Key Points
Question
Is intranasal esketamine hydrochloride an efficacious treatment option for patients with treatment-resistant depression?
Findings
In this randomized, double-blind clinical trial of 67 adults with treatment-resistant depression, significant improvement of depressive symptoms, assessed by the Montgomery-Åsberg Depression Rating Scale total score, was observed after 1 week with intranasal esketamine, 28 to 84 mg administered twice weekly, with a significant ascending dose-response relationship. Improvement appeared to be sustained with reduced dosing frequency for up to 9 weeks.
Meaning
Results of this first clinical trial of intranasal esketamine for treatment-resistant depression support study in larger trials.
Abstract
Importance
Approximately one-third of patients with major depressive disorder (MDD) do not respond to available antidepressants.
Objective
To assess the efficacy, safety, and dose-response of intranasal esketamine hydrochloride in patients with treatment-resistant depression (TRD).
Design, Setting, and Participants
This phase 2, double-blind, doubly randomized, delayed-start, placebo-controlled study was conducted in multiple outpatient referral centers from January 28, 2014, to September 25, 2015. The study consisted of 4 phases: (1) screening, (2) double-blind treatment (days 1-15), composed of two 1-week periods, (3) optional open-label treatment (days 15-74), and (4) posttreatment follow-up (8 weeks). One hundred twenty-six adults with a DSM-IV-TR diagnosis of MDD and history of inadequate response to 2 or more antidepressants (ie, TRD) were screened, 67 were randomized, and 60 completed both double-blind periods. Intent-to-treat analysis was used in evaluation of the findings.
Interventions
In period 1, participants were randomized (3:1:1:1) to placebo (n = 33), esketamine 28 mg (n = 11), 56 mg (n = 11), or 84 mg (n = 12) twice weekly. In period 2, 28 placebo-treated participants with moderate-to-severe symptoms were rerandomized (1:1:1:1) to 1 of the 4 treatment arms; those with mild symptoms continued receiving placebo. Participants continued their existing antidepressant treatment during the study. During the open-label phase, dosing frequency was reduced from twice weekly to weekly, and then to every 2 weeks.
Main Outcomes and Measures
The primary efficacy end point was change from baseline to day 8 (each period) in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score.
Results
Sixty-seven participants (38 women, mean [SD] age, 44.7 [10.0] years) were included in the efficacy and safety analyses. Change (least squares mean [SE] difference vs placebo) in MADRS total score (both periods combined) in all 3 esketamine groups was superior to placebo (esketamine 28 mg: −4.2 [2.09], P = .02; 56 mg: −6.3 [2.07], P = .001; 84 mg: −9.0 [2.13], P < .001), with a significant ascending dose-response relationship (P < .001). Improvement in depressive symptoms appeared to be sustained (−7.2 [1.84]) despite reduced dosing frequency in the open-label phase. Three of 56 (5%) esketamine-treated participants during the double-blind phase vs none receiving placebo and 1 of 57 participants (2%) during the open-label phase had adverse events that led to study discontinuation (1 event each of syncope, headache, dissociative syndrome, and ectopic pregnancy).
Conclusions and Relevance
In this first clinical study to date of intranasal esketamine for TRD, antidepressant effect was rapid in onset and dose related. Response appeared to persist for more than 2 months with a lower dosing frequency. Results support further investigation in larger trials.
Trial Registration
clinicaltrials.gov identifier: NCT01998958
This randomized clinical trial compares the effects of 3 dose levels of intranasal esketamine with placebo in patients with treatment-resistant depression.
Introduction
Major depressive disorder (MDD) is a common and disabling illness, with a lifetime prevalence of approximately 20% in the United States. Major depressive disorder impairs socio-occupational functioning and increases suicide risk, adverse sequelae of other common comorbid medical conditions (eg, cardiovascular disease, type 2 diabetes, and obesity), and mortality. Limitations of currently available antidepressant therapies include delayed onset of efficacy and low remission rates after multiple courses of pharmacotherapy.
Research on mood disorder pathophysiology implicated abnormalities in glutamatergic transmission, along with synaptic and dendritic atrophy, in neural circuits that modulate emotional behavior. Several studies have shown antidepressant efficacy with the N-methyl-d-aspartate (NMDA) receptor antagonist, ketamine. One limitation of ketamine for treating depression is that it may require intravenous administration, reducing its applicability in outpatient settings.
Esketamine, the S-enantiomer of ketamine, has a higher affinity for the NMDA receptor than the R-enantiomer and is being developed as an intranasal formulation for therapy in treatment-resistant depression (TRD). Rapid onset of antidepressant effects has been observed following intravenous administration of esketamine. We report findings from a study of intranasal esketamine, assessing its efficacy and safety compared with placebo in individuals with TRD.
Methods
Population
The study enrolled medically stable (based on physical examination, medical history, vital signs, and 12-lead electrocardiogram performed at screening) adults (aged 20-64 years) with a diagnosis of MDD, according to the DSM-IV-TR.
All participants had TRD, defined as inadequate response to 2 or more antidepressants (assessed by Massachusetts General Hospital Antidepressant Treatment Response Questionnaire), with at least 1 inadequate response in the current depression episode. Otherwise, an antidepressant failure from a prior episode was acceptable. All participants continued the antidepressants they were receiving at study entry during the trial. At screening and before the dose on day 1, eligible participants had a score of 34 or more on the 30-item, clinician-rated Inventory of Depressive Symptomatology, corresponding to moderate to severe depression. Key exclusion criteria included recent or current suicidal ideation with intent to act, suicidal behavior, or homicidal ideation or intent, diagnosis of bipolar or related disorders, intellectual disability, psychotic disorder, MDD with psychosis, posttraumatic stress disorder, obsessive-compulsive disorder, substance/alcohol use disorders in the past year, and recent use of cannabis (more inclusion/exclusion criteria reported in the eAppendix in Supplement 1).
Independent review boards (United States: Sterling Institutional Review Board, University of Pennsylvania Institutional Review Board, Hartford Healthcare Institutional Review Board, and Western Institutional Review Board) and an independent ethics committee (Belgium: Ethisch Comité O.L. Vrouwenziekenhuis) approved the study protocol and amendments. The study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, consistent with Good Clinical Practices and applicable regulatory requirements. All individuals provided written informed consent before participating in the study. Financial compensation was provided.
Design
This phase 2, 2-panel, double-blind, doubly randomized, delayed-start, placebo-controlled study (a variant of sequential parallel comparison design) was conducted from January 28, 2014, to September 25, 2015. In panel A, reported herein, 14 study sites (13 in the United States, 1 in Belgium) enrolled participants. The study protocol is available in Supplement 2.
The study consisted of 4 phases: (1) screening; (2) double-blind treatment (days 1-15), composed of two 1-week periods (period 1, period 2); (3) optional open-label treatment (days 15-74) with tapering of intranasal dosing frequency; and (4) posttreatment follow-up (8 weeks). Based on prior studies of ketamine in which efficacy was reported after 1 to 2 doses, the duration of each period in the double-blind phase was 1 week, during which time it was expected that efficacy could be achieved. This design allowed evaluation of the dose(s) needed to proceed to evaluation in phase 3. The purpose of the open-label, flexible-dose phase was to evaluate the effect of less-frequent dosing on sustaining efficacy.
At the beginning of double-blind period 1, eligible participants were randomized (3:1:1:1) to intranasal placebo or esketamine 28, 56, or 84 mg, twice weekly (days 1 and 4) based on the first of 2 computer-generated randomization schedules (period 1 and period 2). Randomization was balanced by using randomly permuted blocks and stratified by study center. At the end of period 1, those randomized to placebo who had moderate to severe symptoms (assessed by the 16-item Quick Inventory of Depressive Symptomatology-Self Report [QIDS-SR16] total score: moderate, 11-16; severe, >16) were rerandomized (1:1:1:1) to intranasal esketamine 28, 56, or 84 mg or placebo twice weekly (days 8 and 11); those having mild or no symptoms continued placebo. To maintain the blinding, all participants completed an identical process before entry into period 2, whether or not they were rerandomized. Regardless of response in the double-blind phase, all participants were eligible to enter the optional open-label phase. Esketamine, 56 mg, was administered on the first day of the open-label phase (study day 15); subsequent doses could be adjusted (range, 28-84 mg) based on the investigator’s clinical judgment, with administration twice weekly for the first 2 weeks, weekly for the next 3 weeks, then every 2 weeks thereafter.
Study Drug and Administration
Study drug was provided in a disposable nasal spray device containing 200 μL of solution (ie, 2 sprays). Each device delivered either esketamine hydrochloride, 16.14 (14 mg of esketamine base) per 100-μL spray or placebo. To maintain blinding, the placebo solution (intranasal solution of water for injection) had a bittering agent (denatonium benzoate) added to simulate the taste of esketamine intranasal solution. As described above, the antidepressant that participants had been receiving immediately before study entry was continued unchanged.
On each dosing day during the double-blind phase, participants self-administered 1 spray of study drug (esketamine or placebo) into each nostril at 3 points, each separated by 5 minutes. In the open-label phase, depending on the dose selected, participants self-administered 1 spray of esketamine into each nostril at 1, 2, or 3 points (corresponding to 28, 56, or 84 mg, respectively), each separated by 5 minutes.
Efficacy Assessments
Efficacy was assessed with the Montgomery-Åsberg Depression Rating Scale (MADRS) on days 1 (predose and 2 hours postdose), 2, 8 (predose), 9, and 15, using the MADRS structured interview guide.
Overall illness severity was assessed on the Clinical Global Impression of Severity scale. Participants assessed their severity of anxiety on the Generalized Anxiety Disorder 7-item scale (eTables 1 and 2 in Supplement 1).
Safety Assessments
Adverse events were monitored throughout the study. Other safety assessments (ie, laboratory tests, vital signs, physical examination) were performed at prespecified time points. Vital signs, the Clinician Administered Dissociative States Scale (CADSS), and the 4-item positive symptom subscale from the Brief Psychiatric Rating Scale were assessed predose, at 40 minutes, and 2 hours postdose.
Statistical Analysis
Efficacy data were analyzed in intent-to-treat analysis sets for each period and phase. The intent-to-treat analysis sets included all participants who received at least 1 dose of study medication during that period or phase and had baseline and at least 1 postbaseline MADRS total score within that period or phase. Safety data were analyzed in period 1, period 2, double-blind, and open-label data sets for all participants receiving at least 1 dose of study medication.
Efficacy End Points and Analyses
The primary efficacy end point—change from baseline (predose, day 1 in each period) to end point (day 8 in each period) in MADRS total score—was analyzed using the analysis of covariance model. For period 1, the model included treatment and country as factors and baseline MADRS total score as a covariate. For period 2, the model included treatment and country as factors, period 2 baseline QIDS-SR16 score (moderate or severe), and period 2 baseline MADRS total score as a continuous covariate.
Given the consistency between periods 1 and 2 results, esketamine dose groups were compared with placebo using a combined test on the weighted test statistics for each period in the double-blind treatment phase. A dose-response analysis on the primary efficacy end point was performed using data combined from both periods. The multiple comparison procedure modeling methodology was performed.
Sample Size Determination
Sample size was determined based on the following differences between intranasal esketamine and placebo for mean change from baseline in MADRS total score: 9-point treatment difference was assumed for period 1 (day 8), 7-point treatment difference for period 2 (day 15) was assumed for individuals with a moderate QIDS-SR16 score, and 9-point treatment difference for period 2 (day 15) was assumed for individuals with a severe QIDS-SR16 score.
Based on the results of an intravenous esketamine study, it was estimated that 40% of placebo-receiving participants would have a moderate QIDS-SR16 score and 55% would have a severe QIDS-SR16 score at the end of period 1 (day 8 predose). Additional assumptions for the sample size calculation included SD of 10, 92.5% power for the combined data from day 8 and day 15, overall 1-sided significance level of .05, and 5% dropout rate for period 1. It was calculated that this panel of the doubly randomized, outcome-based design required 60 individuals to be randomly assigned to treatment on day 1 in a 3:1:1:1 ratio (30 in the placebo group and 10 per intranasal esketamine dose group). Statistical analysis was performed using SAS, version 9.2 (SAS Institute Inc).
Results
Participants
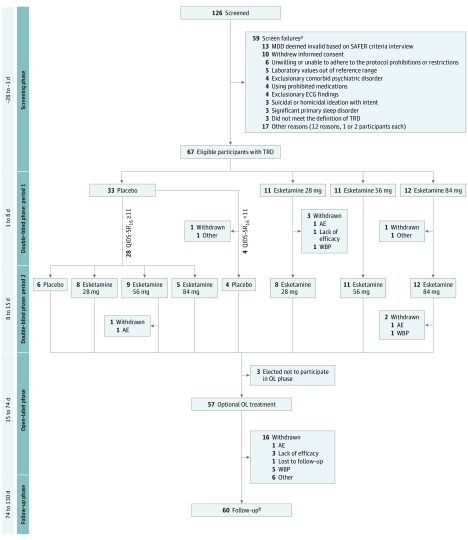
A total of 126 individuals were screened, 67 of whom met the eligibility criteria and were randomized (38 women, mean [SD] age, 44.7 [10.0] years). Of 33 participants randomized to placebo in period 1, 28 (85%) had a QIDS-SR16 score of 11 or higher at the end of period 1 and thus were randomly reassigned to esketamine or placebo in period 2 (Figure 1). Most randomized participants (63 of 67 [94%]) completed period 1 and the 2-week double-blind phase (ie, periods 1 and 2 combined, 60 of 67 [90%], hereafter termed completers). Of these completers, 57 entered the open-label phase, with 51 (89%) subsequently entering the follow-up phase, 41 (80%) of whom completed the week 8 follow-up visit.
Figure 1. Disposition of Participants.
Seven participants started the follow-up phase earlier than day 74, having received 2 weeks of study drug during the open-label phase of the study. AE indicates adverse event; ECG, electrocardiogram; MDD, major depressive disorder; OL, open-label; QIDS-SR16, 16-item Quick Inventory of Depressive Symptoms–Self-Report; SAFER, State vs Trait, Assessibility, Face Validity, Ecological Validity, Rule of Three Ps; TRD, treatment-resistant depression; WBP, withdrawal by participant.
aParticipants could have multiple reasons for being a screen failure.
bParticipants entered the follow-up phase if they did not choose to withdraw from the study.
The treatment groups were similar with respect to demographic and baseline clinical characteristics (eTable 3 in Supplement 1). Forty-three (64%) participants reported only 1 antidepressant treatment failure in the current episode (in addition to 1 in prior episodes), 15 (22%) had 2 treatment failures, and 9 (13%) reported 3 or more antidepressant failures. Twenty-six (39%) participants reported use of atypical antipsychotics as an adjunctive treatment of MDD before study entry.
Efficacy
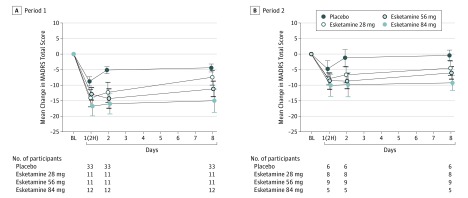
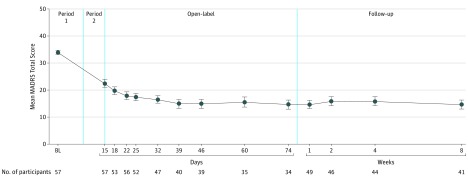
The mean MADRS total score decreased from baseline to day 8 in period 1 and from day 8 to day 15 in period 2 in all groups, with greater improvement in all esketamine dose groups compared with placebo (least squares mean difference ranging from −5.0 to −10.5 in period 1 and from −3.1 to −6.9 in period 2) (Table 1). Change from baseline in the MADRS total score was statistically significantly greater in all 3 esketamine groups than in the placebo group after 1 week of treatment; the ascending dose-response relationship also was significant. Response was rapid in onset (Figure 2; eFigure 1 in Supplement 1) and appeared to increase over time during repeated dosing, as evidenced by a decrease in the mean MADRS total score during the open-label phase (mean [SE] change from open-label baseline to day 74, −7.2 [1.84]). In addition, improvement in mean MADRS ratings persisted over the 8-week follow-up phase (without additional esketamine doses) in participants who remained in the study (Figure 3).
Table 1. MADRS Total Score: Change From Baseline to 2 Hours, 24 Hours, and Period End Point.
| Variablea | Placebo | Esketamine 28 mg | Esketamine 56 mg | Esketamine 84 mg |
|---|---|---|---|---|
| Period 1 | ||||
| No. | 33 | 11 | 11 | 12 |
| MADRS total score at baseline, mean (SD) | 35.0 (5.18) | 31.3 (3.80) | 33.2 (6.26) | 35.0 (4.22) |
| Change at 2 h | ||||
| LS mean change (SE) | −9.7 (1.76) | −16.4 (2.76) | −14.3 (2.70) | −17.6 (2.60) |
| LS mean difference from placebo (SE) | −6.7 (3.03) | −4.6 (2.96) | −7.9 (2.84) | |
| P value | .02 | .06 | .003 | |
| Responders, No. (%) | 6 (18) | 6 (55) | 4 (36) | 7 (58) |
| Remitters, No. (%) | 1 (3) | 3 (27) | 2 (18) | 3 (25) |
| Change at 24 h | ||||
| LS mean change (SE) | −5.7 (1.79) | −14.8 (2.80) | −15.7 (2.74) | −16.4 (2.64) |
| LS mean difference from placebo (SE) | −9.1 (3.08) | −10.0 (3.00) | −10.7 (2.88) | |
| P value | .002 | <.001 | <.001 | |
| Responders, No. (%) | 1 (3) | 4 (36) | 3 (27.3) | 5 (42) |
| Remitters, No. (%) | 0 | 4 (36) | 2 (18) | 3 (25) |
| Change at study period end point | ||||
| LS mean change (SE) | −4.9 (1.74) | −9.8 (2.72) | −12.4 (2.66) | −15.3 (2.56) |
| LS mean difference from placebo (SE) | −5.0 (2.99) | −7.6 (2.91) | −10.5 (2.79) | |
| P value | .05 | .006 | <.001 | |
| Responders, No. (%) | 2 (6) | 1 (9) | 2 (18) | 5 (42) |
| Remitters, No. (%) | 1 (3) | 1 (9) | 1 (9) | 3 (25) |
| Period 2b | ||||
| No. | 6 | 8 | 9 | 5 |
| MADRS total score at baseline, mean (SD) | 29.3 (5.79) | 31.3 (7.09) | 34.9 (6.13) | 30.4 (4.67) |
| Change at 2 h | ||||
| LS mean change (SE) | −6.8 (3.74) | −10.3 (3.18) | −11.7 (3.22) | −11.6 (3.44) |
| LS mean difference from placebo (SE) | −3.5 (3.82) | −4 (3.92) | −4.9 (4.36) | |
| P value | .18 | .11 | .14 | |
| Responders, No. (%) | 1 (17) | 1 (13) | 2 (22) | 2 (40) |
| Remitters, No. (%) | 1 (17) | 1 (13) | 0 | 2 (40) |
| Change at 24 h | ||||
| LS mean change (SE) | −4.1 (4.09) | −8.9 (3.48) | −10.2 (3.52) | −11.6 (3.76) |
| LS mean difference from placebo (SE) | −4.8 (4.18) | −6.1 (4.29) | −7.5 (4.77) | |
| P value | .13 | .09 | .07 | |
| Responders, No. (%) | 0 | 0 | 1 (11) | 2 (40) |
| Remitters, No. (%) | 0 | 0 | 0 | 1 (20) |
| Change at study period end point | ||||
| LS mean change (SE) | −4.5 (2.92) | −7.6 (2.49) | −8.9 (2.51) | −11.4 (2.68) |
| LS mean difference from placebo (SE) | −3.1 (2.99) | −4.4 (3.06) | −6.9 (3.41) | |
| P value | .15 | .08 | .03 | |
| Responders, No. (%) | 0 | 1 (13) | 0 | 1 (20) |
| Remitters, No. (%) | 0 | 1 (13) | 0 | 1 (20) |
| Periods 1 and 2 Combined | ||||
| Mean difference from placebo (SE) | −4.2 (2.09) | −6.3 (2.07) | −9.0 (2.13) | |
| 90% CI for mean difference vs placebo | −7.67 to −0.79 | −9.71 to −2.88 | −12.53 to −5.52 | |
| Combined period test statistic | −2.02 | −3.04 | 4.24 | |
| P value | .02 | .001 | <.001 | |
Abbreviations: LS, least squares; MADRS, Montgomery-Åsberg Depression Rating Scale.
Period 1 (days 1-8) and period 2 (days 8-15) are discussed in the Design section of the Methods and shown in the vertical axis of Figure 1.
The study samples reported for period 2 include only the placebo nonresponsive participants rerandomized following period 1.
Figure 2. Mean Change in Montgomery-Åsberg Depression Rating Scale (MADRS) Total Score Over Time in Double-Blind Phase.
Changes shown in periods 1 (A) and 2 (B). Period 2 consisted only of participants who had received placebo in period 1 and had moderate to severe symptoms (n = 28). Period 1 (days 1-8) and period 2 (days 8-15) are discussed in the Design section of the Methods and shown in the vertical axis of Figure 1. BL indicates baseline; 2H, 2 hours post dose. Error bars indicate SE.
Figure 3. MADRS Total Score: Mean Change in Montgomery-Åsberg Depression Rating Scale (MADRS) Total Score From Baseline to Follow-up End Point for Participants Who Entered the Open-Label Phase.
Period 1 (days 1-8), period 2 (days 8-15), open-label period (days 15-74), and the follow-up period (days 74-130) are discussed in the Design section of the Methods and shown in the vertical axis of Figure 1. BL indicates baseline; error bars, SE.
For completers who received 2 weeks of the same treatment in the double-blind phase, the mean decrease in the MADRS total score was greater in each esketamine dose group compared with placebo at day 15, with the magnitude of decrease directly related to dose (treatment differences relative to placebo of −12.5, −8.3, and −6.0 for esketamine 84 mg, 56 mg, and 28 mg, respectively). Efficacy appeared to be better sustained between drug administrations with the 2 higher doses (Figure 1).
Among those who received the same treatment for both periods and completed the double-blind phase, the proportion of responders (defined as ≥50% improvement from baseline in MADRS total score) in each esketamine dose group was numerically higher than in the placebo group at the period 2 end point (28 mg: 38% [3 of 8], 56 mg: 36% [4 of 11], 84 mg: 50% [5 of 10], and placebo: 10% [1 of 10]). A similar trend for remission (defined as MADRS total score ≤10) was observed across groups. Among completers who received the same treatment in both periods, more participants who received the 2 higher esketamine doses compared with placebo achieved remission after 2 weeks of treatment (13% [1 of 8], 27% [3 of 11], and 40% [4 of 10] in the 28-mg, 56-mg, and 84-mg groups, respectively, and 10% [1 of 10], in the placebo group). Response and remission rates at the end of the open-label and follow-up phases are presented by type of treatment in the double-blind and open-label phases in Table 2.
Table 2. Response and Remission Rates for Participants Who Completed the OL and Follow-up Phasesa.
| Variable | Placebo/Placebo/OL Esketamine (n = 10)b | Placebo/Esketamine/OL Esketamine (n = 20) | Esketamine/Esketamine/OL Esketamine (n = 27) | Total (n = 57) |
|---|---|---|---|---|
| Response Ratec | ||||
| OL end point, day 74, No. | 6 | 10 | 18 | 34 |
| ≥50% Improvement, No. (%) | 6 (100) | 5 (50) | 11 (61) | 22 (65) |
| Week 8 (follow-up), No. | 7 | 12 | 22 | 41 |
| ≥50% Improvement, No. (%) | 5 (71) | 3 (25) | 15 (68) | 23 (56) |
| Remission Ratec | ||||
| OL end point, day 74, No. | 6 | 10 | 18 | 34 |
| No, No. (%) | 4 (67) | 6 (60) | 13 (72) | 23 (68) |
| Yes, No. (%) | 2 (33) | 4 (40) | 5 (28) | 11 (32) |
| Week 8 (follow-up), No. | 7 | 12 | 22 | 41 |
| No, No. (%) | 3 (43) | 9 (75) | 12 (55) | 24 (59) |
| Yes, No. (%) | 4 (57) | 3 (25) | 10 (46) | 17 (42) |
Abbreviation: OL, open-label.
The follow-up phase includes data from 7 participants enrolled under the original version of the protocol in which participants received 2 weeks of study drug during the OL phase of the study and data from 50 participants enrolled under a protocol amendment in which participants received up to 9 weeks of study drug during the OL phase of the study. Percentages calculated with the number of participants per a visit as denominator; percentage change calculated based on period 1 baseline.
Esketamine was given as esketamine hydrochloride.
Response: Montgomery-Åsberg Depression Rating Scale (MADRS) total score ≥50%; remission: MADRS total score ≤10.
Safety
Three of 56 (5%) esketamine-treated participants during the double-blind phase (compared with none receiving placebo) and 1 of 57 (2%) during the open-label phase had adverse events leading to discontinuation of the study drug (1 event each of syncope, headache, dissociative syndrome, and ectopic pregnancy). During the double-blind phase, the 3 most common treatment-emergent adverse events observed among esketamine-treated participants were dizziness, headache, and dissociative symptoms; the frequency of each was more than 2-fold higher for esketamine than for placebo (eTable 4 in Supplement 1). A dose-response trend was noted for dizziness and nausea, but not for other adverse events. The type and frequency of adverse events reported in the open-label phase were similar to those in the double-blind phase; events reported for more than 10% of 57 open-label participants included dizziness (22 [39%]), dysgeusia (13 [23%]), nausea (9 [16%]), headache (8 [14%]), and sedation (6 [11%]). Overall, 14 of 57 (25%) participants reported transient dissociative symptoms. Most adverse events occurring on dosing days were transient and either mild or moderate in severity. No death was reported.
Most of the esketamine-treated participants manifested transient elevations in blood pressure (maximum mean change: systolic, 19.0 mm Hg; diastolic, 10.3 mm Hg) and heart rate (maximum mean change: 9.4 bpm) on dosing days. Maximum blood pressure values were observed in most cases at 10 or 40 minutes after the dose (systolic: 199 mm Hg; diastolic: 115 mm Hg); elevated values typically returned to the value observed before dosing by 2 hours after the dose (eFigures 2 and 3 in Supplement 1). A dose effect was not observed for heart rate, although the greatest mean increases from baseline during both periods were observed in the 84-mg esketamine group.
Perceptual changes and/or dissociative symptoms, as measured by the CADSS, began shortly after the start of intranasal dosing, peaked at approximately 30 to 40 minutes, and resolved by 2 hours (eFigure 4 in Supplement 1). Perceptual changes/dissociative symptoms attenuated in all dose groups with repeated dosing. No participant manifested symptoms suggestive of psychosis based on the Brief Psychiatric Rating Scale positive assessment.
Discussion
We observed a significant and clinically meaningful treatment effect (vs placebo) with 28-mg, 56-mg, and 84-mg doses of esketamine, as evidenced by change in the MADRS total score, with a significant relationship between esketamine dose and antidepressant response observed after 1 week of treatment. Duration of efficacy appeared to be shorter with the 28-mg dose administered twice weekly. Results from the open-label phase suggest that improvement in depressive symptoms can be sustained with lower frequency (weekly or every 2 weeks) of esketamine administration. The size of the medication-placebo difference was substantial from baseline to 1 week and was larger than the mean difference from placebo seen at 6 to 8 weeks in antidepressant studies in the US Food and Drug Administration database. The majority of participants maintained improvement over the 2-month follow-up phase.
The 56- and 84-mg intranasal doses of esketamine produce plasma esketamine levels that are in the pharmacokinetic range achieved by intravenous administration of esketamine, 0.2 mg/kg, which produced a similar clinical outcome as reported for intravenous ketamine, 0.5 mg/kg (consistent with higher affinity for NMDA receptors compared with arketamine).
In what we believe to be the first study of intranasal esketamine for TRD, efficacy and safety were compared with placebo using a double-blind, doubly randomized, delayed-start design. This design allowed for a smaller sample size to assess efficacy, dose-response, and safety than a standard parallel-group design, while preserving a low chance of type 2 error to avoid missing the efficacy signal. The key aim of the design was to include only placebo-receiving participants from period 1 who required treatment in period 2 and to rerandomize them to receive 1 of 3 intranasal esketamine doses or intranasal placebo. At the end of the trial, efficacy data from both randomizations (day 1 and day 8) were combined in an integrated analysis. Given the rerandomized placebo, nonresponders were expected to have a lower placebo response; this approach was used to mitigate high placebo responses observed in psychiatric clinical trials. The consistency in results obtained from the period 1 and period 2 samples supports their combination using weights as discussed by Chi et al, although caution is required in interpretation due to the small sample size.
In general, the esketamine doses evaluated in this study (28, 56, and 84 mg) appeared to be safe, with no new or unexpected safety concerns observed. Overall, transient increases in blood pressure after the dose, particularly increases in systolic blood pressure, support an increase in cardiac output as the underlying mechanism, consistent with previous reports for ketamine. Analysis of perceptual change symptoms (measured by CADSS assessment) suggests that onset begins shortly after initiation of esketamine and resolution occurs by 2 hours after administration. These symptoms were dose dependent and attenuated with repeated administration. In contrast, antidepressant efficacy did not attenuate across administrations.
Limitations
Generalizability of the study findings is limited by the small sample size and enrollment criteria that excluded individuals with a history of psychotic symptoms, substance/alcohol use disorders, recent use of cannabis, or significant medical comorbidities. Also excluded were individuals having current suicidal ideation with intent—a group that was evaluated in a separate study. Difficulty blinding esketamine, despite adding a bittering agent to placebo to mimic the taste of esketamine, is another limitation.
Conclusions
Intranasal esketamine administered at doses of 28, 56, and 84 mg appeared to be efficacious in treating TRD. There was evidence of robust and durable efficacy in the double-blind treatment phase (56 and 84 mg). Improvement in depressive symptoms persisted over the open-label phase, despite reduced dosing frequency, and for up to 2 months after cessation of esketamine dosing. Results support further investigation of intranasal efficacy of esketamine for the treatment of TRD in larger trials. A phase 3 study evaluating the necessary frequency of dosing and duration of effect is under way.
eAppendix. Materials and Methods
eTable 1. GAD-7: ANCOVA Analysis of Change from Baseline to Study End Point
eTable 2. CGI-S: ANCOVA Analysis of Change from Baseline to Study End Point
eTable 3. Demographics and Baseline Characteristics
eTable 4. Summary of Most Frequently Reported Treatment-Emergent Adverse Events (Double-Blind Safety Analysis Data Set)
eFigure 1. Mean (±SE) MADRS Total Score Over Time in Double-Blind Phase
eFigure 2. Mean Systolic Blood Pressure Over Time by Period for Participants who Received the Same Treatment for Both Periods During the Double-Blind Phase
eFigure 3. Mean Diastolic Blood Pressure Over Time by Period for Participants who Received the Same Treatment for Both Periods During the Double-Blind Phase
eFigure 4. Mean CADSS Total Score Over Time for Participants who Received the Same Treatment for Both Periods
Protocol.
References
- 1.Kessler RC, Bromet EJ. The epidemiology of depression across cultures. Annu Rev Public Health. 2013;34:119-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells KB, Stewart A, Hays RD, et al. The functioning and well-being of depressed patients: results from the Medical Outcomes Study. JAMA. 1989;262(7):914-919. [PubMed] [Google Scholar]
- 4.Daly EJ, Trivedi MH, Wisniewski SR, et al. Health-related quality of life in depression: a STAR*D report. Ann Clin Psychiatry. 2010;22(1):43-55. [PubMed] [Google Scholar]
- 5.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med. 2001;63(4):619-630. [DOI] [PubMed] [Google Scholar]
- 6.Katon WJ. Clinical and health services relationships between major depression, depressive symptoms, and general medical illness. Biol Psychiatry. 2003;54(3):216-226. [DOI] [PubMed] [Google Scholar]
- 7.Penninx BW. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci Biobehav Rev. 2017;74(Pt B):277-286. [DOI] [PubMed] [Google Scholar]
- 8.Rahe C, Khil L, Wellmann J, Baune BT, Arolt V, Berger K. Impact of major depressive disorder, distinct subtypes, and symptom severity on lifestyle in the BiDirect Study. Psychiatry Res. 2016;245:164-171. [DOI] [PubMed] [Google Scholar]
- 9.Walker ER, McGee RE, Druss BG. Mortality in mental disorders and global disease burden implications: a systematic review and meta-analysis. JAMA Psychiatry. 2015;72(4):334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The American Psychiatric Association. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. http://psychiatryonline.org/doi/book/10.1176/appi.books.9780890426807. Published 2010. Accessed August 23, 2016.
- 11.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med. 2016;22(3):238-249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351-354. [DOI] [PubMed] [Google Scholar]
- 13.Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864. [DOI] [PubMed] [Google Scholar]
- 14.Kavalali ET, Monteggia LM. Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry. 2012;169(11):1150-1156. [DOI] [PubMed] [Google Scholar]
- 15.Murrough JW, Iosifescu DV, Chang LC, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB; APA Council of Research Task Force on Novel Biomarkers and Treatments . Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172(10):950-966. [DOI] [PubMed] [Google Scholar]
- 17.Salvadore G, Singh JB. Ketamine as a fast acting antidepressant: current knowledge and open questions. CNS Neurosci Ther. 2013;19(6):428-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domino EF. Taming the ketamine tiger: 1965. Anesthesiology. 2010;113(3):678-684. [DOI] [PubMed] [Google Scholar]
- 19.Singh JB, Fedgchin M, Daly E, et al. Intravenous esketamine in adult treatment-resistant depression: a double-blind, double-randomization, placebo-controlled study. Biol Psychiatry. 2016;80(6):424-431. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 21.Chandler GM, Iosifescu DV, Pollack MH, Targum SD, Fava M. RESEARCH: Validation of the Massachusetts General Hospital Antidepressant Treatment History Questionnaire (ATRQ). CNS Neurosci Ther. 2010;16(5):322-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rush AJ, Gullion CM, Basco MR, Jarrett RB, Trivedi MH. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26(3):477-486. [DOI] [PubMed] [Google Scholar]
- 23.Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73-82. [DOI] [PubMed] [Google Scholar]
- 24.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 25.Chi GYH, Li Y, Liu Y, Lewin D, Lim P. On clinical trials with a high placebo rate. Contemp Clin Trials Commun. 2016;2:34-53. doi: 10.1016/j.conctc.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fava M, Evins AE, Dorer DJ, Schoenfeld DA. The problem of the placebo response in clinical trials for psychiatric disorders: culprits, possible remedies, and a novel study design approach. Psychother Psychosom. 2003;72(3):115-127. [DOI] [PubMed] [Google Scholar]
- 27.Chen YF, Yang Y, Hung HM, Wang SJ. Evaluation of performance of some enrichment designs dealing with high placebo response in psychiatric clinical trials. Contemp Clin Trials. 2011;32(4):592-604. [DOI] [PubMed] [Google Scholar]
- 28.Fava M, Mischoulon D, Iosifescu D, et al. A double-blind, placebo-controlled study of aripiprazole adjunctive to antidepressant therapy among depressed outpatients with inadequate response to prior antidepressant therapy (ADAPT-A Study). Psychother Psychosom. 2012;81(2):87-97. [DOI] [PubMed] [Google Scholar]
- 29.Chen YF, Zhang X, Tamura RN, Chen CM. A sequential enriched design for target patient population in psychiatric clinical trials. Stat Med. 2014;33(17):2953-2967. [DOI] [PubMed] [Google Scholar]
- 30.Doros G, Pencina M, Rybin D, Meisner A, Fava M. A repeated measures model for analysis of continuous outcomes in sequential parallel comparison design studies. Stat Med. 2013;32(16):2767-2789. [DOI] [PubMed] [Google Scholar]
- 31.Huang X, Tamura RN. Comparison of test statistics for the sequential parallel design. Stat Biopharm Res. 2010;2(1):42-50. [Google Scholar]
- 32.Ivanova A, Qaqish B, Schoenfeld DA. Optimality, sample size, and power calculations for the sequential parallel comparison design. Stat Med. 2011;30(23):2793-2803. [DOI] [PubMed] [Google Scholar]
- 33.Papakostas GI, Shelton RC, Zajecka JM, et al. L-methylfolate as adjunctive therapy for SSRI-resistant major depression: results of two randomized, double-blind, parallel-sequential trials. Am J Psychiatry. 2012;169(12):1267-1274. [DOI] [PubMed] [Google Scholar]
- 34.Tamura RN, Huang X. An examination of the efficiency of the sequential parallel design in psychiatric clinical trials. Clin Trials. 2007;4(4):309-317. [DOI] [PubMed] [Google Scholar]
- 35.Rybin D, Doros G, Pencina MJ, Fava M. Placebo non-response measure in sequential parallel comparison design studies. Stat Med. 2015;34(15):2281-2293. [DOI] [PubMed] [Google Scholar]
- 36.Tamura RN, Huang X, Boos DD. Estimation of treatment effect for the sequential parallel design. Stat Med. 2011;30(30):3496-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573-583. [DOI] [PubMed] [Google Scholar]
- 38.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [DOI] [PubMed] [Google Scholar]
- 39.Williams JB, Kobak KA. Development and reliability of a structured interview guide for the Montgomery Åsberg Depression Rating Scale (SIGMA). Br J Psychiatry. 2008;192(1):52-58. [DOI] [PubMed] [Google Scholar]
- 40.Guy W. ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publ No ADM 76–338). Rockville, MD: US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976:218-222. [Google Scholar]
- 41.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. [DOI] [PubMed] [Google Scholar]
- 42.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). J Trauma Stress. 1998;11(1):125-136. [DOI] [PubMed] [Google Scholar]
- 43.Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10:799-812. [Google Scholar]
- 44.Bretz F, Pinheiro JC, Branson M. Combining multiple comparisons and modeling techniques in dose-response studies. Biometrics. 2005;61(3):738-748. [DOI] [PubMed] [Google Scholar]
- 45.Liu Q, Lim P, Singh J, Lewin D, Schwab B, Kent J. Doubly randomized delayed-start design for enrichment studies with responders or nonresponders. J Biopharm Stat. 2012;22(4):737-757. [DOI] [PubMed] [Google Scholar]
- 46.Khan A, Fahl Mar K, Faucett J, Khan Schilling S, Brown WA. Has the rising placebo response impacted antidepressant clinical trial outcome? data from the US Food and Drug Administration 1987-2013. World Psychiatry. 2017;16(2):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White PF, Schüttler J, Shafer A, Stanski DR, Horai Y, Trevor AJ. Comparative pharmacology of the ketamine isomers. Studies in volunteers. Br J Anaesth. 1985;57(2):197-203. [DOI] [PubMed] [Google Scholar]
- 48.clinicaltrials.gov A Double-Blind Study to Assess the Efficacy and Safety of Intranasal Esketamine for the Rapid Reduction of the Symptoms of Major Depressive Disorder, Including Suicide Ideation, in Participants Who Are Assessed to Be at Imminent Risk For Suicide. NCT02133001. https://clinicaltrials.gov/ct2/show/NCT02133001. Accessed November 6, 2017.
- 49.clinicaltrials.gov. A Study of Intranasal Esketamine Plus an Oral Antidepressant for Relapse Prevention in Adult Participants With Treatment-resistant Depression (SUSTAIN-1). NCT02493868. https://clinicaltrials.gov/ct2/show/NCT02493868. Accessed November 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Materials and Methods
eTable 1. GAD-7: ANCOVA Analysis of Change from Baseline to Study End Point
eTable 2. CGI-S: ANCOVA Analysis of Change from Baseline to Study End Point
eTable 3. Demographics and Baseline Characteristics
eTable 4. Summary of Most Frequently Reported Treatment-Emergent Adverse Events (Double-Blind Safety Analysis Data Set)
eFigure 1. Mean (±SE) MADRS Total Score Over Time in Double-Blind Phase
eFigure 2. Mean Systolic Blood Pressure Over Time by Period for Participants who Received the Same Treatment for Both Periods During the Double-Blind Phase
eFigure 3. Mean Diastolic Blood Pressure Over Time by Period for Participants who Received the Same Treatment for Both Periods During the Double-Blind Phase
eFigure 4. Mean CADSS Total Score Over Time for Participants who Received the Same Treatment for Both Periods
Protocol.