for end-stage liver disease; DDLT: Deceased-donor liver transplant; LDLT: Living-donor liver transplant.
There were no differences in terms of blood transfusion amount, operation time, cold ischemic time or warm ischemic time among the groups, suggesting that the operative procedures were similar in the groups. Moreover, no differences were found in intensive care unit stay (P = 0.283), hospital stay (P = 0.251), hospital mortality (P = 0.626), or postoperative complication (P = 0.702). Pathology of explants showed that the 3 groups had similar numbers of tumor nodules but the tumors in Group C were larger (P = 0.003), and thus more patients in Group C were beyond the Milan criteria (P = 0.010). Furthermore, Group C had more cases of poor differentiation (P = 0.017) and vascular permeation (P = 0.003).
No patients were lost to follow-up in the study period. The 3 groups had similar follow-up period (P = 0.242). More patients in Group C had graft loss (P = 0.033), and hence this group had poorer 5-year graft survival (P = 0.038) and 5-year patient survival (P = 0.029) (Figure 1). Most patients died of recurrent HCC. The 5 year-disease-free survival was 84.3% in Group A, 80.1% in Group B, and 61.1% in Group C. Disease-free survival was similar in Groups A and B (P = 0.813) but significantly different between Groups A and C (P = 0.004) and between Groups B and C (P = 0.006) (Figure 2).
Figure 1.
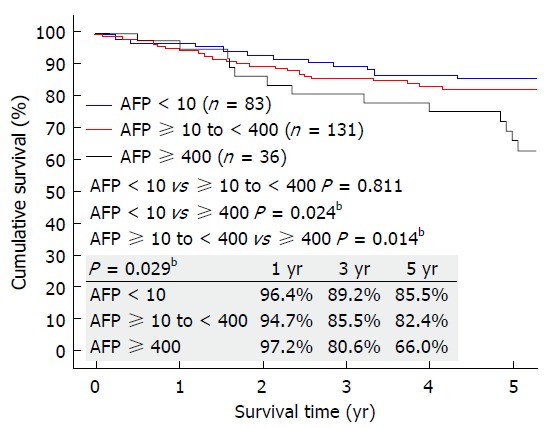
Overall survival of patients with preoperative AFP < 10 ng/mL, ≥ 10 to < 400 ng/mL, and ≥ 400 ng/mL. AFP: Alpha-fetoprotein. bP < 0.01.
Figure 2.
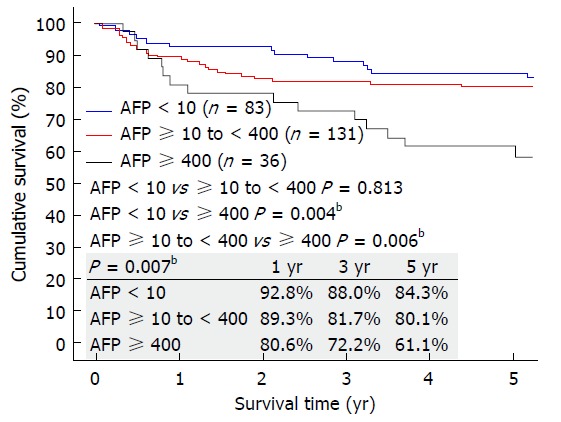
Disease-free survival of patients with preoperative AFP < 10 ng/mL, ≥ 10 to < 400 ng/mL, and ≥ 400 ng/mL. AFP: Alpha-fetoprotein. bP < 0.01.
Disease recurrence and ROC curve analysis
Recurrence of HCC was identified in 42 patients (42/250 = 16.8%). The ability of preoperative AFP to predict HCC recurrence was analyzed by ROC curve. Area under the curve for AFP was 0.685. Among the cut-off values with sufficient specificity, the cut-off point with the highest C-index was chosen as the optimal cut-off value for subsequent analysis. The selected cut-off value was 54 ng/mL for AFP (C-index 0.685; 95% confidence interval 0.592-0.779; sensitivity 0.595; specificity 0.687) (Figure 3).
Figure 3.
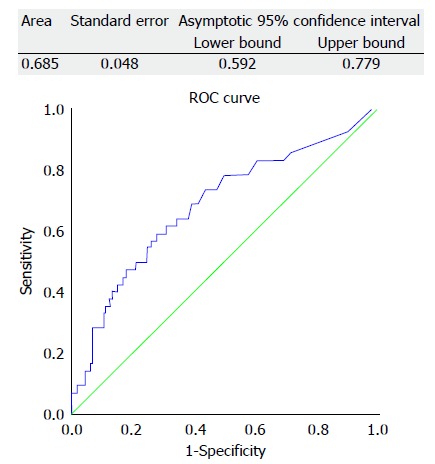
Receiver operating characteristic curve for alpha-fetoprotein in predicting hepatocellular carcinoma recurrence after liver transplant.
Further analysis was performed to identify the lowest AFP level that could affect patient survival. On discriminative analysis, AFP value of 105 ng/mL was identified as the level that could affect the overall survival of the patients. Patients with AFP ≤ 105 ng/mL (Group D) were compared with patients with AFP >105 ng/mL (Group E) (Table 2). Patients in Group E were younger (P = 0.017). When it comes to preoperative comorbidity, underlying cause of cirrhosis, MELD score, operative details, postoperative complication and hospital stay, no significant differences were seen. However, Group E had poorer 5-year graft survival (P = 0.024), patient survival (P = 0.045) (Figure 4), and disease-free survival (P = 0.006) (Figure 5). Looking into the details of the pathological results of the 2 groups, it was clear that the tumors in Group E had worse pathology. Group E had larger tumors (P = 0.017) and fewer cases of well differentiation (15.71% vs 32.78%; P = 0.001), while vascular permeation was more common in this group (44.29% vs 26.67%; P = 0.014) (Table 2).
Table 2.
Comparison of the two subgroups, Group D and Group E
| Group D (n = 180) | Group E (n = 70) | P value | |
| Age (yr) | 56 (38-67) | 53.5 (3-72) | 0.017 |
| Male:Female | 150:30 | 59:11 | 0.855 |
| Diagnosis: | |||
| Cirrhosis | |||
| Cryptogenic | 4 | 1 | |
| Hepatitis B | 136 | 45 | |
| Hepatitis C | 19 | 10 | |
| Alcoholic | 1 | 1 | |
| Hepatitis B + C | 1 | 2 | |
| Alcoholic + hepatitis C | 1 | 1 | |
| Alcoholic + hepatitis B | 1 | 0 | |
| Autoimmune | 1 | 0 | |
| Wilson's disease | 0 | 0 | |
| Preoperative MELD score | 12 (6-35) | 12 (6-43) | 0.972 |
| Waiting time (d) | 58.5 (1-2735) | 32 (1-1874) | 0.183 |
| Blood transfusion (units) | 3 (0-56) | 4(0-32) | 0.988 |
| Fresh frozen plasma transfusion (units) | 6 (0-30) | 6 (0-22) | 0.798 |
| Platelet transfusion (units) | 6 (0-30) | 8 (0-32) | 0.708 |
| Operation time (min) | 654 (333-1110) | 716.5 (300-1273) | 0.151 |
| Cold ischemic time (min) | 137.5 (60-652) | 127.5 (66-633) | 0.195 |
| Warm ischemic time (min) | 51 (25-108) | 53 (28-93) | 0.308 |
| Hospital stay (d) | 15.5 (0-132) | 16 (7-48) | 0.497 |
| Intensive care unit stay (d) | 3 (0-42) | 3 (2-30) | 0.806 |
| Follow-up (mo) | 87.2 (0.0-210.8) | 75.4 (3.8-206.8) | 0.173 |
| Hospital mortality | 4 (2.2%) | 0 | 0.486 |
| LDLT: DDLT | 112:68 | 54:16 | 0.025 |
| Explant Milan Within:Beyond | 116:60 | 39:30 | 0.170 |
| Explant UCSF Within:Beyond | 135:41 | 49:20 | 0.354 |
| No. of tumor in explant | 1.5 (1-multiple) | 1 (1-20) | 0.551 |
| Largest size of tumor in explant (cm) | 2.85 (0.90-7.00) | 3.5 (0.25-19.5) | 0.017 |
| Differentiation | 0.0001 | ||
| Well | 59 | 11 | |
| Moderate | 91 | 41 | |
| Poor | 6 | 10 | |
| Undifferentiated | 0 | 2 | |
| Unknown | 20 | 5 | |
| Vascular permeation | 0.014 | ||
| No | 124 | 35 | |
| Yes | 48 | 31 | |
| Unknown | 4 | 3 | |
| Graft loss | 37 (20.6%) | 24 (34.3%) | 0.023 |
| Patient status Alive:Dead | 143:37 | 47:23 | 0.041 |
| Graft survival, yr | 0.024 | ||
| 1 | 95.00% | 94.30% | |
| 3 | 87.20% | 81.40% | |
| 5 | 83.80% | 72.30% | |
| Patient survival, yr | 0.045 | ||
| 1 | 95.60% | 95.70% | |
| 3 | 87.20% | 82.90% | |
| 5 | 83.80% | 73.80% | |
| Disease-free survival, yr | 0.006 | ||
| 1 | 91.10% | 84.30% | |
| 3 | 85.00% | 75.70% | |
| 5 | 82.20% | 70.00% | |
| Postoperative early complication by Clavien grading | 0.798 | ||
| No | 90 | 38 | |
| I | 35 | 17 | |
| II | 16 | 4 | |
| IIIA | 19 | 6 | |
| IIIB | 11 | 3 | |
| IVA | 6 | 2 | |
| IVB | 0 | 0 | |
| V | 3 | 0 |
Figure 4.
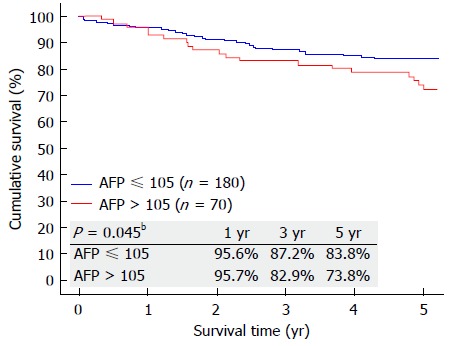
Overall survival of patients with preoperative alpha-fetoprotein ≤ 105 ng/mL and > 105 ng/mL. AFP: Alpha-fetoprotein. bP < 0.01.
Figure 5.
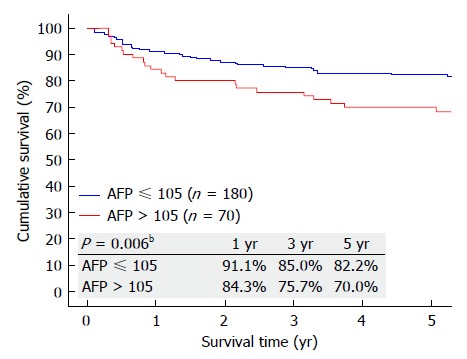
Disease-free survival of patients with preoperative alpha-fetoprotein ≤ 105 ng/mL and > 105 ng/mL. AFP: Alpha-fetoprotein. bP < 0.01.
DISCUSSION
AFP has been used as a tumor marker for HCC. Its elevation depends on pathological characteristics, including tumor size and degree of differentiation of tumor cells. It is a well-established surrogate of tumor biology, as it correlates with histological grading and vascular invasion[12,13,22,23]. The presence of microvascular invasion and poor differentiation of tumor cells are associated with recurrence in patients within and beyond various transplant criteria[24-29]. However, most of the time, preoperative histological results are not available, and therefore prediction of disease recurrence and overall survival cannot be made preoperatively.
AFP is an oncogene protein produced by HCC. Currently, an AFP level of > 400 ng/mL together with a liver mass with characteristic features is a diagnostic feature of HCC. Serum AFP is a well-established prognostic marker of increased tumor virulence in HCC[12,13,30-35]. It has also been shown to be associated with increased risk of waitlist dropout[13,36,37] and post-LT recurrence[12,30-35,38-40]. AFP level has been integrated into a number of transplant criteria, including the Hangzhou criteria[41], the extended Toronto criteria[42], the “total tumor volume”[43], and the Kyoto criteria[44].
Regarding LT for HCC, disease recurrence is a major concern. Extrahepatic metastasis is a clear contraindication to LT as it represents systemic disease, which cannot be cured by LT. The presence of macrovascular invasion has been shown to be an independent risk factor for recurrence and associated with worsened survival[25,45], and therefore is considered a contraindication to LT in the Milan, UCSF and “up-to-seven” criteria[1,5,14]. Poor tumor biology (poor cellular differentiation and presence of microvascular invasion) is associated with an increased risk of tumor recurrence. However, most of the time, the tumor biology cannot be known before operation. Liver biopsy of the target lesion can be an important tool for identifying tumor differentiation and microvascular invasion. It has been proposed that liver biopsy should be included into the Toronto criteria for any number and any size of HCC lesion[42]. While AFP is known to be a well-established surrogate of tumor biology for its correlation with histological grading and vascular invasion[12,13,22,23], it has been used for risk stratification, with elevation of AFP associated with a higher incidence of disease recurrence. Unfortunately, there is no exact cut-off value as an absolute value for contraindication to LT.
AFP is associated with vascular invasion and intrahepatic metastasis is not expressed in well-differentiated HCC[46]. AFP is considered an independent prognostic factor which correlates with histological differentiation[12,47]. This was also reflected in our patients. In Group A, the median time to operation was longer and the outcome was better, suggesting that this group had more favorable tumor biology. Microvascular invasion has been proven to be a strong predictor of outcome (liver resection or LT) for HCC patients[48-51]. Unfortunately, tumor differentiation and microvascular status require histological proof, and most of the time they can only be known after operation. Our patients were divided into 3 main groups, with normal, high and very high preoperative AFP levels. Results showed that higher AFP level was associated with tumor recurrence and correlated with tumor differentiation and microvascular invasion. It was apparent that when the AFP value was lower, the chance of poor differentiation and vascular permeation was also lower. However, this could have been affected by the fact that Group C had more patients beyond the Milan criteria. ROC analysis was performed to assess the ability of preoperative AFP in predicting HCC recurrence after LT. According to C-index analysis based on ROC, the optimal cut-off value was set at 54 ng/mL. However, on further discriminative analysis, 105 ng/mL was set as the cut-off value and it demonstrated significant survival difference despite same amounts of patients who were within the Milan and UCSF criteria. This suggested the importance of using AFP as one of the preoperative surrogate markers to evaluate LT candidates, as it represents additional information on identifying high-risk patients preoperatively so as to predict the risk of recurrence and to let patients have realistic anticipation regarding their long-term outcomes.
HCC patients within the Milan criteria consistently have a 10%-15% risk of disease recurrence after LT[1]. However, strictly following the rules would turn down a substantial number of patients who could benefit from LT and get a cure, even with reasonable disease-free and overall survival. Therefore, allowance is given mainly based on tumor number and size[5,35,52]. It is hoped that further developed selection tools with expanded criteria can identify patients with low risk of recurrence. Applying AFP cut-off could have resulted in the exclusion of certain patients who could have reasonable survival but were at higher risk of tumor recurrence as compared with patients with a lower AFP level. However, using AFP alone to predict the subsequent disease course would result in bias in selection of patients for LT, since patients with the same AFP level can have similar disease-free survival as well as overall survival. Therefore, in listing patients for LT, other factors should also be considered, such as established criteria and tumor status assessed by PET. Fluorine-18-fluorodeoxyglucose PET is able to pick up poorly differentiated HCC[53], while 11C-choline PET has strong avidity for HCC, particularly well differentiated and moderately differentiated tumors[54-56]. Both ways provide preoperative information without the need for biopsy of the liver tumor. For patients who have poor liver function that precludes liver resection, ablative therapy and transarterial chemoembolization, palliative treatment would be the only option if they are denied LT. For these patients, LT should not be ruled out if a relatively inferior survival outcome is acceptable to them. Since LDLT is almost exclusively performed among family members, oftentimes they would accept a relatively inferior survival outcome of the recipient, given a reasonable donor operative outcome.
The findings of this study may not be universally applicable, as AFP 105 ng/mL is a value calculated from our cohort of 250 patients. Moreover, this is a retrospective cohort study with inevitable selection bias. Furthermore, the different levels of AFP (normal, high and very high) were arbitrarily defined. Before adopting AFP as a decision-making tool based on current selection criteria, we have to balance the risk of disease recurrence (hence overall survival) and the patients’ expectation. Still, it is hoped that this study can shed some light on the importance of adding AFP to the armamentarium of assessment tools for LT listing.
HCC patients with a high preoperative AFP level had inferior survival after LT. AFP level of 54 ng/mL was associated with disease recurrence, and AFP level of 105 ng/mL was found to be the cut-off value for overall survival difference.
ARTICLE HIGHLIGHTS
Research background
Liver transplantation is the best treatment option for hepatocellular carcinoma. However, only patients’ tumor criteria should fit the current adopted selection criteria. Most of the criteria are morphological descriptions, including size and number, with the recently added alpha-fetoprotein in some of the updated criteria.
Research motivation
We hoped to identify the cutoff value of alpha-fetoprotein in predicting disease recurrence and overall survival. Apart from using size and number as the selection criteria for liver transplantation, the additional use of alpha-fetoprotein might be able to give practical prediction of disease recurrence.
Research objectives
The objective of this study is to investigate the impact of alpha-fetoprotein on the long-term recurrence rate and overall survival of recipients of liver transplantation for hepatocellular carcinoma.
Research methods
Data of adult patients who received liver transplantation for hepatocellular carcinoma at our hospital from January 2000 to December 2013 were reviewed. Data of included patients were analyzed. We defined the different levels of alpha-fetoprotein as normal (< 10 ng/mL), high (≥ 10 to < 400 ng/mL) and very high (≥ 400 ng/mL). The patients were divided into these 3 groups accordingly. Group comparison was then made.
Research results
Alpha-fetoprotein level was normal in 83 patients, high in 131 patients, and very high in 36 patients. The commonest etiology was hepatitis-B-related cirrhosis. The Model for End-stage Liver Disease scores in these groups were similar (median, 13 vs 13 vs 12; P = 0.745). Patients with normal alpha-fetoprotein level had longer time to operation (median, 94 vs 31 vs 35 d; P = 0.001). The groups were similar in hospital mortality (P = 0.626) and postoperative complication (P = 0.702). Pathology of explants showed that the 3 groups had similar numbers of tumor nodules, but patients with very high alpha-fetoprotein level had bigger tumors (P = 0.003). This group also had a bigger proportion of patients who were beyond Milan criteria (P = 0.010). Poor differentiation and vascular permeation were commoner in this group (P = 0.017 and P = 0.003 respectively). It also had poorer 5-year overall survival (P = 0.029) and disease-free survival (P = 0.007). Receiver operating characteristic area under the curve for alpha-fetoprotein in predicting tumor recurrence was 0.685. The selected cut-off value was 54 ng/mL (C-index 0.685; 95%CI: 0.592-0.779; sensitivity 0.595; specificity 0.687). On discriminative analysis, alpha-fetoprotein value of 105 ng/mL was shown to affect the overall survival of the patients.
Research conclusions
This study showed that patients with high preoperative alpha-fetoprotein levels had poorer post-transplant survival. An alpha-fetoprotein level of 54 ng/mL was associated with disease recurrence, and 105 ng/mL was found to be the cutoff value for overall survival difference. These findings would be useful when considering liver transplantation for patients with a high alpha-fetoprotein level. Currently, there is no definite cutoff value of alpha-fetoprotein for ideal oncological outcomes in hepatocellular carcinoma. With the above alpha-fetoprotein values, superior long-term disease-free and overall survival will be achievable if liver transplantation is offered to patients with a lower preoperative alpha-fetoprotein level. The additional use of alpha-fetoprotein will allow better prediction of the long-term survival outcome, and hence affect the future practice in selection of patients for liver transplantation.
Research perspectives
Selection of patients for liver transplantation should not be based on morphological criteria alone. Other biomarkers such as alpha-fetoprotein should be added to the criteria currently used.
Footnotes
Institutional review board statement: Institutional review board approval was not required for this retrospective study since treatments given to patients were not influenced by the study and the clinical data used in the study are anonymous.
Informed consent statement: Patients’ consent to this retrospective study was not required since treatments given to patients were not influenced by the study and no individual patients would be identified as the clinical data used in the study are anonymous.
Conflict-of-interest statement: None of the authors has any conflict of interest.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Peer-review started: November 24, 2017
First decision: December 12, 2017
Article in press: February 5, 2018
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Marino IRR, Qin JM S- Editor: Cui LJ L- Editor: A E- Editor: Li RF
Contributor Information
Wong Hoi She, Department of Surgery, University of Hong Kong, Hong Kong, China.
Albert Chi Yan Chan, Department of Surgery and State Key Laboratory for Liver Research, University of Hong Kong, Hong Kong, China.
Tan To Cheung, Department of Surgery and State Key Laboratory for Liver Research, University of Hong Kong, Hong Kong, China.
Chung Mau Lo, Department of Surgery and State Key Laboratory for Liver Research, University of Hong Kong, Hong Kong, China.
Kenneth Siu Ho Chok, Department of Surgery and State Key Laboratory for Liver Research, University of Hong Kong, Hong Kong, China.
References
- 1.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 2.Herrero JI, Sangro B, Pardo F, Quiroga J, Iñarrairaegui M, Rotellar F, Montiel C, Alegre F, Prieto J. Liver transplantation in patients with hepatocellular carcinoma across Milan criteria. Liver Transpl. 2008;14:272–278. doi: 10.1002/lt.21368. [DOI] [PubMed] [Google Scholar]
- 3.Ito T, Takada Y, Ueda M, Haga H, Maetani Y, Oike F, Ogawa K, Sakamoto S, Ogura Y, Egawa H, et al. Expansion of selection criteria for patients with hepatocellular carcinoma in living donor liver transplantation. Liver Transpl. 2007;13:1637–1644. doi: 10.1002/lt.21281. [DOI] [PubMed] [Google Scholar]
- 4.Kneteman NM, Oberholzer J, Al Saghier M, Meeberg GA, Blitz M, Ma MM, Wong WW, Gutfreund K, Mason AL, Jewell LD, et al. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl. 2004;10:1301–1311. doi: 10.1002/lt.20237. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 6.Onaca N, Davis GL, Goldstein RM, Jennings LW, Klintmalm GB. Expanded criteria for liver transplantation in patients with hepatocellular carcinoma: a report from the International Registry of Hepatic Tumors in Liver Transplantation. Liver Transpl. 2007;13:391–399. doi: 10.1002/lt.21095. [DOI] [PubMed] [Google Scholar]
- 7.Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, Grant DR, Greig PD, Shapiro AM, Kneteman NM. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008;14:1107–1115. doi: 10.1002/lt.21484. [DOI] [PubMed] [Google Scholar]
- 8.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7:2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 9.Cillo U, Burra P, Mazzaferro V, Belli L, Pinna AD, Spada M, Nanni Costa A, Toniutto P; I-BELT (Italian Board of Experts in the Field of Liver Transplantation) A Multistep, Consensus-Based Approach to Organ Allocation in Liver Transplantation: Toward a “Blended Principle Model”. Am J Transplant. 2015;15:2552–2561. doi: 10.1111/ajt.13408. [DOI] [PubMed] [Google Scholar]
- 10.Chevret S, Trinchet JC, Mathieu D, Rached AA, Beaugrand M, Chastang C. A new prognostic classification for predicting survival in patients with hepatocellular carcinoma. Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire. J Hepatol. 1999;31:133–141. doi: 10.1016/s0168-8278(99)80173-1. [DOI] [PubMed] [Google Scholar]
- 11.Farinati F, Rinaldi M, Gianni S, Naccarato R. How should patients with hepatocellular carcinoma be staged? Validation of a new prognostic system. Cancer. 2000;89:2266–2273. [PubMed] [Google Scholar]
- 12.Duvoux C, Roudot-Thoraval F, Decaens T, Pessione F, Badran H, Piardi T, Francoz C, Compagnon P, Vanlemmens C, Dumortier J, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143:986–994.e3; quiz e14-e15. doi: 10.1053/j.gastro.2012.05.052. [DOI] [PubMed] [Google Scholar]
- 13.Hameed B, Mehta N, Sapisochin G, Roberts JP, Yao FY. Alpha-fetoprotein level > 1000 ng/mL as an exclusion criterion for liver transplantation in patients with hepatocellular carcinoma meeting the Milan criteria. Liver Transpl. 2014;20:945–951. doi: 10.1002/lt.23904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 15.Chok KS, Chan SC, Cheung TT, Chan AC, Fan ST, Lo CM. Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg. 2011;35:2058–2062. doi: 10.1007/s00268-011-1146-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai WC, Chan SC, Chok KS, Cheung TT, Sharr WW, Chan AC, Tsang SH, Fung JY, Poon RT, Fan ST, et al. Good longterm survival after primary living donor liver transplantation for solitary hepatocellular carcinomas up to 8 cm in diameter. HPB (Oxford) 2014;16:749–757. doi: 10.1111/hpb.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan SC, Sharr WW, Chok KS, Chan AC, Lo CM. Wait and transplant for stage 2 hepatocellular carcinoma with deceased-donor liver grafts. Transplantation. 2013;96:995–999. doi: 10.1097/TP.0b013e3182a339a7. [DOI] [PubMed] [Google Scholar]
- 18.Fan ST, Lo CM, Liu CL. Technical refinement in adult-to-adult living donor liver transplantation using right lobe graft. Ann Surg. 2000;231:126–131. doi: 10.1097/00000658-200001000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. Calculation of child and adult standard liver volume for liver transplantation. Hepatology. 1995;21:1317–1321. [PubMed] [Google Scholar]
- 20.Lo CM, Fan ST, Liu CL, Wong J. Hepatic venoplasty in living-donor liver transplantation using right lobe graft with middle hepatic vein. Transplantation. 2003;75:358–360. doi: 10.1097/01.TP.0000046527.19422.3E. [DOI] [PubMed] [Google Scholar]
- 21.Chok KS, Fung JY, Chan AC, Dai WC, Sharr WW, Cheung TT, Chan SC, Lo CM. Comparable Short- and Long-term Outcomes in Living Donor and Deceased Donor Liver Transplantations for Patients With Model for End-stage Liver Disease Scores ≥35 in a Hepatitis-B Endemic Area. Ann Surg. 2017;265:173–177. doi: 10.1097/SLA.0000000000001671. [DOI] [PubMed] [Google Scholar]
- 22.Grąt M, Krasnodębski M, Patkowski W, Wronka KM, Masior Ł, Stypułkowski J, Grąt K, Krawczyk M. Relevance of Pre-Transplant α-fetoprotein Dynamics in Liver Transplantation for Hepatocellular Cancer. Ann Transplant. 2016;21:115–124. doi: 10.12659/aot.894644. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, Yamaoka Y, Belghiti J, Lauwers GY, Poon RT, et al. Tumor size predicts vascular invasion and histologic grade: Implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 24.Ciccarelli O, Lai Q, Goffette P, Finet P, De Reyck C, Roggen F, Sempoux C, Doffagne E, Reding R, Lerut J. Liver transplantation for hepatocellular cancer: UCL experience in 137 adult cirrhotic patients. Alpha-foetoprotein level and locoregional treatment as refined selection criteria. Transpl Int. 2012;25:867–875. doi: 10.1111/j.1432-2277.2012.01512.x. [DOI] [PubMed] [Google Scholar]
- 25.Finkenstedt A, Vikoler A, Portenkirchner M, Mülleder K, Maglione M, Margreiter C, Moser P, Vogel W, Bale R, Freund M, et al. Excellent post-transplant survival in patients with intermediate stage hepatocellular carcinoma responding to neoadjuvant therapy. Liver Int. 2016;36:688–695. doi: 10.1111/liv.12966. [DOI] [PubMed] [Google Scholar]
- 26.Gunay Y, Guler N, Yaprak O, Dayangac M, Akyildiz M, Altaca G, Yuzer Y, Tokat Y. Living Donor Liver Transplantation Outcomes for Hepatocellular Carcinoma Beyond Milan or UCSF Criteria. Indian J Surg. 2015;77:950–956. doi: 10.1007/s12262-014-1078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perea Del Pozo E, Bernal Bellido C, Sendín Matín M, Cepeda Franco C, Álamo Martínez JM, Suarez Artacho G, Marín Gómez LM, Padillo Ruiz J, Gomez Bravo MÁ. Recurrent Hepatocellular Carcinoma After Liver Transplantation: Analysis of Risk Factors. Transplant Proc. 2016;48:2990–2993. doi: 10.1016/j.transproceed.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 28.Vasuri F, Malvi D, Rosini F, Baldin P, Fiorentino M, Paccapelo A, Ercolani G, Pinna AD, Golfieri R, Morselli-Labate AM, et al. Revisiting the role of pathological analysis in transarterial chemoembolization-treated hepatocellular carcinoma after transplantation. World J Gastroenterol. 2014;20:13538–13545. doi: 10.3748/wjg.v20.i37.13538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wan P, Xia Q, Zhang JJ, Li QG, Xu N, Zhang M, Chen XS, Han LZ. Liver transplantation for hepatocellular carcinoma exceeding the Milan criteria: a single-center experience. J Cancer Res Clin Oncol. 2014;140:341–348. doi: 10.1007/s00432-013-1576-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agopian VG, Harlander-Locke M, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Finn RS, Tong M, Hiatt JR, Busuttil RW. A novel prognostic nomogram accurately predicts hepatocellular carcinoma recurrence after liver transplantation: analysis of 865 consecutive liver transplant recipients. J Am Coll Surg. 2015;220:416–427. doi: 10.1016/j.jamcollsurg.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 31.Berry K, Ioannou GN. Serum alpha-fetoprotein level independently predicts posttransplant survival in patients with hepatocellular carcinoma. Liver Transpl. 2013;19:634–645. doi: 10.1002/lt.23652. [DOI] [PubMed] [Google Scholar]
- 32.Lai Q, Avolio AW, Manzia TM, Sorge R, Agnes S, Tisone G, Berloco PB, Rossi M. Combination of biological and morphological parameters for the selection of patients with hepatocellular carcinoma waiting for liver transplantation. Clin Transplant. 2012;26:E125–E131. doi: 10.1111/j.1399-0012.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 33.Mailey B, Artinyan A, Khalili J, Denitz J, Sanchez-Luege N, Sun CL, Bhatia S, Nissen N, Colquhoun SD, Kim J. Evaluation of absolute serum α-fetoprotein levels in liver transplant for hepatocellular cancer. Arch Surg. 2011;146:26–33. doi: 10.1001/archsurg.2010.295. [DOI] [PubMed] [Google Scholar]
- 34.Sotiropoulos GC, Molmenti EP, Lösch C, Beckebaum S, Broelsch CE, Lang H. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur J Med Res. 2007;12:527–534. [PubMed] [Google Scholar]
- 35.Toso C, Asthana S, Bigam DL, Shapiro AM, Kneteman NM. Reassessing selection criteria prior to liver transplantation for hepatocellular carcinoma utilizing the Scientific Registry of Transplant Recipients database. Hepatology. 2009;49:832–838. doi: 10.1002/hep.22693. [DOI] [PubMed] [Google Scholar]
- 36.Merani S, Majno P, Kneteman NM, Berney T, Morel P, Mentha G, Toso C. The impact of waiting list alpha-fetoprotein changes on the outcome of liver transplant for hepatocellular carcinoma. J Hepatol. 2011;55:814–819. doi: 10.1016/j.jhep.2010.12.040. [DOI] [PubMed] [Google Scholar]
- 37.Soriano A, Varona A, Gianchandani R, Moneva ME, Arranz J, Gonzalez A, Barrera M. Selection of patients with hepatocellular carcinoma for liver transplantation: Past and future. World J Hepatol. 2016;8:58–68. doi: 10.4254/wjh.v8.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agopian VG, Morshedi MM, McWilliams J, Harlander-Locke MP, Markovic D, Zarrinpar A, Kaldas FM, Farmer DG, Yersiz H, Hiatt JR, et al. Complete pathologic response to pretransplant locoregional therapy for hepatocellular carcinoma defines cancer cure after liver transplantation: analysis of 501 consecutively treated patients. Ann Surg. 2015;262:536–545; discussion 543-545. doi: 10.1097/SLA.0000000000001384. [DOI] [PubMed] [Google Scholar]
- 39.Guzman G, Alagiozian-Angelova V, Layden-Almer JE, Layden TJ, Testa G, Benedetti E, Kajdacsy-Balla A, Cotler SJ. p53, Ki-67, and serum alpha feto-protein as predictors of hepatocellular carcinoma recurrence in liver transplant patients. Mod Pathol. 2005;18:1498–1503. doi: 10.1038/modpathol.3800458. [DOI] [PubMed] [Google Scholar]
- 40.Todo S, Furukawa H; Japanese Study Group on Organ Transplantation. Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240:451–459; discussion 459-461. doi: 10.1097/01.sla.0000137129.98894.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85:1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 42.Sapisochin G, Goldaracena N, Laurence JM, Dib M, Barbas A, Ghanekar A, Cleary SP, Lilly L, Cattral MS, Marquez M, et al. The extended Toronto criteria for liver transplantation in patients with hepatocellular carcinoma: A prospective validation study. Hepatology. 2016;64:2077–2088. doi: 10.1002/hep.28643. [DOI] [PubMed] [Google Scholar]
- 43.Toso C, Meeberg G, Hernandez-Alejandro R, Dufour JF, Marotta P, Majno P, Kneteman NM. Total tumor volume and alpha-fetoprotein for selection of transplant candidates with hepatocellular carcinoma: A prospective validation. Hepatology. 2015;62:158–165. doi: 10.1002/hep.27787. [DOI] [PubMed] [Google Scholar]
- 44.Kaido T, Ogawa K, Mori A, Fujimoto Y, Ito T, Tomiyama K, Takada Y, Uemoto S. Usefulness of the Kyoto criteria as expanded selection criteria for liver transplantation for hepatocellular carcinoma. Surgery. 2013;154:1053–1060. doi: 10.1016/j.surg.2013.04.056. [DOI] [PubMed] [Google Scholar]
- 45.Andreou A, Bahra M, Schmelzle M, Öllinger R, Sucher R, Sauer IM, Guel-Klein S, Struecker B, Eurich D, Klein F, et al. Predictive factors for extrahepatic recurrence of hepatocellular carcinoma following liver transplantation. Clin Transplant. 2016;30:819–827. doi: 10.1111/ctr.12755. [DOI] [PubMed] [Google Scholar]
- 46.Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–1134. doi: 10.1053/jhep.2001.29202. [DOI] [PubMed] [Google Scholar]
- 47.Yaprak O, Akyildiz M, Dayangac M, Demirbas BT, Guler N, Dogusoy GB, Yuzer Y, Tokat Y. AFP level and histologic differentiation predict the survival of patients with liver transplantation for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:256–261. doi: 10.1016/s1499-3872(12)60157-x. [DOI] [PubMed] [Google Scholar]
- 48.Izumi R, Shimizu K, Ii T, Yagi M, Matsui O, Nonomura A, Miyazaki I. Prognostic factors of hepatocellular carcinoma in patients undergoing hepatic resection. Gastroenterology. 1994;106:720–727. doi: 10.1016/0016-5085(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 49.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 50.Liver Cancer Study Group of Japan. Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann Surg. 1990;211:277–287. [PMC free article] [PubMed] [Google Scholar]
- 51.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227:424–432. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marsh JW, Dvorchik I, Bonham CA, Iwatsuki S. Is the pathologic TNM staging system for patients with hepatoma predictive of outcome? Cancer. 2000;88:538–543. doi: 10.1002/(sici)1097-0142(20000201)88:3<538::aid-cncr7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.Pant V, Sen IB, Soin AS. Role of 18F-FDG PET CT as an independent prognostic indicator in patients with hepatocellular carcinoma. Nucl Med Commun. 2013;34:749–757. doi: 10.1097/MNM.0b013e3283622eef. [DOI] [PubMed] [Google Scholar]
- 54.Cheung TT, Chan SC, Ho CL, Chok KS, Chan AC, Sharr WW, Ng KK, Poon RT, Lo CM, Fan ST. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17:1218–1225. doi: 10.1002/lt.22362. [DOI] [PubMed] [Google Scholar]
- 55.Cheung TT, Ho CL, Lo CM, Chen S, Chan SC, Chok KS, Fung JY, Yan Chan AC, Sharr W, Yau T, et al. 11C-acetate and 18F-FDG PET/CT for clinical staging and selection of patients with hepatocellular carcinoma for liver transplantation on the basis of Milan criteria: surgeon’s perspective. J Nucl Med. 2013;54:192–200. doi: 10.2967/jnumed.112.107516. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto Y, Nishiyama Y, Kameyama R, Okano K, Kashiwagi H, Deguchi A, Kaji M, Ohkawa M. Detection of hepatocellular carcinoma using 11C-choline PET: comparison with 18F-FDG PET. J Nucl Med. 2008;49:1245–1248. doi: 10.2967/jnumed.108.052639. [DOI] [PubMed] [Google Scholar]


