Abstract
Objectives
To explore global changes in the prescription of analgesic drugs over time in the international long‐term care (LTC) population.
Design
Systematic review.
Setting
We included original research articles in English, published and unpublished, that included number of participants, country and year(s) of data collection, and prescription of analgesics (analgesics not otherwise specified, opioids, acetaminophen; scheduled only, or scheduled plus as needed (PRN)).
Participants
LTC residents.
Measurements
We searched PubMed, EMBASE, CINAHL, International Pharmaceutical Abstracts, PsycINFO, Cochrane, Web of Science, Google Scholar, using keywords for LTC facilities and analgesic medication; hand‐searched references of eligible papers; correspondence. Studies were quality rated using an adapted Newcastle‐Ottawa scale. Pearson correlation coefficients were generated between percentage of residents prescribed an analgesic and year of data collection. If available, we investigated changes in acetaminophen and opioid prescriptions.
Results
Forty studies met inclusion criteria. A moderate correlation (0.59) suggested that scheduled prescription rates for analgesics have increased over time. Similar findings were reflected in scheduled prescriptions for acetaminophen and opioids. No increase was seen when analyzing scheduled plus PRN analgesics. Use of opioids (scheduled plus PRN) appears to have increased over time.
Conclusion
Worldwide, use of opioids and acetaminophen has increased in LTC residents. Research is needed to explore whether this reflects appropriate pain management for LTC residents and if PRN medication is used effectively.
Keywords: analgesics, pain, nursing home, dementia
A long‐term care (LTC) facility is an institution providing accommodation, meals, 24‐hour staffing, and in some cases 24‐hour nursing care. In 2011, in the United States, 3.9% of individuals aged 65 and older received LTC,1 similar to other developed countries.2, 3
It is suggested that LTC residents are undertreated for pain4, 5, 6; common painful diseases affecting LTC residents include musculoskeletal disorders, cancer, pressure sores, and neuropathies.7, 8 A large European study estimated that pain affected 48.4% of LTC residents, with 12.0% reporting uncontrolled pain,9 consistent with other countries,5, 6 including a U.S. study that found that 23.0% of residents reporting persistent pain did not receive scheduled analgesics.10 Dementia is often underdiagnosed in this population11; cognitively impaired residents may not remember, understand, or communicate their pain, presenting a complex challenge for care staff assessing pain.12, 13 Poorly managed pain can lead to distress, poor quality of life,14, 15 worsening cognition, and depression.16, 17
Prescribers should take a stepwise approach from nonopioids, used for mild to moderate pain (e.g., acetaminophen, considered a first‐line treatment because it is well tolerated) to opioids, generally used for severe acute pain or chronic pain but with risk of side effects such as sedation, constipation, nausea, and vomiting. In older adults multimorbidity and polypharmacy increase the likelihood of adverse events.8, 18, 19
Review Aims
Our aim was to investigate whether, and how, international prescribing patterns of analgesic medication for LTC residents have changed over time. Specific objectives were to explore changes in the prescription of analgesic drugs, explore changes in prescribing of opioids and acetaminophen; and examine changes in scheduled medications and scheduled plus as‐needed (pro re nata (PRN)) medications.
Method
Search Strategy
We used a three‐step search strategy. To refine the search terms, an initial limited search of PubMed was run, followed by analysis of the text words and Medical Subject Heading terms contained in the title, abstract, and index of identified papers. Then a search was run using identified key words and index terms (for LTC facilities and analgesics; see Appendix S1) across included databases until December 2016 (PubMed (including Medline, 1966–present), EMBASE (1947–present), CINAHL (1937–present), International Pharmaceutical Abstracts (1970–present), PsycINFO (1880s–present), Cochrane (1898–present), Web of Science (1900–present) and Google Scholar). There were no restrictions on country. Finally, references of included articles were hand searched.
Eligibility Criteria
Original research articles reporting prescribing of analgesics in LTC facilities were included. Single case studies and studies not published in English were excluded.
Setting
We included LTC facilities (residential homes (institution with board, meals, 24‐hour staffing), nursing homes (as before plus 24‐hour nurse coverage), group dwellings (if deemed suitable based on description)). We excluded assisted living accommodations, sheltered accommodations, retirement apartments, and hospitals.
Study Population
Included participants were residents in an eligible setting where the majority of participants were aged 55 and older in studies that did not focus on a specific illness or condition. A study population was ineligible if it consisted of newly admitted (admission <3 months) residents; those diagnosed with a specific illness, those receiving palliative care, individuals who were included only if they were deemed to be in pain; individuals who were included only because of polypharmacy; incidence of adverse drug event; incidence of fall or recent hospital admission; if dementia or cognitive impairment were excluded; mild cognitive impairment or severe cognitive impairment only; or where residents with severe impairment were excluded, and the number of residents in the excluded population exceeded the number of included participants.
Data
One reviewer (FL) independently screened titles, abstracts, and full‐text articles and extracted the number or percentage of residents prescribed analgesics (including analgesic‐antipyretics), opioids, or acetaminophen; the total number of participants; if available the number of LTC facilities; and year and country of data collection. Data were ineligible if prescriptions included drugs that were potentially not for analgesia (e.g., MO1 drug class) or analgesics combined with other medications, such as disease‐modifying antirheumatic products; only PRN data were available; medication was recorded only if the drug was administered within a specific time window (unless daily, when it was counted as scheduled only); or only weighted percentages were given. If authors indicated that they had collected relevant but unpublished information, they were contacted. There was no restriction on study design. Randomized controlled trials were included if baseline data were published. For longitudinal studies, data were analyzed from the first time point that was at least 3 months after admission to the LTC facility to avoid confounding variables associated with newly admitted residents.
Data Extraction and Quality Checking
Two researchers independently extracted and reviewed data (FL, RB). Eligible studies were assessed for methodological validity using a 5‐point scale (Appendix S2) adapted from the Newcastle‐Ottawa scale20 and Boyle scale.21 Studies were deemed strong, moderate, or weak (adapted from Boyle21) by rating representativeness of the target cohort, adequacy and standardization of data collection tools, participation rate, and inclusion of cluster sampling in analysis. If a study did not account for cluster sampling, it was demoted by 1 quality rating. If answers were unclear, the authors were contacted. If they could not be reached, we used the lowest score for that item. Final scores were resolved through discussion and with a third independent author (ELS).
Analysis
The percentage of residents prescribed analgesics was calculated to one decimal place. Data were specified as scheduled drugs only or scheduled plus PRN; if not explicitly mentioned, they were deemed to be scheduled plus PRN. Articles that included scheduled medications and scheduled plus PRN medications or published data from 2 time points were divided into “cohorts” for separate analysis. Analgesic medications were coded using the Anatomical Therapeutic Chemical classification system22 (Appendix S3).
We quantified study heterogeneity (I 2 > 75% is considerable heterogeneity). If the data were statistically viable, we planned to meta‐analyze them, but if that was not possible, we planned to generate correlation coefficients using the Pearson correlation. The Pearson correlation is sensitive to outliers, so we planned to exclude extreme outliers, identified from the scatter plot, if there was sufficient clinical justification to do so based on the original article's discussion. Stata version 14 (Stata Corp., College Station, TX) was used.
Results
Of 14,323 citations reviewed, 40 studies were included (Figure 1). From the 40 studies, 50 cohorts were eligible. Supplementary Appendix S4 describes study characteristics and quality ratings.
Figure 1.
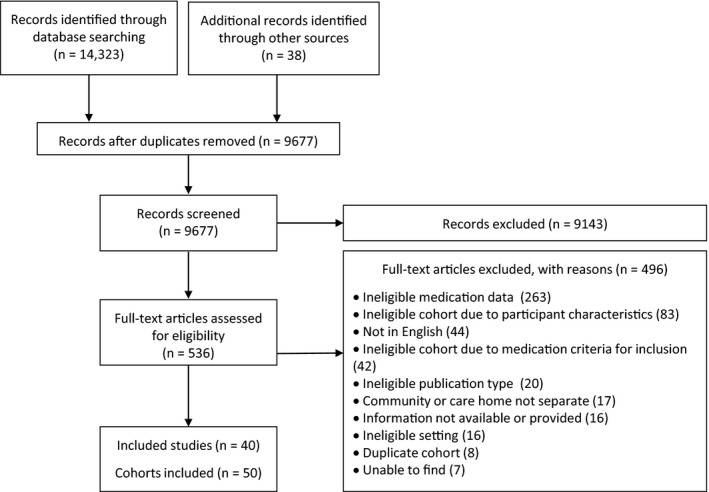
Flow diagram of study selection.
Data were divided according to prescription type: scheduled only (n = 15) or scheduled plus PRN (n = 35). For scheduled only, the median number of participants per study was 551 (range 215–7,309). For scheduled plus PRN prescriptions, the median was 595 (range 13–16,126).
Data were available from 16 countries. One study included data from across Europe (excluding Italy). The countries with the most cohorts were Australia (n = 8), Norway (n = 7), and the United States (n = 6). All other cohorts were from Europe, North America, and Australia. We were unable to meta‐analyze because of heterogeneity (prescriptions of scheduled analgesics I 2 = 99.1, scheduled plus PRN analgesics, I 2 = 99.8).
Quality Rating
Six cohorts were scored as being of strong quality, 20 as moderate, and 24 as weak. The main reasons for low scores were authors not using cluster sampling and lack of detail about data collection methods.
Analgesics
Temporal Changes in Prescriptions of Scheduled Analgesics
Fifteen cohorts were eligible (Table 1) (data drawn from 17,670 residents and at least 490 LTC facilities in 8 countries). Two studies,23, 24 accounting for 7,545 residents, did not provide the number of included LTC facilities.
Table 1.
Cohorts Included in Analysis of Scheduled Analgesic Prescribing Rates
| Study | Year Data Collection Ended | Country | Residents Prescribed Regular Analgesics, % (n = 18,867) |
|---|---|---|---|
| Hoffmann and Schmiemann26,a | 2015 | Germany | 33.7 |
| Tan, Visvanathan54,a,b | 2014 | Australia | 75.2 |
| Bauer, Pitzer55,b | 2012 | Austria | 52 |
| Veal, Bereznicki23,b | 2012 | Australia | 62.8 |
| Sandvik, Selbaek19,a,b | 2011 | Norway | 57.6 |
| Kölzsch, Wulff27 | 2010 | Germany | 32 |
| Krüger, Folkestad56,b | 2008 | Norway | 54.8 |
| Lövheim, Karlsson24,a,b | 2006 | Sweden, Finland | 60.6 |
| Reynolds, Hanson32 | 2004 | United States | 32 |
| Sandvik, Selbaek19,a,b | 2004 | Norway | 45 |
| Decker, Culp57 | 2003 | United States | 45.6 |
| Smalbrugge, Jongenelis33,a,b | 2001 | Netherlands | 45.9 |
| Sandvik, Selbaek19,a,b | 2000 | Norway | 34.9 |
| Nygaard, Naik58,a,b | 1997 | Norway | 29.9 |
| Nygaard and Naik25 | 1996 | Norway | 23 |
Acetaminophen data available.
Opioid data available.
Figure 2 suggests that, between 1996 and 2015, analgesic prescribing increased in LTC facilities. Data from a Norwegian study show that 23% of residents were prescribed scheduled analgesics in 1996, compared with 57.6% in 2011.19, 25 Two studies, both from Germany, reported lower levels: one26 reported that 33.7% of residents were prescribed scheduled analgesics in 2014, and another27 reported a 32% prescription rate in 2010. The correlation between prescription prevalence and final year of data collection was 0.59, showing a moderate positive trend.
Figure 2.
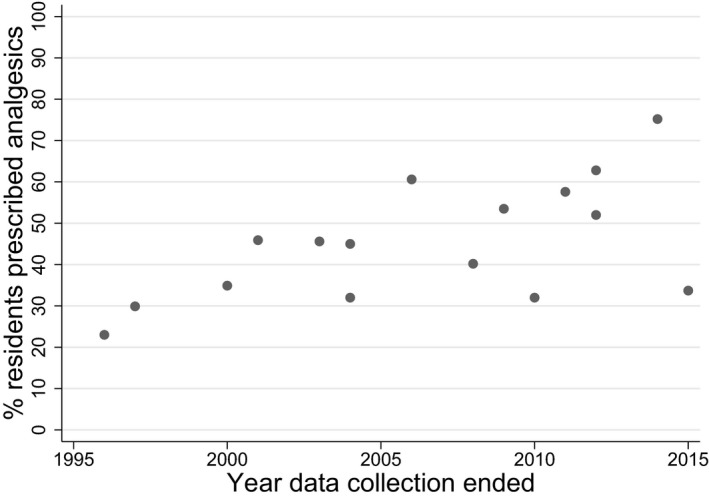
Percentage of residents prescribed scheduled analgesic medication over time.
Temporal Changes in Prescriptions of Scheduled Opioids and Acetaminophen
Ten studies included data on opioid prescriptions (correlation coefficients (Rs) = 0.94), and eight on acetaminophen prescriptions (Rs = 0.93, excluding one outlier that reported very low acetaminophen use (2.5%)). The number of scheduled prescriptions of opioids and acetaminophen has increased over time.
Temporal Changes in Prescriptions of Scheduled Plus PRN Analgesics
Thirty‐one cohorts were eligible (73,938 residents, at least 526 LTC facilities in 16 countries plus Europe, excluding Italy; Table 2). There were 10 cohorts, accounting for 46,211 residents, that did not provide the number of LTC facilities included.
Table 2.
Cohorts Included in Analysis of Scheduled Plus As‐Needed Analgesic Prescribing Rates
| Study | Year Data Collection Ended | Country | Residents Prescribed Regular Analgesics, % (n = 73,938) |
|---|---|---|---|
| Hoffmann and Schmiemann26,a | 2015 | Germany | 73.8 |
| Lövheim (2017, personal communication, 3 April)a,b | 2013 | Sweden | 66.6 |
| Onder, Vetrano30,b | 2013 | Europe, not including Italy | 28 |
| Onder, Vetrano30,b | 2013 | Italy | 16 |
| Bauer, Pitzer55 | 2012 | Austria | 83 |
| Kaasalainen, Wickson‐Griffiths59 | 2012 | Canada | 90 |
| Veal, Bereznicki23 | 2012 | Australia | 90.8 |
| Taxis, Kochen60,b | 2009 | Australia, Netherlands | 80.8 |
| Boerlage, Masman41,b | 2008 | Netherlands | 45.8 |
| Lövheim (2017, personal communication, 3 April)a,b | 2007 | Sweden | 62.8 |
| Stafford, Alswayan61 | 2007 | Australia | 56.8 |
| Torvik, Kaasa62,b | 2006 | Norway | 54.7 |
| Carey, De Wilde63 | 2005 | United Kingdom | 60.6 |
| Elseviers, Vander Stichele64 | 2005 | Belgium | 41.5 |
| Roughead, Gilbert65,a | 2005 | Australia | 53.8 |
| Reynolds, Hanson32 | 2004 | United States | 68.6 |
| Bergman, Olsson66,b | 2003 | Sweden | 61.5 |
| Snowdon, Day67 | 2003 | Australia | 63.6 |
| Jervis, Shore28 | 2002 | United States | 95 |
| Smalbrugge, Jongenelis33 | 2001 | Netherlands | 54.5 |
| Jyrkka, Vartiainen68 | 1998 | Finland | 54 |
| King69,a,b | 1997 | Australia | 74 |
| O'Grady and Weedle70 | 1997 | Ireland | 20 |
| Kaasalainen, Middleton29 | 1996 | Canada | 95 |
| Neutel, Perry71 | 1996 | Canada | 33.5 |
| Van Dijk, de Vries72,b | 1995 | Netherlands | 53 |
| King69,a,b | 1994 | Australia | 60.9 |
| Ferrell, Ferrell73 | 1990 | United States | 78 |
| Vander Stichele, Mestdagh74 | 1990 | Belgium | 26 |
| Williams, Nichol31 | 1990 | United States | 38.3 |
| Passmore, Crawford75 | 1989 | Northern Ireland | 24.8 |
| Hatton76 | 1987 | England | 43 |
| Nolan and O'Malley77 | 1987 | Ireland | 27 |
| Yakabowich, Keeley78 | 1987 | Canada | 58.5 |
| Primrose, Capewell79 | 1984 | Scotland | 32 |
Acetaminophen data available.
Opioid data available.
Because the scatter plot did not suggest a trend, it was not appropriate to run a correlation. Scheduled plus PRN prescriptions have not changed since 1984. Several studies24, 28, 29 show very high prescribing rates (>90%). One of the most recent studies (from 2013) reported the lowest prescribing rate (16%).30 Of the four U.S. studies, the earliest (1990) reported that 38.3% of residents were prescribed analgesics,31 compared with 68.6% in 2004.32
Temporal Changes in Prescriptions of Scheduled Plus PRN Opioids and Acetaminophen
For scheduled plus PRN prescriptions for opioids and acetaminophen over time, there was a positive linear trend for opioids over time, with a moderate correlation coefficient (0.48). It appears that scheduled plus PRN prescriptions for opioids have increased. Opioids were prescribed less frequently than acetaminophen.
Discussion
Prescribing Patterns
We have demonstrated a multinational trend of increased prescription of scheduled analgesics, with corroborative findings for acetaminophen and opioids. Intracountry longitudinal studies (e.g., increases in Norway between 2000 and 2011) and intercountry comparisons (in 2000–01, 34.9% of Norwegian residents and 45.9% of Dutch residents were prescribed analgesics, and in 2011–12, 57.6% of Norwegian residents and 62.8% Australian residents were prescribed analgesics) support this finding.19, 23, 33
There does not appear to be a temporal trend for scheduled plus PRN prescribing. This may be because there is no explicit guidance regarding assessment before giving PRN medication12 and individual clinical preference continues to influence prescribing.
As expected, acetaminophen remained the most commonly prescribed analgesic,4, 23, 34 and prescriptions have increased. The exception is Germany, probably because of the frequent use of dipyrone, a drug banned in several other countries because of risk of agranulocytosis.26
Several factors may have influenced increases in opioid prescriptions. Clinicians are more cautious about nonsteroidal antiinflammatory drugs (NSAIDS) and may prescribe opioids as an alternative. A Finnish study saw a reduction in NSAID use in LTC facilities from 13.0% in 2003 to 2.6% in 2011,35 as did a Norwegian study (6.8% in 2000 to 3.2% in 2011), alongside increases in opioids and acetaminophen.19 Concerns have been expressed that opioids are used for their sedative effect, not just pain.13, 35 Another concern is that opioids may be wrongly prescribed for neuropathic pain, for which an adjuvant drug may be more effective; the prevalence of adjuvant drugs does not match the prevalence of neuropathic pain.19, 35
More detailed studies have identified that strong opioids are used more than weak opioids.4, 19, 36 The introduction of buprenorphine and fentanyl patches may have contributed to use of strong opioids.37 A Danish study reported that nursing home residents were more likely to receive transdermal opioids.13 Their use may be appealing because of ease of administration,38 but U.S. and U.K. guidelines advise that extended‐release opioids should not be the first choice because of negative side effects.18, 38, 39, 40
Quality Rating
The ranges of prescribing prevalence were similar for high‐ and low‐quality studies. It is troubling that there were so few high‐quality studies (6 out of 50 cohorts). There was no clear indication that higher‐quality studies produced mutually consistent results in terms of prescribing prevalence, which may be because of the heterogeneity of samples and settings.
Cultural Factors
Several studies found a low prevalence of analgesic use. In Italy, 24% of residents reporting pain did not receive analgesics, and authors commented that medication was neither appropriately nor effectively managing pain.30 A Dutch study reported that 38% of residents in “substantial” pain received no analgesics, noting that pain was not included in national nursing home performance indicators.41 Another study reported remarkably low analgesic use in Poland. Only 28.8% of residents received analgesics, and only 21.4% of these received scheduled pain relief. Authors commented that pain is not routinely assessed in nursing homes.42 Where low analgesic use is reported, authors often describe a cultural climate that does not prioritize pain assessment. In Italy, where low rates of analgesic prescriptions are reported,4 nonpharmacological analgesia is used more frequently, as it is in Finland.
Limitations
Sample sizes varied greatly, from primary data collection studies involving 1 LTC facility to databases of thousands. One doctor or practice typically manages LTC prescribing, which is thus subject to individual preferences. Data from a small number of facilities may indicate less typical prescribing patterns than a larger sample and contribute to the high levels of observed heterogeneity. Conversely, it can be more difficult to ensure reliability of database records because they depend on accurate input from the LTC facility.43 There were no studies from South America, Africa, or Asia, and conclusions are not generalizable outside Western Europe, North America, and Australia. Lastly, it has been suggested that neuropathic pain, estimated to be present in 8% to 11% of elderly and nursing home populations,44, 45 is often treated inappropriately. This review has not explored prescriptions of neuropathic analgesics because they may be prescribed for other conditions, and most studies do not collect information on prescribing indications.
Clinical and Policy Implications
Many countries have shifted from NSAID use, and in their place other analgesics may be prescribed. In Australia, 2005 national prescribing guidelines, which highlighted good practice in pain management in residential care,23, 46 may be influencing increasing analgesic use, and a UK increase in fentanyl use may have occurred after its licensing for noncancer pain in 2002. There has been growing interest in pain in individuals with dementia and LTC facilities highlighting undertreatment,9, 12 leading to greater use of assessment tools and treatment guidelines.8, 18, 47 Furthermore, there has been more research into behavioral and psychological symptoms of dementia and pain.48, 49 These studies, combined with policy pressure to limit use of psychotropics, such as the Omnibus Budget Reconciliation Act of 1987, may have contributed to the increase in analgesic prescriptions, particularly opioids.50, 51
Future Research Needed
An increase in analgesic prescribing does not necessarily mean that residents are receiving the most appropriate treatment,36 and more frequent pain assessment does not necessarily equate to more analgesia.52 Medication is often prescribed as needed, and administration depends upon staff and their ability to assess pain accurately. This is particularly relevant for cognitively impaired residents who cannot communicate their pain; regular prescriptions may ensure that this population is at less risk of undertreatment.53 Research into using clinical decision‐making algorithms (with stepped treatment approaches), greater collaboration between professionals such as pharmacists and palliative care nurses, and developing interventions to empower and engage the whole care team involved in regularly assessing pain and evaluating pain management strategies could address the disconnect between recognizing and treating pain.37
Conclusion
This is the first systematic review to investigate changes in prescribing patterns of analgesics in the international LTC population. We included data from all studies reporting analgesic use and demonstrated that increases in prescribing seen in smaller studies are representative of an international upward trend, providing a context for current prescribing practices in LTC facilities and insight into the influence of research focus and policy changes.
Supporting information
Appendix S1. Search terms used in each database
Appendix S2. Adapted quality rating scale
Appendix S3. Anatomical Therapeutic Chemical codes used to describe analgesics included in cohorts
Appendix S4. Table of included cohorts: study characteristics and quality ratings
Acknowledgments
Financial Disclosure: F. La Frenais's PhD is supported by the Marie Curie Palliative Care Research Department and the MARQUE Programme (Chief Investigator Professor Gill Livingston), which is funded by the National Institute for Health Research/Economic and Social Research Council (Grant Reference 512810).
Conflict of Interest: None.
Author Contributions: FL, ES, PS: Conception and design of review, development of search strategy. FL: Study selection, data extraction from included studies. FL, RB: Quality rating and data extraction. FL, ES, VV: Data analysis. FL., ES, PS, VV: Interpretation and discussion of results. FL: Drafting the manuscript. ES, PS: Revision of the manuscript. All authors approved the final version of the article.
Sponsor's Role: None.
References
- 1. OECD Health Policy Studies, OECD Publishing . A Good Life in Old Age? Monitoring and Improving Quality in Long‐Term Care. Paris: OECD Health Policy Studies, OECD Publishing, 2013. [Google Scholar]
- 2. Knapp M, Comas‐Herrera A, Somani A et al. Dementia: International Comparisons. London, UK: Personal Social Services Research Unit, London School of Economics and Political Science and the Institute of Psychiatry, King's College London, 2007. [Google Scholar]
- 3. Changes in the Older Resident Care Home Population . Between 2001 and 2011. London: Office for National, Statistics, 2014. [Google Scholar]
- 4. Lukas A, Mayer B, Fialová D et al. Treatment of pain in European nursing homes: Results from the Services and Health for Elderly in Long TERm Care (SHELTER) study. J Am Med Dir Assoc 2013;14:821–831. [DOI] [PubMed] [Google Scholar]
- 5. Hughes L, Hanslip J, Witham M. Centrally active prescribing for nursing home residents‐how are we doing? Eur Geriatr Med 2012;3:304–307. [Google Scholar]
- 6. Könner F, Budnick A, Kuhnert R et al. Interventions to address deficits of pharmacological pain management in nursing home residents‐A cluster‐randomized trial. Eur J Pain 2015;19:1331–1341. [DOI] [PubMed] [Google Scholar]
- 7. Ahn H, Stechmiller J, Fillingim R et al. Bodily pain intensity in nursing home residents with pressure ulcers: Analysis of national minimum data set 3.0. Res Nurs Health 2015;38:207–212. [DOI] [PubMed] [Google Scholar]
- 8. AGS Panel . Pharmacological management of persistent pain in older persons. J Am Geriatr Soc 2009;57:1331. [DOI] [PubMed] [Google Scholar]
- 9. Lukas A, Mayer B, Fialová D et al. Pain characteristics and pain control in European nursing homes: Cross‐sectional and longitudinal results from the Services and Health for Elderly in Long TERm care (SHELTER) Study. J Am Med Dir Assoc 2013;14:421–428. [DOI] [PubMed] [Google Scholar]
- 10. Lapane KL, Quilliam BJ, Chow W et al. Pharmacologic management of non‐cancer pain among nursing home residents. J Pain Symptom Manage 2013;45:33–42. [DOI] [PubMed] [Google Scholar]
- 11. Buhr GT, Kuchibhatla M, Clipp EC. Caregivers' reasons for nursing home placement: Clues for improving discussions with families prior to the transition. Gerontologist 2006;46:52–61. [DOI] [PubMed] [Google Scholar]
- 12. Barry HE, Parsons C, Passmore AP et al. Pain in care home residents with dementia: An exploration of frequency, prescribing and relatives' perspectives. Int J Geriatr Psychiatry 2014;30:55–63. [DOI] [PubMed] [Google Scholar]
- 13. Jensen‐Dahm C, Gasse C, Astrup A et al. Frequent use of opioids in patients with dementia and nursing home residents: A study of the entire elderly population of Denmark. Alzheimer's Dement 2015;11:691–699. [DOI] [PubMed] [Google Scholar]
- 14. Corbett A, Husebo BS, Achterberg WP et al. The importance of pain management in older people with dementia. Br Med Bull 2014;111:139–148. [DOI] [PubMed] [Google Scholar]
- 15. Katz N. The impact of pain management on quality of life. J Pain Symptom Manage 2002;24(1 Suppl):S38–S47. [DOI] [PubMed] [Google Scholar]
- 16. Parmelee PA, Katz IR, Lawton MP. The relation of pain to depression among institutionalized aged. J Gerontol 1991;46:15–21. [DOI] [PubMed] [Google Scholar]
- 17. Fishbain DA, Cutler R, Rosomoff HL et al. Chronic pain‐associated depression: Antecedent or consequence of chronic pain? A review. Clin J Pain 1997;13:116–137. [DOI] [PubMed] [Google Scholar]
- 18. Abdulla A, Adams N, Bone M et al. Guidance on the management of pain in older people. Age Ageing 2013;42:i1–i57. [DOI] [PubMed] [Google Scholar]
- 19. Sandvik R, Selbaek G, Kirkevold O et al. Analgesic prescribing patterns in Norwegian nursing homes from 2000 to 2011: Trend analyses of four data samples. Age Ageing 2016;45:54–60. [DOI] [PubMed] [Google Scholar]
- 20. Wells G, Shea B, O'Connell D et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses, 2000. [on‐line]. Available at http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Accessed June 3, 2016.
- 21. Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Ment Health 1998;1:37–39. [Google Scholar]
- 22. World Health Organization . Analgesics Norway: WHO Collaborating Centre for Drug Statistics; 2015. [on‐line]. Available at http://www.whocc.no/atc_ddd_index/?code=N02&showdescription=yes Accessed May 29, 2016.
- 23. Veal FC, Bereznicki LR, Thompson AJ et al. Pharmacological management of pain in Australian aged care facilities. Age Ageing 2014;43:851–856. [DOI] [PubMed] [Google Scholar]
- 24. Lövheim H, Karlsson S, Gustafson Y. The use of central nervous system drugs and analgesics among very old people with and without dementia. Pharmacoepidemiol Drug Saf 2008;17:912–918. [DOI] [PubMed] [Google Scholar]
- 25. Nygaard H, Naik M. Drug use in homes for the aged. A comparison between mentally intact and mentally impaired residents. Aging (Milan, Italy) 1999;11:186–193. [PubMed] [Google Scholar]
- 26. Hoffmann F, Schmiemann G. Pain medication in German nursing homes: A whole lot of metamizole. Pharmacoepidemiol Drug Saf 2016;25:646–651. [DOI] [PubMed] [Google Scholar]
- 27. Kölzsch M, Wulff I, Ellert S et al. Deficits in pain treatment in nursing homes in Germany: A cross‐sectional study. Eur J Pain 2012;16:439–446. [DOI] [PubMed] [Google Scholar]
- 28. Jervis LL, Shore J, Hutt E et al. Suboptimal pharmacotherapy in a tribal nursing home. J Am Med Dir Assoc 2007;8:1–7. [DOI] [PubMed] [Google Scholar]
- 29. Kaasalainen S, Middleton J, Knezacek S et al. Pain and cognitive status in the institutionalized elderly: Perceptions & interventions. J Gerontol Nurs 1998;24:24–31; quiz 50–51. [DOI] [PubMed] [Google Scholar]
- 30. Onder G, Vetrano DL, Cherubini A et al. Prescription drug use among older adults in Italy: A country‐wide perspective. J Am Med Dir Assoc 2014;15:531.e11–e15. [DOI] [PubMed] [Google Scholar]
- 31. Williams BR, Nichol MB, Lowe B et al. Medication use in residential care facilities for the elderly. Ann Pharmacother 1999;33:149–155. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds KS, Hanson LC, DeVellis RF et al. Disparities in pain management between cognitively intact and cognitively impaired nursing home residents. J Pain Sympt Manag 2008;35:388–396. [DOI] [PubMed] [Google Scholar]
- 33. Smalbrugge M, Jongenelis LK, Pot AM et al. Pain among nursing home patients in the Netherlands: Prevalence, course, clinical correlates, recognition and analgesic treatment—an observational cohort study. BMC Geriatr 2007;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miu DKY, Chan KC. Under‐detection of pain in elderly nursing home residents with moderate to severe dementia. J Clin Gerontol Geriatr 2014;5:23–27. [Google Scholar]
- 35. Pitkala KH, Juola AL, Hosia H et al. Eight‐year trends in the use of opioids, other analgesics, and psychotropic medications among institutionalized older people in Finland. J Am Med Dir Assoc 2015;16:973–978. [DOI] [PubMed] [Google Scholar]
- 36. Ruscitto A, Smith BH, Guthrie B. Changes in opioid and other analgesic use 1995–2010: Repeated cross‐sectional analysis of dispensed prescribing for a large geographical population in Scotland. Eur J Pain 2015;19:59–66. [DOI] [PubMed] [Google Scholar]
- 37. Achterberg W. Pain management in long‐term care: Are we finally on the right track? Age Ageing 2016;45:7–8. [DOI] [PubMed] [Google Scholar]
- 38. Vadivelu N, Hines RL. Management of chronic pain in the elderly: Focus on transdermal buprenorphine. Clin Interv Aging 2008;3:421–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. NICE . Palliative Care for Adults: Strong Opioids for Pain Relief. NICE Clinical Guideline, Contract No.: 140. UK: NICE, 2012. [Google Scholar]
- 40. Dowell D, Haegerich T, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain United States, 2016. [Google Scholar]
- 41. Boerlage A, Masman A, Tibboel D et al. Is pain measurement a feasible performance indicator for Dutch nursing homes? A cross‐sectional approach. Pain Manag Nurs 2013;14:36–40. [DOI] [PubMed] [Google Scholar]
- 42. Neumann‐Podczaska A, Nowak T, Suwalska A et al. Analgesic use among nursing homes residents, with and without dementia, in Poland. Clin Interv Aging 2016;11:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lix LM, Yan L, Blackburn D et al. Agreement between administrative data and the Resident Assessment Instrument Minimum Dataset (RAI‐MDS) for medication use in long‐term care facilities: A population‐based study. BMC Geriatr 2015;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kollenburg EG, Lavrijsen J, Verhagen SC et al. Prevalence, causes, and treatment of neuropathic pain in Dutch nursing home residents: A retrospective chart review. J Am Geriatr Soc 2012;60:1418–1425. [DOI] [PubMed] [Google Scholar]
- 45. Torrance N, Smith BH, Bennett MI et al. The epidemiology of chronic pain of predominantly neuropathic origin. Results from a general population survey. J Pain 2006;7:281–289. [DOI] [PubMed] [Google Scholar]
- 46. The Australian Pain Society . Pain in Residential Aged Care Facilities Management Strategies. Sydney: The Australian Pain Society, 2005. [Google Scholar]
- 47. Corbett A, Achterberg W, Husebo B et al. An international road map to improve pain assessment in people with impaired cognition: The development of the Pain Assessment in Impaired Cognition (PAIC) meta‐tool. BMC Neurol 2014;14:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sampson EL, White N, Lord K et al. Pain, agitation, and behavioural problems in people with dementia admitted to general hospital wards: A longitudinal cohort study. Pain 2015;156:675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tosato M, Lukas A, van der Roest HG et al. Association of pain with behavioral and psychiatric symptoms among nursing home residents with cognitive impairment: Results from the SHELTER study. Pain 2012;153:305–310. [DOI] [PubMed] [Google Scholar]
- 50. Banerjee S. The Use of Antipsychotic Medication for People with Dementia: Time for Action. London: Department of Health, 2009. [Google Scholar]
- 51. Hawes C, Mor V, Phillips CD et al. The OBRA‐87 nursing home regulations and implementation of the Resident Assessment Instrument: Effects on process quality. J Am Geriatr Soc 1997;45:977–985. [DOI] [PubMed] [Google Scholar]
- 52. Petyaeva A, Kajander M, Lawrence V et al. Feasibility of a staff training and support programme to improve pain assessment and management in people with dementia living in care homes. Int J Geriatr Psychiatry 2017. May 5. Available at http://www.onlinelibrary.wiley.com/doi/10.1002/gps.4727/full Accessed October 11, 2017. [DOI] [PubMed] [Google Scholar]
- 53. Veal FC, Bereznicki LR, Thompson AJ et al. Use of opioid analgesics in older Australians. Pain Med 2015;16:1519–1527. [DOI] [PubMed] [Google Scholar]
- 54. Tan EC, Visvanathan R, Hilmer SN et al. Analgesic use and daytime sleepiness in residents with and without dementia in residential aged care facilities. Drugs Aging 2015;32:1045–1053. [DOI] [PubMed] [Google Scholar]
- 55. Bauer U, Pitzer S, Schreier MM et al. Pain treatment for nursing home residents differs according to cognitive state—a cross‐sectional study. BMC Geriatr 2016;16:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Krüger K, Folkestad M, Geitung J‐T et al. Psychoactive drugs in seven nursing homes. Prim Health Care Res Dev 2012;13:244–254. [DOI] [PubMed] [Google Scholar]
- 57. Decker SA, Culp KR, Cacchione PZ. Evaluation of musculoskeletal pain management practices in rural nursing homes compared with evidence‐based criteria. Pain Manag Nurs 2009;10:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nygaard HA, Naik M, Ruths S et al. Nursing‐home residents and their drug use: A comparison between mentally intact and mentally impaired residents. Eur J Clin Pharmacol 2003;59:463–469. [DOI] [PubMed] [Google Scholar]
- 59. Kaasalainen S, Wickson‐Griffiths A, Akhtar‐Danesh N et al. The effectiveness of a nurse practitioner‐led pain management team in long‐term care: A mixed methods study. Int J Nurs Stud 2016;62:156–167. [DOI] [PubMed] [Google Scholar]
- 60. Taxis K, Kochen S, Wouters H et al. Cross‐national comparison of medication use in Australian and Dutch nursing homes. Age Ageing 2017;46:320–323. Erratum in: Age Ageing 2017 Jan 13. [DOI] [PubMed] [Google Scholar]
- 61. Stafford AC, Alswayan MS, Tenni PC. Inappropriate prescribing in older residents of Australian care homes. J Clin Pharm Ther 2011;36:33–44. [DOI] [PubMed] [Google Scholar]
- 62. Torvik K, Kaasa S, Kirkevold Ø et al. Pain in patients living in Norwegian nursing homes. Palliat Med 2008;23:8–16. [DOI] [PubMed] [Google Scholar]
- 63. Carey IM, De Wilde S, Harris T et al. What factors predict potentially inappropriate primary care prescribing in older people? Drugs Aging 2008;25:693–706. [DOI] [PubMed] [Google Scholar]
- 64. Elseviers MM, Vander Stichele RR, Van Bortel L. Drug utilization in Belgian nursing homes: Impact of residents' and institutional characteristics. Pharmacoepidemiol Drug Saf 2010;19:1041–1048. [DOI] [PubMed] [Google Scholar]
- 65. Roughead EE, Gilbert AL, Woodward MC. Medication use by Australian war veterans in residential aged‐care facilities. J Pharm Pract Res 2008;38:14–18. [Google Scholar]
- 66. Bergman Å, Olsson J, Carlsten A et al. Evaluation of the quality of drug therapy among elderly patients in nursing homes: A computerized pharmacy register analysis. Scand J Prim Health Care 2007;25:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Snowdon J, Day S, Baker W. Audits of medication use in Sydney nursing homes. Age Ageing 2006;35:403–408. [DOI] [PubMed] [Google Scholar]
- 68. Jyrkka J, Vartiainen L, Hartikainen S et al. Increasing use of medicines in elderly persons: A five‐year follow‐up of the Kuopio 75 + Study. Eur J Clin Pharmacol 2006;62:151–158. [DOI] [PubMed] [Google Scholar]
- 69. King MA. Medication Care: Databases, Drug Use and Outcomes. Brisbane, Australia: University of Queensland, 2003. [Google Scholar]
- 70. O'Grady M, Weedle P. A descriptive study of drug therapy and cost for elderly residents in a nursing home. Irish Med J 1997;91:172–174. [PubMed] [Google Scholar]
- 71. Neutel CI, Perry S, Maxwell C. Medication use and risk of falls. Pharmacoepidemiol Drug Saf 2002;11:97–104. [DOI] [PubMed] [Google Scholar]
- 72. Van Dijk K, de Vries CS, Van den Berg P et al. Drug utilisation in Dutch nursing homes. Eur J Clin Pharmacol 2000;55:765–771. [DOI] [PubMed] [Google Scholar]
- 73. Ferrell BA, Ferrell BR, Osterweil D. Pain in the nursing home. J Am Geriatr Soc 1990;38:409–414. [DOI] [PubMed] [Google Scholar]
- 74. Vander Stichele R, Mestdagh J, Van Haecht C et al. Medication utilization and patient information in homes for the aged. Eur J Clin Pharmacol 1992;43:319–321. [DOI] [PubMed] [Google Scholar]
- 75. Passmore A, Crawford V, Beringer T et al. Determinants of drug utilization in an elderly population in North and West Belfast. Pharmacoepidemi Drug Saf 1995;4:147–160. [Google Scholar]
- 76. Hatton P. “Primum non nocere”—an analysis of drugs prescribed to elderly patients in private nursing homes registered with Harrogate Health Authority. Care Elderly 1990;2:166–169. [Google Scholar]
- 77. Nolan L, O'Malley K. The need for a more rational approach to drug prescribing for elderly people in nursing homes. Age Ageing 1989;18:52–56. [DOI] [PubMed] [Google Scholar]
- 78. Yakabowich MR, Keeley G, Montgomery PR. Impact of a formulary on personal care homes in Manitoba. Can Med Assoc J 1994;150:1601–1610. [PMC free article] [PubMed] [Google Scholar]
- 79. Primrose WR, Capewell AE, Simpson GK et al. Prescribing patterns observed in registered nursing homes and long‐stay geriatric wards. Age Ageing 1987;16:25–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Search terms used in each database
Appendix S2. Adapted quality rating scale
Appendix S3. Anatomical Therapeutic Chemical codes used to describe analgesics included in cohorts
Appendix S4. Table of included cohorts: study characteristics and quality ratings


