Key Points
Question
Are varicose veins associated with an increased risk of developing deep venous thrombosis (DVT), pulmonary embolism (PE), or peripheral artery disease (PAD)?
Findings
In this retrospective cohort study from Taiwan that included 425 968 adults, the presence of varicose veins was associated with a significantly increased risk of incident DVT (hazard ratio [HR], 5.30), PE (HR, 1.73), and PAD (HR, 1.72).
Meaning
This study found a significant association between varicose veins and DVT, and less clear potential associations with PE and PAD; further research is needed to understand whether the association with DVT is causal.
Abstract
Importance
Varicose veins are common but rarely associated with serious health risks. Deep venous thrombosis (DVT), pulmonary embolism (PE), and peripheral artery disease (PAD) are also vascular diseases but associated with serious systemic effects. Little is known about the association between varicose veins and the incidence of other vascular diseases including DVT, PE, and PAD.
Objective
To investigate whether varicose veins are associated with an increased risk of DVT, PE, or PAD.
Design, Setting, and Participants
A retrospective cohort study using claims data from Taiwan’s National Health Insurance program. Patients aged 20 years and older with varicose veins were enrolled from January 1, 2001-December 31, 2013, and a control group of patients without varicose veins were matched by propensity score. Patients previously diagnosed with DVT, PE, or PAD were excluded. Follow-up ended December 31, 2014.
Exposures
Presence of varicose veins.
Main Outcomes and Measures
Incidence rates of DVT, PE, and PAD were assessed in people with and without varicose veins. Cox proportional hazards models were used to estimate relative hazards, with the control group as reference.
Results
There were 212 984 patients in the varicose veins group (mean [SD] age, 54.5 [16.0] years; 69.3% women) and 212 984 in the control group (mean [SD] age, 54.3 [15.6] years; 70.3% women). The median follow-up duration was 7.5 years for DVT, 7.8 years for PE, and 7.3 years for PAD for patients with varicose veins, and for the control group, follow-up duration was 7.6 years for DVT, 7.7 years for PE, and 7.4 years for PAD. The varicose veins group had higher incidence rates than the control group for DVT (6.55 vs 1.23 per 1000 person-years [10 360 vs 1980 cases]; absolute risk difference [ARD], 5.32 [95% CI, 5.18-5.46]), for PE (0.48 for the varicose veins group vs 0.28 for the control group per 1000 person-years [793 vs 451 cases]; ARD, 0.20 [95% CI, 0.16-0.24]), and for PAD (10.73 for the varicose veins group vs 6.22 for the control group per 1000 person-years [16 615 vs 9709 cases]; ARD, 4.51 [95% CI, 4.31-4.71]). The hazard ratios for the varicose veins group compared with the control group were 5.30 (95% CI, 5.05-5.56) for DVT, 1.73 (95% CI, 1.54-1.94) for PE, and 1.72 (95% CI, 1.68-1.77) for PAD.
Conclusions and Relevance
Among adults diagnosed with varicose veins, there was a significantly increased risk of incident DVT; the findings for PE and PAD are less clear due to the potential for confounding. Whether the association between varicose veins and DVT is causal or represents a common set of risk factors requires further research.
This cohort study uses claims data from Taiwan’s National Health Insurance program to investigate associations between varicose veins and risk of deep venous thrombosis (DVT), pulmonary embolism (PE), and peripheral artery disease (PAD).
Introduction
Varicose veins are common in the general population. The prevalence of varicose veins varies widely. Approximately 23% of adults have been reported to have varicose veins in the United States.
Varicose veins are rarely associated with serious health risks. In contrast, deep venous thrombosis (DVT), pulmonary embolism (PE), and peripheral artery disease (PAD) are vascular diseases that are associated with serious systemic effects. Patients with varicose veins have increased levels of inflammatory and prothrombotic markers. Inflammation is thought to be associated with the pathophysiology of DVT, PE, and PAD. Because of the high prevalence of varicose veins, elucidating potential associations between varicose veins and health-threatening diseases is important. Previous studies evaluating the association of varicose veins with venous and arterial disease were cross-sectional or case-control studies, had relatively small sample sizes, and did not verify the diagnosis of varicose veins. Limited data are available from longitudinal cohort studies to investigate the association between varicose veins and the subsequent incidence of vascular diseases.
This study investigated whether varicose veins are associated with an increased risk of incident PAD, DVT, and PE in a nationwide population-based cohort study in Taiwan.
Methods
Data Source
This retrospective cohort study used claims data from the National Health Insurance (NHI) program linked to the National Register of Deaths in Taiwan (eMethods in the Supplement). The death registry contains date of death, which was required information for follow-up because death was treated as one of the censoring events. The enrollment rate in the NHI program was 96% of the entire population in 1996 and 99% in 2015, and death registration is mandatory. This study was approved by the institutional review board at Chang-Gung Memorial Hospital, and the need for informed consent was waived.
Identification of Study Patients and Validation of Varicose Veins Diagnosis
We identified patients diagnosed with varicose veins from January 1, 2001-December 31, 2013, according to the International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes 454.xx for an outpatient or inpatient claim. The index date was defined as the date of diagnosis. Patients were excluded if they were younger than 20 years of age at the index date or if they had a medical history of PAD, DVT, or PE before or on the index date (see eTable 1 in the Supplement for ICD-9-CM codes). To assess the validity of the 454 code corresponding to the diagnosis of varicose veins, we reviewed a random sample of outpatient medical records from 3 branches of Chang-Gung Memorial Hospital (Taipei, Linkou, and Taoyuan) that provide health care services to patients with diverse characteristics. There were 239 patients in this sample, which represented 1.5% of all patients (n=16 028) diagnosed with varicose veins, of whom 39 could not be evaluated due to incomplete or unidentifiable medical records. The validation criterion was met in 196 of the remaining 200 patients, with a positive predictive value of 98% (95% CI, 0.95%-0.99%) (see eMethods in the Supplement). The worst-case positive predictive value was 82% (95% CI, 0.77%-0.87%) if all 39 patients actually did not have varicose veins.
Identification of the Control Group and Propensity Score Analysis
We randomly selected a control group without varicose veins from all participants enrolled in the NHI. Individuals in the control group were matched with those in the varicose veins group at a 4:1 ratio based on year of birth, sex, and the calendar year of index date. The participants in the control group were free of PAD, DVT, and PE before and on the index date. Propensity score matching was performed to balance confounders between the 2 groups. A logistic regression model was used to estimate the exposure propensity score (ie, probability of having varicose veins) for each participant. In the model, status of varicose veins (yes/no) was regressed on potential confounders including age, sex, the calendar year of index date, the number of outpatient visits in the past year, and comorbidities including hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, malignancy, heart failure, ischemic heart disease, stroke, and chronic renal insufficiency. We created propensity score–matched pairs by matching each patient with varicose veins to a participant without varicose veins using the greedy-matching algorithm. Comorbidities were defined as the presence of diagnosis codes (eTable 1 in the Supplement) in at least 2 outpatient claims or 1 inpatient claim before the index date.
Study Outcomes and Follow-up
The study outcomes were PAD, DVT, and PE, defined as at least 2 outpatient visits or 1 hospital admission with the relevant diagnosis codes (eTable 1 in the Supplement). All patients were monitored from the index date until the earliest occurrence of PAD, DVT, and PE; death; or end of the study (December 31, 2014). We ascertained the study outcomes using NHI claims and vital status data from the National Register of Deaths. The 3 end points were analyzed separately; that is, a patient with DVT who later developed another end point was also counted as a case in the analysis of the other end point.
Negative Controls and Falsification End Points
Negative control exposure and falsification end points (or negative control outcomes) have been used to detect residual confounding and bias due to unobserved confounders. For negative control exposure, we used hemangioma, a vascular condition similar to varicose veins but unlikely to cause PAD, DVT, or PE. We hypothesized that an association between hemangioma and PAD, DVT, or PE may imply possible unmeasured confounding. We identified patients diagnosed with hemangioma (ICD-9-CM; 228.0x) in at least 2 outpatient claims or 1 hospitalization from 2001 through 2013 and randomly selected a nonhemangioma control group using propensity score matching for the same variables used when selecting the controls for the patients with varicose veins. All patients were free of PAD, DVT, or PE before and on the index date.
We used 2 falsification end points, lung cancer (identified using the cancer registry ICD-O-3, C33-C34) and hyperlipidemia (ICD-9-CM; 272.xx), which are not associated with varicose veins according to any known pathophysiologic mechanisms. Thus, an association between varicose veins and these end points may suggest the presence of uncontrolled confounders, such as smoking (a known risk factor for lung cancer) and obesity ( a known risk factor for hyperlipidemia).
Statistical Analysis
We compared baseline characteristics between groups using standardized mean difference, a measure of distribution not sensitive to sample size. This measure is calculated as the absolute difference in means or proportions of a variable between 2 groups divided by a pooled standard deviation of that variable in the 2 groups. A standardized mean difference of less than 0.1 is typically considered to indicate adequate balance in variables between groups. Kaplan-Meier curves were generated showing cumulative probabilities of experiencing the events (DVT, PE, and PAD) over time and the differences between the 2 groups were tested using a log-rank test. The hazard ratios (HRs) with 95% CIs associated with varicose veins were estimated using Cox proportional hazard models with a robust variance estimator that accounted for the matched nature in the propensity score–matched sample. Age- and sex-stratified analyses were performed to assess whether the association varied with age and sex. The interaction effects of age and sex were examined using the likelihood ratio test comparing models excluding and including the interaction term. We also conducted additional analyses with unadjusted and adjusted Cox proportional hazard models to control for potential confounding factors (sex, age, index year, number of outpatient visits during the year before index date, and comorbidities including hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, malignancy, heart failure, ischemic heart disease, stroke, and chronic renal insufficiency) to assess the HRs in patients with varicose veins compared with the age-, sex-, and calendar year–matched controls.
The proportional hazard assumption was tested by including product terms between the predictors and a function of follow-up time in the models. The assumptions were met for PAD and PE but not for DVT. We further conducted analyses using increments of follow-up duration (ie, >1 year, >2 years, and >5 years) (eTable 2 in the Supplement). The HRs of DVT decreased substantially after 1 year of follow-up and remained stable thereafter. Therefore, we performed additional analyses restricting the follow-up duration to more than 1 year.
In analyses of the negative control exposures, Cox models were used for a sample consisting of patients with hemangioma and matched controls. In analyses of the falsification outcomes, the dependent variables used in the Cox model were lung cancer and hyperlipidemia instead of DVT, PE, or PAD.
Two analyses were conducted to evaluate the potential effect of unmeasured confounders. First, we used the method reported by Greenland and Lash to estimate how large the association between smoking and varicose veins would need to be to remove the observed association between varicose veins and DVT, PE, and PAD. The required parameters for this approach, which included estimated smoking prevalence in the control group and the strength of associations between smoking and DVT, PE, and PAD, were obtained from the literature (Methods section of eAppendix in the Supplement). Second, we performed sensitivity analysis using a confounding function approach, which described the degree of the entire effect of all unmeasured and unrecognized confounders. The relative risks (RRs) of DVT, PE, and PAD were calculated using a log-binomial model with inverse probability of treatment weighting (IPTW) using the propensity score. The marginal RRs obtained from IPTW were corrected for various values of confounding functions, c(0) and c(1). These parameters are counterfactual; c(0) represents a hypothetical RR of the outcome (DVT, PE, or PAD) comparing those who actually did and did not have varicose veins but assuming none had varicose veins; c(1) represents a hypothetical RR of the outcome comparing those who actually did and did not have varicose veins but assuming all had varicose veins. The values of c(0) and c(1) were then varied to estimate the effect of unmeasured confounding required to give an adjusted RR. For example, c(0)=c(1) denotes the assumption that there were no unmeasured effect modifiers, and c(0) greater than 1 and c(1) greater than 1 implies that the exposed participants would have had a greater risk of the potential outcome than unexposed participants, if they had the same exposure status.
Missing or invalid data on age, sex, and vital status were noted in 2% of the patients, and these patients were excluded from the analyses. SAS software version 9.4 was used to perform all analyses except confounding function analyses, for which R software was applied. Statistical tests were 2-sided, and a P value of less than .05 was considered to indicate statistical significance.
Results
Baseline Characteristics of the Study Patients
There were 239 616 patients aged 20 years and older with varicose veins at diagnosis who were identified from 2001 through 2013. We excluded those diagnosed with PAD, DVT, or PE before the index date (n=26 632), and classified the remaining 212 984 patients in the varicose veins group. The mean (SD) age at diagnosis was 54.5 (16.0) years, and 69.3% were women (Table 1). Distributions of age and sex at diagnosis were not different between the varicose veins group and the 2 control groups. The patients with varicose veins had higher prevalence of chronic obstructive pulmonary disease and more medical visits than the age-, sex-, and calendar year–matched control group (n=851 936). Propensity score matching resulted in 212 984 matched pairs of patients with varicose veins and controls. The baseline characteristics were well balanced between the groups after matching. The median follow-up time for the specific study outcomes ranged from 7.3 to 7.8 years in the varicose veins group and 7.4 to 7.7 years in the propensity score–matched controls.
Table 1. Baseline Characteristics of Patients With Varicose Veins vs Patients in the Control Group.
| Varicose Veins Group (n=212 984) | Control Group Matched on Age, Sex, and Calendar Year (n=851 936) | Standardized Difference | Control Group Matched on Propensity Score (n=212 984) | Standardized Difference | |
|---|---|---|---|---|---|
| Women, No. (%) | 147 643 (69.3) | 590 572 (69.3) | 0.0000 | 149 632 (70.3) | 0.0203 |
| Age, mean (SD), y | 54.5 (16.0) | 54.5 (16.0) | 0.0002 | 54.3 (15.6) | 0.0141 |
| Age, No. (%), y | |||||
| 20-34 | 26 797 (12.6) | 107 074 (12.6) | 0.0004 | 26 810 (12.6) | 0.0002 |
| 35-44 | 32 457 (15.2) | 129 983 (15.3) | 0.0005 | 32 235 (15.1) | 0.0029 |
| 45-54 | 47 791 (22.4) | 191 572 (22.5) | 0.0011 | 48 054 (22.6) | 0.003 |
| 55-64 | 43 720 (20.5) | 174 503 (20.5) | 0.0011 | 44 711 (21.0) | 0.0115 |
| 65-74 | 36 714 (17.2) | 146 616 (17.2) | 0.0007 | 37 901 (17.8) | 0.0147 |
| ≥75 | 25 505 (12.0) | 102 188 (12.0) | 0.0006 | 23 273 (10.9) | 0.0329 |
| Comorbidity, No. (%)a | |||||
| Hypertension | 68 969 (32.4) | 248 108 (29.1) | 0.0707 | 68 521 (32.3) | 0.0045 |
| Chronic obstructive pulmonary disease | 54 278 (25.5) | 169 358 (19.9) | 0.1342 | 53 192 (25.0) | 0.0117 |
| Hyperlipidemia | 46 237 (21.7) | 156 921 (18.4) | 0.0822 | 45 520 (21.4) | 0.0082 |
| Ischemic heart disease | 36 975 (17.4) | 117 192 (13.8) | 0.0996 | 35 273 (16.6) | 0.0213 |
| Diabetes | 27 319 (12.8) | 107 062 (12.6) | 0.0078 | 26 328 (12.4) | 0.014 |
| Malignancy | 13 263 (6.2) | 42 909 (5.0) | 0.0517 | 11 729 (5.5) | 0.0307 |
| Chronic renal insufficiency | 11 213 (5.3) | 38 906 (4.6) | 0.0323 | 9332 (4.4) | 0.0412 |
| Atrial fibrillation | 3823 (1.8) | 10 076 (1.2) | 0.0506 | 2768 (1.3) | 0.0401 |
| Heart failure | 3002 (1.4) | 10 595 (1.2) | 0.0145 | 2175 (1.0) | 0.0354 |
| Stroke | 3322 (1.6) | 18 824 (2.2) | 0.0323 | 2753 (1.3) | 0.0412 |
| Utilization of Health Care Serviceb | |||||
| No. of outpatient visits | |||||
| Mean (SD) | 26.1 (21.9) | 18.9 (19.5) | 0.3471 | 24.7 (20.1) | 0.0647 |
| Median (IQR) | 21 (11-35) | 14 (5-27) | 30 (10-34) | ||
| <5 Visits, No. (%) | 18 764 (8.8) | 20 8671 (24.5) | 0.4306 | 18 791 (8.8) | 0.0004 |
| 5 to <14 Visits, No. (%) | 49 453 (23.2) | 210 969 (24.8) | 0.0362 | 49 718 (23.3) | 0.0029 |
| 14 to <27 Visits, No. (%) | 63 597 (29.9) | 214 258 (25.1) | 0.1056 | 64 185 (30.1) | 0.006 |
| ≥27 Visits, No. (%) | 81 170 (38.1) | 218 038 (25.6) | 0.2711 | 80 290 (37.7) | 0.0085 |
| No. of hospital admissions | |||||
| Mean (SD) | 0.19 (0.6) | 0.16 (0.6) | 0.1055 | 0.19 (0.7) | 0.0506 |
| Median (range) | 0 (0-32) | 0 (0-37) | 0 (0-37) | ||
| 0 Admissions, No. (%) | 184 435 (86.6) | 763 706 (89.6) | 0.0943 | 186 397 (87.5) | 0.0274 |
| 1 Admission, No. (%) | 21 666 (10.2) | 63 256 (7.4) | 0.0971 | 19 314 (9.1) | 0.0375 |
| ≥2 Admissions, No. (%) | 6883 (3.2) | 24 974 (3.0) | 0.0174 | 7273 (3.4) | 0.0102 |
| Years of Follow-up, Median (IQR) | |||||
| Deep venous thrombosis | 7.5 (4.0-10.8) | 7.6 (4.2-10.9) | 0.0318 | 7.6 (4.2-10.9) | 0.0263 |
| Pulmonary embolism | 7.8 (4.4-11.1) | 7.7 (4.3-11.0) | 0.0262 | 7.7 (4.2-11.0) | 0.0308 |
| Peripheral artery disease | 7.3 (3.7-10.7) | 7.5 (3.9-10.8) | 0.0304 | 7.4 (3.8-10.7) | 0.0156 |
Abbreviation: IQR, interquartile range.
Measured before the index date.
Measured during the year before the index date.
Varicose Veins and Incidence of Venous Thromboembolism
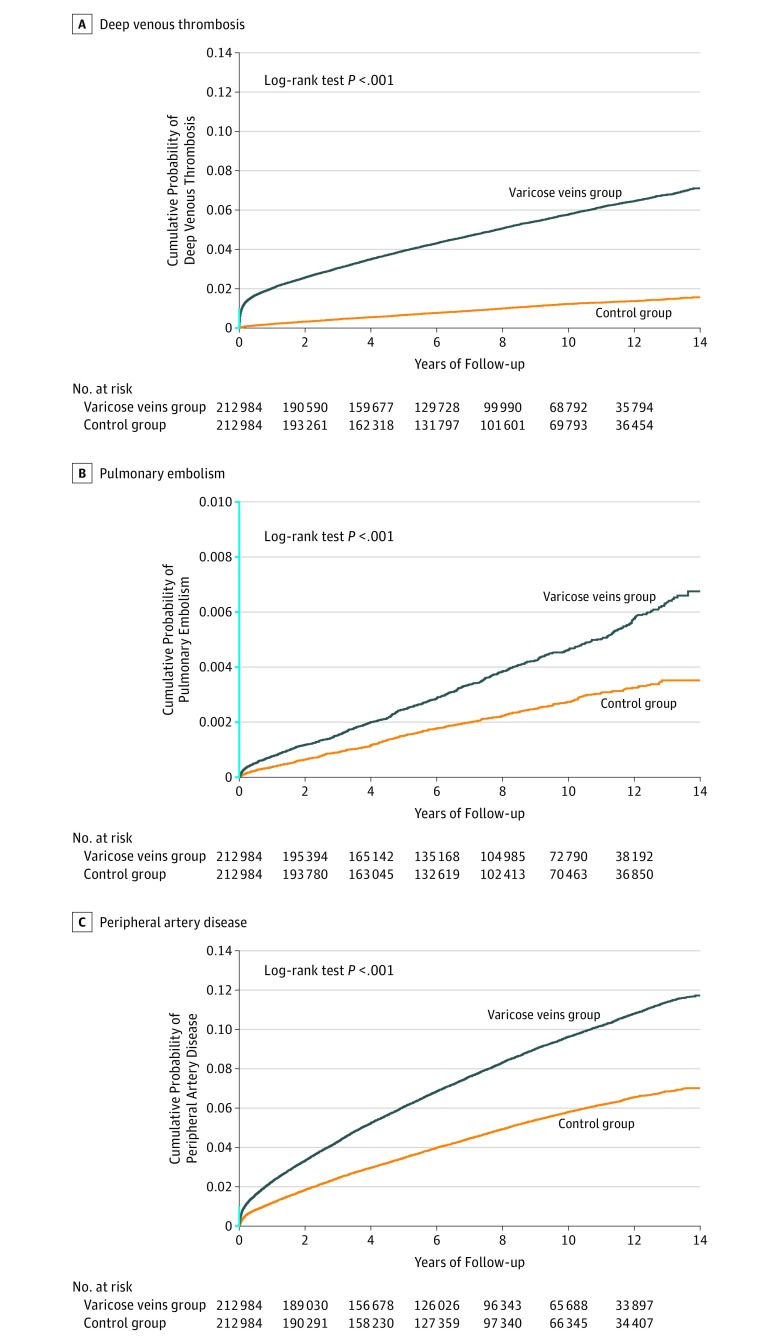
The Kaplan-Meier curves of cumulative probability of developing an event showed increased risk of DVT and PE in patients with varicose veins compared with propensity score–matched controls (Figure; P<.001 for both DVT and PE). Table 2 shows incidence rates and HRs of DVT, PAD, and PE for the varicose veins group and propensity score–matched controls. The incidence of DVT was higher among the patients with varicose veins (6.55 [95% CI, 6.42-6.68] per 1000 person-years) than in the control group (1.23 [95% CI, 1.18-1.29] per 1000 person-years); absolute risk difference, 5.32 [95% CI, 5.18-5.46]). In the Cox model, the HR for the association of varicose veins with DVT was 5.30 (95% CI, 5.05-5.56) for the patients with varicose veins compared with the control group. In the analysis restricting the follow-up duration to at least 1 year, the overall HR decreased to 3.98 (95% CI, 3.77-4.21). Age- and sex-stratified analyses showed that varicose veins were associated with an increased risk of DVT in all groups, and the HRs differed significantly by age and sex (P for interaction <.001 for both) (eTable 3 in the Supplement). The HR was greatest among the patients aged 20 to 34 years and decreased with increasing age. The age- and sex-specific HRs were lower but remained statistically significant in the analyses restricting follow-up duration to at least 1 year (eTable 4 in the Supplement).
Figure. Time to Occurrence of Deep Venous Thrombosis, Pulmonary Embolism, and Peripheral Artery Disease in the Varicose Veins Group and the Propensity Score–Matched Control Group.
The segment of the y-axis shown in blue indicates range from 0 to 0.01.
A, Median duration of follow-up was 7.5 years (interquartile range [IQR], 4.0-10.8) for the varicose veins group and 7.6 years (IQR, 4.2-10.9) for the control group.
B, Median duration of follow-up was 7.8 years (IQR, 4.4-11.1) for the varicose veins group and 7.7 years (IQR, 4.2-11.0) for the control group.
C, Median duration of follow-up was 7.3 years (IQR, 3.7-10.7) for the varicose veins group and 7.4 years (IQR, 3.8-10.7) for the control group.
Table 2. Hazard Ratios of the Incidence of Deep Venous Thrombosis, Pulmonary Embolism, and Peripheral Artery Disease Associated With Varicose Veins.
| Varicose Veins Group | Control Group | Absolute Risk Difference/1000 Person-Years (95% CI) | Hazard Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)a | No. of Patients | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)a | |||
| DVT | ||||||||||
| Cox model using propensity score–matched controls | 212 984 | 10 360 | 1 581 466 | 6.55 (6.42-6.68) | 212 984 | 1980 | 1 605 243 | 1.23 (1.18-1.29) | 5.32 (5.18-5.46) | 5.30 (5.05-5.56) |
| Multivariable-adjusted modelb | 212 984 | 10 360 | 1 581 466 | 6.55 (6.42-6.68) | 851 936 | 6942 | 6 440 345 | 1.08 (1.05-1.10) | 5.47 (5.34-5.60) | 5.39 (5.22-5.56) |
| DVT (restricted to length of follow-up ≥1 y) | ||||||||||
| Cox model using propensity score–matched controls | 208 648 | 6060 | 1 371 818 | 4.42 (4.31-4.53) | 211 403 | 1545 | 1 393 182 | 1.11 (1.05-1.17) | 3.31 (3.19-3.43) | 3.98 (3.77-4.21) |
| Multivariable-adjusted modelb | 208 648 | 6060 | 1 371 818 | 4.42 (4.31-4.53) | 847 026 | 5488 | 5 591 184 | 0.98 (0.96-1.01) | 3.44 (3.33-3.55) | 3.99 (3.84-4.14) |
| PE | ||||||||||
| Cox model using propensity score–matched controls | 212 984 | 793 | 1 639 174 | 0.48 (0.45-0.52) | 212 984 | 451 | 1 613 354 | 0.28 (0.25-0.31) | 0.20 (0.16-0.24) | 1.73 (1.54-1.94) |
| Multivariable-adjusted modelb | 212 984 | 793 | 1 639 174 | 0.48 (0.45-0.52) | 851 936 | 1521 | 6 469 344 | 0.24 (0.22-0.25) | 0.24 (0.20-0.28) | 1.75 (1.60-1.91) |
| PAD | ||||||||||
| Cox model using propensity score–matched controls | 212 984 | 16 615 | 1 547 861 | 10.73 (10.57-10.90) | 212 984 | 9709 | 1 561 421 | 6.22 (6.09-6.34) | 4.51 (4.31-4.71) | 1.72 (1.68-1.77) |
| Multivariable-adjusted modelb | 212 984 | 16 615 | 1 547 861 | 10.73 (10.57-10.90) | 851 936 | 32725 | 6 296 328 | 5.20 (5.14-5.25) | 5.53 (5.36-5.70) | 1.76 (1.72-1.79) |
Abbreviations: DVT, deep venous thrombosis; PAD, peripheral artery disease; PE, pulmonary embolism.
Exact CIs for Possion distribution.
The analysis was performed using the sample of age-, sex-, and calendar year–matched controls. Models were adjusted for sex, age, index year, number of outpatient visits during the year before index date, and comorbidities including hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, malignancy, heart failure, ischemic heart disease, stroke, and chronic renal insufficiency.
The incidence of PE was higher among participants with varicose veins (0.48 [95% CI, 0.45-0.52] per 1000 person-years) compared with those in the control group (0.28 [95% CI, 0.25-0.31] per 1000 person-years); absolute risk difference, 0.20 (95% CI, 0.16-0.24) (Table 2). The HR for the association of varicose veins with PE was 1.73 (95% CI, 1.54-1.94). The association did not significantly differ by sex or age (P values for interaction effect, .36 for sex and .62 for age) (eTable 5 in the Supplement). Similar results were observed in the multivariable Cox proportional hazard models comparing the varicose veins group with the age-, sex-, and calendar year–matched group (eTables 6-8 in the Supplement).
Varicose Veins and Incidence of Peripheral Artery Disease
The probability of developing PAD was higher in patients with varicose veins than in the propensity score–matched controls over the follow-up period (Figure, P<.001). The incidence of PAD was higher in the varicose veins group (10.73 [95% CI, 10.57-10.90] per 1000 person-years) than in the propensity score–matched control group (6.22 [95% CI, 6.09-6.34] per 1000 person-years); absolute risk difference, 4.51 (95% CI, 4.31-4.71), irrespective of age and sex (Table 2; eTable 9 in the Supplement). Overall, the HR for the patients with varicose veins was 1.72 (95% CI 1.68-1.77) compared with the control group. The HR was higher among men than in women (P value for interaction <.001), and lower with increasing age (P value for interaction <.001) except for the patients aged 75 years or older. Using a multivariable Cox model with the age-, sex-, and calendar year–matched control group as the reference standard revealed similar results (eTable 10 in the Supplement).
Negative Control Exposure and Falsification End Points
Between 2001 and 2013, we identified 117 762 patients with hemangioma (Table 3). In analysis using propensity score–matched controls, the HRs associated with hemangioma were 1.09 (95% CI, 0.99-1.20) for DVT, 1.02 (95% CI, 0.81-1.28) for PE, and 0.82 (95% CI, 0.78-0.86) for PAD. We performed unadjusted- and multivariable-adjusted analyses using the age-, sex-, and calendar year–matched controls. The unadjusted Cox models revealed HRs of 1.50 (95% CI, 1.35-1.66) for DVT, 1.50 (95% CI, 1.17-1.93) for PE, and 1.00 (95% CI, 0.95-1.06) for PAD; however, the HR for DVT (1.25 [95% CI, 1.12-1.40]) and the HR for PE (1.14 [95% CI, 0.88-1.48]) decreased after multivariable adjustments.
Table 3. Hazard Ratios of the Incidence of the Study End Points Associated With Hemangioma (Negative Controls).
| Patients With Hemangioma | Control Group | Absolute Risk Difference/1000 Person-Years (95% CI)/ | Hazard Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patients | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)a | No. of Patients | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)a | |||
| DVT | ||||||||||
| Unadjusted Cox model | 117 762 | 886 | 877 224 | 1.01 (0.94 to 1.08) | 117 762 | 592 | 877 098 | 0.67 (0.62 to 0.73) | 0.34 (0.25 to 0.42) | 1.50 (1.35 to 1.66) |
| Multivariable-adjusted modelb | 117 762 | 886 | 877 224 | 1.01 (0.94 to 1.08) | 117 762 | 592 | 877 098 | 0.67 (0.62 to 0.73) | 0.34 (0.25 to 0.42) | 1.25 (1.12 to 1.40) |
| Cox model using propensity score–matched sample | 116 607 | 877 | 868 419 | 1.01 (0.94 to 1.08) | 116 607 | 795 | 856 236 | 0.93 (0.87 to 0.995) | 0.08 (−0.01 to 0.17) | 1.09 (0.99 to 1.20) |
| PE | ||||||||||
| Unadjusted Cox model | 117 762 | 155 | 881 040 | 0.18 (0.15 to 0.21) | 117 762 | 103 | 879 561 | 0.12 (0.10 to 0.14) | 0.06 (0.02 to 0.09) | 1.50 (1.17 to 1.93) |
| Multivariable-adjusted modelb | 117 762 | 155 | 881 040 | 0.18 (0.15 to 0.21) | 117 762 | 103 | 879 561 | 0.12 (0.10 to 0.14) | 0.06 (0.02 to 0.09) | 1.14 (0.88 to 1.48) |
| Cox model using propensity score–matched sample | 116 607 | 150 | 872 214 | 0.17 (0.15 to 0.20) | 116 607 | 145 | 859 510 | 0.17 (0.14 to 0.20) | 0.00 (−0.04 to 0.04) | 1.02 (0.81 to 1.28) |
| PAD | ||||||||||
| Unadjusted Cox model | 117 762 | 2877 | 867 230 | 3.32 (3.20 to 3.44) | 117 762 | 2864 | 865 277 | 3.31 (3.19 to 3.43) | 0.01 (−0.16 to 0.18) | 1.002 (0.95 to 1.06) |
| Multivariable-adjusted modelb | 117 762 | 2877 | 867 230 | 3.32 (3.20 to 3.44) | 117 762 | 2864 | 865 277 | 3.31 (3.19 to 3.43) | 0.01 (−0.16 to 0.18) | 0.82 (0.78 to 0.87) |
| Cox model using propensity score–matched sample | 116 607 | 2845 | 858 542 | 3.31 (3.19 to 3.44) | 116 607 | 3415 | 842 622 | 4.05 (3.92 to 4.19) | −0.74 (−0.92 to −0.56) | 0.82 (0.78 to 0.86) |
Abbreviations: DVT, deep venous thrombosis; PAD, peripheral artery disease; PE, pulmonary embolism.
Exact CIs for Poisson distribution.
Model was adjusted for age, sex, index year, hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, malignancy, heart failure, ischemic heart disease, stroke, chronic renal insufficiency, number of outpatient visits, and varicose veins.
Analyses of the falsification end points showed a weak association between varicose veins and increased risk of hyperlipidemia (HR in the propensity score–matched model, 1.16 [95% CI, 1.14-1.18]; HR in the multivariable-adjusted model, 1.20 [95% CI, 1.19-1.22]). However, a small yet statistically significant inverse association was observed for lung cancer (HR in propensity score–matched model, 0.80 [95% CI, 0.74-0.86]; HR in the multivariable-adjusted model, 0.81 [95% CI, 0.76-0.86]) (Table 4).
Table 4. Hazard Ratios of the Incidence of Lung Cancer and Hyperlipidemia (Falsification End Points) Associated With Varicose Veins.
| Patients With Varicose Veins | Control Group | Absolute Risk Difference/1000 Person-Years (95% CI) | Hazard Ratio (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Patientsa | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)b | No. of Patientsa | No. of Cases | No. of Person-Years | Incidence/1000 Person-Years (95% CI)b | |||
| Lung cancerc | ||||||||||
| Propensity score–matched controls | 169 198 | 1169 | 1 146 330 | 1.02 (0.96 to 1.08) | 163 069 | 1397 | 1 089 406 | 1.28 (1.22 to 1.35) | −0.26 (−0.35 to −0.17) | 0.80 (0.74 to 0.86) |
| Age-, sex-, and calendar year–matched controls | ||||||||||
| Unadjusted model | 169 198 | 1169 | 1 146 330 | 1.02 (0.96 to 1.08) | 645 190 | 4669 | 4 319 384 | 1.08 (1.05 to 1.11) | −0.06 (−0.13 to 0.01) | 0.94 (0.89 to 1.01) |
| Multivariable-adjusted modeld | 169 198 | 1169 | 1 146 330 | 1.02 (0.96 to 1.08) | 645 190 | 4669 | 4 319 384 | 1.08 (1.05 to 1.11) | −0.06 (−0.13 to 0.01) | 0.81 (0.76 to 0.86) |
| Hyperlipidemia | ||||||||||
| Propensity score–matched controls | 166 747 | 41 057 | 1 106 405 | 37.11 (36.75 to 37.47) | 159 807 | 34 301 | 1 080 204 | 31.75 (31.42 to 32.09) | 5.35 (4.86 to 5.85) | 1.16 (1.14 to 1.18) |
| Age-, sex-, and calendar year–matched controls | ||||||||||
| Unadjusted model | 166 747 | 41 057 | 1 106 405 | 37.11 (36.75 to 37.47) | 563 225 | 105 207 | 4 023 615 | 26.15 (25.99 to 26.31) | 10.96 (10.57 to 11.35) | 1.40 (1.38 to 1.42) |
| Multivariable-adjusted modeld | 166 747 | 41 057 | 1 106 405 | 37.11 (36.75 to 37.47) | 563 225 | 105 207 | 4 023 615 | 26.15 (25.99 to 26.31) | 10.96 (10.57 to 11.35) | 1.20 (1.19 to 1.22) |
The number of patients was not the same as that in the analysis of study end points because patients with cancer before index date were excluded from analysis of lung cancer and those with hyperlipidemia before index date were excluded from analysis of hyperlipidemia.
Exact CIs for Poisson distribution.
In the analyses, the recruitment of the patients with varicose veins ended on December 31, 2011, because lung cancer was defined using the cancer registry, which was available until the end of 2012.
Models were adjusted for sex, age, index year, number of outpatient visits in the year before the index date, and comorbidities including hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, malignancy, heart failure, ischemic heart disease, stroke, and chronic renal insufficiency.
Analysis of the Potential Effect of Unmeasured Confounders
Details of the results of external adjustments for smoking are provided in the Results section of the eAppendix in the Supplement. In all scenarios, the smoking-adjusted RRs for DVT were greater than 2.5. To achieve a substantial reduction in the observed associations for PE and PAD (RR, 1.06 for each), the RR for the associations between smoking and these end points would have to be 5, and the smoking prevalence in the varicose veins group would need to be 2.4-fold greater than that in the general population.
The RRs obtained using IPTW were 5.52 for DVT, 1.91 for PE, and 1.79 for PAD. In sensitivity analyses (eFigure in the Supplement), these RRs were corrected for hypothetical confounding functions c(0) and c(1), which shows that the RRs decrease as the values of the confounding functions increase. In absence of effect modifiers and unmeasured confounders, the value of c(1)=c(0) equals to 1. For example, for DVT, assuming c(0)=c(1), the unmeasured confounding-adjusted RR would be equal to 1 if the confounding function was at least 5.52 (ie, patients with varicose veins would have to be at least 5.52 times more likely to have DVT than the controls, if they had the same exposure status, to remove the entire observed association between varicose veins and DVT). eFigure (in the Supplement) shows all combinations c(0) of c(1) up to a value of 2.0, none of which would bring the corrected RR to 1 for DVT. However, for PE and PAD, some combinations in this range would bring the corrected RR to 1.
Discussion
In this large, population-based cohort study, varicose veins were associated with a significantly increased risk of DVT, PE, and PAD. Previous epidemiological studies have examined the association between varicose veins and the risk of DVT. A cross-sectional study in Germany reported a 7-fold higher prevalence of DVT in 2357 patients with varicose veins, based solely on ICD codes without additional verification of the diagnosis. A case-control study that enrolled 401 patients with DVT and 431 control patients aged 70 years or older reported a 1.6- to 10.5-fold increase in the odds of DVT associated with varicose veins, depending on clinical characteristics such as varicose veins, leg ulcers, and leg edema. The present study was a longitudinal study that revealed a strong association between varicose veins and DVT. The overall HR of DVT decreased to 3.98 when the analysis was restricted to a follow-up duration of 1 year or more, indicating that the HR was higher within the first year after the diagnosis of varicose veins.
A population-based study in Finland of 888 patients with varicose veins and 2006 control patients found statistically significant increased odds of developing arterial disease (PAD, angina pectoris, myocardial infarction, or cerebrovascular disease) in the patients with varicose veins (odds ratio = 2). Authors of this report suggested that varicose veins and arterial disease may have a common cause. In the present study, the association between varicose veins and PAD remained after controlling for hypertension, diabetes, chronic obstructive pulmonary disease, hyperlipidemia, and malignancy, suggesting that the increased risk of PAD could not be attributed solely to these confounders.
There are a several possible explanations for the findings in this study. Animal models have shown higher concentrations of macrophages, monocytes, neutrophils, lymphocytes, and matrix metalloproteinases in venous valves exposed to high pressure for prolonged periods. Patients with chronic venous diseases have increased leukocyte adhesion and more activated leukocytes, as well as higher levels of inflammatory and prothrombotic markers. Riva et al reported that inflammation may promote a prothrombotic state that may facilitate venous thromboembolism. Lee et al reported higher levels of hematological factors including fibrinogen, von Willebrand factor, tissue plasminogen activator (tPA), and fibrin D-dimer in patients with diabetes and PAD compared with those without PAD.
Patients with varicose veins have increased levels of inflammatory and prothrombotic markers. The inflammatory processes may cause the study outcomes (DVT, PAD, and PE), of which the pathophysiology may be due to the inflammatory process.
Limitations
There are several limitations to this study. First, claims data do not include information for patients who do not seek medical assistance. Varicose veins are a lesser-known disease in the older generation compared with other life‐threatening diseases such as stroke and diabetes in Taiwan. In addition, the unsightly appearance of varicose veins may embarrass the relatively conservative Taiwanese patients, thereby increasing reluctance to seek medical assistance, a phenomenon also reported among Asian patients in the United Kingdom. Therefore, results of the present study may only reflect the risk among patients with more severe varicose veins requiring medical treatment. This might also explain the higher HR for DVT during the first year after the diagnosis of varicose veins because patients with severe varicose veins may have been at greater risk of developing DVT. Second, causal inferences cannot be made as this was an observational study and thus prone to bias due to uncontrolled confounders. In addition, information on some potential confounders, such as smoking and obesity were not available in the claims data, and these factors may have confounded the observed association between varicose veins and DVT, PE, and PAD. The 2 falsification end points were statistically associated with varicose veins, and the negative controls exposure (hemangioma) also was associated with PAD. These observations suggested that unmeasured confounding was likely to have existed. The magnitude of the association between varicose veins and PE and PAD was small, and therefore, it may more likely be due to residual or unmeasured confounding. In the external adjustment for smoking, extreme scenarios were required to remove the associations between varicose veins and PE and PAD and were unlikely on the basis of data reported in previous studies. However, when considering the overall effect of unmeasured confounders, a confounding function of 2 to explain the observed associations between varicose veins and PAD and PE may be plausible. A recent study showed that the joint effect of multiple unmeasured confounders could account for an association at this strength.
Third, the control group may have included patients with varicose veins who did not seek medical care. This misclassification may have resulted in an underestimation of the association. Fourth, diagnostic evaluations for PAD are more likely to occur in patients with varicose veins because of their leg symptoms. This bias may partially explain the association between varicose veins and PAD. Fifth, misclassification of DVT, PE, and PAD may have occurred because the accuracy of these diagnosis codes in the NHI claims have not been validated. Validation studies are needed to assess the effect of misclassification of these end points on the magnitude of the observed associations. Sixth, the study could not examine whether greater severity of varicose veins was associated with higher risk because varicose vein severity was not available.
Conclusions
Among adults diagnosed with varicose veins, there was a significantly increased risk of incident DVT; the findings for PE and PAD are less clear due to the potential for confounding. Whether the association between varicose veins and DVT is causal or represents a common set of risk factors requires further research.
eAppendix. Methods, Results, References
eTable 1. Diagnosis codes
eTable 2. Associations between varicose veins and subsequent risk of deep vein thrombosis, pulmonary embolism and peripheral artery disease by duration of follow-up
eTable 3. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 4. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins in analysis restricted to a length of follow-up of at least one year
eTable 5. Incidence rates and hazard ratios for pulmonary embolism in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 6. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 7. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins in analysis restricted to a length of follow-up of at least one year
eTable 8. Incidence rates and hazard ratios for pulmonary embolism in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 9. Incidence rates and hazard ratios for peripheral artery disease in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 10. Incidence rates and hazard ratios for peripheral artery disease in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eFigure. Sensitivity analysis for the relative risk (RR) of DVT, PE and PAD calculated using inverse probability of treatment weighting (IPTW).
Abbreviations List
- DVT
deep venous thrombosis
- PAD
peripheral artery disease
- PE
pulmonary embolism
References
- 1.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175-184. [DOI] [PubMed] [Google Scholar]
- 2.Maurins U, Hoffmann BH, Lösch C, Jöckel KH, Rabe E, Pannier F. Distribution and prevalence of reflux in the superficial and deep venous system in the general population—results from the Bonn Vein Study, Germany. J Vasc Surg. 2008;48(3):680-687. [DOI] [PubMed] [Google Scholar]
- 3.Hamdan A. Management of varicose veins and venous insufficiency. JAMA. 2012;308(24):2612-2621. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37(5):1047-1053. [DOI] [PubMed] [Google Scholar]
- 5.Piazza G. Varicose veins. Circulation. 2014;130(7):582-587. [DOI] [PubMed] [Google Scholar]
- 6.Poredos P, Spirkoska A, Rucigaj T, Fareed J, Jezovnik MK. Do blood constituents in varicose veins differ from the systemic blood constituents? Eur J Vasc Endovasc Surg. 2015;50(2):250-256. [DOI] [PubMed] [Google Scholar]
- 7.Riva N, Donadini MP, Ageno W. Epidemiology and pathophysiology of venous thromboembolism: similarities with atherothrombosis and the role of inflammation. Thromb Haemost. 2015;113(6):1176-1183. [DOI] [PubMed] [Google Scholar]
- 8.Lee AJ, MacGregor AS, Hau CM, et al. . The role of haematological factors in diabetic peripheral arterial disease: the Edinburgh Artery Study. Br J Haematol. 1999;105(3):648-654. [DOI] [PubMed] [Google Scholar]
- 9.Engbers MJ, Karasu A, Blom JW, Cushman M, Rosendaal FR, van Hylckama Vlieg A. Clinical features of venous insufficiency and the risk of venous thrombosis in older people. Br J Haematol. 2015;171(3):417-423. [DOI] [PubMed] [Google Scholar]
- 10.Müller-Bühl U, Leutgeb R, Engeser P, Achankeng EN, Szecsenyi J, Laux G. Varicose veins are a risk factor for deep venous thrombosis in general practice patients. Vasa. 2012;41(5):360-365. [DOI] [PubMed] [Google Scholar]
- 11.National Health Insurance Administration, Ministry of Health and Welfare, Taiwan National Health Insurance in Taiwan 2015-2016 Annual Report. https://www.nhi.gov.tw/english/Content_List.aspx?n=8FC0974BBFEFA56D&topn=ED4A30E51A609E49. Accessed November 18, 2017.
- 12.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. A comparison of 12 algorithms for matching on the propensity score. Stat Med. 2014;33(6):1057-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21(3):383-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA. 2013;309(3):241-242. [DOI] [PubMed] [Google Scholar]
- 16.Mamdani M, Sykora K, Li P, et al. . Reader’s guide to critical appraisal of cohort studies: 2. BMJ. 2005;330(7497):960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med. 2013;32(16):2837-2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenland S, Lash TL. Analysis of unmeasured confounders—external adjustment In: Rothman KJ, Greenland S, Lash TL, eds. Modern Epidemiology. 3rd ed Philadelphia, PA: Lippincott Williams & Wilkins; 2008:348-351. [Google Scholar]
- 19.Lu L, Mackay DF, Pell JP. Meta-analysis of the association between cigarette smoking and peripheral arterial disease. Heart. 2014;100(5):414-423. [DOI] [PubMed] [Google Scholar]
- 20.Cheng YJ, Liu ZH, Yao FJ, et al. . Current and former smoking and risk for venous thromboembolism: a systematic review and meta-analysis. PLoS Med. 2013;10(9):e1001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chang FC, Hu TW, Lo SY, Yu PT, Chao KY, Hsiao ML. Quit smoking advice from health professionals in Taiwan: the role of funding policy and smoker socioeconomic status. Tob Control. 2010;19(1):44-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasza J, Wolfe R, Schuster T. Assessing the impact of unmeasured confounding for binary outcomes using confounding functions. Int J Epidemiol. 2017;46(4):1303-1311. [DOI] [PubMed] [Google Scholar]
- 23.Cornfield J, Haenszel W, Hammond EC, Lilienfeld AM, Shimkin MB, Wynder EL. Smoking and lung cancer: recent evidence and a discussion of some questions. J Natl Cancer Inst. 1959;22(1):173-203. [PubMed] [Google Scholar]
- 24.Mäkivaara LA, Ahti TM, Luukkaala T, Hakama M, Laurikka JO. Persons with varicose veins have a high subsequent incidence of arterial disease: a population-based study in Tampere, Finland. Angiology. 2007;58(6):704-709. [DOI] [PubMed] [Google Scholar]
- 25.Bergan JJ, Schmid-Schönbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488-498. [DOI] [PubMed] [Google Scholar]
- 26.Sam RC, Hobbs SD, Darvall KA, et al. . Chronic venous disease in a cohort of healthy UK Asian men. Eur J Vasc Endovasc Surg. 2007;34(1):92-96. [DOI] [PubMed] [Google Scholar]
- 27.Groenwold RH, Sterne JA, Lawlor DA, Moons KG, Hoes AW, Tilling K. Sensitivity analysis for the effects of multiple unmeasured confounders. Ann Epidemiol. 2016;26(9):605-611. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Methods, Results, References
eTable 1. Diagnosis codes
eTable 2. Associations between varicose veins and subsequent risk of deep vein thrombosis, pulmonary embolism and peripheral artery disease by duration of follow-up
eTable 3. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 4. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins in analysis restricted to a length of follow-up of at least one year
eTable 5. Incidence rates and hazard ratios for pulmonary embolism in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 6. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 7. Incidence rates and hazard ratios for deep vein thrombosis in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins in analysis restricted to a length of follow-up of at least one year
eTable 8. Incidence rates and hazard ratios for pulmonary embolism in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 9. Incidence rates and hazard ratios for peripheral artery disease in patients with varicose veins vs. propensity score-matched controls presented overall and by sex and age at diagnosis of varicose veins
eTable 10. Incidence rates and hazard ratios for peripheral artery disease in patients with varicose veins vs. age-, sex-, calendar year-matched controls presented overall and by sex and age at diagnosis of varicose veins
eFigure. Sensitivity analysis for the relative risk (RR) of DVT, PE and PAD calculated using inverse probability of treatment weighting (IPTW).