Abstract
Objective
To determine the human dose‐response relationship between a stepwise increase in arterial oxygen tension and its associated changes in DO2 and sublingual microcirculatory perfusion.
Methods
Fifteen healthy volunteers breathed increasing oxygen fractions for 10 minutes to reach arterial oxygen tensions of baseline (breathing air), 20, 40, 60 kPa, and max kPa (breathing oxygen). Systemic hemodynamics were measured continuously by the volume‐clamp method. At the end of each period, the sublingual microcirculation was assessed by SDF.
Results
Systemic DO2 was unchanged throughout the study (P slope = .8). PVD decreased in a sigmoidal fashion (max −15% while breathing oxygen, SD18, P slope = .001). CI decreased linearly (max −10%, SD10, P slope < .001) due to a reduction in HR (max −10%, SD7, P slope = .009). There were no changes in stroke volume or MAP. Most changes became apparent above an arterial oxygen tension of 20 kPa.
Conclusions
In healthy volunteers, supraphysiological arterial oxygen tensions have no effect on systemic DO2. Sublingual microcirculatory PVD decreased in a dose‐dependent fashion. All hemodynamic changes appear negligible up to an arterial oxygen tension of 20 kPa.
Keywords: dose response, healthy volunteers, hyperoxia, microcirculation, oxygen, oxygen delivery
Abbreviations
- BSA
body surface area
- CaO2
arterial oxygen content
- CI
cardiac index
- DO2
oxygen delivery
- FIO2
fraction of inspired oxygen
- Hb
hemoglobin
- HR
heart rate
- ICU
intensive care unit
- IFD
intermittent flow density
- MAP
mean arterial pressure
- MFI
microvascular flow index
- PaO2
arterial partial pressure of oxygen
- PPV
proportion of perfused vessels
- PVD
perfused vessel density
- SaO2
arterial oxygen saturation
- SDF
sidestream darkfield imaging
- SVI
stroke volume index
- SVR
systemic vascular resistance
- SVRI
systemic vascular resistance index
- VD
vessel density
1. INTRODUCTION
Supplemental oxygen is administered to patients with arterial hypoxemia to ensure sufficient DO2 to organs. However, in clinical practice, physicians are inclined to administer oxygen profusely, even in patients who are not hypoxemic.1, 2, 3 As a result, supraphysiological oxygen tensions (hyperoxia) are frequently encountered.4 Restoring normal arterial oxygen tensions (PaO2) is obviously beneficial in hypoxemic patients, but it is uncertain whether oxygen supplementation beyond normoxia is safe and actually improves DO2.
Hyperoxia may increase ICU mortality5, 6, 7 and myocardial infarct size.8 On the other hand, moderate hyperoxia may alleviate organ dysfunction after cardiac arrest.9 In mechanically ventilated ICU patients, the (retrospective) relation between the degree of hyperoxia and mortality is U‐shaped, with a nadir around 15‐20 kPa.10 Potential adverse effects of hyperoxia may occur via microvascular constriction11, 12 and a reduction in cardiac output.13, 14, 15, 16 However, findings regarding such effects are ambiguous.17, 18 The reduced perfusion and cardiac output may lead to a net loss of DO2 that had been found in some,19, 20, 21 but not all studies.22, 23, 24
The evidence for hyperoxia causing microvascular constriction mostly comes from animal studies. In humans, the effects of hyperoxia on the microvasculature consists of indirect measures, such as an increase in SVR15, 25, 26, 27 or a reduction in peripheral blood flow.28, 29, 30, 31 Recently, a direct effect of hyperoxia on the sublingual microcirculation was shown.32 In this study, a marked decrease in PVD (−30%) was observed, when 10 healthy volunteers breathed pure oxygen for 30 minutes. However, as with most studies on hyperoxia, only 2 inspired oxygen concentrations were studied; air (21% O2) and pure oxygen (100%). Although this comparison creates the highest contrast, its clinical relevancy is limited. An FIO2 of 1.0 is rarely used in daily practice to avoid the direct toxicity of pure oxygen to the lungs. Second, the PaO2s that arise from breathing pure oxygen by healthy volunteers is not comparable to the ones in patients with existing lung pathology. As a result, the relation between hemodynamic effects of oxygen and PaO2 at clinically relevant doses remains unknown.
Only a few groups investigated the dose‐response effect of oxygen on the cardiovascular system15, 26 and none directly visualized the microcirculation. It is therefore currently unknown at which PaO2 the microcirculatory effects of hyperoxia start to occur and what the nature of the dose‐response effect is.
The aim of this study was to determine the dose‐response relationship between a stepwise increase in PaO2 and its associated changes in DO2 and sublingual microcirculatory perfusion.
2. MATERIALS AND METHODS
2.1. Study design and ethical approval
Single‐blind, cross‐over physiological study with healthy volunteers performed at the ICU of the VU University Medical Centre (Amsterdam, the Netherlands). The study protocol was approved by the Dutch Central Committee on Research Involving Human Subjects (NL5816602916) and conformed to the standards set by the Declaration of Helsinki.
2.2. Subjects
Volunteers were recruited through social media and were eligible for participation if they were 18 years or older and had no medical history of pulmonary or cardiovascular disease. A modified Allen test was performed to assess arterial competency, and subjects without a patent ulnar artery were not included. Subjects were included after written informed consent was obtained.
2.3. Protocol
2.3.1. Preparation
Subjects lay in a semirecumbent position in a temperature‐controlled room at the ICU. After application of a local anesthetic (lidocaine), the radial artery was cannulated for blood sampling and blood pressure measurements. A finger cuff was placed on the index or middle finger for continuous measurement of hemodynamic parameters by the volume‐clamp method, according to the manufacturer's instructions (Nexfin®, BMEYE, Amsterdam, the Netherlands). Finally, subjects were fitted with a noninvasive ventilation mask coupled to a SERVO‐I mechanical ventilator (Maquet, Rastatt, Germany). The ventilator was set to provide zero continuous positive airway pressure or pressure support. When the subjects were accustomed to the setup (~15 minutes after radial artery cannulation), the intervention and measurements were started.
2.3.2. Intervention
The FIO2 was adjusted to reach target PaO2s of baseline (kPa while breathing air), 20, 40, 60 kPa, and max kPa (while breathing pure oxygen) during 5 separate phases. Five minutes into each phase, arterial blood gas analysis was performed and the FIO2 was adjusted once if PaO2 was not at the intended target. After an additional 5 minutes, a second arterial blood gas was taken. When all study measurements were performed (see below), the subject rested 5‐10 minutes before moving on to the next PaO2 target. Subjects knew they would inspire FIO2s between 21%‐100%, but were unaware of the predetermined stepwise increase. Monitors and the control of FIO2 were not visible for the participants.
2.4. Measurements
At the end of each period, the NIV mask was removed and the sublingual microcirculation was visualized immediately (within one minute) with SDF (MicroVision Medical BV, Amsterdam, the Netherlands). In SDF imaging, green light is emitted from the device which is then absorbed by the Hb present in erythrocytes. SDF therefore relies on the presence of Hb to visualize blood vessels. Three to 5 sites were recorded and analyzed in accordance with the latest quality recommendations.33 After acquisition, the video files were stored for blinded offline semiquantitative analysis with the Automated Vascular Analysis software 3.1 (MicroVision Medical BV). In short, a grid of 5 equidistant vertical and horizontal lines is placed on top of the recording. Vessels crossing these lines are counted and classified as having either continuous, slow/sluggish, intermittent, or no flow. Vascular density (VD) is reported as the total number of vessels per mm of grid. PVD is comprised of vessels showing only continuous or slow/sluggish flow. Although not regularly reported, we also calculated the number of intermittent perfused vessels (IFD) in a similar fashion. All recordings and analyses were carried out by the same operator (BS). All data reported pertain to small vessels with a diameter of 20 μm or less.
Heart rate, CI, SVI, and SVRI were measured continuously during the entire experiment. MAP was measured via the arterial line. The average of the last 2 minutes of each exposure was used for statistics.
Blood gas and metabolite parameters were measured on‐site with an ABL800 FLEX analyzer (Radiometer, Copenhagen, Denmark).
2.5. Calculations
Systemic DO2 index was calculated by multiplying CI with the CaO2. For the latter, the following formula was used; CaO2 = (Hb [g/dL] × 10 × 1.36 × SaO2) + (0.0031 × [PaO2 (kPa) × 7.5]).
2.6. Statistics
All values are reported as mean and standard deviation unless stated otherwise. Dose‐response relations were primarily fitted with a linear regression based on the parameters at each kPa target. An additional nonlinear regression was performed if deemed warranted based on visual inspection. Fit performance was assessed visually and by means of the Sy.x statistic (standard deviation of errors in regression). For all data, we tested whether the slope was statistically different from zero. All graphs and statistics were carried out with GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA).
3. RESULTS
3.1. Volunteers and measurements
Baseline characteristics of the 15 included volunteers are listed in Table 1. All participants gave written informed consent and completed the entire protocol without adverse events. On average, the duration of the study was 95 minutes (SD 8). In one subject, continuous measurement of hemodynamic parameters by the volume‐clamp method was omitted because a stable, valid waveform could not be obtained (due to peripheral vasoconstriction). Sublingual measurements and MAP values obtained through the arterial line did not differ from the other participants. Therefore, the sublingual data of this participant were included in the final analysis.
Table 1.
Baseline characteristics
| Variable | Participants |
|---|---|
| Number | 15 |
| Gender (male/female) | 7/8 |
| Age (y) | 30 (9) |
| Height (cm) | 175 (9) |
| Weight (kg) | 71 (11) |
| BSA (m2) | 1.85 (0.21) |
BSA, body surface area.
3.2. Intervention/Respiration/Blood gas
The obtained PaO2s during the 5 periods were 14 kPa (SD 1.3), 20 kPa (SD 1.8), 39 kPa (SD 3.0), 57 kPa (SD 4.9), and 73 kPa (SD 4.1). The FIO2s required to reach these oxygen tensions was 21% (SD 0), 29 (SD 2), 55 (SD 3), 80 (SD 5), and 100 (SD 0), respectively. Blood gas analysis revealed no changes in PaCO2 or pH. Glucose and lactate decreased slightly during the study period but remained within normal ranges.
3.3. Systemic hemodynamic effects
The absolute values for all systemic hemodynamics at each study period are reported in Table 2. Hyperoxia resulted in a linear decrease in CI (Figure 1, P slope = .009). The largest decrease occurred while breathing pure oxygen (max −10%, SD 10). This decrease was due to a similar reduction in HR (max −10%, SD 7, P slope = .009), as stroke volume remained unchanged (P slope = .75). MAP was not affected by hyperoxia (P slope = .68). SVRI increased slightly over the entire PaO2 range (Figure 2) to a maximum of +7% (SD 8, P slope = .009). On visual inspection, the SVRI data fitted a second order polynomial equation better than a linear one, although the Sy.x statistics were identical (8.1).
Table 2.
Measurements
| Period | Air | T1 | T2 | T3 | Oxygen | P * |
|---|---|---|---|---|---|---|
| Intervention (n = 15) | ||||||
| Target (kPa) | – | 20 (1.5) | 40 (1.5) | 60 (1.5) | – | – |
| PaO2 (kPa) | 14 (1.3) | 20 (1.8) | 39 (3.0) | 57 (4.9) | 73 (4.1) | – |
| FIO2 (%) | 21 (0) | 29 (2) | 55 (3) | 80 (5) | 100 (0) | – |
| Arterial blood gas (n = 15) | ||||||
| SaO2 (%) | 98 (1) | 99 (1) | 100 (0) | 100 (1) | 100 (1) | <.001 |
| PaCO2 (kPa) | 4.7 (0.7) | 4.8 (0.6) | 4.6 (0.8) | 4.8 (0.5) | 4.8 (0.5) | .98 |
| pH | 7.4 (.04) | 7.4 (.03) | 7.5 (.05) | 7.4 (.04) | 7.4 (.03) | .51 |
| Hb (mmol L−1) | 8.6 (0.8) | 8.5 (0.9) | 8.6 (0.8) | 8.6 (0.7) | 8.6 (0.7) | .84 |
| Glucose (mmol L−1) | 6.3 (1.3) | 6.1 (1.2) | 6.0 (1.0) | 5.7 (0.8) | 5.6 (0.6) | .028 |
| Lactate (mmol L−1) | 1.0 (0.6) | 0.9 (0.4) | 0.9 (0.4) | 0.8 (0.3) | 0.7 (0.2) | .015 |
| Microcirculation (n = 15) | ||||||
| VD (n/mm) | 8.0 (0.9) | 8.0 (1.0) | 8.0 (1.8) | 7.6 (1.2) | 6.9 (1.5) | .02 |
| PVD (n/mm) | 7.8 (1.0) | 7.8 (1.0) | 7.6 (1.6) | 6.9 (1.0) | 6.5 (1.5) | .001 |
| IFD (n/mm) | 0.07 (.09) | 0.16 (.14) | 0.30 (.31) | 0.46 (.32) | 0.23 (.27) | <.001 |
| PPV (%) | 98 (2.2) | 97 (1.9) | 96 (3.8) | 91 (3.7) | 93 (8.3) | <.001 |
| MFI | 3.0 (.09) | 2.9 (.15) | 2.9 (.22) | 2.8 (.29) | 2.7 (.31) | <.001 |
| Hemodynamics (n = 14) | ||||||
| HR (bpm) | 64 (7) | 62 (8) | 60 (8) | 58 (7) | 58 (7) | .009 |
| SVI (mL min−1m−2) | 56 (7) | 57 (7) | 58 (8) | 58 (7) | 57 (8) | .75 |
| CI (L min−1m−2) | 3.6 (0.7) | 3.5 (0.6) | 3.4 (0.6) | 3.3 (0.6) | 3.3 (0.6) | <.001 |
| SVRI (dyn·s cm−5 m−2) | 2142 (359) | 2199 (368) | 2236 (426) | 2317 (431) | 2288 (408) | .009 |
| MAP (mm Hg) | 98 (16) | 98 (14) | 98 (15) | 98 (13) | 95 (13) | .68 |
| DO2 (n = 14) | ||||||
| CaO2 (mL/L) | 192 (18) | 194 (20) | 200 (18) | 206 (16) | 208 (17) | <.001 |
| DO2 (mL min−1m−2) | 684 (141) | 676 (132) | 686 (133) | 695 (141) | 679 (145) | .83 |
Data are presented as mean (SD). VD, vessel density; PVD, perfused vessel density; PPV, proportion of perfused vessels; MFI, microvascular flow index; IFD, intermittent flow density; HR, heart rate; MAP, mean arterial pressure; SVI, stroke volume index; CI, cardiac index; SVRI, systemic vascular resistance index; CaO2, arterial oxygen content; DO2, oxygen delivery. *P‐values for slope.
Figure 1.
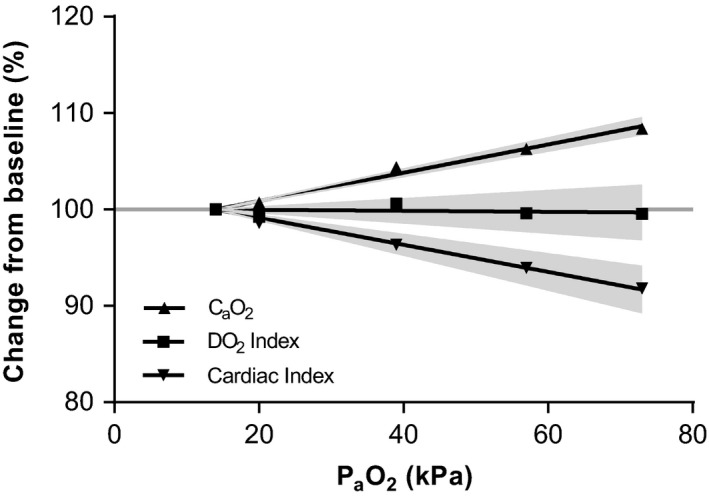
Relation between oxygen content, delivery, and CI. Oxygen content increased linearly with increasing PaO2. Inversely, CI decreased, which resulted in a stable DO 2I over the entire PaO2 range. Gray areas indicate 95% confidence intervals of the fitted curve. PaO2, arterial oxygen tension; CaO2, arterial oxygen content; DO 2I, arterial oxygen delivery index; CI, Cardiac index
Figure 2.
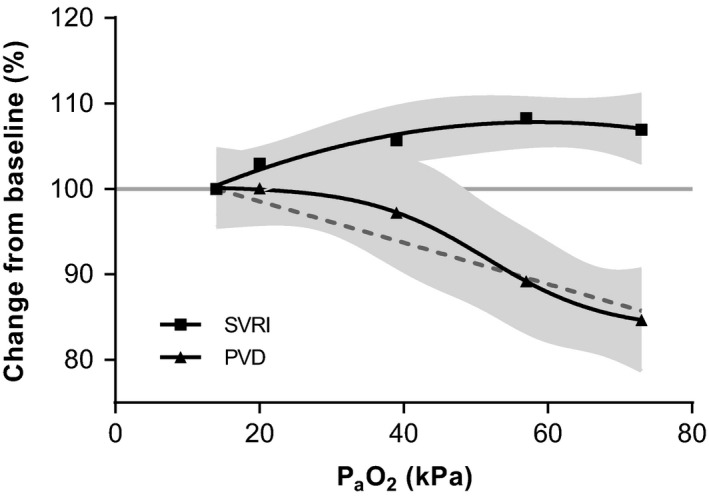
Dose‐response for PVD and SVRI. Sublingual PVD decreases in a sigmoidal fashion upon increase PaO2. SVRI shows the largest increase up to 54 kPa. Gray areas indicate 95% confidence intervals of the fitted curves. For PVD, the dotted line represents the best‐fit line based on linear regression (line is plotted without 95% CI). PaO2, arterial oxygen tension; SVRI, systemic vascular resistance index; PVD, perfused vessel density; CI, Cardiac index
3.4. Oxygen delivery
The oxygen content of arterial blood increased linearly to a maximum of +8% (SD 3, P slope < .0001) when breathing pure oxygen (Table 2, Figure 1). Systemic DO2 index remained unaltered (P slope = .83).
3.5. Sublingual microcirculation
PaO2 reduced, in a dose‐dependent fashion, vascular density (VD) and perfused vascular density (PVD). Compared to measurements performed at baseline, VD changed with +1% (SD 13), 0% (SD 18), −4% (SD 12), and −13% (SD 17, P = .005) at a PaO2 of 20, 39, 57, and 73 kPa, respectively. Similarly, PVD changed with +0% (SD 15), −3% (SD 18), −11%(SD 13), and −15% (SD 18, P = .003). The data could be fitted with both a straight (P slope < .0001) and a sigmoidal line. The standard deviation of the values around the regression line (Sy.x) was 13.6 and 13.7, respectively. On visual inspection, the sigmoidal curve was a better fit (Figure 2). The number of vessels showing intermittent flow increased linearly up to 57 kPa, but was then relatively reduced at 73 kPa. A representative image of the microcirculation while breathing room air is shown in Figure 3A. When breathing oxygen, the number of perfused vessels was reduced and blood flow became stagnant or intermittent, visible as “dotted” vessels in Figure 3B.
Figure 3.
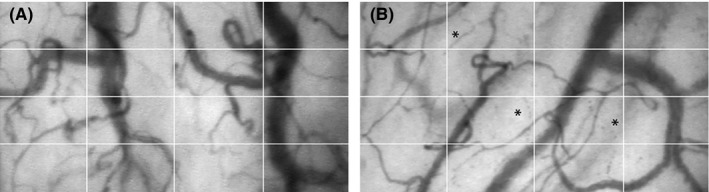
Sublingual microcirculation. Representative images of the sublingual microcirculation acquired with the SDF device. Compared to breathing air (A), oxygen supplementation (B) decreased overall VD (vessels crossing the white grid) and caused interrupted flow (asterisk). SDF, sidestream darkfield imaging; VD, vessel density
4. DISCUSSION
The main finding of this study is that in healthy volunteers, supplemental oxygen does not alter DO2, while sublingual PVD decreased in a sigmoidal fashion as PaO2 was increased stepwise from 14 up to 73 kPa. Hyperoxia decreased CI, by a reduction in HR rather than stroke volume, and increased SVRI. MAP was unchanged.
In this healthy volunteer population, the increase in CaO2 caused by an increase in PaO2 was negated by a simultaneous reduction in CI. Two previous studies in healthy volunteers on hemodynamic effects of oxygen showed a slight decrease in DO2, 24, 34 while another showed no effect.35 In patients, a similar heterogeneity has been seen as DO2 was reduced in 2 studies,22, 23 but remained unaltered in 2 others.17, 19 An important conclusion from our study is that, in nonhypoxemic individuals, an intended increase in DO2 is not achieved by any level of normobaric oxygen supplementation.
We found a significant reduction in sublingual PVD simultaneous to a stepwise increase in PaO2 in this group of healthy individuals. Changes in the sublingual microcirculation in response to an increase in the FIO2 to 1.0 also occur in patients after coronary artery bypass surgery and in a cohort of mixed ICU patients (postcardiac arrest, neurological defects, polytrauma, sepsis).18, 36 Critical illness is associated with (regional) disturbances in microcirculatory perfusion.37 It is possible that systemic oxygen‐induced changes in blood flow further impair regional perfusion and cause a mismatch between DO2 and demand.38 For example, in an animal model for severe coronary artery stenosis, hyperoxia was found to exacerbate myocardial ischemia due to coronary vasoconstriction.39 In our study, we found no evidence for cellular hypoxia (as indicated by lactate), but this may be different in the critically ill with pre‐existing perfusion defects. Either way, in terms of oxygenation, there appears to be no clear advantage to supraphysiological oxygen tensions; in a best‐case scenario, there is no effect on DO2, and in a worst‐case, an unintended reduction.
This is the first study in which dose‐dependent effects of oxygen on the sublingual microcirculation are described. The relation between PaO2 and PVD could be fitted with both a linear and a sigmoidal curve. Both models performed similarly based on the standard deviation of errors in regression. However, the graphical presentation of the data pleads for a sigmoidal relation between PaO2 and PVD. Also, a nonlinear relation is corroborated by observations carried out in hamsters,40, 41 rats,42 and rabbits.43 In another study with healthy volunteers, a reduction in PVD of approximately 30%32 was found, which is much larger than the effect (of ~15%) found in our study. Discrepancies may be explained by differences in study design: prolonged exposure to hyperoxia (30 vs 10 minutes) and an acute exposure to pure oxygen (vs the gradual increase in our study). The SVR, which is an indicator of vasoconstriction, shows a sharp increase after 5‐10 minutes and then further increases by a small amount over the course of an hour in healthy volunteers25, 44 and postoperative critically ill patients.17 SVR does not show a larger increase when volunteers are acutely16, 24, 26, 27, 45, 46, 47, 48 or chronically exposed to oxygen.15, 35, 49, 50, 51 The difference in effect size may therefore be partially explained by the exposure time.
The exact mechanism behind the constrictive effect of high arterial oxygen tensions is currently unclear. Reactive oxygen species (ROS) are one possible candidate, considering that in vitro, as PO2 increases, so does the production of superoxide.52 Superoxide reacts heavily with the vasodilator nitric oxide, reducing its bioavailability and thereby causing vasoconstriction. This has been shown directly in porcine coronary arteries53 and indirectly in human studies; where the scavenging of ROS by infusion of high levels of vitamin C reduced or prevented hyperoxic constriction.11, 12, 54 However, the involvement of ROS is not found uniformly.25, 55 Intravital studies in hamsters, rat, and mice suggest that other pathways may be involved, including inactivation of calcium56, 57 and potassium channels57, 58 or the alteration of the metabolism of arachidonic acid.59, 60 For instance, high PO2 was found to decrease the activity of the enzyme cyclooxygenase, reducing the formation of dilating prostaglandins from arachidonic acid.61, 62, 63 Conversely, the production of the vasoconstrictor 20‐HETE from arachidonic acid by the CYP‐450 pathway was shown to be increased by hyperoxia.64, 65, 66 These observations are however not mutually exclusive, different mechanisms of hyperoxic vasoconstriction may be present depending on the vascular bed and/or species under investigation.
Our study shows a small imbalance between the reduction in PVD and SVRI. At higher PaO2s (>57 kPa), the fractional decrease in PVD was larger than the increase in SVRI. Based on the data from our study, we can only speculate why this is the case. One possible explanation is heterogeneity between microvascular beds: Capillary recruitment in the sublingual area may decrease, while recruitment in other areas/organs remains unaltered or increases. As a result, the effect of increasing PaO2 on the PVD in the sublingual microcirculation may be larger than on SVR. In anaesthetized dogs, hyperoxia has been shown to redistribute blood flow to the kidney, liver, and intestines, while blood flow to the myocardium, pancreas, and skeletal muscle decreases.67 The amount of redistribution may vary at different PaO2s. Another explanation is recruitment of arteriovenous shunts, which provide relatively less resistance to flow than smaller arterioles/capillaries and therefore mitigate the increase in SVR. Shunting may also explain the decrease in VO2 that is seen in some studies after oxygen administration.17, 68 A bypass of metabolic active tissue will elevate venous PO2, reducing the arteriovenous oxygen difference used to calculate VO2. However, we did not take venous blood samples to calculate VO2, as this was beyond the scope of our study.
In our study, the majority of hyperoxia‐induced changes became apparent above an oxygen tension of 20 kPa. From a hemodynamic and microcirculatory point of view, arterial oxygen tensions up to 20 kPa could therefore be suggested as “permissive hyperoxia.” Interestingly, the range of 10‐20 kPa is retrospectively associated with lower mortality in critically ill populations5, 10 and with improved organ function after cardiac arrest.9 Slight hyperoxia (up to a PaO2 of 20 kPa) may thus be beneficial, because its influence on perfusion is possibly negligible. However, even within this range, one prospective study in ICU patients showed reduced mortality in a conservative oxygen group (median PaO2 of 11.5 kPa) compared to a conventional oxygen group (median PaO2 of 13.5 kPa). Beside some methodological issues, it should be noted that the study was stopped prematurely due to slow inclusion and was therefore underpowered for a mortality endpoint.69, 70 Large randomized controlled studies in specific critically ill populations are required to determine whether slight hyperoxia is beneficial or not.
In healthy volunteers, supraphysiological arterial oxygen tensions, in the range of 14‐73 kPa, have no effect on systemic DO2; however, sublingual microcirculatory PVD decreased in a dose‐dependent fashion. Simultaneous with the increase in CaO2, cardiac output decreased due to a decline in HR rather than stroke volume. SVRI increased slightly, while MAP remained unaltered. All hemodynamic changes appear negligible up to a PaO2 of 20 kPa.
4.1. Limitations
Our study has several limitations. For one, we used a noninvasive measurement of systemic hemodynamics which is less precise than the gold standard thermodilution method. The true effect size of hyperoxia on systemic hemodynamics may therefore be different.
Second, we included a relatively low number of participants, but the study was adequately powered given the prepost study design.
Third, we performed the study in a single‐blind fashion due to the incremental oxygen exposure. This approach was chosen because of the possible residual effects of oxygen inhalation on hemodynamics for up to 30 minutes.27 The risk of operator bias was reduced by blinded analysis of the microvascular recordings and the use of a set time‐period for the averaging of hemodynamic variables.
Fourth, we did not include a control group that exclusively inhaled air during the study. This means we cannot exclude the possibility of time or comfort related changes in hemodynamics (eg, a reduction in HR due to increased comfort, rather than oxygen). However, we think it is highly unlikely that an increased level of comfort toward the end of the study is a factor in our results; all volunteers had ample time to adjust to the measurements before the start of the study and they showed no signs of anxiety at any time (eg, raised blood pressure). Our results on HR, MAP and stroke volume are in line with several other studies performed in healthy volunteers.34, 35, 45, 49, 51, 71, 72 HR and sublingual microcirculatory perfusion decreased dose‐dependently throughout the entire protocol; if there was no interaction with the intervention and the effect was solely due to increased comfort, we would have expected the effect to stabilize after an initial 20 or 30 minutes. Also, we are unaware of any mechanism that may link comfort with reduced sublingual perfusion. However, we cannot completely exclude a partial effect of comfort in our results; therefore, we advise future studies to include either a time control (eg, a group inhaling air only) or a phase with return to baseline (eg, air after oxygen exposure).
Fifth, the sublingual microcirculation may not be representative for other parts of the human body. However, we chose to investigate the sublingual area with SDF because of its noninvasive nature and has been used extensively in studies with the critically ill; it is a clinically relevant area as it is correlated with mortality and organ failure in patients with cardiogenic shock73 and sepsis74 and with postoperative complications after abdominal surgery.75 Also, the sublingual area is perfused directly from the carotis externa, making it very closely related to the central circulation.
PERSPECTIVE
Despite decades of research into the cardiovascular effects of hyperoxia, the effects of clinically relevant high arterial oxygen tensions (hyperoxia) on systemic DO2 and sublingual microcirculatory perfusion are currently unknown.
In this arterial oxygen tension guided study, we found that in healthy volunteers, any level of normobaric oxygen supplementation has no effect on systemic DO2. Simultaneously, sublingual PVD was substantially decreased in the hyperoxic range of 20‐73 kPa.
Due to the prevalence of hyperoxia in critically ill patients, these findings warrant studies to determine whether hyperoxia exacerbates pre‐existing microcirculatory defects.
CONFLICT OF INTEREST
The authors declare that they have no conflicting interests.
ACKNOWLEDGMENTS
We would like to thank all the volunteers for their participation and E. Alberts for her assistance during the experiments.
Smit B, Smulders YM, Eringa EC, et al. Hyperoxia does not affect oxygen delivery in healthy volunteers while causing a decrease in sublingual perfusion. Microcirculation. 2018;25:e12433 https://doi.org/10.1111/micc.12433
REFERENCES
- 1. de Graaff AE, Dongelmans DA, Binnekade JM, de Jonge E. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2 . Intensive Care Med. 2011;37:46‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Self‐reported attitudes versus actual practice of oxygen therapy by ICU physicians and nurses. Ann Intensive Care. 2014;4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eastwood GM, Reade MC, Peck L, Jones D, Bellomo R. Intensivists’ opinion and self‐reported practice of oxygen therapy. Anaesth Intensive Care. 2011;39:122‐126. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28:647‐654. [DOI] [PubMed] [Google Scholar]
- 5. Eastwood G, Bellomo R, Bailey M, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38:91‐98. [DOI] [PubMed] [Google Scholar]
- 6. Girardis M, Busani S, Damiani E, et al. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the oxygen‐ICU randomized clinical trial. JAMA. 2016;316:1583‐15889. [DOI] [PubMed] [Google Scholar]
- 7. Panwar R, Hardie M, Bellomo R, et al. Conservative versus liberal oxygenation targets for mechanically ventilated patients. A pilot multicenter randomized controlled trial. Am J Respir Crit Care Med. 2016;193:43‐51. [DOI] [PubMed] [Google Scholar]
- 8. Stub D, Smith K, Bernard S, et al. Air versus oxygen in ST‐segment elevation myocardial infarction. Circulation. 2015;131:2143‐2150. [DOI] [PubMed] [Google Scholar]
- 9. Elmer J, Scutella M, Pullalarevu R, et al. The association between hyperoxia and patient outcomes after cardiac arrest: analysis of a high‐resolution database. Intensive Care Med. 2015;41:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care. 2008;12:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mak S, Egri Z, Tanna G, Colman R, Newton GE. Vitamin C prevents hyperoxia‐mediated vasoconstriction and impairment of endothelium‐dependent vasodilation. Am J Physiol Heart Circ Physiol. 2002;282:H2414‐H2421. [DOI] [PubMed] [Google Scholar]
- 12. McNulty PH, Robertson BJ, Tulli MA, et al. Effect of hyperoxia and vitamin C on coronary blood flow in patients with ischemic heart disease. J Appl Physiol. 2007;102:2040‐2045. [DOI] [PubMed] [Google Scholar]
- 13. Harten JM, Anderson KJ, Kinsella J, Higgins MJ. Normobaric hyperoxia reduces cardiac index in patients after coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2005;19:173‐175. [DOI] [PubMed] [Google Scholar]
- 14. Gao Z, Spilk S, Momen A, et al. Vitamin C prevents hyperoxia‐mediated coronary vasoconstriction and impairment of myocardial function in healthy subjects. Eur J Appl Physiol. 2012;112:483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bak Z, Sjöberg F, Rousseau A, Steinvall I, Janerot‐Sjoberg B. Human cardiovascular dose‐response to supplemental oxygen. Acta Physiol. 2007;191:15‐24. [DOI] [PubMed] [Google Scholar]
- 16. Sinski M, Lewandowski J, Przybylski J, et al. Deactivation of carotid body chemoreceptors by hyperoxia decreases blood pressure in hypertensive patients. Hypertens Res. 2014;37:858‐862. [DOI] [PubMed] [Google Scholar]
- 17. Reinhart K, Bloos F, König F, Bredle D, Hannemann L. Reversible decrease of oxygen consumption by hyperoxia. Chest. 1991;99:690‐694. [DOI] [PubMed] [Google Scholar]
- 18. Helmerhorst HJF, de Wilde RB, Lee DH, et al. Hemodynamic effects of short‐term hyperoxia after coronary artery bypass grafting. Ann Intensive Care. 2017;7:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reinhart K, Spies CD, Meier‐Hellmann A, et al. N‐acetylcysteine preserves oxygen consumption and gastric mucosal pH during hyperoxic ventilation. Am J Respir Crit Care Med. 1995;151:773‐779. [DOI] [PubMed] [Google Scholar]
- 20. Koshy A, Moreau R, Cerini R, et al. Effects of oxygen inhalation on tissue oxygenation in patients with cirrhosis. Evidence for an impaired arterial baroreflex control. J Hepatol. 1989;9:240‐245. [DOI] [PubMed] [Google Scholar]
- 21. Daly WJ, Behnke RH. Hemodynamic consequences of oxygen breathing in left ventricular failure. Circulation. 1963;27:252‐256. [DOI] [PubMed] [Google Scholar]
- 22. Spies C, Giese C, Meier‐Hellmann A, et al. The effect of prophylactically administered n‐acetylcysteine on clinical indicators for tissue oxygenation during hyperoxic ventilation in cardiac risk patients. Anaesthesist. 1996;45:343‐350. [DOI] [PubMed] [Google Scholar]
- 23. Rossi P, Tauzin L, Weiss M, et al. Could hyperoxic ventilation impair oxygen delivery in septic patients? Clin Physiol Funct Imaging. 2007;27:180‐184. [DOI] [PubMed] [Google Scholar]
- 24. Andersen A, Hillestad L. Hemodynamic responses to oxygen breathing and the effect of pharmacological blockade. Acta Med Scand. 1970;188:419‐424. [DOI] [PubMed] [Google Scholar]
- 25. Waring WS, Thomson AJ, Adwani SH, et al. Cardiovascular effects of acute oxygen administration in healthy adults. J Cardiovasc Pharmacol. 2003;42:245‐250. [DOI] [PubMed] [Google Scholar]
- 26. Rousseau A, Bak Z, Janerot‐Sjöberg B, Sjöberg F. Acute hyperoxaemia‐induced effects on regional blood flow, oxygen consumption and central circulation in man. Acta Physiol Scand. 2005;183:231‐240. [DOI] [PubMed] [Google Scholar]
- 27. Gole Y, Gargne O, Coulange M, et al. Hyperoxia‐induced alterations in cardiovascular function and autonomic control during return to normoxic breathing. Eur J Appl Physiol. 2011;111:937‐946. [DOI] [PubMed] [Google Scholar]
- 28. Rousseau A, Steinwall I, Woodson RD, Sjöberg F. Hyperoxia decreases cutaneous blood flow in high‐perfusion areas. Microvasc Res. 2007;74:15‐22. [DOI] [PubMed] [Google Scholar]
- 29. Rousseau A, Tesselaar E, Henricson J, Sjöberg F. Prostaglandins and radical oxygen species are involved in microvascular effects of hyperoxia. J Vasc Res. 2010;47:441‐450. [DOI] [PubMed] [Google Scholar]
- 30. Yamazaki F. Hyperoxia attenuates endothelial‐mediated vasodilation in the human skin. J Physiol Sci. 2007;57:81‐84. [DOI] [PubMed] [Google Scholar]
- 31. Yamazaki F, Takahara K, Sone R, Johnson JM. Influence of hyperoxia on skin vasomotor control in normothermic and heat‐stressed humans. J Appl Physiol. 2007;103:2026‐2033. [DOI] [PubMed] [Google Scholar]
- 32. Orbegozo Cortés D, Puflea F, Donadello K, et al. Normobaric hyperoxia alters the microcirculation in healthy volunteers. Microvasc Res. 2015;98:23‐28. [DOI] [PubMed] [Google Scholar]
- 33. De Backer D, Hollenberg S, Boerma C, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bodetoft S, Carlsson M, Arheden H, Ekelund U. Effects of oxygen inhalation on cardiac output, coronary blood flow and oxygen delivery in healthy individuals, assessed with MRI. Eur J Emerg Med. 2011;18:25‐30. [DOI] [PubMed] [Google Scholar]
- 35. Karetzky MS, Keighley JF, Mithoefer JC. The effect of oxygen administration on gas exchange and cardiopulmonary function in normal subjects. Respir Physiol. 1971;12:361‐370. [DOI] [PubMed] [Google Scholar]
- 36. Donati A, Damiani E, Zuccari S, et al. Effects of short‐term hyperoxia on erythropoietin levels and microcirculation in critically ill patients: a prospective observational pilot study. BMC Anesthesiol. 2017;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klijn E, Den Uil CA, Bakker J, Ince C. The heterogeneity of the microcirculation in critical illness. Clin Chest Med. 2008;29:643‐654. [DOI] [PubMed] [Google Scholar]
- 38. Iscoe S, Beasley R, Fisher JA. Supplementary oxygen for nonhypoxemic patients: O2 much of a good thing? Crit Care. 2011;15:305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Guensch DP, Fischer K, Shie N, Lebel J, Friedrich MG. Hyperoxia exacerbates myocardial ischemia in the presence of acute coronary artery stenosis in swine. Circ Cardiovasc Interv. 2015;8:e002928. [DOI] [PubMed] [Google Scholar]
- 40. Klitzman B, Damon DN, Gorczynski RJ, Duling BR. Augmented tissue oxygen supply during striated muscle contraction in the hamster. Relative contributions of capillary recruitment, functional dilation, and reduced tissue PO2 . Circ Res. 1982;51:711‐721. [DOI] [PubMed] [Google Scholar]
- 41. Duling BR. Microvascular responses to alterations in oxygen tension. Circ Res. 1972;31:481‐489. [DOI] [PubMed] [Google Scholar]
- 42. Prewitt RL, Johnson PC. The effect of oxygen on arteriolar red cell velocity and capillary density in the rat cremaster muscle. Microvasc Res. 1976;12:59‐70. [DOI] [PubMed] [Google Scholar]
- 43. Shibata M, Ichioka S, Ando J, Togawa T, Kamiya A. Nonlinear regulation of capillary perfusion in relation to ambient pO2 changes in skeletal muscle. Eur J Appl Physiol. 2005;94:352‐355. [DOI] [PubMed] [Google Scholar]
- 44. Thomson AJ, Drummond GB, Waring WS, Webb DJ, Maxwell SRJ. Effects of short‐term isocapnic hyperoxia and hypoxia on cardiovascular function. J Appl Physiol. 2006;101:809‐816. [DOI] [PubMed] [Google Scholar]
- 45. Daly WJ, Bondurant S. Effects of oxygen breathing on the heart rate, blood pressure, and cardiac index of normal men‐resting, with reactive hyperemia, and after atropine. J Clin Invest. 1962;41:126‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fagoni N, Sivieri A, Antonutto G, et al. Cardiovascular responses to dry resting apnoeas in elite divers while breathing pure oxygen. Respir Physiol Neurobiol. 2015;219:1‐8. [DOI] [PubMed] [Google Scholar]
- 47. Barratt‐Boyes BG, Wood EH. Cardiac output and related measurements and pressure values in the right heart and associated vessels, together with an analysis of the hemo‐dynamic response to the inhalation of high oxygen mixtures in healthy subjects. J Lab Clin Med. 1958;51:72‐90. [PubMed] [Google Scholar]
- 48. Kenmure AC, Murdoch WR, Hutton I, Cameron AJ. Hemodynamic effects of oxygen at 1 and 2 Ata pressure in healthy subjects. J Appl Physiol. 1972;32:223‐226. [DOI] [PubMed] [Google Scholar]
- 49. Harten JM, Anderson KJ, Angerson WJ, Booth MG, Kinsella J. The effect of normobaric hyperoxia on cardiac index in healthy awake volunteers. Anaesthesia. 2003;58:885‐888. [DOI] [PubMed] [Google Scholar]
- 50. Foster GL, Casten GG, Reeves TJ. The effects of oxygen breathing in patients with acute myocardial infarction. Cardiovasc Res. 1969;3:179‐189. [DOI] [PubMed] [Google Scholar]
- 51. Anderson KJ, Harten JM, Booth MG, Kinsella J. The cardiovascular effects of inspired oxygen fraction in anaesthetized patients. Eur J Anaesthesiol. 2005;22:420‐425. [DOI] [PubMed] [Google Scholar]
- 52. Jones CI, Han Z, Presley T, et al. Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite. Am J Physiol Cell Physiol. 2008;295:C180‐C191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pasgaard T, Stankevicius E, Jørgensen MM, et al. Hyperoxia reduces basal release of nitric oxide and contracts porcine coronary arteries. Acta Physiol (Oxf). 2007;191:285‐296. [DOI] [PubMed] [Google Scholar]
- 54. Ranadive S, Joyner M, Walker B, Taylor J, Casey D. Effect of hyperoxia on contraction‐induced forearm blood flow dynamics in young healthy adults. FASEB J. 2014;28:1106‐1113. [Google Scholar]
- 55. Milone SD, Newton GE, Parker JD. Hemodynamic and biochemical effects of 100% oxygen breathing in humans. Can J Physiol Pharmacol. 1999;77:124‐130. [PubMed] [Google Scholar]
- 56. Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L‐type Ca2 + channels. Am J Physiol. 1998;274:H2018‐H2024. [DOI] [PubMed] [Google Scholar]
- 57. Ngo AT, Riemann M, Holstein‐Rathlou N‐H, Torp‐Pedersen C, Jensen LJ. Significance of K(ATP) channels, L‐type Ca2+ channels and CYP450‐4A enzymes in oxygen sensing in mouse cremaster muscle arterioles in vivo. BMC Physiol. 2013;13:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ngo AT, Jensen LJ, Riemann M, Holstein‐Rathlou N‐H, Torp‐Pedersen C. Oxygen sensing and conducted vasomotor responses in mouse cremaster arterioles in situ. Pflugers Arch. 2010;460:41‐53. [DOI] [PubMed] [Google Scholar]
- 59. Stuart MJ, Setty Y, Walenga RW, Graeber JE, Ganley C. Effects of hyperoxia and hypoxia on vascular prostacyclin formation in vitro. Pediatrics. 1984;74:548‐553. [PubMed] [Google Scholar]
- 60. Yamaja Setty BN, Walenga RW, Stuart MJ. Kinetic analyses of the effects of hyperoxia and hypoxia on vascular cyclooxygenase activity in vitro. Biochem Biophys Res Commun. 1984;125:170‐176. [DOI] [PubMed] [Google Scholar]
- 61. Frisbee JC, Krishna UM, Falck JR, Lombard JH. Role of prostanoids and 20‐HETE in mediating oxygen‐induced constriction of skeletal muscle resistance arteries. Microvasc Res. 2001;62:271‐283. [DOI] [PubMed] [Google Scholar]
- 62. Messina EJ, Sun D, Koller A, Wolin MS, Kaley G. Increases in oxygen tension evoke arteriolar constriction by inhibiting endothelial prostaglandin synthesis. Microvasc Res. 1994;48:151‐160. [DOI] [PubMed] [Google Scholar]
- 63. Win TS, Marshall JM. Contribution of prostaglandins to the dilation that follows isometric forearm contraction in human subjects: effects of aspirin and hyperoxia. J Appl Physiol. 2005;99:45‐52. [DOI] [PubMed] [Google Scholar]
- 64. Harder DR, Narayanan J, Birks EK, et al. Identification of a putative microvascular oxygen sensor. Circ Res. 1996;79:54‐61. [DOI] [PubMed] [Google Scholar]
- 65. Lombard JH, Kunert MP, Roman RJ, et al. Cytochrome P‐450 omega‐hydroxylase senses O2 in hamster muscle, but not cheek pouch epithelium, microcirculation. Am J Physiol. 1999;276:H503‐H508. [DOI] [PubMed] [Google Scholar]
- 66. Kunert MP, Roman RJ, Alonso‐Galicia M, Falck JR, Lombard JH. Cytochrome P‐450 omega‐hydroxylase: a potential O(2) sensor in rat arterioles and skeletal muscle cells. Am J Physiol Heart Circ Physiol. 2001;280:H1840‐H1845. [DOI] [PubMed] [Google Scholar]
- 67. Meier J, Pape A, Kleen M, et al. Regional blood flow during hyperoxic haemodilution. Clin Physiol Funct Imaging. 2005;25:158‐165. [DOI] [PubMed] [Google Scholar]
- 68. Lauscher P, Lauscher S, Kertscho H, Habler O, Meier J. Hyperoxia reversibly alters oxygen consumption and metabolism. Sci World J. 2012;2012:410321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Spoelstra‐de Man AME, Oudemans‐van Straaten HM, Girbes ARJ. Oxygen supplementation among patients in the intensive care unit. JAMA. 2017;317:646. [DOI] [PubMed] [Google Scholar]
- 70. Ferguson ND. Oxygen in the ICU. JAMA. 2016;316:1553. [DOI] [PubMed] [Google Scholar]
- 71. Ley S, Puderbach M, Risse F, et al. Impact of oxygen inhalation on the pulmonary circulation: assessment by magnetic resonance (MR)‐perfusion and MR‐flow measurements. Invest Radiol. 2007;42:283‐290. [DOI] [PubMed] [Google Scholar]
- 72. Kim YK, Jun IG, Kim SR, et al. Using 100% oxygen does not alter the cardiovascular autonomic regulation during non‐invasively simulated haemorrhage in healthy volunteers. J Int Med Res. 2008;36:227‐236. [DOI] [PubMed] [Google Scholar]
- 73. De Backer D, Creteur J, Dubois MJ, Sakr Y, Vincent JL. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am Heart J. 2004;147:91‐99. [DOI] [PubMed] [Google Scholar]
- 74. Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825‐1831. [DOI] [PubMed] [Google Scholar]
- 75. Jhanji S, Lee C, Watson D, Hinds C, Pearse RM. Microvascular flow and tissue oxygenation after major abdominal surgery: association with post‐operative complications. Intensive Care Med. 2009;35:671‐677. [DOI] [PubMed] [Google Scholar]


