Summary
Dengue virus (DENV) infection is considered one of the most important mosquito‐borne diseases. It causes a spectrum of illness that could be due to qualitative and/or quantitative difference(s) of the natural killer (NK) cell responses during acute DENV infection. This view prompted us to perform a detailed phenotypic comparative characterization of NK cell subsets from DENV‐infected patients with dengue fever (DF), patients with dengue haemorrhagic fever (DHF) and healthy controls. The activation/differentiation molecules, CD69 and CD57 and a variety of tissue homing molecules were analysed on the CD56hi CD16− and CD56lo CD16+ NK cells. Although there was no increase in the frequency of the total NK cells during DENV infection compared with the healthy individuals, there was a significant increase in the frequency of the CD56hi CD16− subset and the frequency of CD69 expression by both NK cell subsets during the febrile phase of infection. We also found an increase in the frequencies of cells expressing CD69 and CD57 in the CD56lo CD16+ subset compared with those in the CD56hi CD16− subset. Moreover, although the CD56lo CD16+ subset contained a high frequency of cells expressing skin‐homing markers, the CD56hi CD16− subset contained a high frequency of cells expressing bone marrow and lymph node trafficking markers. Interestingly, no differences of these NK cell subsets were noted in samples from patients with DF versus those with DHF. These findings suggest that activation and differentiation and the patterns of tissue homing molecules of the two major NK cell subsets are different and that these might play a critical role in the immune response against acute DENV infection.
Keywords: cell trafficking, dengue infection, innate immunity, natural killer cells
Abbreviations
- DENV
dengue virus
- DF
dengue fever
- DHF
dengue haemorrhagic fever
- NK cell
natural killer cell
Introduction
Dengue is a mosquito‐borne flavivirus disease that is responsible for infecting almost 400 million individuals annually with a vast majority being infants and children living in tropical and sub‐tropical Asian and Latin American countries.1 Dengue virus (DENV) is transmitted to humans by infected female mosquitoes mainly of the species Aedes aegypti, the mosquito that also transmits Zika virus, yellow fever virus and Chikungunya virus. DENV infection causes a broad spectrum of clinical manifestations, ranging from asymptomatic, relatively mild dengue fever (DF) to severe disease with vascular leakage, shock and multi‐organ failure, traditionally referred to as dengue haemorrhagic fever (DHF)/dengue shock syndrome (DSS), which can lead to death.2 There are four distinct, but antigenically related, serotypes (DENV‐1, ‐2, ‐3, ‐4) that cause dengue infection.3 Primary infection with one serotype is usually benign and provides significant protection against the same serotype with only partial cross‐protection against other serotypes. However, patients with a secondary heterotypic infection are at least 40–80 times more likely to develop DHF/DSS.4 Although significant advances have been made in our understanding of dengue pathogenesis, it is still difficult to predict whether an acutely infected individual will develop a severe form of disease. However, both viral and host genetics along with the characteristics of the innate and acquired host immune responses appear to be involved in the disease outcomes.5, 6 Despite the fact that there is a large body of data with regards to dengue‐specific humoral and cellular immune responses and their role in both disease pathogenesis and protection,4 much less is known about the role of innate immunity. Upon infection with DENV, the virus infects and replicates within the Langerhans cells, macrophages and dendritic cells in the skin followed by spread of the virus throughout the body.7 The innate immune response plays an intrinsic role at the level of these infected cells by recruiting and activating innate immune cells that have the potential to eliminate the virus at the early stage of infection and promote the development of adaptive immune responses.8 The signalling system of type‐1 interferon derived from these infected cells is known to play a crucial role in the activation of innate immune cells including natural killer (NK) cells, which may play an important role in limiting viral replication during acute DENV infection.
Natrual killer cells are an innate immune lymphocyte cell type that comprise up to 15% of all human peripheral blood mononuclear cells (PBMC), and 5–20% of the PBMC lymphocyte population. Traditionally, human NK cells in PBMCs are phenotypically characterized using standard polychromatic flow cytometric techniques, as a cell lineage that does not express CD3 within the gated population of lymphoid cells and by the expression of CD56 (CD3− CD56+). The NK cells can be further divided into subsets based on the expression levels of CD56 and CD16. Hence, there are CD56lo CD16+ and the CD56hi CD16− cells that constitute the two major subsets as well as a minor population of cells that are CD56hi CD16+ NK cells, which are thought to represent an intermediate stage of NK cell maturation.9 The CD56lo CD16+ NK cell subset constitutes up to 90% of total NK cells; it expresses perforin and killer immunoglobulin‐like receptors and is considered to be the subset with a cytotoxic potential that can be recruited into inflammatory tissues in response to chemokine gradients.10 The other CD56hi CD16− NK cell subset is conventionally known as the cytokine‐producing NK cells, and these are rare in the circulation but dominant in lymph nodes and other tissues.11 NK cell activity is transiently increased following DENV infection.12 The DENV envelope protein activates NK cells by binding to the NKp44 activating receptor on the NK cells.13 Furthermore, the activation of NK cells and the subsequent release of intracellular cytotoxic granules have been associated with mild clinical disease.14 These data suggest that NK cells may particularly play an important role during acute infection in the defence against DENV infection.
The aim of this study was to describe more precisely the frequencies of NK cell subsets, their expression of markers of cell activation and those that are associated with tissue‐specific homing in efforts to define a role for these subsets during acute DENV infection. In addition, it was the objective to determine if these phenotypic markers would help to distinguish benign DENV illness from the more severe form of DENV infection including DHF/DSS. Although we found no difference in the frequencies of NK cell subsets based on disease severity, the two major NK cell subsets showed differences in the frequencies of cells expressing activation markers and those associated with tissue‐specific homing. Hence, there was an increase in the frequencies of activation markers and those associated with skin homing by the CD56lo CD16+ NK cell subset and an increase in the frequencies of the CD56hi CD16− NK cell subset that express cell surface markers that are associated with preferential homing to the bone marrow, lymph nodes and central nervous system. These findings suggest that these changes in NK cell subsets may contribute to the tissue‐specific pathology during acute dengue infection.
Materials and methods
Study population and sample collection
Subjects enrolled in this study included those that were clinically classified by the physicians as highly suspected of dengue infection. Dengue‐infected patients were diagnosed and the stage of the disease was classified using routine laboratory measurements including complete blood count, urine and blood chemistry, and clinical records by the attending physicians. Confirmation of DENV infection was performed by a serotype‐specific RT‐PCR and other serological tests including the NS1 ELISA test. Based on the severity of illness as defined by the World Health Organization (1997), the patients were classified as DF (n = 14) and DHF (n = 22) stages 1–4. The patient samples were collected from the paediatric wards at Ramathibodi Hospital and Siriraj Hospital, Mahidol University, Bangkok, Thailand. Samples from healthy volunteers (n = 15) were used as controls. Patients' demographics and characterizations are shown in Table 1. The blood samples were collected in sodium citrate and the protocols were all ethically approved by Siriraj Institutional Review Board, Faculty of Medicine Siriraj Hospital, Mahidol University (approval number Si 092/2010).
Table 1.
Subject demographics and disease characteristics
| Subjects | Demographic data | Laboratory data | Number of patients infected with dengue virus serotype | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of samples | Gender (male:female) | Age, years | Haematocrit | Platelets | ALT | 1 | 2 | 3 | 4 | |
| Dengue fever | 14 | 9 : 5 | 11 (5–19) | 40·9 (36·3–44·5) | 146 071·4 (57 000–321 000) | 74·7 (17–173) | 5 | 5 | 4 | 0 |
| Dengue haemorrhagic fever | 22 | 8 : 14 | 11 (5–19) | 39·8 (24·4–57·5) | 96 227·3 (9000–290 000) | 54·1 (13–130) | 9 | 2 | 10 | 1 |
| Healthy individuals | 15 | 7 : 8 | 13 (10–19) | |||||||
Identification of dengue virus serotype
Plasma samples from DENV‐infected patients were subjected to RNA extraction using a QIAamp viral RNA extraction kit (Qiagen, Hilden, Germany) as per the manufacturer's instructions. Dengue virus serotypes (DEN 1−4) were identified by a multiplex nested RT‐PCR. Briefly, the viral RNA was reverse transcribed to cDNA with Env (E) primers and a multiplex‐nested PCR was performed using a set of four primer pairs specific for sequences within the E region of each DENV serotype (see Supplementary material, Table S1).
Monoclonal antibodies and reagents
The staining panels consisted of the following fluorochrome‐conjugated anti‐human monoclonal antibodies (mAbs): fluorescein isothiocyanate (FITC) ‐conjugated mAb to HLA‐DR (MHC class II cell surface receptor; clone L243), phycoerythrin (PE) ‐conjugated mAb to CD57 (terminal differentiation marker of NK cells; clone HCD57), peridinin chlorophyll protein (PerCP) ‐conjugated mAb to CD45 (pan‐human leucocyte antigen; clone 2D1), PE/Cyanine7 (PE‐Cy7) ‐conjugated mAb to CD3 (T‐cell marker; clone SK7) and CD19 (B‐cell marker; clone HIB19), allophycocyanin (APC) ‐conjugated mAb‐CD7 (transmembrane protein; clone CD7‐6B7), Alexa Fluor® 700 (A700)‐ conjugated mAb to CD56 (NK cell marker; clone HCD56), APC‐Cy7‐conjugated mAb to CD14 (monocyte marker; clone M5E2), Pacific Blue‐conjugated mAb to CD69 (activation marker of NK cell; clone FN50) and BV510‐conjugated mAb to CD16 (FcγIII receptor; clone 3G8). The PE‐conjugated mAbs associated with tissue specific homing used included those with predominant homing to the gut tissues such as CD103 (clone Ber‐ACT8), CCR9 (clone 112509) and Beta7 (clone FIB504); CXCR4 (clone 12G5), CD122 (clone TUGh4), CD132 (clone TU27) and CD137 (clone 4B4‐1) for bone marrow (BM)‐homing receptors; ICOS (clone C398.4A) for a lung‐homing receptor; CD29 (clone HUTS‐21) for a central nervous system‐homing receptor; CCR10 (clone 6588‐5), which is known as a skin‐homing receptor; CCR7 (clone 150503) and CD62L (clone SK11) for lymph node‐homing receptors; CXCR3 (clone 1C6/CXCR3), CCR2 (clone 48607) and CCR5 (clone 2D7/CCR5) for inflamed tissue‐homing receptors. The mAbs purchased from BD Biosciences (San Jose, CA) included anti‐HLA‐DR‐FITC, anti‐CD45‐PerCP and PE‐labelled mAbs to CD103, CXCR4, CD62L, CD29, CCR5 and CXCR3; the PE‐labelled mAbs to CCR9 and CCR7 were purchased from R&D Systems (Minneapolis, MN); the PE‐labelled mAb to ICOS was purchased from eBioscience (San Diego, CA) and the remaining mAbs were purchased from Biolegend (San Diego, CA).
Immunofluorescent staining
One hundred microlitres of whole blood was dispensed in a 12 × 75‐mm polystyrene tube containing saturated concentrations of each of the following mAbs: HLA‐DR‐FITC, CD57‐PE, CD45‐PerCP, CD3‐PE‐Cy7, CD19‐PE‐Cy7, CD7‐APC, CD56‐A700, CD14‐APC‐Cy7, CD69‐Pacific Blue and CD16‐BV510. After 15 min of incubation at room temperature in the dark, the stained cells were re‐suspended in 2 ml of 1× FACSlysing solution (BD Biosciences) then incubated for another 15 min. After centrifugation at 450 g for 5 min, the supernatant fluid was discarded. The stained cell pellets were washed with 500 μl of 1× FACSlysing solution (BD) and incubated for 1 min, followed by the addition of 2 ml of PBS and centrifugation. Finally, the stained samples were re‐suspended in 300 μl of PBS and kept at 4° before analysis using a BD LSRFortessa flow cytometer (BD Immunocytometry Division, Mountain View, CA). For the analysis of tissue‐specific homing markers, the staining procedure used was the same as described above except that CD57‐PE was replaced by the following mAbs: CCR2, CCR5, CCR7, CCR9, CCR10, CD29, CD62L, CD103, CD122, CD132, CD137, CXCR3, CXCR4, ICOS and Beta7.
Flow cytometric analysis
The NK cell subsets were analysed with linear amplification of the FSC‐H and SSC‐H signals and logarithmic amplification of the fluorescence channels. Cells stained with FITC‐, PE‐, PerCP‐ and PE‐Cy7‐conjugated mAbs were excited using a 488‐nmblue laser, the long red APC, A700 and APC‐Cy7 were excited by a 635‐nmred diode laser, whereas the violet Pacific Blue and BV510 were excited by a 405‐nmviolet laser. Acquisition of all events of the stained cells in the bivariant FSC‐H/SSC‐H was performed. The FSC‐H/FSC‐A, SSC‐W/SSC‐H and FSC‐W/FSC‐H were used to discriminate doublets from single cells. The mononuclear cells were identified by SSC‐A/CD45. The monocyte population was deleted from analysis by gating out cells that were strongly positive for the CD14 cell surface molecule confirmed by using FSC‐A/SSC‐A. NK cells were identified by cells that were negative for CD3 and CD19, the cell surface markers of T and B lymphocytes, respectively. The gating strategy for the identification of NK cells and its subsets is shown in Fig. 1. Hence, after gating out CD14+, CD3+ and CD19+ cells, the HLA‐DR+/CCR7+ cells were selected to distinguish NK cells from dendritic cells.15, 16 The total number of NK cells within this gated population varied from 3000 to 30 000 events. The two major subsets of NK cells were identified as CD56hi CD16− and CD56lo CD16+ (Fig. 1). The frequencies of activated and terminally differentiated NK cell subsets were defined by CD69 and CD57, respectively. In addition, the frequencies of each NK subset that expressed each of the tissue‐specific homing markers were also analysed. The data were acquired using the BD LSRFortessa and analysed using the bd cellquest pro software (Ashland, OR).
Figure 1.
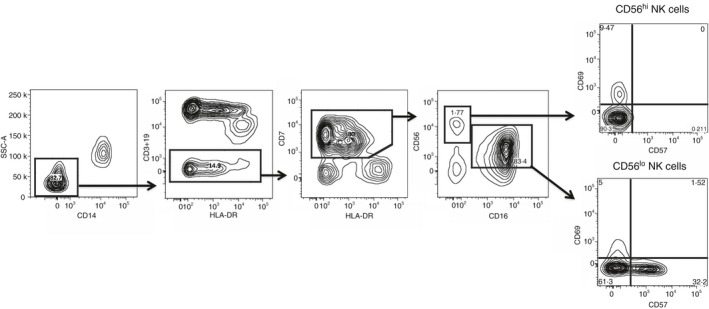
Gating strategy used for the natural killer (NK) cells in peripheral blood. Dot plots show the two major populations of the NK cell subsets, which are CD56hi CD16− and CD56lo CD16+ NK cells on the gated population of CD14−/CD3−/CD19−/HLA− DR−/CD7+ cells.
Statistical analysis
All descriptive statistics were performed using prism software. Data for each assay were expressed as the mean ± SD of the number of samples within each category of patients or controls. Comparisons of statistical difference between parameters were performed using the non‐parametric Mann–Whitney U‐test. The threshold for statistical significance for all comparisons was chosen as P < 0·05.
Results
Frequency of CD56hi CD16− NK cell subset increases during DENV infection
Using polychromatic flow cytometry, we performed phenotypic characterization of the frequencies of NK cells and their subsets in peripheral blood samples from DENV‐infected patients and compared the results obtained from healthy individuals.
As can be seen, there were no significant differences in the frequency of total NK cells between DF patients, DHF patients and healthy individuals (Fig. 2a). However, as seen in Fig. 2(b), whereas the frequencies of the CD56hi CD16− NK cell subset increased significantly in samples from the DF and DHF patients compared with the healthy individuals (P < 0·05 and P = 0·02, respectively) the frequency of the CD56lo CD16+ NK cell subset decreased in samples from the DF patients compared with the healthy individuals (P = 0·04) (Fig. 2c).
Figure 2.
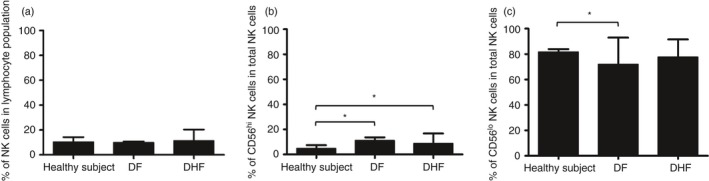
Comparison of the frequencies of natural killer (NK) cells. Bar graphs demonstrating the frequency of total NK cells (a), and the frequency of the CD56hi CD16− NK cell subset (b) and CD56lo CD16+ NK cell subset (c) in total NK cell population in samples from healthy controls (n = 15), dengue fever (DF) patients (n = 14) and dengue haemorrhagic fever (DHF) patients (n = 22). Although there were no significant changes in the frequencies of total NK cells during dengue virus (DENV) infection, the frequencies of CD56hi CD16− NK cells from DF and DHF patients were significantly increased (P < 0·05 and P = 0·02, respectively), and the frequencies of CD56lo CD16+ NK cells in DENV‐infected patients was decreased, especially in DF patients (P = 0·04). (*P < 0·05, Mann–Whitney U‐test).
Magnitude of NK cell activation during acute DENV infection is not distinguished by severity of DENV infection
As expected, acute DENV infection led to increases in the frequencies of CD69‐expressing NK cells. As seen in Fig. 3(a,b), there were significant increases in the frequencies of CD69‐expressing CD56hi CD16− and CD56lo CD16+ NK cells in samples from DENV‐infected patients compared with control healthy individuals (P < 0·001 and P = 0·001, respectively). The increases in NK cell activation were essentially similar in samples from both DF and DHF patients, suggesting a lack of correlation between the frequencies of activated NK cells and severity of the disease.
Figure 3.
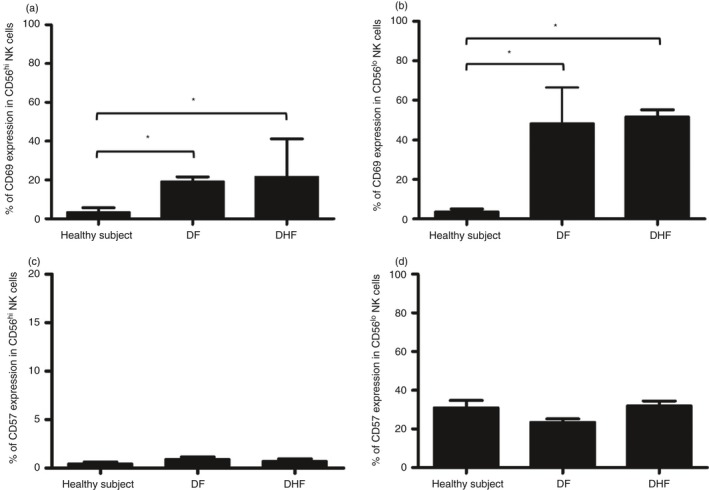
CD69 and CD57 expression on natural killer (NK) cell subsets in samples from patients with different dengue virus (DENV) severity. Bar graphs show the frequency of CD69+ CD56hi CD16− NK cells (a) CD69+ CD56lo CD16+ NK cells (b) CD57+ CD56hi CD16− NK cells and (c) CD57+ CD56lo CD16+ NK cells (d) in samples from healthy individuals (n = 15), dengue fever (DF) patients (n = 14) and dengue haemorrhagic fever (DHF) patients (n = 22). (*P < 0·05, Mann–Whitney U‐test).
The finding that viral infections could contribute to NK cell differentiation prompted us to analyse the frequencies of cells expressing CD57, a terminal differentiation marker. The results of these analyses showed that the frequencies of CD57‐expressing CD56hi CD16− and CD56lo CD16+ NK cell subsets in samples from both DF and DHF patients were similar to that noted for samples from healthy controls (Fig. 3c,d). Frequencies of CD69‐expressing and CD57‐expressing CD56lo CD16+ NK cell subsets were significantly higher than those in the CD56hi CD16− NK cell subset (P < 0·001 for both; see Supplementary material, Fig. S1).
Kinetics of the NK cell activation and differentiation during acute DENV infection
The increased frequencies of NK cells that express the CD69 activation marker during acute DENV infection prompted us to examine in more detail the kinetics by which such increases occur and its relationship to the expression of CD57, the marker of terminally differentiated cells. We therefore determined NK cell subsets in DENV‐infected patients on different days of fever ranging from day −3 (D−3) to day +3 (D+3). Samples collected on D−3 to D−1 were considered as representing the febrile phase, those collected during D0 to D+1 represented the defervescence phase, and those collected during D+2 to D+3 represented the recovery phase. As shown in Fig. 4(a,b), the frequencies of CD69‐expressing CD56hi CD16− (P = 0·003) and CD56lo CD16+ (P < 0·001) NK cell subsets peaked during the febrile phase of DENV infection. The frequencies of CD69‐expressing NK cell subsets subsequently decreased slightly in samples representing the recovery phase. The analysis of CD57‐expressing cells, on the other hand showed a trend towards an increase in the expression of CD57 by NK cells, particularly the CD56lo CD16+ NK cells during the recovery phase of infection but the values obtained did not reach statistical significance (Fig. 4c,d). Overall, these data suggest that during DENV infection, NK cells are activated during the early phase for rapid response against the virus, and differentiate to the final stage of NK cells during the late phase of infection.
Figure 4.
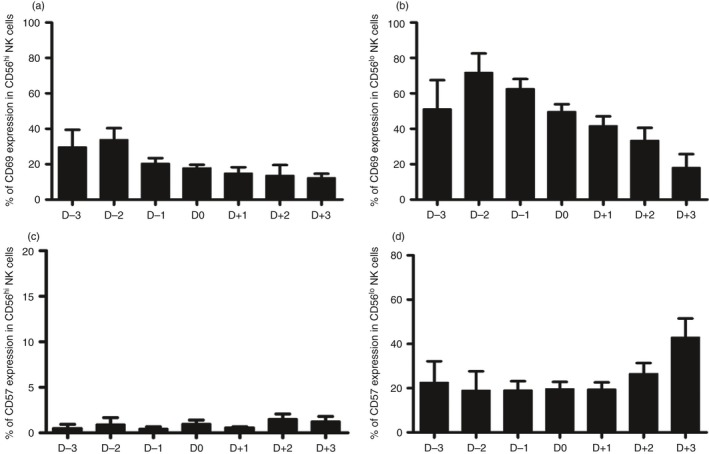
Kinetics of natural killer (NK) cell activation and differentiation during acute dengue virus (DENV) infection. Bar graphs show the frequency of CD69‐expressing CD56hi CD16− NK cells (a) and CD56lo CD16+ NK cells (b), and the percentage of CD56hi CD16− NK cells (c) and CD56lo CD16+ NK cells (d) that express CD57 in samples from day −3 (D−3) to day +3 (D+3) during DENV infection.
Expression of homing molecules of the NK cell subsets during DENV infection
It is generally known that a variety of chemokine receptors are expressed during NK cell activation that serve to mobilize these cells to distinct tissue compartments in vivo. There is, at present, limited knowledge as to the nature of these chemokine receptors expressed by NK cells during acute DENV infection. It is reasoned that the results of such studies may help in our understanding of the kinetics by which tissues that potentially serve as the major targets of DENV induced inflammation during acute infection. We therefore examined a series of homing markers that serve to direct cells to distinct tissue compartments and included those that guide cells to home to the gastro‐intestinal tissues (CD103, CCR9 and Beta7), those that guide cells to the bone marrow (CD122, CD132, CD137 and CXCR4), those that are known to be lymph node seeking (CCR7, CD62L), the skin (CCR10), the lungs (ICOS), central nervous system (CD29), and those that home to inflamed tissues in general (CCR2, CCR5 and CXCR3). We investigated the expression of these homing markers on NK cell subsets on samples from DENV‐infected patients and compared these values with those obtained on samples from healthy individuals.
The expression of homing markers on the surface of the CD56hi CD16− and CD56lo CD16+ NK cell subsets varied in both DENV‐infected patients (D) and healthy individuals (H). First of all, the homing markers that were expressed at low to ultra‐low levels by both subsets of NK cells in both healthy individuals and dengue‐infected patients included CXCR4, ICOS, CD137, CD103, CD29, CCR9, CCR7 and CCR2 (Fig. 5). The major differences noted between healthy individuals and dengue patients was the decreased frequencies of both NK cell subsets that expressed CXCR3 (P < 0·05) and increased frequencies of both subsets that expressed CCR10 (P < 0·001). However, there was no significant difference in the level of expression of any of these homing molecules between the DF and DHF patients (see Supplementary material, Fig. S2).
Figure 5.
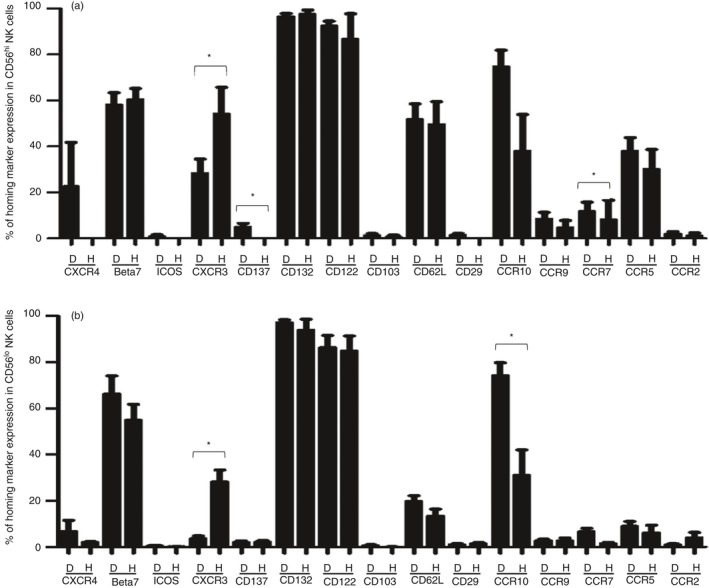
Expression of homing molecules on activated natural killer (NK) cell subsets in dengue virus (DENV) ‐infected patients. Bar graphs show the frequency of homing markers expressed by activated CD56hi CD16− NK cells (a) and CD56lo CD16+ NK cells (b) in samples from DENV‐infected patients (D) and healthy individuals (H). (*P < 0·05, Mann–Whitney U‐test).
These data indicate that the most distinguishing characteristics in homing marker expression between DENV‐infected patients and healthy individuals was the increased expression of the homing markers to bone marrow, lymph nodes and skin by both NK cell subsets in DENV‐infected patients compared with controls. A decreased frequency of CXCR3 (marker of inflammation) expression was also observed in both NK cell subsets; however, the significance of this decrease remains unclear.
Discussion
In addition to B‐ and T‐cell adaptive immunity, the innate immune response constitutes the first line of protection against pathogenic microorganisms and plays an important role during the early control of viral infections. NK cells are innate lymphoid cells specialized in recognizing and destroying virus‐infected cells during the early phase of infections. In this study, we extensively characterized the expression of activation and differentiation markers of the NK cell subsets in peripheral blood collected from young patients during acute DENV infection. This study most likely represents patients who have been exposed previously to either DENV or other cross‐reactive flaviviruses and so are secondary or tertiary infections. Therefore, these data need to be interpreted with this in mind.
As opposed to previous studies that demonstrated increased levels of NK cells in acute DENV‐infected patients,14, 17 we did not detect any difference in the frequency of the total NK cells among the DF and DHF patients compared with the healthy controls. The reasons for this contradictory result are not clear but a number of variable factors have been previously identified and could be the basis. These include the development of mechanisms that lead to evasion of the human innate immune system by previous exposure to dengue or other related flaviviruses, variations in the levels of poorly neutralizing serotype cross‐reactive antibodies known to induce antibody‐dependent enhancement, cross‐reactive T cells and rapid and massive virus‐specific plasmablast response.18, 19, 20, 21 DENV‐infected patients showed a significantly higher frequency of the CD56hi CD16− NK cell subset concomitantly with a lower frequency of the CD56lo CD16+ NK cell subset than the healthy individuals but there was no significant difference in the frequency of these two major NK cell subsets between the DF and DHF patients. There are increasing numbers of articles describing the activation of CD56hi CD16− NK cells in many diseases.22, 23, 24 The cells with phenotypic characteristics of CD56hi NK cells were increased significantly in patients with a positive tuberculin skin test compared with patients with overt tuberculosis and normal individuals.25 The same phenomenon was also observed in patients who were chronically infected with hepatitis C virus (HCV), in which the proportion of CD56hi CD16− among total NK cells was increased and these immunoregulatory NK cells produced more interferon‐γ than HCV resolvers and normal uninfected controls.26 The significant increase of CD56hi CD16− in these patients might contribute to T‐cell polarization, as in the case of HCV, and protect from active tuberculosis by secreting high amounts of interferon‐γ by CD56hi CD16− NK cells. In addition, the proportion of NK cells and their subsets that express the early activation marker, CD69, significantly increased in the DENV‐infected patients compared with the healthy controls, but there was no significant difference in frequency of CD69+ NK cells between DF and DHF patients. This activation of the NK cells was transient as the values slightly decreased to baseline level a few days after disease onset. These results were consistent with other studies demonstrating an elevation of the frequency of CD69+ NK cells during the acute phase of DENV infection,13, 14, 27 particularly in children who developed DHF.28, 29 The NK cell activation has been observed not only in primary DENV infection in mice,17 but also in response to influenza and hepatitis B virus (HBV) infection, and shown to correlate with human immunodeficiency virus 1 (HIV‐1) disease progression.22, 23, 24 There was an increase of CD57 expression in NK cells during infection especially by the CD56lo CD16+ NK cell subset and the population was slightly increased, suggesting that the less differentiated CD56lo CD16+ NK cells become more mature as exemplified by the expression of CD57 during the recovery phase of infection. The CD57 expression is associated with NK cell maturation and function, which includes high cytolytic activity and cytokine production. Increased frequencies of CD57+ NK cells have been reported in patients with a variety of viral infections such as cytomegalovirus, HIV, Chikungunya virus, HBV and HCV.30, 31, 32, 33 Interestingly, CD57 is also considered as a marker of memory NK cells that can expand rapidly in response to the appropriate viral antigens. This is supported by the finding that the NK cells directly recognize mouse cytomegalovirus and result in extensive proliferation of this cell lineage and leads to the generation of long‐lived NK cells with characteristics that are similar to that noted for memory T cells.34 Taken together, these data suggest that both activation and maturation of NK cells are induced during the acute phase of DENV infection.
The NK cells are one of the earliest immune cell types rapidly recruited to sites of infection. They leave the bone marrow and reside in the blood, spleen, liver and various organs.35 It is reasonable to assume that more information on the expression of homing markers by the NK cells, particularly during acute infection, may provide some clues with regards to where these cells originate and target. Using flow cytometry techniques, we analysed the expression of a series of homing markers for many tissues or organs on activated peripheral blood CD56hi CD16− and CD56lo CD16+ cells, the two major NK cell subsets in samples from DF/DHF patients and, for comparison, healthy individuals. A high frequency of cells expressing the homing molecules that promote the trafficking of cells to the bone marrow and lymph nodes were shown to be expressed by the activated population of the cytokine‐synthesizing CD56hi CD16− NK cell subset. On the other hand a high frequency of cells expressing the homing molecule that promotes trafficking to the skin was found on the activated CD56lo CD16+ cytolytic NK cell subset. These data appear to suggest that the predominant homing of these NK cells (particularly activated NK cells) appears to be directed to both the bone marrow, skin and possibly the lymph nodes. These tissue‐specific localizations are consistent with the generally accepted finding of DENV infection being a ‘bone‐breaking disease’ and a disease that often results in ‘petechiae’ following resolution of infection. We reason that elevated levels of these homing markers enhance the influx of the NK cells to those specific tissue sites that may be involved in protection by limiting viral replication or exacerbation of DENV pathogenesis that would otherwise occur by the overt triggering of cytokine production that might facilitate the infection. We showed that CD56hi CD16− NK cells retained the homing receptors expression for migration into the bone marrow and lymph nodes, which are known as important organs for NK cell development.36, 37 Previous observations indicate that various NK cell developmental stages can be found in different tissues and organs, including bone marrow, lymph nodes, thymus, liver and spleen. The bone marrow has been considered as the primary site of NK cell development38, 39 with precursors that are derived from the pluripotent CD34+ haematopoietic precursor cells. These cells migrate to lymph nodes to differentiate into CD56hi CD16− and CD56lo CD16+ NK cells, respectively.37, 40, 41 Long‐term bone marrow cultures with bone marrow‐derived CD34+ haematopoietic precursor cells and stroma are able to support the in vitro differentiation of NK cells, and the depletion of bone marrow causes NK cell deficiency in mice.42, 43, 44, 45 The CD56hi CD16− NK cells commonly express homing molecules that facilitate their migration to secondary lymphoid organs, as exemplified by the finding that 90% of the NK cells in lymph nodes are CD56hi CD16− NK cells.40, 46, 47 During DENV infection, skin is the first encounter between DENV and the immune system as the viruses are transmitted by mosquitoes and enter the body via the skin.7, 48 Langerhans cells are the targets for DENV replication and the viruses then travel to the lymph nodes and activate immune responses. Our findings showed that a high frequency of the CD56lo CD16+ NK cells express CCR10, which suggests that the migration of CD56lo CD16+ NK cells to the skin may play an important role in the response to DENV infection. The CD56lo CD16+ NK cell subset has potent cytolytic activity, so they might suppress viral replication by destroying the target cells at the inflammatory tissue site during acute infection.49 Activated CD56lo CD16+ NK cells release perforin and granzyme, two cytotoxic proteins in the secretory granules that can trigger apoptosis of infected cells. Our results showed lower expression of the CXCR3 molecule in both NK cell subsets when compared with healthy controls and this was in accordance with previous studies especially in CD56hi CD16− NK cell subset.50, 51 Several studies have previously shown an involvement of the CXCR3 chemokine during inflammation as the CXCR3‐deficient mice have significantly higher mortality rates and viral loads in the brain after DENV infection than the wild‐type mice.52 One possible explanation for this finding is that CXCR3‐expressing NK cells might accumulate in the inflammatory tissues, so we could not detect them in the peripheral blood. Supporting evidence in other viral infections shows that lymph nodes and cerebrospinal fluid are enriched with CXCR3+ CD8 T cells and also its cognate ligands CXCL9 and CXCL10 compared with the levels in the blood during the early phase of HIV infection.53, 54, 55, 56
In conclusion, our study demonstrates an important role of NK cells in the response against the early phase of DENV infection. Although we could not observe any association between the NK cell subsets and the severity of DENV disease, we found an increase in the frequency of activated NK cells, especially in the CD56lo CD16+ NK cell subset during acute infection. The two major subsets of NK cells also appear to express chemokines that facilitate homing to specific tissues as exemplified by the finding of the preferential residency of CD56hi CD16− NK cell subset in the bone marrow and possibly in lymph nodes, whereas the CD56lo CD16+ NK cell subset migrates to the skin. These results may provide a better understanding of dengue pathogenesis and provide food for thought with regard to the development of prognostic markers and effective treatment for DENV infection.
Author contribution
RK performed the experiments, analysed the data and wrote the manuscript. LK and SL contributed to sample preparation and helped in data interpretation. KT, NA, SY, AC and KC provided dengue blood samples. AAA provided a conceptual framework and contributed to the final version of the manuscript. NO co‐supervised and designed the experiments. KP supervised the research and wrote the manuscript.
Disclosures
The authors declare no conflict of interest.
Supporting information
Figure S1. Comparison of CD69 and CD57 expression between natural killer (NK) cell subsets in samples from dengue virus (DENV) ‐infected patients.
Figure S2. Comparison of homing molecules expression on natural killer (NK) cell subsets between dengue fever (DF) and dengue haemorrhagic fever (DHF) patients.
Table S1. PCR primers for dengue serotyping.
Acknowledgements
This research project was supported by grant from the NIH grant no. 1R01AI099385‐01 and the Thailand Research Fund (TRF) – Distinguished Research Professor Grant, grant no. DPG5980001. LW and NO were also supported by the Chalermprakiat Foundation, Faculty of Medicine Siriraj Hospital, Mahidol University. Both RK and SL were supported by the TRF‐Royal Golden Jubilee Ph.D. programme.
References
- 1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL et al The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Dengue 2009 [WWW document]. URL http://www.cdc.gov/dengue/clinicallab/clinical.html [accessed 6 September 2014].
- 3. Ligon BL. Dengue fever and dengue hemorrhagic fever: a review of the history, transmission, treatment, and prevention. Semin Pediatr Infect Dis 2005; 16:60–5. [DOI] [PubMed] [Google Scholar]
- 4. Nielsen DG. The relationship of interacting immunological components in dengue pathogenesis. Virol J 2009; 6:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine 2011; 29:7221–8. [DOI] [PubMed] [Google Scholar]
- 6. Yacoub S, Mongkolsapaya J, Screaton G. The pathogenesis of dengue. Curr Opin Infect Dis 2013; 26:284–9. [DOI] [PubMed] [Google Scholar]
- 7. Briant L, Despres P, Choumet V, Misse D. Role of skin immune cells on the host susceptibility to mosquito‐borne viruses. Virology 2014; 464–465:26–32. [DOI] [PubMed] [Google Scholar]
- 8. Tosi MF. Innate immune responses to infection. J Allergy Clin Immunol 2005; 116:241–9; quiz 50. [DOI] [PubMed] [Google Scholar]
- 9. Beziat V, Duffy D, Quoc SN, Le Garff‐Tavernier M, Decocq J, Combadiere B et al CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol 2011; 186:6753–61. [DOI] [PubMed] [Google Scholar]
- 10. Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ 2008; 15:226–33. [DOI] [PubMed] [Google Scholar]
- 11. Poli A, Michel T, Theresine M, Andres E, Hentges F, Zimmer J. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 2009; 126:458–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shresta S, Kyle JL, Snider HM, Basavapatna M, Beatty PR, Harris E. Interferon‐dependent immunity is essential for resistance to primary dengue virus infection in mice, whereas T‐ and B‐cell‐dependent immunity are less critical. J Virol 2004; 78:2701–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hershkovitz O, Rosental B, Rosenberg LA, Navarro‐Sanchez ME, Jivov S, Zilka A et al NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J Immunol 2009; 183:2610–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Azeredo EL, De Oliveira‐Pinto LM, Zagne SM, Cerqueira DI, Nogueira RM, Kubelka CF. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin Exp Immunol 2006; 143:345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rabinowich H, Lin WC, Herberman RB, Whiteside TL. Signaling via CD7 molecules on human NK cells. Induction of tyrosine phosphorylation and β1 integrin‐mediated adhesion to fibronectin. J Immunol 1994; 153:3504–13. [PubMed] [Google Scholar]
- 16. Milush JM, Lopez‐Verges S, York VA, Deeks SG, Martin JN, Hecht FM et al CD56negCD16+ NK cells are activated mature NK cells with impaired effector function during HIV‐1 infection. Retrovirology 2013; 10:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shresta S, Kyle JL, Robert Beatty P, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology 2004; 319:262–73. [DOI] [PubMed] [Google Scholar]
- 18. Pagni S, Fernandez‐Sesma A. Evasion of the human innate immune system by dengue virus. Immunol Res 2012; 54:152–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Halstead SB, O'Rourke EJ. Antibody‐enhanced dengue virus infection in primate leukocytes. Nature 1977; 265:739–41. [DOI] [PubMed] [Google Scholar]
- 20. Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A et al Rapid and massive virus‐specific plasmablast responses during acute dengue virus infection in humans. J Virol 2012; 86:2911–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Priyamvada L, Cho A, Onlamoon N, Zheng NY, Huang M, Kovalenkov Y et al B cell responses during secondary dengue virus infection are dominated by highly cross‐reactive, memory‐derived plasmablasts. J Virol 2016; 90:5574–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hwang I, Scott JM, Kakarla T, Duriancik DM, Choi S, Cho C et al Activation mechanisms of natural killer cells during influenza virus infection. PLoS One 2012; 7:e51858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Littwitz E, Francois S, Dittmer U, Gibbert K. Distinct roles of NK cells in viral immunity during different phases of acute Friend retrovirus infection. Retrovirology 2013; 10:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zheng Q, Zhu YY, Chen J, Ye YB, Li JY, Liu YR et al Activated natural killer cells accelerate liver damage in patients with chronic hepatitis B virus infection. Clin Exp Immunol 2015; 180:499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barcelos W, Sathler‐Avelar R, Martins‐Filho OA, Carvalho BN, Guimaraes TM, Miranda SS et al Natural killer cell subpopulations in putative resistant individuals and patients with active Mycobacterium tuberculosis infection. Scand J Immunol 2008; 68:92–102. [DOI] [PubMed] [Google Scholar]
- 26. Golden‐Mason L, Madrigal‐Estebas L, McGrath E, Conroy MJ, Ryan EJ, Hegarty JE et al Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut 2008; 57:1121–8. [DOI] [PubMed] [Google Scholar]
- 27. Tsai JJ, Jen YH, Chang JS, Hsiao HM, Noisakran S, Perng GC. Frequency alterations in key innate immune cell components in the peripheral blood of dengue patients detected by FACS analysis. J Innate Immun 2011; 3:530–40. [DOI] [PubMed] [Google Scholar]
- 28. Green S, Pichyangkul S, Vaughn DW, Kalayanarooj S, Nimmannitya S, Nisalak A et al Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J Infect Dis 1999; 180:1429–35. [DOI] [PubMed] [Google Scholar]
- 29. Homchampa P, Sarasombath S, Suvatte V, Vongskul M. Natural killer cells in dengue hemorrhagic fever/dengue shock syndrome. Asian Pac J Allergy Immunol 1988; 6:95–102. [PubMed] [Google Scholar]
- 30. Gratama JW, Kluin‐Nelemans HC, Langelaar RA, den Ottolander GJ, Stijnen T, D'Amaro J et al Flow cytometric and morphologic studies of HNK1+ (Leu 7+) lymphocytes in relation to cytomegalovirus carrier status. Clin Exp Immunol 1988; 74:190–5. [PMC free article] [PubMed] [Google Scholar]
- 31. Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X et al Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119:2665–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hong HS, Eberhard JM, Keudel P, Bollmann BA, Ballmaier M, Bhatnagar N et al HIV infection is associated with a preferential decline in less‐differentiated CD56dim CD16+ NK cells. J Virol 2010; 84:1183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petitdemange C, Becquart P, Wauquier N, Beziat V, Debre P, Leroy EM et al Unconventional repertoire profile is imprinted during acute chikungunya infection for natural killer cells polarization toward cytotoxicity. PLoS Pathog 2011; 7:e1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lopez‐Verges S, Milush JM, Pandey S, York VA, Arakawa‐Hoyt J, Pircher H et al CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+ NK‐cell subset. Blood 2010; 116:3865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shi FD, Ljunggren HG, La Cava A, Van Kaer L. Organ‐specific features of natural killer cells. Nat Rev Immunol 2011; 11:658–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Caligiuri MA. Human natural killer cells. Blood 2008; 112:461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Freud AG, Caligiuri MA. Human natural killer cell development. Immunol Rev 2006; 214:56–72. [DOI] [PubMed] [Google Scholar]
- 38. Solomon T, Dung NM, Vaughn DW, Kneen R, Thao LT, Raengsakulrach B et al Neurological manifestations of dengue infection. Lancet 2000; 355:1053–9. [DOI] [PubMed] [Google Scholar]
- 39. Spits H, Lanier LL, Phillips JH. Development of human T and natural killer cells. Blood 1995; 85:2654–70. [PubMed] [Google Scholar]
- 40. Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ et al A human CD34+ subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity 2005; 22:295–304. [DOI] [PubMed] [Google Scholar]
- 41. Di Santo JP. Natural killer cell developmental pathways: a question of balance. Annu Rev Immunol 2006; 24:257–86. [DOI] [PubMed] [Google Scholar]
- 42. Srour EF, Brandt JE, Briddell RA, Leemhuis T, van Besien K, Hoffman R. Human CD34+ HLA‐DR‐ bone marrow cells contain progenitor cells capable of self‐renewal, multilineage differentiation, and long‐term in vitro hematopoiesis. Blood Cells 1991; 17:287–95. [PubMed] [Google Scholar]
- 43. Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood 1992; 80:2221–9. [PubMed] [Google Scholar]
- 44. Seaman WE, Gindhart TD, Greenspan JS, Blackman MA, Talal N. Natural killer cells, bone, and the bone marrow: studies in estrogen‐treated mice and in congenitally osteopetrotic (mi/mi) mice. J Immunol 1979; 122:2541–7. [PubMed] [Google Scholar]
- 45. Miller JS, Alley KA, McGlave P. Differentiation of natural killer (NK) cells from human primitive marrow progenitors in a stroma‐based long‐term culture system: identification of a CD34+7+ NK progenitor. Blood 1994; 83:2594–601. [PubMed] [Google Scholar]
- 46. Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR et al Unique subpopulations of CD56+ NK and NK‐T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol 2001; 166:6477–82. [DOI] [PubMed] [Google Scholar]
- 47. Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII‐positive and negative natural killer cells. J Immunol 1989; 143:3183–91. [PubMed] [Google Scholar]
- 48. Rivino L, Kumaran EA, Thein TL, Too CT, Gan VC, Hanson BJ et al Virus‐specific T lymphocytes home to the skin during natural dengue infection. Sci Transl Med 2015; 7:278ra35. [DOI] [PubMed] [Google Scholar]
- 49. Maghazachi AA. G protein‐coupled receptors in natural killer cells. J Leukoc Biol 2003; 74:16–24. [DOI] [PubMed] [Google Scholar]
- 50. Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer‐cell subsets. Trends Immunol 2001; 22:633–40. [DOI] [PubMed] [Google Scholar]
- 51. Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA et al The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig‐like receptors and become cytolytic. J Immunol 2004; 172:1455–62. [DOI] [PubMed] [Google Scholar]
- 52. Hsieh MF, Lai SL, Chen JP, Sung JM, Lin YL, Wu‐Hsieh BA et al Both CXCR3 and CXCL10/IFN‐inducible protein 10 are required for resistance to primary infection by dengue virus. J Immunol 2006; 177:1855–63. [DOI] [PubMed] [Google Scholar]
- 53. Foley JF, Yu CR, Solow R, Yacobucci M, Peden KW, Farber JM. Roles for CXC chemokine ligands 10 and 11 in recruiting CD4+ T cells to HIV‐1‐infected monocyte‐derived macrophages, dendritic cells, and lymph nodes. J Immunol 2005; 174:4892–900. [DOI] [PubMed] [Google Scholar]
- 54. Li Q, Schacker T, Carlis J, Beilman G, Nguyen P, Haase AT. Functional genomic analysis of the response of HIV‐1‐infected lymphatic tissue to antiretroviral therapy. J Infect Dis 2004; 189:572–82. [DOI] [PubMed] [Google Scholar]
- 55. Triozzi PL, Bresler HS, Aldrich WA. HIV type 1‐reactive chemokine‐producing CD8+ and CD4+ cells expanded from infected lymph nodes. AIDS Res Hum Retroviruses 1998; 14:643–9. [DOI] [PubMed] [Google Scholar]
- 56. Shacklett BL, Cox CA, Wilkens DT, Karl Karlsson R, Nilsson A, Nixon DF et al Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV‐1 infection. J Infect Dis 2004; 189:2202–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison of CD69 and CD57 expression between natural killer (NK) cell subsets in samples from dengue virus (DENV) ‐infected patients.
Figure S2. Comparison of homing molecules expression on natural killer (NK) cell subsets between dengue fever (DF) and dengue haemorrhagic fever (DHF) patients.
Table S1. PCR primers for dengue serotyping.


