Abstract
The IL‐23/Th17 axis has been implicated in the development of autoimmune diseases, such as rheumatoid arthritis (RA) and psoriatic arthritis (PsA). RA and PsA are heterogeneous diseases with substantial burden on patients. Increasing evidence suggests that the IL‐23 signaling pathway may be involved in the development of autoimmunity and erosive joint damage. IL‐23 can act either directly or indirectly on bone forming osteoblasts as well as on bone resorbing osteoclasts. As IL‐23 regulates the activity of cells of the bone, it is conceivable that in addition to inflammation‐mediated joint erosion, IL‐23 may play a role in physiological bone remodeling. In this review, we focus on the role of IL‐23 in autoimmune arthritis in patients and murine models, and provide an overview of IL‐23 producing and responding cells in autoimmune arthritic joints. In addition, we discuss the role of IL‐23 on bone forming osteoblasts and bone resorbing osteoclasts regarding inflammation‐mediated joint damage and bone remodeling. At last, we briefly discuss the clinical implications of targeting this pathway for joint damage and systemic bone loss in autoimmune arthritis.
Keywords: Auto‐immune arthritis, IL‐23, Joint damage, Osteoblasts, Osteoclasts
Introduction
Interleukin‐23 (IL‐23), a member of the IL‐12 cytokine family, is a heterodimeric cytokine, which consists of an IL‐12p40 subunit, shared with IL‐12, and an IL‐23 specific p19 subunit 1. The receptor for IL‐23 consists of IL‐23Rα in complex with IL‐12Rβ1, which also serves as a subunit for the IL‐12 receptor 2. Although structurally similar to IL‐12, IL‐23 has the unique ability of amplifying and stabilizing the proliferation of IL‐17 secreting T helper‐17 (Th17) cells 3. In fact, exposure of Th17 cells to IL‐23 drives their pathogenic phenotype 4, 5. These pathogenic Th17 cells are characterized by their master regulator RORγt and production of pro‐inflammatory cytokines such as IL‐17A, IL‐17F, IL‐22, GM‐CSF and are able to promote their lineage commitment through autocrine IL‐21 production 6, 7. Furthermore, these cells express the chemokine receptor CCR6, which enables them to migrate toward sites of inflammation in response to the chemokine CCL20 8, 9.
In recent years, it has become clear that the IL‐23/Th17 pathway plays a crucial role in many inflammatory autoimmune diseases including psoriasis, psoriatic arthritis (PsA), rheumatoid arthritis (RA) and systemic lupus erythematosus 10, 11, 12. Both RA and PsA are disorders with distinct clinical phenotypes, resulting from complex interactions between genetic and environmental factors such as smoking or infections. Although there are some similarities between RA and PsA including the occurrence of erosive joint inflammation and systemic bone loss, there are also important differences 13. For instance, PsA displays features of spondyloarthropathy such as new bone formation and enthesitis, while RA does not. Furthermore, both diseases affect different anatomical joints and in addition to the joint, PsA targets the skin, eyes and the spine 13.
Another difference is the occurrence of autoantibodies such as rheumatoid factor and anti‐citrullinated protein antibodies (ACPAs), which are specific to RA, but not to PsA. Although the IL‐23 signaling pathway is implicated in both RA and PsA, its involvement in the pathogenesis of these disorders may be diverse as demonstrated by clinical studies where targeting IL‐23 has different outcomes 14, 15. In PsA, treatment with anti‐IL‐23 antibodies have shown beneficial effects but not in RA so far. Another finding supporting this hypothesis, is the notion that polymorphisms in the IL‐23 receptor (IL‐23R) have been linked to susceptibility for psoriasis and PsA 16, 17, 18, but are still a matter of debate in RA (Table 1) 19, 20, 21, 22, 23, 24, 25, 26, 27.
Table 1.
An overview of studies on IL‐23R polymorphisms in RA
| IL‐23R SNP | Association with RA | Study population | Number of patients | Number of controls | Study reference |
|---|---|---|---|---|---|
| rs1004819 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| rs7517847 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| rs10489629 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| No | Algerian | 343 | 323 | Louahchi et al. 20 | |
| rs11209026 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| No | North American | 1136 | 1797 | Chang et al. 21 | |
| No | Dutch | 596 | 705 | Chang et al. 21 | |
| Yes | Egyptian | 120 | 120 | Hamdy et al. 22 | |
| No | Polish | 89 | 125 | Bogunia‐Kubik et al. 25 | |
| No | Algerian | 343 | 323 | Louahchi et al. 20 | |
| rs1343151 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| No | Algerian | 343 | 323 | Louahchi et al. 20 | |
| rs10889677 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| Yes | Hungarian | 412 | 220 | Faragó et al. 23 | |
| Yes | Brazilian | 127 | 134 | Da Silva et al. 24 | |
| No | Egyptian | 120 | 120 | Hamdy et al. 22 | |
| rs11209032 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| rs1495965 | No | Spanish | 322 | 342 | Orozco et al. 26 |
| No | Korean | 1204 | 979 | Park et al. 19 | |
| rs2201841 | No | Korean | 1204 | 979 | Park et al. 19 |
| No | New Zealand | 855 | 557 | Hollis‐Moffatt et al. 27 | |
| Yes | Hungarian | 412 | 220 | Faragó et al. 23 | |
| No | Egyptian | 120 | 120 | Hamdy et al. 22 | |
| rs7530511 | No | North American | 1136 | 1797 | Chang et al. 21 |
| No | Dutch | 596 | 705 | Chang et al. 21 | |
| rs1884444 | No | Hungarian | 412 | 220 | Faragó et al. 23 |
Meta‐analyses are not included.
In this review, we focus on the role of IL‐23 in the development of autoimmune arthritis and give an outline of IL‐23 producing and responding cells in arthritic joints. In addition, we review on the role of IL‐23 on bone forming and bone resorbing cells in relation to erosive joint damage and bone remodeling. At last, we discuss the implications of targeting the IL‐23 signaling pathway for joint damage and systemic bone loss.
IL‐23 signaling pathway
The biologically active IL‐23 is composed of IL‐23p19 linked through a disulphide‐bond to IL‐12p40 and signals through the IL‐23R in complex with IL‐12Rβ1 1, 2. IL‐23R associates constitutively with Janus Kinase 2 (JAK2) and IL‐12Rβ1 interacts with Tyrosine kinase 2 (Tyk2) 2. In a ligand dependent manner, IL‐23R associates with STAT3, resulting in STAT3 phosphorylation and activation 2, 28. Activated STAT3 homodimerizes and translocates into the nucleus and induces expression of the transcription factor RORγt which can activate transcription of downstream cytokines such as IL‐17A, IL‐17F, IL‐22, Csf2 29. In addition to these pro‐inflammatory cytokines, the chemokine receptor CCR6, often used as an identification marker for Th17 cells 8, and its ligand CCL20 are downstream of the IL‐23 pathway 30. Interestingly, the IL‐23R is another downstream target of the IL‐23 pathway, resulting in a positive feedback loop and further promoting the pathogenic activity of this pathway 31.
IL‐23 producing and responding cells in autoimmune arthritic joints
Both RA and PsA are characterized by synovitis due to infiltration of immune cells including T cells, B cells, dendritic cells, monocytes, macrophages and hyper‐proliferation of synovial fibroblasts. These cells interact via direct cell‐cell contact and/or by secretion of inflammatory cytokines including IL‐23 in the joint (Fig. 1). The IL‐23p19 protein is abundantly present in RA synovial fibroblasts 32, 33. However, these cells do not express functional IL‐23. This was demonstrated by the finding that heterodimeric IL‐23 protein is not detected in co‐cultures of human Th17 cells with RA synovial fibroblasts and neutralizing IL‐23 has no effect on IL‐17 or IL‐6 levels in these co‐cultures 34. On the other hand, dendritic cells are a source of IL‐23 in the joint as RA synovial dendritic cells co‐express both p19 and p40 subunits 33. This is in line with another study which demonstrated that CD1c+ myeloid dendritic cells (mDCs) were abundantly present in synovial fluid from RA patients and produce IL‐23, IL‐12, IL‐33 and IL‐1β in vitro 35. Other producers of IL‐23 are synovial macrophages as the expression of functional IL‐23 by RA synovial macrophages is induced upon TLR2 stimulation in vitro 32, 36.
Figure 1.
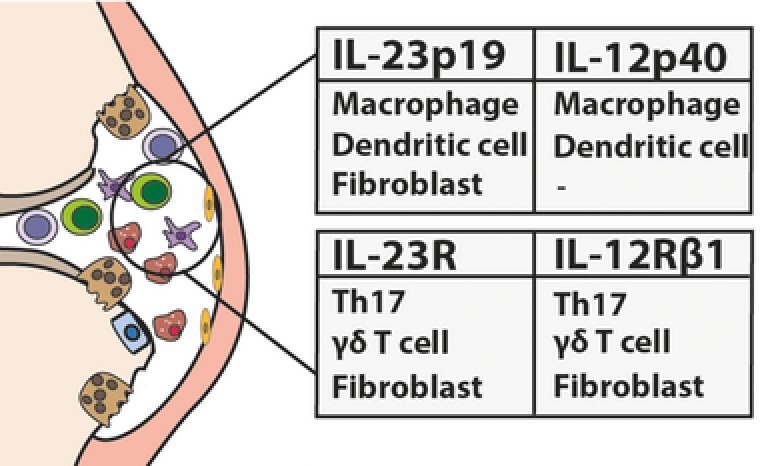
An overview of the reported immune cells in the RA or PsA joints which express IL‐23 or IL‐23R subunits. Both IL‐23 subunits (p19 and p40) are expressed by macrophages and dendritic cells, while fibroblasts express only the p19‐subunit of IL‐23. Expression of both subunits for the IL‐23R is found so far on synovial Th17 cells, γδ T cells, and fibroblasts.
In addition to IL‐23 producing cells, the presence of IL‐23 responding cells in the joints of autoimmune arthritis patients is reported (Fig. 1). RA synovial fibroblasts express IL‐23R as they respond to IL‐23 by increasing their receptor activator of NF‐κB ligand (RANKL) expression 37. Furthermore, CCR6+ Mucosal associated invariant T cells (MAIT cells) have been detected in the synovial fluid of RA patients 38. However, it remains to be elucidated whether these cells express the IL‐23R. In addition, IL‐23R+ Th17 cells are detected in PsA synovial fluid 39, 40 and IFNy+, IL‐17+ γδ T cells are found enriched in the synovial fluid compared to peripheral blood 41.
Inflammation and ossification of the entheseal tissue (the region where tendon fibers or ligaments attach to the bone) is characteristic for PsA. In a mouse model of spondyloarthritis (SpA), IL‐23 has been reported to be involved in the induction of entheseal inflammation through its actions on enthesis‐resident IL‐23R+CD3+CD4−CD8− lymphocytes 42. These cells are possibly tissue‐resident IL‐23R+ γδ T cells and have been shown to accumulate at inflammatory sites and to be the main IL‐17‐producing cells in the enthesis of mice 43.
IL‐23: a major player in early autoimmune arthritis
Serum levels of IL‐23 are increased in both RA and PsA patients and correlate with their disease activity 44, 45. Furthermore, IL‐23 and IL‐17A producing cells are present in autoimmune arthritic synovium 33, 46, 47, 48, 49, while IL‐17 producing γδ T cells are elevated in the skin of PsA patients. In the skin, secretion of IL‐17 and IL‐22 promotes keratinocyte differentiation and hyper‐proliferation which results in aggravation of psoriasis 50. In addition to induction of synovitis and psoriasis, animal studies have suggested a role for IL‐23 in supporting the development of enthesitis 42, 51.
Experimental models have played a pivotal role in investigating the role of IL‐23 in arthritis 52. In vivo overexpression of IL‐23 results in systemic inflammation and chronic arthritis 53, while depletion of this cytokine completely protects mice from arthritis in the collagen‐induced arthritis (CIA) model 54. In this model, IL‐23 is required for the development of pathogenic Th17 cells 54. This indicates that IL‐23 is crucial for the development of CIA. However, after onset of arthritis the requirement for IL‐23 is limited. This was demonstrated by the finding that IL‐23 inhibition did not prevent full‐blown disease after onset of CIA 55. The mechanism behind the IL‐23‐mediated induction of autoimmunity was reported in a recent study, which demonstrated that IL‐23 is essential for CIA onset through the reduction of sialylation in autoantibodies 56. IL‐23 can thereby control the inflammatory activity of autoantibodies. Autoantibody sialylation is reduced by cytokines of Th17 cells, IL‐21 and IL‐22, which may act on plasma cells. Previous studies demonstrated that IL‐23 is required for the induction of IL‐22 in Th17 cells 57. In line with this, a role for IL‐22 in the regulation of autoantibody formation has been reported showing less severe CIA in IL‐22−/− mice with decreased serum autoantibody titers, germinal centers and germinal center B cell numbers 58. Interestingly, reduced sialylation of antibodies is also detected in asymptomatic ACPA+ individuals who developed RA within 12 months compared to those who did not develop RA within this period 56.
These findings suggest that IL‐23 is essential in disease onset through generation of pathogenic Th17 cells including Th17 cytokines, which are involved in regulation of autoantibody producing cells. In this context, IL‐23 may be a driver of RA onset by mediating a shift toward a pro‐inflammatory antibody repertoire.
In RA, relapses often occur in patients who have achieved remission 59. With the antigen‐induced arthritis model, arthritic flares can be mimicked. Interestingly, in the antigen‐induced arthritis (AIA) model, blockade of IL‐23 reduced disease severity after T‐cell‐mediated arthritic flare 55. The mechanism behind this is not fully understood. However, relapses occur as a consequence of memory T cell reactivation and may resemble early disease onset which is driven by pathogenic Th17 cells downstream of IL‐23 60. This suggests that in addition to autoimmune arthritis development, IL‐23 may be important for driving disease relapses.
IL‐23, osteoclasts, and bone loss
Juxta‐articular bone damage around inflamed joints starts during the early phases of RA and is a radiological characteristic of autoimmune arthritis. In fact, the most progression in bone damage is detected during the first year of disease and bone erosions are associated with more severe disease course and increased disability 61. Local and generalized bone loss during autoimmune arthritis may be accelerated as a result of increased pro‐inflammatory cytokine production, such as IL‐23, and potentially by autoantibodies including ACPAs, which contribute to increased formation of bone resorbing cells. Bone resorbing osteoclasts play a crucial role in the development and progression of bone loss and are directly or indirectly under the influence of the immune system 62, 63. The finding that numerous osteoclasts are present in the inflamed synovium 64, suggests that both the precursor cells and the required stimulatory factors for osteoclast differentiation may also be present in the joint itself.
Both animal and human studies have demonstrated pro‐osteoclastogenic roles for the IL‐23 pathway. IL‐23 induces the formation of pathogenic autoantibodies during the early development of CIA 56 and may thereby further promote bone erosion. Pathogenic APCAs are involved in bone loss as ACPA positivity correlates to reduced bone mineral density (BMD) in both the spine and the hip in early RA patients 65. This may be explained by the notion that ACPAs directly activate osteoclasts by binding to citrullinated vimentin, which is present on osteoclasts and their precursor cells 65, 66, 67.
Other indirect actions of IL‐23 on osteoclasts are mediated through T cells, synovial fibroblasts and osteoclast precursor cells. Osteoclasts emerge from hematopoietic myeloid precursor cells and require RANK signaling for their differentiation. In this context, IL‐23 stimulates osteoclastogenesis by enhancing RANK expression in osteoclast precursor cells 68 and RANKL on T cells and RA synovial fibroblasts (Fig. 2) 37, 69. However, it should be noted that IL‐23 has also been reported to reduce osteoclastogenesis via the induction of GM‐CSF in murine T cells, which can inhibit osteoclast formation 70. This indicates that although IL‐23 has mainly pro‐osteoclastogenic roles, it can also suppress osteoclast formation.
Figure 2.

Schematic overview of the role of IL‐23 on osteoclast formation. IL‐23 can stimulate osteoclastogenesis in several ways: (i) increase of RANK expression on osteoclast precursor cells; (ii) increase of RANKL expression on T‐helper cells or fibroblasts; (iii) activation of DAP12 ITAMs. IL‐23 may also induce pathogenic ACPAs which can stimulate osteoclastogenesis. IL‐23 indirectly inhibits osteoclasts through GM‐CSF. Pointed arrows indicate stimulatory actions of IL‐23 and blunt arrows show suppressive effects. Dashed lines indicate indirect effects of IL‐23.
In addition to inducing the RANKL pathway, IL‐23 acts on osteoclast precursors through activation of DNAX activating protein of 12kDa‐ (DAP12) ITAMs to stimulate osteoclast formation independent of RANKL 71. Accordingly, bone marrow cells of IL‐23p19−/− mice have reduced differentiating capacity toward osteoclasts and less dentine resorptive activity in vitro 53. In line with the in vitro studies, overexpression of IL‐23 leads to arthritis and systemic bone loss in mice 53, 72, whereas inflammation‐mediated bone destruction is less pronounced with reduced osteoclast formation in mice lacking IL‐17 or IL‐23 73, 74, 75.
Together these findings suggest that IL‐23 has mainly pro‐osteoclastogenic capacity via both RANKL/RANK dependent and independent pathways, thereby aggravating joint damage and systemic bone loss.
IL‐23, osteoblasts, and bone formation
A distinguishing feature between RA and PsA is the occurrence of new bone formation in the form of syndesmophytes (inside spinal ligament) and enthesophytes (at the attachment of tendons or ligaments to the bone) in PsA 76. Although the role of IL‐23 in osteoclasts has been studied extensively, studies on its role in bone forming osteoblasts are limited and report mainly indirect effects of IL‐23 on these cells (Fig. 3). Messenger RNA expression of IL‐23Rα subunit is found on murine osteoblasts, but no protein expression could be detected 77. Supporting this, no effect of IL‐23 stimulation on osteoblasts was shown and IL‐23p19−/− osteoblasts were not functionally impaired in vitro 70. Nevertheless, IL‐23 can exert indirect effects on osteoblasts through downstream cytokines such as IL‐17A or IL‐22 42, 78.
Figure 3.
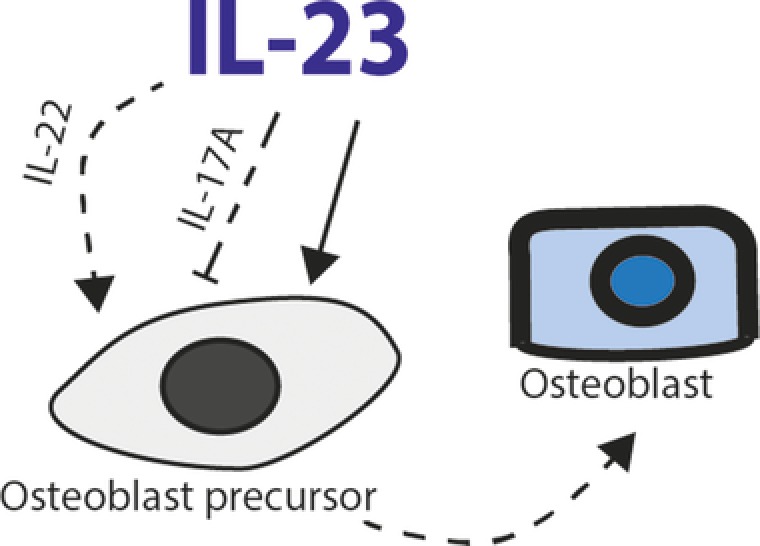
Schematic overview of the role of IL‐23 on osteoblast precursor cells. IL‐23 acts directly on osteoblast precursor cells to stimulate formation of osteoblasts. IL‐23 can indirectly inhibit or stimulate osteoblast formation via IL‐17 or IL‐22 respectively. Pointed arrows indicate stimulatory actions of IL‐23 and blunt arrows show suppressive effects. Dashed lines indicate indirect effects of IL‐23.
IL‐17 can inhibit osteoblast formation by increasing antagonists of the Wnt/B‐catenin pathway. This pathway promotes Runx2, which is the key transcription factor for osteoblast development. An antagonist of the Wnt pathway, secreted frizzled related protein 1 (sFRP1), is induced in differentiating osteoblasts upon in vitro IL‐17A stimulation. This increase in sFPRP1 contributes to impaired osteoblast formation 78. Accordingly, arthritic IL‐17−/− mice develop increased periosteal bone formation 78. In line with the experimental study, sFRP1 is increased in RA synovial fluid compared to osteoarthritis and correlates with increased synovial IL‐17A 79. Interestingly, in vitro stimulation of Th17 cells with sFRP1 results in increased IL‐17A production and IL‐23R expression. This suggests that there may be a positive feedback loop between IL‐17 and sFRP1.
Another Wnt antagonist, Dickkopf‐1 (DKK‐1), is also induced by IL‐17A together with TNFα in murine synovial fibroblasts 78. DKK‐1 is increased in RA joint compared to osteoarthritic joint and correlates with disease activity 80 and decreased BMD 81. The findings of dysregulated expression of Wnt antagonists may also explain the absence of bone repair in RA joint.
In PsA patients, co‐occurrence of joint erosion and new bone formation is often observed. Interestingly, serum DKK‐1 levels are lower in PsA compared to RA and healthy controls, potentially contributing to new bone formation 82. This also suggests that there may be a shift in the IL‐17A/IL‐22 balance in PsA compared to RA.
Another cytokine which acts on osteoblasts downstream of the IL‐23 signaling pathway is IL‐22. This cytokine is associated with bone formation and is found to be elevated in synovial fluid of PsA patients compared to patients with osteoarthritis. In this context, systemic overexpression of IL‐23 leads to new entheseal bone formation and osteoblast expansion via upregulation of IL‐22, which induces osteoblast‐related genes in the enthesis. Similar to IL‐23, overexpression of IL‐22 leads to new periosteal bone formation through STAT3 activation and increased expression of genes that regulate bone formation, including the Wnt family members 42. These findings are further supported by a recent study demonstrating that IL‐22 stimulates human mesenchymal stem cell proliferation and migration and increases osteogenic genes such as ALPL and Runx2 83. Interestingly, IL‐23 has also been reported to directly regulate osteoblast formation as its binding to the IL‐23R on human mesenchymal stem cells leads to increased expression of osteoblast‐related genes and formation of osteoblasts in vitro (Fig. 3) 84.
To summarize, IL‐23 has pleiotropic roles on bone forming osteoblasts either by directly acting on the precursors of these cells or through induction of IL‐17 and IL‐22.
A role for IL‐23 in physiological bone remodeling?
In healthy individuals, bone forming osteoblasts and bone resorbing osteoclasts maintain bone homeostasis through balanced activity. In vivo studies using IL‐23p19−/− mice have reported contradictory findings about the role of IL‐23 in bone homeostasis. Illustrating this, Sato et al. reported no bone abnormalities in 12 weeks old IL‐17−/− and IL‐23p19−/− mice 73. In contrast, Quinn et al. did find bone defects in 12 and 26 weeks old IL‐23p19−/− mice as shown by lower trabecular BMD 70. Along this line, IL‐23p19−/− mice had shorter femurs and histological analysis of the tibial growth plate region revealed that IL‐23p19−/− mice had smaller hypertrophic zones. This is possibly due to increased resorption of the hypertrophic cartilage by osteoclasts. This increased activity of osteoclasts may be explained by the finding that under normal condition IL‐23 can inhibit osteoclast formation through induction of GM‐CSF production in T cells 70.
Similarly to the study by Quinn et al., data from Adamopoulos et al. suggested that IL‐23 might have a role in bone remodeling. However, these authors observed a slight increase in bone mass of 26 weeks old IL‐23p19−/− mice which may have resulted from impaired osteoclastogenesis in the absence of IL‐23 53. Interestingly, bone defects of IL‐23p19−/− mice are not congenital as no abnormalities were found in 4 and 8 weeks old mice 53, 70.
An explanation for these different findings may be the use of different mouse strains, differences in gut microbiota of the mice or the sensitivity of the equipment used for the analysis of the bone. Nevertheless, despite the differences observed, these studies suggest that IL‐23 signaling may play a role in bone homeostasis. However, further studies are required to confirm this and to unravel the potential mechanism.
Targeting the IL‐17/23 pathway during autoimmune arthritis: clinical implications
IL‐23 is required for the maintenance, stability and pathogenicity of Th17 cells, which are well known key effectors in inflammation and tissue damage in several autoimmune diseases. Therefore, targeting this pathway through biologic disease modifying anti‐rheumatic drugs (bDMARDs) including antibodies against IL‐17A or IL‐23 might be beneficial as they have strong anti‐inflammatory properties. Currently, treatment with anti‐TNFα biologicals for autoimmune arthritis has proven beneficial in both dampening of the inflammation and reduction of bone loss. Aggressive anti‐inflammatory treatment of early RA patients with synthetic and biologic DMARDs results in reduced rate of annual bone loss in 2–10 years period of follow up compared to 0–2 years 85.
While TNF‐α inhibitors have shown efficacy in treatment of autoimmune arthritis, there is still a substantial proportion of patients who remain unresponsive to these drugs or suffer from loss of efficacy over time. Therefore in recent years, biologicals targeting the IL‐23/IL‐17A pathway have emerged as alternative therapy. IL‐17A inhibition with Secukinumab showed moderate clinical improvement in rheumatoid arthritis. In a phase II clinical trial, Sekukinumab demonstrated improved efficacy in reducing disease activity (DAS28) over placebo in patients with inadequate response to methotrexate at week 12 86. However, the primary endpoint, a 20% improvement in disease activity according to the American College of Rheumatology (ACR20), was not met in this study. In contrast to this, Secukinumab demonstrated ACR20 achievement at week 24 in a phase III study with RA patients who responded inadequately to TNFα inhibitors 87. Nevertheless, IL‐17A inhibition did not have an additional benefit over Abatacept (a CTLA‐4‐Ig fusion protein that prevents CD80/86 interaction with CD28 receptor) 87, which is already approved by the FDA for RA treatment.
Treatment of RA patients with IL‐23 inhibitors has so far not shown clinical benefit. A recent randomized placebo controlled phase II study showed no treatment benefit of ustekinumab, a monoclonal anti‐IL‐12/23 p40 antibody, and Guselkumab, a monoclonal anti‐IL‐23p19 antibody, over placebo treatment in patients with active RA on methotrexate 14.
These findings suggest that the role of IL‐23 in established RA is limited. However, IL‐23 may be essential in the early autoimmune development including the production of pathogenic autoantibodies, which is demonstrated to be IL‐23‐dependent 56. In addition, IL‐23 may be an important driver of disease relapse in patients as suggested by experimental studies since IL‐23 plays a role in reactivation of memory T cells that are involved in arthritic flares 55. Therefore, future research should reveal whether targeting the IL‐23 signaling pathway in RA patients can prevent an arthritic relapse.
In contrast to RA, both anti‐IL‐17A or anti‐IL‐23 treatment (Secukinumab and Ustekinumab, respectively) have shown beneficial effects in psoriasis and PsA and are currently approved for treatment of both disorders 15, 88. Ustekinumab treatment resulted in sustained inhibition of radiographic progression of joint damage in patients with active PsA 89. Likewise, a phase III clinical trial demonstrated less joint damage progression at week 24 and 52 in PsA patients treated with Secukinumab compared to those receiving placebo 90. Guselkumab is currently under study with active PsA patients in a phase II trial and has so far yielded improvement in joint symptoms, physical function, psoriasis, enthesitis and quality of life for patients undergoing this clinical trial 91. It would be of interest in future long‐term studies to investigate if targeting IL‐23/IL‐17 also inhibits systemic loss of BMD and how it would affect new bone formation in patients with inflammatory arthritis.
The finding that anti‐IL‐23 biologicals are effective in established PsA but not in RA, points toward a difference in the immunopathology of both diseases. In established RA, the requirement for the IL‐23/IL‐17 pathway is limited compared to its role in the early autoimmune phase of the disease and possibly also during arthritic relapses.
In addition to targeting IL‐23 and IL‐17A for the treatment of erosive inflammatory arthritis, an anti‐RANKL monoclonal antibody (Denosumab) is approved for patients with osteoporosis, and inhibited bone erosion and systemic bone loss at 12 months compared with placebo in a phase II study with RA patients 92.
Conclusion
Increasing evidence suggests that the IL‐23 pathway may act as a checkpoint during autoimmune arthritis development, where it can shift the balance of subclinical inflammation in favor of autoimmunity. On the other hand, in established RA the role of this pathway might be limited as indicated by clinical and experimental studies which report lack of efficacy of anti‐IL‐23 treatment during the effector phase of disease. However, few experimental studies have suggested that this pathway is involved in the reactivation of memory T cells which may drive disease relapses. This may offer new possibilities of using anti‐IL‐23 biologicals to suppress or even prevent relapses in these patients.
Chronic arthritis leads to joint damage due to increased activation of bone resorbing cells. Several studies have demonstrated that IL‐23 acts on bone resorbing osteoclasts and bone forming osteoblasts by either directly targeting precursors of these cells or through induction of downstream cytokines such as IL‐17A and IL‐22. IL‐23 can exert pro‐osteoclastogenic effects via IL‐17A, while it may play a role in bone formation by inducing IL‐22. The role of IL‐23 in physiological bone remodeling together with its underlying mechanism still remains to be fully elucidated.
Author contributions
W.R. performed literature research, prepared the review lay‐out and wrote the review. M.vD. revised the manuscript. E.L. prepared the review lay‐out and revised the manuscript.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Abbreviations
- ACPAs
anti‐citrullinated protein antibodies
- BMD
bone mineral density
- CIA
collagen‐induced arthritis
- IL‐23R
IL‐23 receptor
- PsA
psoriatic arthritis
- RA
rheumatoid arthritis
- RANK
receptor activator of NF‐κB
- Th17
T helper‐17
Acknowledgements
This work was funded by the Dutch Arthritis Association (Reumafonds, no. 13‐3‐403) to E.L. We thank Wendy Dankers for her help with the figures.
References
- 1. Oppmann, B. , Lesley, R. , Blom, B. , Timans, J. C. , Xu, Y. , Hunte, B. , Vega, F. et al, Novel p19 protein engages IL‐12p40 to form a cytokine, IL‐23, with biological activities similar as well as distinct from IL‐12. Immunity 2000. 13: 715–725. [DOI] [PubMed] [Google Scholar]
- 2. Parham, C. , Chirica, M. , Timans, J. , Vaisberg, E. , Travis, M. , Cheung, J. , Pflanz, S. et al, A receptor for the heterodimeric cytokine IL‐23 is composed of IL‐12Rbeta1 and a novel cytokine receptor subunit, IL‐23R. J. Immunol. 2002. 168: 5699–5708. [DOI] [PubMed] [Google Scholar]
- 3. Stritesky, G. L. , Yeh, N. and Kaplan, M. H. , IL‐23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008. 181: 5948–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burkett, P. R. , Meyer zu Horste, G. and Kuchroo, V. K. , Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J. Clin. Invest. 2015. 125: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croxford, A. L. , Mair, F. and Becher, B. , IL‐23: one cytokine in control of autoimmunity. Eur. J. Immunol. 2012. 42: 2263–2273. [DOI] [PubMed] [Google Scholar]
- 6. Wei, L. , Laurence, A. , Elias, K. M. and O'Shea, J. J. , IL‐21 is produced by Th17 cells and drives IL‐17 production in a STAT3‐dependent manner. J. Biol. Chem. 2007. 282: 34605–34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee, Y. , Awasthi, A. , Yosef, N. , Quintana, F. J. , Xiao, S. , Peters, A. , Wu, C. et al, Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012. 13: 991–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Paulissen, S. M. , van Hamburg, J. P. , Dankers, W. and Lubberts, E. , The role and modulation of CCR6+ Th17 cell populations in rheumatoid arthritis. Cytokine 2015. 74: 43–53. [DOI] [PubMed] [Google Scholar]
- 9. Hirota, K. , Yoshitomi, H. , Hashimoto, M. , Maeda, S. , Teradaira, S. , Sugimoto, N. , Yamaguchi, T. et al, Preferential recruitment of CCR6‐expressing Th17 cells to inflamed joints via CCL20 in rheumatoid arthritis and its animal model. J. Exp. Med. 2007. 204: 2803–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai, H. , He, F. , Tsokos, G. C. and Kyttaris, V. C. , IL‐23 limits the production of IL‐2 and promotes autoimmunity in lupus. J. Immunol. 2017. 199: 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sivanesan, D. , Beauchamp, C. , Quinou, C. , Lee, J. , Lesage, S. , Chemtob, S. , Rioux, J. D. et al, IL23R (Interleukin 23 Receptor) variants protective against inflammatory bowel diseases (IBD) display loss of function due to impaired protein stability and intracellular trafficking. J. Biol. Chem. 2016. 291: 8673–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lubberts, E. , The IL‐23‐IL‐17 axis in inflammatory arthritis. Nat Rev Rheumatol 2015. 11: 415–429. [DOI] [PubMed] [Google Scholar]
- 13. Veale, D. J. and Fearon, U. , What makes psoriatic and rheumatoid arthritis so different? RMD Open 2015. 1: e000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smolen, J. S. , Agarwal, S. K. , Ilivanova, E. , Xu, X. L. , Miao, Y. , Zhuang, Y. , Nnane, I. et al, A randomised phase II study evaluating the efficacy and safety of subcutaneously administered ustekinumab and guselkumab in patients with active rheumatoid arthritis despite treatment with methotrexate. Ann. Rheum. Dis. 2017. 76: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritchlin, C. , Rahman, P. , Kavanaugh, A. , McInnes, I. B. , Puig, L. , Li, S. , Wang, Y. et al, Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐month and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Ann. Rheum. Dis. 2014. 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Safrany, E. , Szell, M. , Csongei, V. , Jaromi, L. , Sipeky, C. , Szabo, T. , Kemeny, L. et al, Polymorphisms of the IL23R gene are associated with psoriasis but not with immunoglobulin A nephropathy in a Hungarian population. Inflammation 2011. 34: 603–608. [DOI] [PubMed] [Google Scholar]
- 17. Popadic, S. , Ramic, Z. , Medenica, L. , Pravica, V. and Popadic, D. , IL‐23R gene polymorphism rs2201841 is associated with psoriatic arthritis. Int. J. Immunogenet. 2014. 41: 335–337. [DOI] [PubMed] [Google Scholar]
- 18. Indhumathi, S. , Rajappa, M. , Chandrashekar, L. , Ananthanarayanan, P. H. , Thappa, D. M. and Negi, V. S. , Investigation of association of the IL‐12B and IL‐23R genetic variations with psoriatic risk in a South Indian Tamil cohort. Hum. Immunol. 2016. 77: 54–62. [DOI] [PubMed] [Google Scholar]
- 19. Park, J. H. , Kim, Y. J. , Park, B. L. , Bae, J. S. , Shin, H. D. and Bae, S. C. , Lack of association between interleukin 23 receptor gene polymorphisms and rheumatoid arthritis susceptibility. Rheumatol. Int. 2009. 29: 781–786. [DOI] [PubMed] [Google Scholar]
- 20. Louahchi, S. , Allam, I. , Berkani, L. , Boucharef, A. , Abdesemed, A. , Khaldoun, N. , Nebbab, A. et al, Association study of single nucleotide polymorphisms of IL23R and IL17 in rheumatoid arthritis in the Algerian population. Acta Reumatol Port 2016. 41: 151–157. [PubMed] [Google Scholar]
- 21. Chang, M. , Saiki, R. K. , Cantanese, J. J. , Lew, D. , van der Helm‐van Mil, A. H. , Toes, R. E. , Huizinga, T. W. et al, The inflammatory disease‐associated variants in IL12B and IL23R are not associated with rheumatoid arthritis. Arthritis Rheum. 2008. 58: 1877–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hamdy, G. , Darweesh, H. , Khattab, E. A. , Fawzy, S. , Fawzy, E. and Sheta, M. , Evidence of association of interleukin‐23 receptor gene polymorphisms with Egyptian rheumatoid arthritis patients. Hum. Immunol. 2015. 76: 417–420. [DOI] [PubMed] [Google Scholar]
- 23. Farago, B. , Magyari, L. , Safrany, E. , Csongei, V. , Jaromi, L. , Horvatovich, K. , Sipeky, C. et al, Functional variants of interleukin‐23 receptor gene confer risk for rheumatoid arthritis but not for systemic sclerosis. Ann. Rheum. Dis. 2008. 67: 248–250. [DOI] [PubMed] [Google Scholar]
- 24. Gomes da Silva, I. I. F. , Angelo, H. D. , Rushansky, E. , Mariano, M. H. , Maia, M. M. D. and de Souza, P. R. E. , Interleukin (IL)‐23 receptor, IL‐17A and IL‐17F gene polymorphisms in Brazilian patients with rheumatoid arthritis. Arch. Immunol. Ther. Exp. (Warsz.) 2017. 65: 537–543. [DOI] [PubMed] [Google Scholar]
- 25. Bogunia‐Kubik, K. , Swierkot, J. , Malak, A. , Wysoczanska, B. , Nowak, B. , Bialowas, K. , Gebura, K. et al, IL‐17A, IL‐17F and IL‐23R gene polymorphisms in Polish patients with rheumatoid arthritis. Arch. Immunol. Ther. Exp. (Warsz.) 2015. 63: 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Orozco, G. , Rueda, B. , Robledo, G. , Garcia, A. and Martin, J. , Investigation of the IL23R gene in a Spanish rheumatoid arthritis cohort. Hum. Immunol. 2007. 68: 681–684. [DOI] [PubMed] [Google Scholar]
- 27. Hollis‐Moffatt, J. E. , Merriman, M. E. , Rodger, R. A. , Rowley, K. A. , Chapman, P. T. , Dalbeth, N. , Gow, P. J. et al, Evidence for association of an interleukin 23 receptor variant independent of the R381Q variant with rheumatoid arthritis. Ann. Rheum. Dis. 2009. 68: 1340–1344. [DOI] [PubMed] [Google Scholar]
- 28. Cho, M. L. , Kang, J. W. , Moon, Y. M. , Nam, H. J. , Jhun, J. Y. , Heo, S. B. , Jin, H. T. et al, STAT3 and NF‐kappaB signal pathway is required for IL‐23‐mediated IL‐17 production in spontaneous arthritis animal model IL‐1 receptor antagonist‐deficient mice. J. Immunol. 2006. 176: 5652–5661. [DOI] [PubMed] [Google Scholar]
- 29. Yang, X. O. , Panopoulos, A. D. , Nurieva, R. , Chang, S. H. , Wang, D. , Watowich, S. S. and Dong, C. , STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007. 282: 9358–9363. [DOI] [PubMed] [Google Scholar]
- 30. Manel, N. , Unutmaz, D. and Littman, D. R. , The differentiation of human T(H)‐17 cells requires transforming growth factor‐beta and induction of the nuclear receptor RORgammat. Nat. Immunol. 2008. 9: 641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghoreschi, K. , Laurence, A. , Yang, X. P. , Tato, C. M. , McGeachy, M. J. , Konkel, J. E. , Ramos, H. L. et al, Generation of pathogenic T(H)17 cells in the absence of TGF‐beta signalling. Nature 2010. 467: 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Canete, J. D. , Celis, R. , Yeremenko, N. , Sanmarti, R. , van Duivenvoorde, L. , Ramirez, J. , Blijdorp, I. et al, Ectopic lymphoid neogenesis is strongly associated with activation of the IL‐23 pathway in rheumatoid synovitis. Arthritis Res. Ther. 2015. 17: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brentano, F. , Ospelt, C. , Stanczyk, J. , Gay, R. E. , Gay, S. and Kyburz, D. , Abundant expression of the interleukin (IL)23 subunit p19, but low levels of bioactive IL23 in the rheumatoid synovium: differential expression and Toll‐like receptor‐(TLR) dependent regulation of the IL23 subunits, p19 and p40, in rheumatoid arthritis. Ann. Rheum. Dis. 2009. 68: 143–150. [DOI] [PubMed] [Google Scholar]
- 34. Paulissen, S. M. , van Hamburg, J. P. , Davelaar, N. , Asmawidjaja, P. S. , Hazes, J. M. and Lubberts, E. , Synovial fibroblasts directly induce Th17 pathogenicity via the cyclooxygenase/prostaglandin E2 pathway, independent of IL‐23. J. Immunol. 2013. 191: 1364–1372. [DOI] [PubMed] [Google Scholar]
- 35. Moret, F. M. , Hack, C. E. , van der Wurff‐Jacobs, K. M. , de Jager, W. , Radstake, T. R. , Lafeber, F. P. and van Roon, J. A. , Intra‐articular CD1c‐expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of T cell‐attracting chemokines and spontaneously induce Th1, Th17 and Th2 cell activity. Arthritis Res. Ther. 2013. 15: R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Park, S. Y. , Lee, S. W. , Lee, W. S. , Rhim, B. Y. , Lee, S. J. , Kwon, S. M. , Hong, K. W. et al, RhoA/ROCK‐dependent pathway is required for TLR2‐mediated IL‐23 production in human synovial macrophages: suppression by cilostazol. Biochem. Pharmacol. 2013. 86: 1320–1327. [DOI] [PubMed] [Google Scholar]
- 37. Li, X. , Kim, K. W. , Cho, M. L. , Ju, J. H. , Kang, C. M. , Oh, H. J. , Min, J. K. et al, IL‐23 induces receptor activator of NF‐kappaB ligand expression in fibroblast‐like synoviocytes via STAT3 and NF‐kappaB signal pathways. Immunol. Lett. 2010. 127: 100–107. [DOI] [PubMed] [Google Scholar]
- 38. Gracey, E. , Qaiyum, Z. , Almaghlouth, I. , Lawson, D. , Karki, S. , Avvaru, N. , Zhang, Z. et al, IL‐7 primes IL‐17 in mucosal‐associated invariant T (MAIT) cells, which contribute to the Th17‐axis in ankylosing spondylitis. Ann. Rheum. Dis. 2016. 75: 2124–2132. [DOI] [PubMed] [Google Scholar]
- 39. Benham, H. , Norris, P. , Goodall, J. , Wechalekar, M. D. , FitzGerald, O. , Szentpetery, A. , Smith, M. et al, Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res. Ther. 2013. 15: R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fiocco, U. , Stramare, R. , Martini, V. , Coran, A. , Caso, F. , Costa, L. , Felicetti, M. et al, Quantitative imaging by pixel‐based contrast‐enhanced ultrasound reveals a linear relationship between synovial vascular perfusion and the recruitment of pathogenic IL‐17A‐F+IL‐23+ CD161+ CD4+ T helper cells in psoriatic arthritis joints. Clin. Rheumatol. 2017. 36: 391–399. [DOI] [PubMed] [Google Scholar]
- 41. Guggino, G. , Ciccia, F. , Di Liberto, D. , Lo Pizzo, M. , Ruscitti, P. , Cipriani, P. , Ferrante, A. et al, Interleukin (IL)‐9/IL‐9R axis drives gammadelta T cells activation in psoriatic arthritis patients. Clin. Exp. Immunol. 2016. 186: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sherlock, J. P. , Joyce‐Shaikh, B. , Turner, S. P. , Chao, C. C. , Sathe, M. , Grein, J. , Gorman, D. M. et al, IL‐23 induces spondyloarthropathy by acting on ROR‐gammat+ CD3+CD4‐CD8‐ entheseal resident T cells. Nat. Med. 2012. 18: 1069–1076. [DOI] [PubMed] [Google Scholar]
- 43. Reinhardt, A. , Yevsa, T. , Worbs, T. , Lienenklaus, S. , Sandrock, I. , Oberdorfer, L. , Korn, T. et al, Interleukin‐23‐dependent gamma/delta T cells produce Interleukin‐17 and accumulate in the enthesis, aortic valve, and ciliary body in mice. Arthritis Rheumatol 2016. 68: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 44. Andersen, T. , Hvid, M. , Johansen, C. , Stengaard‐Pedersen, K. , Hetland, M. L. , Horslev‐Petersen, K. , Junker, P. et al, Interleukin‐23 in early disease development in rheumatoid arthritis. Scand. J. Rheumatol. 2015. 44: 438–442. [DOI] [PubMed] [Google Scholar]
- 45. Dalila, A. S. , Mohd Said, M. S. , Shaharir, S. S. , Asrul, A. W. , Low, S. F. , Shamsul, A. S. and Sakthiswary, R. , Interleukin‐23 and its correlation with disease activity, joint damage, and functional disability in rheumatoid arthritis. Kaohsiung J. Med. Sci. 2014. 30: 337–342. [DOI] [PubMed] [Google Scholar]
- 46. van Baarsen, L. G. , Lebre, M. C. , van der Coelen, D. , Aarrass, S. , Tang, M. W. , Ramwadhdoebe, T. H. , Gerlag, D. M. et al, Heterogeneous expression pattern of interleukin 17A (IL‐17A), IL‐17F and their receptors in synovium of rheumatoid arthritis, psoriatic arthritis and osteoarthritis: possible explanation for nonresponse to anti‐IL‐17 therapy? Arthritis Res. Ther. 2014. 16: 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Raychaudhuri, S. P. , Raychaudhuri, S. K. and Genovese, M. C. , IL‐17 receptor and its functional significance in psoriatic arthritis. Mol. Cell. Biochem. 2012. 359: 419–429. [DOI] [PubMed] [Google Scholar]
- 48. Kotake, S. , Udagawa, N. , Takahashi, N. , Matsuzaki, K. , Itoh, K. , Ishiyama, S. , Saito, S. et al, IL‐17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J. Clin. Invest. 1999. 103: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andersson, K. M. , Cavallini, N. F. , Hu, D. , Brisslert, M. , Cialic, R. , Valadi, H. , Erlandsson, M. C. et al, Pathogenic Transdifferentiation of Th17 Cells Contribute to Perpetuation of Rheumatoid Arthritis during Anti‐TNF Treatment. Mol. Med. 2015. 21: 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cai, Y. , Shen, X. , Ding, C. , Qi, C. , Li, K. , Li, X. , Jala, V. R. et al, Pivotal role of dermal IL‐17‐producing gammadelta T cells in skin inflammation. Immunity 2011. 35: 596–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Benham, H. , Rehaume, L. M. , Hasnain, S. Z. , Velasco, J. , Baillet, A. C. , Ruutu, M. , Kikly, K. et al, Interleukin‐23 mediates the intestinal response to microbial beta‐1,3‐glucan and the development of spondyloarthritis pathology in SKG mice. Arthritis Rheumatol 2014. 66: 1755–1767. [DOI] [PubMed] [Google Scholar]
- 52. Razawy, W. , Alves, C. H. , Molendijk, M. , Asmawidjaja, P. S. , Mus, A. M. and Lubberts, E. , Experimental Arthritis Mouse Models Driven by Adaptive and/or Innate Inflammation. Methods Mol. Biol. 2017. 1559: 391–410. [DOI] [PubMed] [Google Scholar]
- 53. Adamopoulos, I. E. , Tessmer, M. , Chao, C. C. , Adda, S. , Gorman, D. , Petro, M. , Chou, C. C. et al, IL‐23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J. Immunol. 2011. 187: 951–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Murphy, C. A. , Langrish, C. L. , Chen, Y. , Blumenschein, W. , McClanahan, T. , Kastelein, R. A. , Sedgwick, J. D. et al, Divergent pro‐ and antiinflammatory roles for IL‐23 and IL‐12 in joint autoimmune inflammation. J. Exp. Med. 2003. 198: 1951–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cornelissen, F. , Asmawidjaja, P. S. , Mus, A. M. C. , Corneth, O. , Kikly, K. and Lubberts, E. , IL‐23 Dependent and Independent Stages of Experimental Arthritis: No Clinical Effect of Therapeutic IL‐23p19 Inhibition in Collagen‐induced Arthritis. PLoS One 2013. 8: e57553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Pfeifle, R. , Rothe, T. , Ipseiz, N. , Scherer, H. U. , Culemann, S. , Harre, U. , Ackermann, J. A. et al, Regulation of autoantibody activity by the IL‐23‐TH17 axis determines the onset of autoimmune disease. Nat. Immunol. 2017. 18: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Mus, A. M. , Cornelissen, F. , Asmawidjaja, P. S. , van Hamburg, J. P. , Boon, L. , Hendriks, R. W. and Lubberts, E. , Interleukin‐23 promotes Th17 differentiation by inhibiting T‐bet and FoxP3 and is required for elevation of interleukin‐22, but not interleukin‐21, in autoimmune experimental arthritis. Arthritis Rheum. 2010. 62: 1043–1050. [DOI] [PubMed] [Google Scholar]
- 58. Corneth, O. B. , Reijmers, R. M. , Mus, A. M. , Asmawidjaja, P. S. , van Hamburg, J. P. , Papazian, N. , Siegers, J. Y. et al, Loss of IL‐22 inhibits autoantibody formation in collagen‐induced arthritis in mice. Eur. J. Immunol. 2016. 46: 1404–1414. [DOI] [PubMed] [Google Scholar]
- 59. El Miedany, Y. , El Gaafary, M. , Youssef, S. , Ahmed, I. , Bahlas, S. , Hegazi, M. and Nasr, A. , Optimizing therapy in inflammatory arthritis: prediction of relapse after tapering or stopping treatment for rheumatoid arthritis patients achieving clinical and radiological remission. Clin. Rheumatol. 2016. 35: 2915–2923. [DOI] [PubMed] [Google Scholar]
- 60. Villanueva, M. T. , Rheumatoid arthritis: IL‐23 assists the transition from autoimmunity to inflammatory disease. Nat Rev Rheumatol 2017. 13: 1. [DOI] [PubMed] [Google Scholar]
- 61. Welsing, P. M. , van Gestel, A. M. , Swinkels, H. L. , Kiemeney, L. A. and van Riel, P. L. , The relationship between disease activity, joint destruction, and functional capacity over the course of rheumatoid arthritis. Arthritis Rheum. 2001. 44: 2009–2017. [DOI] [PubMed] [Google Scholar]
- 62. Braun, T. and Schett, G. , Pathways for bone loss in inflammatory disease. Curr Osteoporos Rep 2012. 10: 101–108. [DOI] [PubMed] [Google Scholar]
- 63. Alves, C. H. , Farrell, E. , Vis, M. , Colin, E. M. and Lubberts, E. , Animal Models of Bone Loss in Inflammatory Arthritis: from Cytokines in the Bench to Novel Treatments for Bone Loss in the Bedside‐a Comprehensive Review. Clin. Rev. Allergy Immunol. 2016. 51: 27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schett, G. , Cells of the synovium in rheumatoid arthritis. Osteoclasts. Arthritis Res. Ther. 2007. 9: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bugatti, S. , Bogliolo, L. , Vitolo, B. , Manzo, A. , Montecucco, C. and Caporali, R. , Anti‐citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis. Arthritis Res. Ther. 2016. 18: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kocijan, R. , Harre, U. and Schett, G. , ACPA and bone loss in rheumatoid arthritis. Curr. Rheumatol. Rep. 2013. 15: 366. [DOI] [PubMed] [Google Scholar]
- 67. Harre, U. , Georgess, D. , Bang, H. , Bozec, A. , Axmann, R. , Ossipova, E. , Jakobsson, P. J. et al, Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J. Clin. Invest. 2012. 122: 1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen, L. , Wei, X. Q. , Evans, B. , Jiang, W. and Aeschlimann, D. , IL‐23 promotes osteoclast formation by up‐regulation of receptor activator of NF‐kappaB (RANK) expression in myeloid precursor cells. Eur. J. Immunol. 2008. 38: 2845–2854. [DOI] [PubMed] [Google Scholar]
- 69. Ju, J. H. , Cho, M. L. , Moon, Y. M. , Oh, H. J. , Park, J. S. , Jhun, J. Y. , Min, S. Y. et al, IL‐23 induces receptor activator of NF‐kappaB ligand expression on CD4+ T cells and promotes osteoclastogenesis in an autoimmune arthritis model. J. Immunol. 2008. 181: 1507–1518. [DOI] [PubMed] [Google Scholar]
- 70. Quinn, J. M. , Sims, N. A. , Saleh, H. , Mirosa, D. , Thompson, K. , Bouralexis, S. , Walker, E. C. et al, IL‐23 inhibits osteoclastogenesis indirectly through lymphocytes and is required for the maintenance of bone mass in mice. J. Immunol. 2008. 181: 5720–5729. [DOI] [PubMed] [Google Scholar]
- 71. Shin, H. S. , Sarin, R. , Dixit, N. , Wu, J. , Gershwin, E. , Bowman, E. P. and Adamopoulos, I. E. , Crosstalk among IL‐23 and DNAX activating protein of 12 kDa‐dependent pathways promotes osteoclastogenesis. J. Immunol. 2015. 194: 316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bouchareychas, L. , Grossinger, E. M. , Kang, M. , Qiu, H. and Adamopoulos, I. E. , Critical Role of LTB4/BLT1 in IL‐23‐Induced Synovial Inflammation and Osteoclastogenesis via NF‐kappaB. J. Immunol. 2017. 198: 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sato, K. , Suematsu, A. , Okamoto, K. , Yamaguchi, A. , Morishita, Y. , Kadono, Y. , Tanaka, S. et al, Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006. 203: 2673–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cornelissen, F. , Mus, A. M. , Asmawidjaja, P. S. , van Hamburg, J. P. , Tocker, J. and Lubberts, E. , Interleukin‐23 is critical for full‐blown expression of a non‐autoimmune destructive arthritis and regulates interleukin‐17A and RORgammat in gammadelta T cells. Arthritis Res. Ther. 2009. 11: R194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pollinger, B. , Junt, T. , Metzler, B. , Walker, U. A. , Tyndall, A. , Allard, C. , Bay, S. et al, Th17 cells, not IL‐17+ gammadelta T cells, drive arthritic bone destruction in mice and humans. J. Immunol. 2011. 186: 2602–2612. [DOI] [PubMed] [Google Scholar]
- 76. Rahimi, H. and Ritchlin, C. T. , Altered bone biology in psoriatic arthritis. Curr. Rheumatol. Rep. 2012. 14: 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Kamiya, S. , Nakamura, C. , Fukawa, T. , Ono, K. , Ohwaki, T. , Yoshimoto, T. and Wada, S. , Effects of IL‐23 and IL‐27 on osteoblasts and osteoclasts: inhibitory effects on osteoclast differentiation. J. Bone Miner. Metab. 2007. 25: 277–285. [DOI] [PubMed] [Google Scholar]
- 78. Shaw, A. T. , Maeda, Y. and Gravallese, E. M. , IL‐17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res. Ther. 2016. 18: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lee, Y. S. , Lee, K. A. , Yoon, H. B. , Yoo, S. A. , Park, Y. W. , Chung, Y. , Kim, W. U. et al, The Wnt inhibitor secreted Frizzled‐Related Protein 1 (sFRP1) promotes human Th17 differentiation. Eur. J. Immunol. 2012. 42: 2564–2573. [DOI] [PubMed] [Google Scholar]
- 80. Diarra, D. , Stolina, M. , Polzer, K. , Zwerina, J. , Ominsky, M. S. , Dwyer, D. , Korb, A. et al, Dickkopf‐1 is a master regulator of joint remodeling. Nat. Med. 2007. 13: 156–163. [DOI] [PubMed] [Google Scholar]
- 81. Rossini, M. , Viapiana, O. , Adami, S. , Fracassi, E. , Idolazzi, L. , Dartizio, C. , Povino, M. R. et al, In patients with rheumatoid arthritis, Dickkopf‐1 serum levels are correlated with parathyroid hormone, bone erosions and bone mineral density. Clin. Exp. Rheumatol. 2015. 33: 77–83. [PubMed] [Google Scholar]
- 82. Fassio, A. , Idolazzi, L. , Viapiana, O. , Benini, C. , Vantaggiato, E. , Bertoldo, F. , Rossini, M. et al, In psoriatic arthritis Dkk‐1 and PTH are lower than in rheumatoid arthritis and healthy controls. Clin. Rheumatol. 2017. [DOI] [PubMed] [Google Scholar]
- 83. El‐Zayadi, A. A. , Jones, E. A. , Churchman, S. M. , Baboolal, T. G. , Cuthbert, R. J. , El‐Jawhari, J. J. , Badawy, A. M. et al, Interleukin‐22 drives the proliferation, migration and osteogenic differentiation of mesenchymal stem cells: a novel cytokine that could contribute to new bone formation in spondyloarthropathies. Rheumatology (Oxford) 2017. 56: 488–493. [DOI] [PubMed] [Google Scholar]
- 84. Tu, B. , Liu, S. , Liu, G. , Yan, W. , Wang, Y. , Li, Z. and Fan, C. , Macrophages derived from THP‐1 promote the osteogenic differentiation of mesenchymal stem cells through the IL‐23/IL‐23R/beta‐catenin pathway. Exp. Cell Res. 2015. 339: 81–89. [DOI] [PubMed] [Google Scholar]
- 85. Haugeberg, G. , Helgetveit, K. B. , Forre, O. , Garen, T. , Sommerseth, H. and Proven, A. , Generalized bone loss in early rheumatoid arthritis patients followed for ten years in the biologic treatment era. BMC Musculoskelet. Disord. 2014. 15: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tlustochowicz, W. , Rahman, P. , Seriolo, B. , Krammer, G. , Porter, B. , Widmer, A. and Richards, H. B. , Efficacy and Safety of Subcutaneous and Intravenous Loading Dose Regimens of Secukinumab in Patients with Active Rheumatoid Arthritis: Results from a Randomized Phase II Study. J. Rheumatol. 2016. 43: 495–503. [DOI] [PubMed] [Google Scholar]
- 87. Blanco, F. J. , Moricke, R. , Dokoupilova, E. , Codding, C. , Neal, J. , Andersson, M. , Rohrer, S. et al, Secukinumab in Active Rheumatoid Arthritis: A Phase III Randomized, Double‐Blind, Active Comparator‐ and Placebo‐Controlled Study. Arthritis Rheumatol 2017. 69: 1144–1153. [DOI] [PubMed] [Google Scholar]
- 88. McInnes, I. B. , Mease, P. J. , Kirkham, B. , Kavanaugh, A. , Ritchlin, C. T. , Rahman, P. , van der Heijde, D. et al, Secukinumab, a human anti‐interleukin‐17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2015. 386: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 89. Kavanaugh, A. , Ritchlin, C. , Rahman, P. , Puig, L. , Gottlieb, A. B. , Li, S. , Wang, Y. et al, Ustekinumab, an anti‐IL‐12/23 p40 monoclonal antibody, inhibits radiographic progression in patients with active psoriatic arthritis: Results of an integrated analysis of radiographic data from the phase 3, multicentre, randomised, double‐blind, placebo‐controlled PSUMMIT‐1 and PSUMMIT‐2 trials. Ann. Rheum. Dis. 2014. 73: 1000–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van der Heijde, D. , Landewe, R. B. , Mease, P. J. , McInnes, I. B. , Conaghan, P. G. , Pricop, L. , Ligozio, G. et al, Brief Report: Secukinumab Provides Significant and Sustained Inhibition of Joint Structural Damage in a Phase III Study of Active Psoriatic Arthritis. Arthritis Rheumatol 2016. 68: 1914–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Deodhar, A. A. , Gottlieb, A. B. , Boehncke, W. H. , Dong, B. , Wang, Y. , Barchuk, W. , Xu, X. et al, Efficacy and safety results of Guselkumab, an anti‐IL23 monoclonal antibody, in patients with active psoriatic arthritis over 24 weeks: a phase 2a, randomized, double‐blind, placebo‐controlled study. Arthritis and rheumatology. Conference: american college of rheumatology/association of rheumatology health professionals annual scientific meeting, ACR/ARHP 2016. United states. Conference start: 20161111. Conference end: 20161116 2017. 68: 4359–4361. [Google Scholar]
- 92. Takeuchi, T. , Tanaka, Y. , Ishiguro, N. , Yamanaka, H. , Yoneda, T. , Ohira, T. , Okubo, N. et al, Effect of denosumab on Japanese patients with rheumatoid arthritis: a dose‐response study of AMG 162 (Denosumab) in patients with RheumatoId arthritis on methotrexate to Validate inhibitory effect on bone Erosion (DRIVE)‐a 12‐month, multicentre, randomised, double‐blind, placebo‐controlled, phase II clinical trial. Ann. Rheum. Dis. 2016. 75: 983–990. [DOI] [PMC free article] [PubMed] [Google Scholar]


