Key Points
Question
Can we identify reproducible symptom subtypes that map onto distinct underlying components of neurocognitive behavior, brain activation, and daily function and/or cut across commonly comorbid mood, anxiety, and trauma disorders?
Findings
In this cross-sectional study of 420 individuals with major depression, panic disorder, and posttraumatic stress disorder, clinical symptoms and associated measurements of neurocognitive behavior, neurophysiological brain activation, and daily functional capacity were assessed, and 6 clusters of symptoms were found to be distributed equivalently across diagnostic categories. The subtypes were strongly expressed in distinct underlying components of neurocognitive performance and brain activation and differentiated clinically meaningful degrees of functional capacity.
Meaning
Identification of transdiagnostic subtypes that are coherent across symptom, behavioral, and neural levels may help disentangle the symptom overlap in conventional psychiatric diagnoses, ultimately guiding tailored treatment choices.
The cross-sectional study demonstrates an approach to identifying clinical and functional subtypes within a transdiagnostic sample via electroencephalography.
Abstract
Importance
The symptoms that define mood, anxiety, and trauma disorders are highly overlapping across disorders and heterogeneous within disorders. It is unknown whether coherent subtypes exist that span multiple diagnoses and are expressed functionally (in underlying cognition and brain function) and clinically (in daily function). The identification of cohesive subtypes would help disentangle the symptom overlap in our current diagnoses and serve as a tool for tailoring treatment choices.
Objective
To propose and demonstrate 1 approach for identifying subtypes within a transdiagnostic sample.
Design, Setting, and Participants
This cross-sectional study analyzed data from the Brain Research and Integrative Neuroscience Network Foundation Database that had been collected at the University of Sydney and University of Adelaide between 2006 and 2010 and replicated at Stanford University between 2013 and 2017. The study included 420 individuals with a primary diagnosis of major depressive disorder (n = 100), panic disorder (n = 53), posttraumatic stress disorder (n = 47), or no disorder (healthy control participants) (n = 220). Data were analyzed between October 2016 and October 2017.
Main Outcomes and Measures
We followed a data-driven approach to achieve the primary study outcome of identifying transdiagnostic subtypes. First, machine learning with a hierarchical clustering algorithm was implemented to classify participants based on self-reported negative mood, anxiety, and stress symptoms. Second, the robustness and generalizability of the subtypes were tested in an independent sample. Third, we assessed whether symptom subtypes were expressed at behavioral and physiological levels of functioning. Fourth, we evaluated the clinically meaningful differences in functional capacity of the subtypes. Findings were interpreted relative to a complementary diagnostic frame of reference.
Results
Four hundred twenty participants with a mean (SD) age of 39.8 (14.1) years were included in the final analysis; 256 (61.0%) were female. We identified 6 distinct subtypes characterized by tension (n=81; 19%), anxious arousal (n=55; 13%), general anxiety (n=38; 9%), anhedonia (n=29; 7%), melancholia (n=37; 9%), and normative mood (n=180; 43%), and these subtypes were replicated in an independent sample. Subtypes were expressed through differences in cognitive control (F5,383 = 5.13, P < .001, ηp2 = 0.063), working memory (F5,401 = 3.29, P = .006, ηp2 = 0.039), electroencephalography-recorded β power in a resting paradigm (F5,357 = 3.84, P = .002, ηp2 = 0.051), electroencephalography-recorded β power in an emotional paradigm (F5,365 = 3.56, P = .004, ηp2 = 0.047), social functional capacity (F5,414 = 21.33, P < .001, ηp2 = 0.205), and emotional resilience (F5,376 = 15.10, P < .001, ηp2 = 0.171).
Conclusions and Relevance
These findings offer a data-driven framework for identifying robust subtypes that signify specific, coherent, meaningful associations between symptoms, behavior, brain function, and observable real-world function, and that cut across DSM-IV-defined diagnoses of major depressive disorder, panic disorder, and posttraumatic stress disorder.
Introduction
Diagnostic criteria defined by the DSM-IV are heterogeneous within each disorder and overlap substantially between disorders,1 as demonstrated by at least 50% of individuals having concurrent diagnoses from more than 1 category of anxiety and mood disorder at a given time.2,3,4,5 Heterogeneity within each disorder manifests not only at the symptom level but also in underlying behavior and physiology, and this limits the opportunity for health care professionals to understand disease mechanisms and to identify valid biomarkers for disease progression and intervention targets. Identifying such biomarkers is an urgent task, given that depression and anxiety have become the leading cause of disability and lost productivity worldwide6 and that only one-third of people recover from treatment.7 In this study, we propose a complementary data-driven approach to uncovering symptom clusters that are coherent across behavioral, physiological, and daily function levels.
In previous data-driven approaches in psychiatry, the focus has typically been on stratification based on symptom type or severity and behavior within a single diagnostic category (eg, schizophrenia,8,9,10 psychotic disorders,11,12 depression,13,14,15,16,17 attention-deficit/hyperactivity disorder,18,19,20 and autism21,22,23). This focus is important for data-driven discovery of subtypes within diagnostic categories but cannot address the need to characterize the heterogeneity and overlap of symptoms across diagnostic categories. Of the available data-driven studies of symptoms across multiple diagnoses, the focus has been on youth transdiagnostic samples.24,25,26 To our knowledge, no study has documented valid symptom clusters in a cohort of adults spanning multiple mood, anxiety, and trauma disorders and integrated symptom cluster data with data from multiple levels of function.
To address these issues, our data-driven approach has 4 corresponding aims. Our first aim was to use unsupervised machine learning to identify naturally occurring transdiagnostic subgroups within representative samples spanning multiple mood, anxiety, and trauma diagnoses, including major depressive disorder (MDD), panic disorder, generalized anxiety disorder, obsessive-compulsive disorder, posttraumatic stress disorder (PTSD), and bipolar disorder type 2. Because of the large overlap in symptoms across these disorders,27,28,29,30 they were considered appropriate for our approach. In this context, the term subgroup refers to transdiagnostic rather than within-diagnostic classification. Second, we sought to assess the robustness and generalizability of the resulting subgroups in an independent validation sample. Third, we integrated multiple sources of data to assess how subgroups differ with respect to independent and external metrics for neurocognitive performance and brain activation. We selected domains that encompass behavior, brain physiology, and self-reported functioning31,32 and that assess broad aspects of neurocognitive and behavioral dysfunction implicated multiple mood, anxiety, and stress disorders.33 Fourth, we evaluated how daily functional capacity varied between the subtypes. Daily functioning was selected as an indicator of clinical meaning because it is considered a primary domain affected in individuals experiencing depression and anxiety symptoms.34 To provide a complementary, theoretically driven diagnostic frame of reference for interpretation, we mapped our data-driven subtypes onto the original categories found in the DSM-IV. We hypothesized that participants would be represented in transdiagnostic, reproducible symptom clusters that map onto specific profiles of neurocognition, brain activation, and daily functional capacity. We further hypothesized that subtypes would cut across diagnostic boundaries.
Methods
Participants
Primary Sample
Participants were recruited systematically by advertisements placed in outpatient and community health settings in the communities surrounding the University of Sydney, Australia, and the University of Adelaide, Australia. These samples are diverse with respect to race/ethnicity, age, and sex, and are therefore representative of the surrounding communities.
Our protocol received independent institutional ethical review board approval of the Human Research Ethical Committees of the Sydney Medical School at the Western Sydney Area Health Service and the University of Sydney prior to recruitment of participants. All participants signed and dated an approved informed consent form. Data are from participants who consented to have their data made available to the Brain Research and Integrative Neuroscience Network Foundation Database,35 for open sharing and secondary analysis by the research community.
We recruited clinical participants who had 1 of 3 primary diagnoses (based on DSM-IV criteria): MDD, PTSD, and panic disorder. Diagnosis was made using the Mini-International Neuropsychiatric Interview36 and Hamilton Depression Rating Scale37 for MDD, the Clinician-Administered PTSD Scale38 and the Structured Clinical Interview for DSM-IV Axis 1 Disorders39 for PTSD, and the Mini-International Neuropsychiatric Interview and Composite International Diagnostic Interview40 for panic disorder.
Comorbid mood and anxiety disorders were present. Of participants with a primary diagnosis of MDD (n = 100), comorbid conditions included PTSD (n = 12; 12%), panic disorder (n = 14; 14%), generalized anxiety disorder (n = 36; 36%), and attention-deficit/hyperactivity disorder (n = 1; 1%). Of participants with PTSD (n = 47), comorbid conditions included MDD (n = 17; 36%) and generalized anxiety disorder (n = 4; 9%). Of participants with panic disorder (n = 53), comorbid conditions included MDD (n = 7; 13%), PTSD (n = 15; 28%), generalized anxiety disorder (n = 5; 9%), dysthymia (n = 3; 6%), obsessive-compulsive disorder (n = 13; 25%), and seasonal affective disorder (n = 31; 59%) (additional data appear in eTable 1 in the Supplement).
Participants were excluded for lifetime and current medical conditions that could affect testing procedures. Exclusion criteria also included lifetime diagnoses of a neurological disorder, brain injury, or any other disorder affecting cognitive, sensory, and/or motor function or the presence of an ongoing substance use disorder (as defined by the DSM-IV).
A total of 497 adults with a mood, anxiety, or trauma disorder (n = 248) or healthy control status (n = 249) were enrolled. Healthy participants, recruited from equivalent communities in each population center, were matched for age and sex to clinical participants on a casewise basis. Those with incomplete symptom data (n = 77) were excluded from reporting, reducing the sample size to 420 participants with a mean (SD) age of 39.8 (14.1) years (range, 18-83 years); 256 (61.0%) were women.
Consistent with our focus on transdiagnostic heterogeneity, individuals were treated as 1 large transdiagnostic sample for the purpose of analysis. Details of recruitment and screening have been published previously35,41 and are documented further in the eMethods in the Supplement.
Independent Validation Sample
Data for the independent validation sample were acquired from a sample of 381 adult participants, of whom 207 (54.3%) were female. The mean (SD) age was 36.7 (14.6) years (range, 18 to 86 years). They were also recruited from community sources and tested at an academic center.32 This transdiagnostic scope of this sample also encompassed MDD, PTSD, panic disorder, multiple comorbid disorders, and healthy control participants. (For comorbidity rates and details of inclusion and exclusion criteria, see the eMethods and eTable 2 in the Supplement.)
Self-Reported Symptoms
Negative mood was assessed by the Depression, Anxiety and Stress Scale, version 21 (DASS-21).42 The DASS-21 is a self-report scale for assessing 3 symptom areas common to mood, anxiety, and stress disorders, including subscales for depression (including low positive affect, low self-esteem, and sense of hopelessness), anxiety (encompassing fear, somatic features, and hyperarousal features) and stress (tension and irritability) (eTable 3 in the Supplement contains details on study measures). Because the DASS-21 is well normed and well established for use in representative community samples43 and racial/ethnic groups44 and because it correlates with other instruments used to measure multiple mood and anxiety symptoms,45 it was appropriate for use with our transdiagnostic samples.
Behavioral Measures of Neurocognition
A standardized behavioral test battery, “IntegNeuro,” was used to assess neurocognitive function. IntegNeuro has demonstrated reliability, validity, and cross-cultural consistency, has established norms for patients aged 6 years to 80 years and older,46,47,48,49 and has demonstrated usefulness for diagnostic groups in case-control studies.50,51,52 The cognitive constructs assessed by IntegNeuro include cognitive control (choice reaction time, switching of attention, verbal interference/Stroop, go/no-go, and maze), working memory (digit span and span of visual memory), language fluency (word generation for words starting with F, A, or S), and response speed (motor tapping).
Neurophysiological Measure of Brain Activation
Electroencephalography (EEG) served as our neurophysiological measure of brain activation. As in prior research on negative mood states,53,54,55,56 EEG data were recorded during resting conditions and during facial emotion-viewing paradigms. Two resting conditions were recorded: a 2-minute span with eyes open, followed by a 2-minute span with eyes closed.
We used well-established facial emotion paradigms for eliciting brain activation responses during the viewing of facial expressions under both conscious (unmasked) and nonconscious (masked) conditions. Facial emotion stimuli depicted expressions of fear, anger, sadness, and happiness, relative to a neutral expression.57 The stimuli included 8 different individuals selected from a standardized series.58 In each paradigm, the stimuli were grouped by the 8 individuals displaying the same emotion, with each grouping repeated 5 times. In the nonconscious condition, facial emotion stimuli were presented below the threshold for conscious sensory detection.
A Neuroscan Compumedics Nuamps system and an Quikcap Electrode System (Compumedics Ltd) were used to record EEG data according to the 10 to 20 electrode international system with 32 channels, including 4 electrooculography channels, an orbicularis oculus channel, and a masseter channel. The details of this well-established protocol have been published previously.41
Informed by prior EEG research on negative mood states, we quantified data for primary frequency bands of focus—α (8–13 Hz) and β (14.5–30 Hz)—and these values were averaged for the frontal region (Fp1, Fp2, F7, F3, Fz, F4, and F8), the central region (FC3, FCz, FC4, C3, Cz, C4, CP3, CPz, and CP4), the temporal regions (T3, T4, T5, and T6), and the parietal/occipital regions (P3, Pz, P4, O1, Oz and O2) bilaterally.41,53,59 We designated θ (4-7.5 Hz) and δ (1.5-3.5 Hz) frequencies as secondary bands of interest, and we clarified in exploratory analyses that there were no significant effects for these bands. Outliers beyond 3 SDs from the mean of power values at each electrode site were mean-replaced. Drawing on prior findings for negative mood states,60 we also quantified frontal α asymmetry according to a subtraction of the natural log transformation of α power for FC4 minus FC3.
Daily Functional Capacity
Daily functional status was assessed using the Brief Risk-Resilience Index for Screening, a 45-question screening tool that includes subindexes assessing capacity for social skills and emotional resilience.34 The association between this instrument and other self-reported measures of functional capacity, including social and occupational functioning and quality of life, has been established by previous research.34
Data Analysis
Unsupervised Machine Learning to Classify Individuals Into Putative Subtypes
Statistical analyses were conducted using the stats, psych, cluster, and factoextra packages in R, version 3.3.2 (R Foundation for Statistical Computing), and NumPy, SciPy, IPython, Jupyter, matplotlib, and scikit-learn packages in Python, version 3.6.2 (Python Software Foundation). We used a principal component analysis on the DASS-21 item-level data for all participants (clinical and control) to reduce the data dimensionality while retaining significant variance across measures. The number of components was determined by interpretation of the scree plot and by the percentage of variance explained. Symptom component scores for all participants (clinical and control) were the inputs to the agglomerative hierarchical clustering with Ward error sum of squares algorithm in the R cluster package; this is a standard and reliable method to identify clusters. The optimal number of clusters was determined using 4 standard methods: the gap statistic,61 the Calinski-Harabasz index,62 the elbow method, and the dendrogram (eResults and eFigures 1-4 in the Supplement). Cluster centers were plotted from 10 000 repeated subsamples to assess robustness of the clustering solution (eFigures 5-7 in the Supplement).
Assessing the Reproducibility of the Clustering Solution in an Independent Validation Sample
To further evaluate robustness and reproducibility, we repeated our clustering methods in the independent validation sample. We performed a principal component analysis using item-level data from the DASS-21 and used the resulting component scores as inputs to the agglomerative hierarchical clustering created with the Ward error sum of squares algorithm, then evaluated how closely the resulting cluster centers matched the original cluster centers.
Expression of Putative Subtypes in Neurocognitive, Neurophysiological, and Daily Function Domains
We evaluated the extent to which subtypes differentiated on the external measures of neurocognition, neurophysiological, and daily functional status. One-way analysis of variance (ANOVA) tests were run to identify significant differences on each measure between the mean scores of the 6 subtypes. Variance explained by the 6-cluster solution was compared with that explained by DSM-IV diagnosis (eTable 4 in the Supplement); post hoc tests were also completed (eFigure 8 and eTable 5 in the Supplement). Redundant variables within each measure were not analyzed to reduce comparisons and retain meaningful results. Multiple comparisons were addressed by using the Bonferroni correction (eMethods in the Supplement). Additional analysis of covariance tests that included comorbidity covariates were run, and variance explained by the 6-cluster solution was compared with variance explained by DSM-IV diagnosis (eTable 6 in the Supplement). For neurocognitive performance, ANOVA tests were run on each of the 9 tests, with a Bonferroni-corrected α level of P = .006. For neurophysiology measures, ANOVAs were run separately for electroencephalographic tests with eyes open and eyes closed and for conscious and nonconscious emotion conditions. In these ANOVAs, dependent variables were the 4 averaged regional power values for both α and β bands; thus, the corrected alpha level was P = .006. An ANOVA was run on the single measure of α asymmetry at P = .05. For self-reported daily function, ANOVAs were run on the 2 functioning domains of social skills and emotional resilience at the corrected alpha level of P = .03.
Results
Unsupervised Machine Learning Algorithms
Principal component analysis of the DASS-21 items revealed 3 components with the orthogonal rotation converging in 6 iterations (eTable 7 in the Supplement). Together, these components accounted for 71.2% of the total variance. Based on the loadings (eResults in the Supplement), the 3 components were named anhedonia, anxious arousal, and tension.
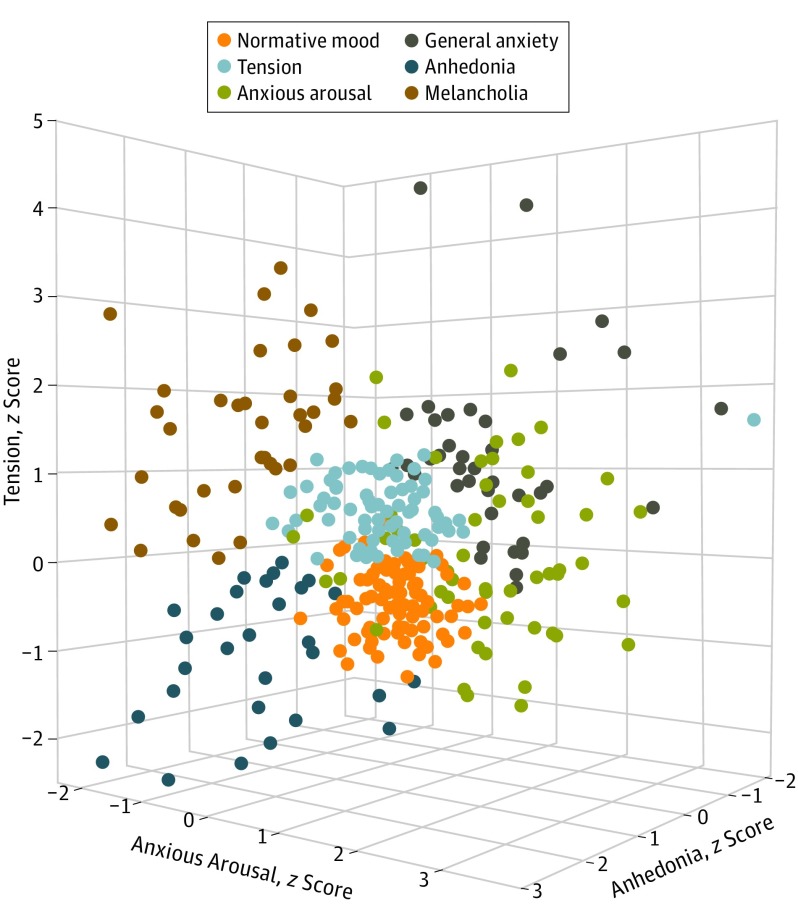
The unsupervised machine learning algorithm identified a 6-cluster solution (Figure 1). Each cluster, or subtype, had a distinct symptom profile (eFigure 9 in the Supplement). Based on each subtype’s mean symptom component scores, they were interpreted as representing the following: normative mood (characterized by low symptom scores on all 3 components) (n = 180); tension (n = 81); anxious arousal (n = 55); general anxiety (n = 38); anhedonia (n = 29); and melancholia (n = 37).
Figure 1. Visual Demonstration of Clustering of Subtypes in 3-Dimensional Space.
Each symptom component is a spatial dimension on the x-axis, y-axis, and z-axis.
Subtypes differed significantly in anhedonia, anxious arousal, and tension, as determined by 1-way analyses of variance (Table 1). Subtypes did not differ significantly with respect to age, sex, or years of education. (See eTable 8 in the Supplement for sex distribution across clusters.)
Table 1. Differences Between Subtype Clusters on Profiles of Symptom Severity Means, Expressed as Mean z Scores for Standardized Comparisons Between Measures.
| Symptom | Type Cluster, Mean (SE) | Test of Difference | ||||||
|---|---|---|---|---|---|---|---|---|
| Normative Mood | Tension | Anxious Arousal | General Anxiety | Anhedonia | Melancholia | F5,414 | P Value | |
| Anhedonia | −0.411 (0.028) | −0.047 (0.062) | 0.69 (0.108) | −0.824 (0.096) | 2.113 (0.098) | 1.185 (0.152) | 170.71 | <.001 |
| Anxious arousal | −0.026 (0.023) | −0.504 (0.040) | 1.842 (0.096) | 0.929 (0.136) | −0.154 (0.149) | −1.217 (0.096) | 228.33 | <.001 |
| Tension | −0.662 (0.025) | 0.468 (0.040) | 0.122 (0.117) | 1.201 (0.172) | −0.958 (0.124) | 1.533 (0.143) | 143.87 | <.001 |
Replication in Independent Sample
In an independent sample, principal component analysis identified the same 3-component solution of anhedonia, anxious arousal, and tension components (eResults and eTable 9 in the Supplement). Hierarchical clustering algorithms, using the 3 component scores as inputs, identified the same 6-cluster solution (eFigures 9 and 10 in the Supplement).
Expression in Behavioral Measures of Neurocognition
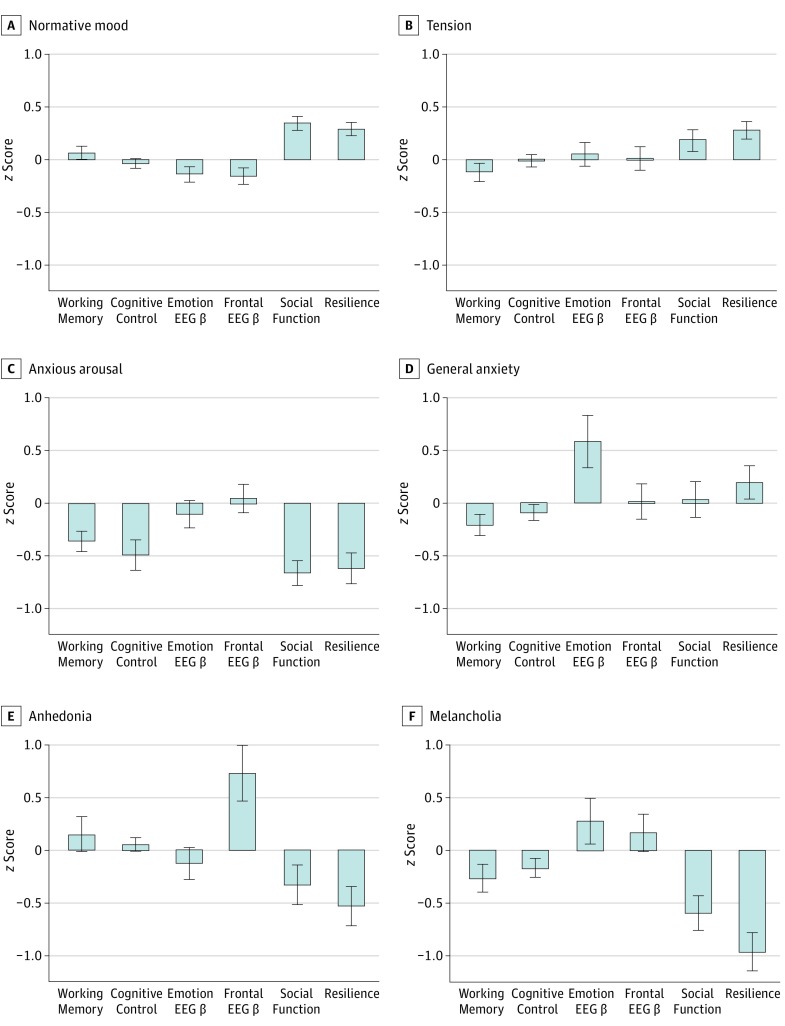
Subtypes were expressed in a differential profile of behavioral performance on tests of neurocognition (Figure 2). Subtypes differed significantly in their cognitive control, as measured by the go/no-go test (z scores were −0.038 for people with normative mood, −0.012 for people with tension, −0.491 for people with anxious arousal, −0.903 for people with general anxiety, 0.057 for people with anhedonia, and −0.163 for people with melancholia; F5,383 = 5.13; P < .001; ηp2 = 0.063). Subtypes also differed by working memory as measured by the digit span test (z scores were for 0.062 for people with normative mood, −0.116 for people with tension, −0.357 for people with anxious arousal, −0.209 for people with general anxiety, 0.158 for people with anhedonia, and −0.260 for people with melancholia; F5,401 = 3.29; P = .01; ηp2 = 0.039). This difference was owing to particularly poor cognitive control and working memory for the anxious arousal subtype.
Figure 2. Clinical-Behavioral-Brain Profiles for Each Subtype.
Cognitive control is measured by the go/no-go paradigm; working memory is measured by the digit span paradigm; emotion electroencephalographic (EEG) β is β power in the parietal/occipital region for the happy condition during the conscious emotion paradigm; frontal electroencephalographic β is mean β power in the frontal region during the eyes-open resting condition; social function is self-reported daily functional capacity in the domain of social skills; and resilience is daily functional capacity in the domain of emotional resilience. All values are expressed in standardized units to facilitate interpretation of profiles across measures. Error bars represent 1 SD from the mean.
Expression in Neurophysiological Measures of Brain Activation
Subtypes were also expressed in a differential profile of resting brain activation, as assessed by the neurophysiological measure EEG power (Figure 2). Subtypes differed in β power across the frontal region (z scores were for −0.160 for people with normative mood, 0.011 for people with tension, −0.047 for people with anxious arousal, −0.013 for people with general anxiety, 0.725 for people with anhedonia, and −0.165 for people with melancholia; F5,357= 3.84; P = .002; ηp2 = 0.051), particularly for the resting, eyes-open condition. This difference was most prominently related to the comparatively elevated β power of the anhedonia subtype (Figure 2). Subtypes were further differentiated by their emotion-evoked brain activation as assessed by EEG power. During the conscious emotion paradigm, subtypes differed in parietooccipital β power for facial expressions of happiness in particular (z scores were for −0.141 for people with normative mood, 0.052 for people with tension, −0.101 for people with anxious arousal, 0.590 for people with general anxiety, −0.127 for people with anhedonia, and 0.273 for people with melancholia; F5,365 = 3.56; P = .004; ηp2 = 0.047) (Figure 2). This profile of elevated β was primarily owing to the general anxiety subtype relative to other subtypes (Figure 2). Subtypes did not differ significantly in the measure of EEG α asymmetry.
Expression in Daily Functional Capacity
Subtypes were expressed clinically through differential profiles of self-reported functioning, assessed by the Brief Risk-Resilience Index for Screening domains of capacity for social skills (z scores were for 0.287 for people with normative mood, 0.280 for people with tension, −0.617 for those with anxious arousal, 0.195 in persons with general anxiety, −0.529 in those with anhedonia, and −0.959 in people with melancholia; F5,414 = 21.33; P < .001; ηp2 = 0.205) and emotional resilience (z scores were for 0.348 for people with normative mood, 0.188 for people with tension, −0.661 for people with anxious arousal, 0.032 for people with general anxiety, −0.329 for persons with anhedonia, and −0.590 for people with melancholia; F5,376 = 15.10; P < .001; ηp2 = 0.171) (Figure 2). The profile of low emotional resilience, in particular, reflected the poorest functioning in the melancholia subtype.
Comparison With Conventional Diagnostic Boundaries
Subtypes were mapped onto the original DSM-IV diagnostic categories to provide a complementary, theoretically driven frame of reference. Subtypes were shown to cut across diagnostic boundaries because frequency distributions showed subtypes were composed of participants from all diagnostic groups, revealing that cluster groups did not represent diagnosis. For instance, individuals with primary diagnoses of MDD and PTSD were distributed across all 6 subtypes (Table 2; eResults, eTable 6, and eFigure 11 in the Supplement).
Table 2. Number of Individuals Classified Into Subtype Clusters per Conventional Diagnosis.
| Diagnosis | No. | ||||||
|---|---|---|---|---|---|---|---|
| Total | Normative Mood | Tension | Anxious Arousal | General Anxiety | Anhedonia | Melancholia | |
| None (control) | 220 | 155 | 57 | 2 | 3 | 1 | 2 |
| MDD | 100 | 6 | 12 | 23 | 9 | 23 | 27 |
| Panic disorder | 53 | 14 | 7 | 18 | 12 | 2 | 0 |
| PTSD | 47 | 5 | 5 | 12 | 14 | 3 | 8 |
Abbreviations: MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
Discussion
This study demonstrates a novel approach to identifying subtypes defined by distinct profiles of symptoms that map onto unique patterns of neurocognition, brain activation, and clinically relevant daily functioning. A machine learning stratification algorithm, blind to diagnosis, identified 6 clusters of individuals based on specific symptom profiles. This 6-cluster solution was replicated in an independent sample, indicating the solution is reproducible. Each type demonstrated a unique profile across domains. Anxious arousal was distinguished by poor daily functioning and the greatest level of neurocognitive impairment compared with most subtypes, particularly in the cognitive control domain. General anxiety was characterized by an elevation in emotion-elicited parietooccipital β power as compared with the normative mood and anxious arousal subtypes, and intact daily functioning. Melancholia, in contrast, was distinguished by the poorest daily functioning, particularly social functioning, compared with the normative mood, tension, and general anxiety subtypes. Anhedonia was distinguished by specific elevations in resting frontal β power, and the tension subtype by average performance across domains despite severe symptoms of tension.
Although previous studies have not examined multidomain profiles across multiple diagnoses, specific aspects of these profiles align with prior findings. Our observation of the poorest neurocognition in the anxious arousal subtype accords with previous within-diagnosis relationships between anxiety and impaired cognition.63,64,65,66 Our results align with the view that functional disability, spanning social and (especially) emotional domains, is a hallmark of melancholia.67,68 The frontal β elevation observed for anhedonia is somewhat surprising, because β waves are often associated with active or anxious thinking.69,70 However, at least 1 previous study has shown a connection between increased β and depression specifically.71 A possible account of the elevated parietal β and somewhat poor working memory of people with general anxiety is a parietally mediated compensation for core working memory dysfunction72; however, in this study, the difference on working performance from other subtypes was not significant. By contrast, the relatively intact status of the tension subgroup may reflect more fundamental compensatory mechanisms or a state of stress that does not reach the level of disrupting daily life.
Our approach demonstrates 1 application of a proposed model for ultimately developing a taxonomy for mental disorders that maps specific symptom profiles onto underlying neurobehavioral dimensions. There are important ways in which this approach should be further refined and expanded. Future studies should use additional data-driven techniques and independent samples to further test the robustness of subtype structures. Longitudinal designs (such as the Minnesota Twin Family Study73) will help determine whether subtypes are stable over time, as has been shown within the diagnosis of major depression for adolescents and older adults.13 To further elucidate the functional anatomical basis of the subtypes, high-density EEG data could be acquired for source localization. With in vivo imaging, we could anchor transdiagnostic subtypes in increasingly proximal measures of underlying brain circuits.74,75 Imaging should also be considered as primary inputs for clustering and validating solutions, alongside symptom measures. The Bipolar and Schizophrenia Network for Intermediate Phenotypes has advanced the identification of biotypes for psychosis based on cognitive and neurophysiological measures,11 and validated with functional and imaging measures.76 It will be important to incorporate information at many different domain levels and examine cross-level interactions77 as a means to tap biological systems implicated in major psychiatric domains. Several foundational studies have used imaging of resting functional brain connectivity to identify neurophysiological biotypes within samples of depressed individuals.78,79,80 These novel approaches have had a profound effect on our understanding of depression and reflect state-of-the-art systems biology research needed to develop valid biotypes.
The clinical utility of new subtype models ultimately rests on their value in helping guide intervention decisions. Because our data-driven approach yields groups of individuals that share symptom, behavioral, and brain activation profiles, it offers one way forward for considering new targets for intervention studies. For example, in light of the general anxiety subtype profiles, 1 such hypothesis would be that behavioral interventions targeting working memory would have a specific association with general anxiety symptoms and would be visible on associated working memory and emotion-elicited EEG β power end points.
Limitations
One limitation of our study is the relatively small number of individuals in each subtype and the overall small size of the sample. Over and beyond replication, larger samples are needed so that stratifications by sex, age, and symptom-defined subgroups retain large enough numbers in important strata. Data from a large number of participants, input into a data set for the machine learning processes used in this study, will allow additional latent constructs in the data to be uncovered.
Furthermore, this study used limited symptom data for subtype determination. These inputs were constrained by the need for common assessments across diagnoses and samples. Future research that expands the sample size and input features and uses complementary features for subtype determination will be essential in establishing a valid and clinically viable taxonomy for mood, anxiety, and trauma disorders.
Our analyses also focused on current Axis 1 diagnoses. Thus, we were unable to include data on the potential contributions of lifetime history of psychiatric disorder or history of substance use and other disorders. Future studies should include a lifetime history to ensure that all relevant domains underlying psychopathology are being tapped.
Conclusions
We demonstrate a data-driven approach for identifying transdiagnostic subtypes that are distinct, reproducible, and expressed across domains of symptoms, neurocognitive functioning, brain activation, and daily functioning. Because the symptom profiles map on to clinically relevant domains, they offer new targets for developing personalized treatments.
eMethods.
eTable 1. Comorbidities in primary sample.
eTable 2. Comorbidities in validation sample.
eTable 3. Study measures.
eTable 4. Partial eta squared values for 6-cluster solution versus DSM diagnosis.
eTable 5. Tukey HSD post-hoc tests.
eTable 6. R-squared for ANCOVAs including comorbidity covariates for 6-cluster solution versus DSM diagnosis.
eTable 7. PCA component loadings for three negative mood components.
eTable 8. Sex distribution by cluster.
eTable 9. PCA component loadings for three negative mood components in validation sample.
eTable 10. Number and percentage of individuals with each diagnosis across subtypes.
eFigure 1. Gap Statistic for cluster number determination.
eFigure 2. Calinski-Harabasz index for cluster number determination.
eFigure 3. Within-cluster sum of squares for cluster number determination.
eFigure 4. Dendrogram of clustering solution.
eFigure 5. Cluster centers plotted from 10,000 repeated subsamples.
eFigure 6. Histogram of cluster centers calculated from repeated subsampling.
eFigure 7. Distribution of adjusted Rand scores.
eFigure 8. Tukey HSD post-hoc tests.
eFigure 9. Clusters in primary and validation samples.
eFigure 10. Symptom profiles in primary and validation samples.
eFigure 11. Distribution of each diagnostic category by subtype.
eResults.
References
- 1.Kessler RC, Gruber M, Hettema JM, Hwang I, Sampson N, Yonkers KA. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38(3):365-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein-Piekarski AN, Williams LM, Humphreys K. A trans-diagnostic review of anxiety disorder comorbidity and the impact of multiple exclusion criteria on studying clinical outcomes in anxiety disorders. Transl Psychiatry. 2016;6(6):e847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somers JM, Goldner EM, Waraich P, Hsu L. Prevalence and incidence studies of anxiety disorders: a systematic review of the literature. Can J Psychiatry. 2006;51(2):100-113. [DOI] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration HHS Publication No. (SMA) 13-4805: Results From the 2012 National Survey on Drug Use and Health: Mental Health Findings. Rockville, MD: US Department of Health and Human Services, 2013: 1-136. [Google Scholar]
- 5.Weissman MM, Bland RC, Canino GJ, et al. The cross-national epidemiology of panic disorder. Arch Gen Psychiatry. 1997;54(4):305-309. [DOI] [PubMed] [Google Scholar]
- 6.Whiteford HA, Degenhardt L, Rehm J, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382(9904):1575-1586. [DOI] [PubMed] [Google Scholar]
- 7.Saveanu R, Etkin A, Duchemin AM, et al. The international study to predict optimized treatment in depression (iSPOT-D): outcomes from the acute phase of antidepressant treatment. J Psychiatr Res. 2014;61:1-12. [DOI] [PubMed] [Google Scholar]
- 8.Brodersen KH, Deserno L, Schlagenhauf F, et al. Dissecting psychiatric spectrum disorders by generative embedding. Neuroimage Clin. 2013;4:98-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geisler D, Walton E, Naylor M, et al. Brain structure and function correlates of cognitive subtypes in schizophrenia. Psychiatry Res. 2015;234(1):74-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun H, Lui S, Yao L, et al. Two patterns of white matter abnormalities in medication-naive patients with first-episode schizophrenia revealed by diffusion tensor imaging and cluster analysis. JAMA Psychiatry. 2015;72(7):678-686. [DOI] [PubMed] [Google Scholar]
- 11.Clementz BA, Sweeney JA, Hamm JP, et al. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry. 2016;173(4):373-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewandowski KE, Sperry SH, Cohen BM, Ongür D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med. 2014;44(15):3239-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lamers F, Burstein M, He JP, Avenevoli S, Angst J, Merikangas KR. Structure of major depressive disorder in adolescents and adults in the US general population. Br J Psychiatry. 2012;201:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rhebergen D, Lamers F, Spijker J, de Graaf R, Beekman AT, Penninx BW. Course trajectories of unipolar depressive disorders identified by latent class growth analysis. Psychol Med. 2012;42(7):1383-1396. [DOI] [PubMed] [Google Scholar]
- 15.van Loo HM, Cai T, Gruber MJ, et al. Major depressive disorder subtypes to predict long-term course. Depress Anxiety. 2014;31(9):765-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milaneschi Y, Lamers F, Peyrot WJ, et al. ; CHARGE inflammation working group . Polygenic dissection of major depression clinical heterogeneity. Mol Psychiatry. 2016;21(4):516-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Loo HM, de Jonge P, Romeijn JW, Kessler RC, Schoevers RA. Data-driven subtypes of major depressive disorder: a systematic review. BMC Med. 2012;10:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costa Dias TG, Iyer SP, Carpenter SD, et al. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Dev Cogn Neurosci. 2015;11:155-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Hulst BM, de Zeeuw P, Durston S. Distinct neuropsychological profiles within ADHD: a latent class analysis of cognitive control, reward sensitivity and timing. Psychol Med. 2015;45(4):735-745. [DOI] [PubMed] [Google Scholar]
- 20.Mostert JC, Hoogman M, Onnink AM, et al. Similar subgroups based on cognitive performance parse heterogeneity in adults with ADHD and healthy controls. J Atten Disord. 2015;pii:1087054715602332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgiades S, Szatmari P, Boyle M, et al. ; Pathways in ASD Study Team . Investigating phenotypic heterogeneity in children with autism spectrum disorder: a factor mixture modeling approach. J Child Psychol Psychiatry. 2013;54(2):206-215. [DOI] [PubMed] [Google Scholar]
- 22.Doshi-Velez F, Ge Y, Kohane I. Comorbidity clusters in autism spectrum disorders: an electronic health record time-series analysis. Pediatrics. 2014;133(1):e54-e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veatch OJ, Veenstra-Vanderweele J, Potter M, Pericak-Vance MA, Haines JL. Genetically meaningful phenotypic subgroups in autism spectrum disorders. Genes Brain Behav. 2014;13(3):276-285. [DOI] [PubMed] [Google Scholar]
- 24.Shanmugan S, Wolf DH, Calkins ME, et al. Common and dissociable mechanisms of executive system dysfunction across psychiatric disorders in youth. Am J Psychiatry. 2016;173(5):517-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olino TM, Klein DN, Lewinsohn PM, Rohde P, Seeley JR. Latent trajectory classes of depressive and anxiety disorders from adolescence to adulthood: descriptions of classes and associations with risk factors. Compr Psychiatry. 2010;51(3):224-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kleinman A, Caetano SC, Brentani H, et al. Attention-based classification pattern, a research domain criteria framework, in youths with bipolar disorder and attention-deficit/hyperactivity disorder. Aust N Z J Psychiatry. 2015;49(3):255-265. [DOI] [PubMed] [Google Scholar]
- 27.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602. [DOI] [PubMed] [Google Scholar]
- 28.Kessler RC, Merikangas KR. The National Comorbidity Survey Replication (NCS-R): background and aims. Int J Methods Psychiatr Res. 2004;13(2):60-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afzali MH, Sunderland M, Teesson M, Carragher N, Mills K, Slade T. A network approach to the comorbidity between posttraumatic stress disorder and major depressive disorder: the role of overlapping symptoms. J Affect Disord. 2017;208:490-496. [DOI] [PubMed] [Google Scholar]
- 30.Zbozinek TD, Rose RD, Wolitzky-Taylor KB, et al. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety. 2012;29(12):1065-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozak MJ, Cuthbert BN. The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology. 2016;53(3):286-297. [DOI] [PubMed] [Google Scholar]
- 32.Williams LM, Goldstein-Piekarski AN, Chowdhry N, et al. Developing a clinical translational neuroscience taxonomy for anxiety and mood disorder: protocol for the baseline-follow up Research domain criteria Anxiety and Depression (“RAD”) project. BMC Psychiatry. 2016;16(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thibodeau R, Jorgensen RS, Kim S. Depression, anxiety, and resting frontal EEG asymmetry: a meta-analytic review. J Abnorm Psychol. 2006;115(4):715-729. [DOI] [PubMed] [Google Scholar]
- 34.Williams LM, Cooper NJ, Wisniewski SR, et al. Sensitivity, specificity, and predictive power of the “Brief Risk-resilience Index for SCreening,” a brief pan-diagnostic web screen for emotional health. Brain Behav. 2012;2(5):576-589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koslow SH, Wang Y, Palmer DM, Gordon E, Williams LM. BRAINnet: A standardized global human brain project. Technol Innov. 2013;15(1):17-28. [Google Scholar]
- 36.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22-33. [PubMed] [Google Scholar]
- 37.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer R, Gibbon M, Williams JBW. Structured Clinical Interview or DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: New York State Psychiatric Institute; 2002. [Google Scholar]
- 40.Kessler RC, Abelson J, Demler O, et al. Clinical calibration of DSM-IV diagnoses in the World Mental Health (WMH) version of the World Health Organization (WHO) Composite International Diagnostic Interview (WMHCIDI). Int J Methods Psychiatr Res. 2004;13(2):122-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gordon E, Palmer DM, Cooper N. EEG alpha asymmetry in schizophrenia, depression, PTSD, panic disorder, ADHD and conduct disorder. Clin EEG Neurosci. 2010;41(4):178-183. [DOI] [PubMed] [Google Scholar]
- 42.Lovibond L. Manual for the Depression Anxiety Stress Scales. Sydney, Australia: Psychology Foundation of Australia; 1995. [Google Scholar]
- 43.Norton PJ. Depression Anxiety and Stress Scales (DASS-21): psychometric analysis across four racial groups. Anxiety Stress Coping. 2007;20(3):253-265. [DOI] [PubMed] [Google Scholar]
- 44.Keogh E, Reidy J. Exploring the factor structure of the Mood and Anxiety Symptom Questionnaire (MASQ). J Pers Assess. 2000;74(1):106-125. [DOI] [PubMed] [Google Scholar]
- 45.Crawford JR, Henry JD. The Depression Anxiety Stress Scales (DASS): normative data and latent structure in a large non-clinical sample. Br J Clin Psychol. 2003;42(pt 2):111-131. [DOI] [PubMed] [Google Scholar]
- 46.Clark CR, Paul RH, Williams LM, et al. Standardized assessment of cognitive functioning during development and aging using an automated touchscreen battery. Arch Clin Neuropsychol. 2006;21(5):449-467. [DOI] [PubMed] [Google Scholar]
- 47.Paul RH, Lawrence J, Williams LM, Richard CC, Cooper N, Gordon E. Preliminary validity of “Integneuro”: a new computerized battery of neurocognitive tests. Int J Neurosci. 2005;115(11):1549-1567. [DOI] [PubMed] [Google Scholar]
- 48.Paul RH, Gunstad J, Cooper N, et al. Cross-cultural assessment of neuropsychological performance and electrical brain function measures: additional validation of an international brain database. Int J Neurosci. 2007;117(4):549-568. [DOI] [PubMed] [Google Scholar]
- 49.Williams LM, Simms E, Clark CR, Paul RH, Rowe D, Gordon E. The test-retest reliability of a standardized neurocognitive and neurophysiological test battery: “neuromarker”. Int J Neurosci. 2005;115(12):1605-1630. [DOI] [PubMed] [Google Scholar]
- 50.Williams LM, Hermens DF, Thein T, et al. Using brain-based cognitive measures to support clinical decisions in ADHD. Pediatr Neurol. 2010;42(2):118-126. [DOI] [PubMed] [Google Scholar]
- 51.Williams LM, Whitford TJ, Flynn G, et al. General and social cognition in first episode schizophrenia: identification of separable factors and prediction of functional outcome using the IntegNeuro test battery. Schizophr Res. 2008;99(1-3):182-191. [DOI] [PubMed] [Google Scholar]
- 52.Hatch A, Madden S, Kohn MR, et al. In first presentation adolescent anorexia nervosa, do cognitive markers of underweight status change with weight gain following a refeeding intervention? Int J Eat Disord. 2010;43(4):295-306. [DOI] [PubMed] [Google Scholar]
- 53.Gatt JM, Kuan SA, Dobson-Stone C, et al. Association between BDNF Val66Met polymorphism and trait depression is mediated via resting EEG alpha band activity. Biol Psychol. 2008;79(2):275-284. [DOI] [PubMed] [Google Scholar]
- 54.Stewart JL, Coan JA, Towers DN, Allen JJ. Resting and task-elicited prefrontal EEG alpha asymmetry in depression: support for the capability model. Psychophysiology. 2014;51(5):446-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Auerbach RP, Stewart JG, Stanton CH, Mueller EM, Pizzagalli DA. Emotion-processing biases and resting EEG activity in depressed adolescents. Depress Anxiety. 2015;32(9):693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pizzagalli DA, Peccoralo LA, Davidson RJ, Cohen JD. Resting anterior cingulate activity and abnormal responses to errors in subjects with elevated depressive symptoms: a 128-channel EEG study. Hum Brain Mapp. 2006;27(3):185-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams LM, Liddell BJ, Rathjen J, et al. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Hum Brain Mapp. 2004;21(2):64-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115(2):137-143. [DOI] [PubMed] [Google Scholar]
- 59.Fingelkurts AA, Fingelkurts AA, Rytsälä H, Suominen K, Isometsä E, Kähkönen S. Composition of brain oscillations in ongoing EEG during major depression disorder. Neurosci Res. 2006;56(2):133-144. [DOI] [PubMed] [Google Scholar]
- 60.Davidson RJ. Anterior electrophysiological asymmetries, emotion, and depression: conceptual and methodological conundrums. Psychophysiology. 1998;35(5):607-614. [DOI] [PubMed] [Google Scholar]
- 61.Tibshirani R, Walther G, Hastie T. Estimating the number of clusters in a data set via the gap statistic. J R Stat Soc Series B Stat Methodol. 2001;63(2):411-423. [Google Scholar]
- 62.Calińskii T, Harabasz J. A dendrite method for cluster analysis. Commun Stat Theory Methods. 1974;3(1):1-27. [Google Scholar]
- 63.Beaudreau SA, O’Hara R. Late-life anxiety and cognitive impairment: a review. Am J Geriatr Psychiatry. 2008;16(10):790-803. [DOI] [PubMed] [Google Scholar]
- 64.Gualtieri CT, Morgan DW. The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry. 2008;69(7):1122-1130. [DOI] [PubMed] [Google Scholar]
- 65.Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336-353. [DOI] [PubMed] [Google Scholar]
- 66.Moran TP. Anxiety and working memory capacity: A meta-analysis and narrative review. Psychol Bull. 2016;142(8):831-864. [DOI] [PubMed] [Google Scholar]
- 67.Day CV, Gatt JM, Etkin A, DeBattista C, Schatzberg AF, Williams LM. Cognitive and emotional biomarkers of melancholic depression: an iSPOT-D report. J Affect Disord. 2015;176:141-150. [DOI] [PubMed] [Google Scholar]
- 68.Day CV, John Rush A, Harris AW, et al. Impairment and distress patterns distinguishing the melancholic depression subtype: an iSPOT-D report. J Affect Disord. 2015;174:493-502. [DOI] [PubMed] [Google Scholar]
- 69.Gerez M, Suárez E, Serrano C, Castanedo L, Tello A. The crossroads of anxiety: distinct neurophysiological maps for different symptomatic groups. Neuropsychiatr Dis Treat. 2016;12:159-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pavlenko V, Chernyi S, Goubkina D. EEG correlates of anxiety and emotional stability in adult healthy subjects. Neurophysiology. 2009;41(5):337-345. [Google Scholar]
- 71.Knott V, Mahoney C, Kennedy S, Evans K. EEG power, frequency, asymmetry and coherence in male depression. Psychiatry Res. 2001;106(2):123-140. [DOI] [PubMed] [Google Scholar]
- 72.de Vries FE, de Wit SJ, Cath DC, et al. Compensatory frontoparietal activity during working memory: an endophenotype of obsessive-compulsive disorder. Biol Psychiatry. 2014;76(11):878-887. [DOI] [PubMed] [Google Scholar]
- 73.Iacono WG, McGue M. Minnesota twin family study. Twin Res. 2002;5(5):482-487. [DOI] [PubMed] [Google Scholar]
- 74.Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3(5):472-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tamminga CA, Pearlson G, Keshavan M, Sweeney J, Clementz B, Thaker G. Bipolar and schizophrenia network for intermediate phenotypes: outcomes across the psychosis continuum. Schizophr Bull. 2014;40(suppl 2):S131-S137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kendler KS. The structure of psychiatric science. Am J Psychiatry. 2014;171(9):931-938. [DOI] [PubMed] [Google Scholar]
- 78.Price RB, Gates K, Kraynak TE, Thase ME, Siegle GJ. Data-driven subgroups in depression derived from directed functional connectivity paths at rest. Neuropsychopharmacology. 2017;42(13):2623-2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Price RB, Lane S, Gates K, et al. Parsing heterogeneity in the brain connectivity of depressed and healthy adults during positive mood. Biol Psychiatry. 2017;81(4):347-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Comorbidities in primary sample.
eTable 2. Comorbidities in validation sample.
eTable 3. Study measures.
eTable 4. Partial eta squared values for 6-cluster solution versus DSM diagnosis.
eTable 5. Tukey HSD post-hoc tests.
eTable 6. R-squared for ANCOVAs including comorbidity covariates for 6-cluster solution versus DSM diagnosis.
eTable 7. PCA component loadings for three negative mood components.
eTable 8. Sex distribution by cluster.
eTable 9. PCA component loadings for three negative mood components in validation sample.
eTable 10. Number and percentage of individuals with each diagnosis across subtypes.
eFigure 1. Gap Statistic for cluster number determination.
eFigure 2. Calinski-Harabasz index for cluster number determination.
eFigure 3. Within-cluster sum of squares for cluster number determination.
eFigure 4. Dendrogram of clustering solution.
eFigure 5. Cluster centers plotted from 10,000 repeated subsamples.
eFigure 6. Histogram of cluster centers calculated from repeated subsampling.
eFigure 7. Distribution of adjusted Rand scores.
eFigure 8. Tukey HSD post-hoc tests.
eFigure 9. Clusters in primary and validation samples.
eFigure 10. Symptom profiles in primary and validation samples.
eFigure 11. Distribution of each diagnostic category by subtype.
eResults.